Microbial Secondary Metabolites and Their Use in Achieving Sustainable Agriculture: Present Achievements and Future Challenges
Abstract
1. Introduction
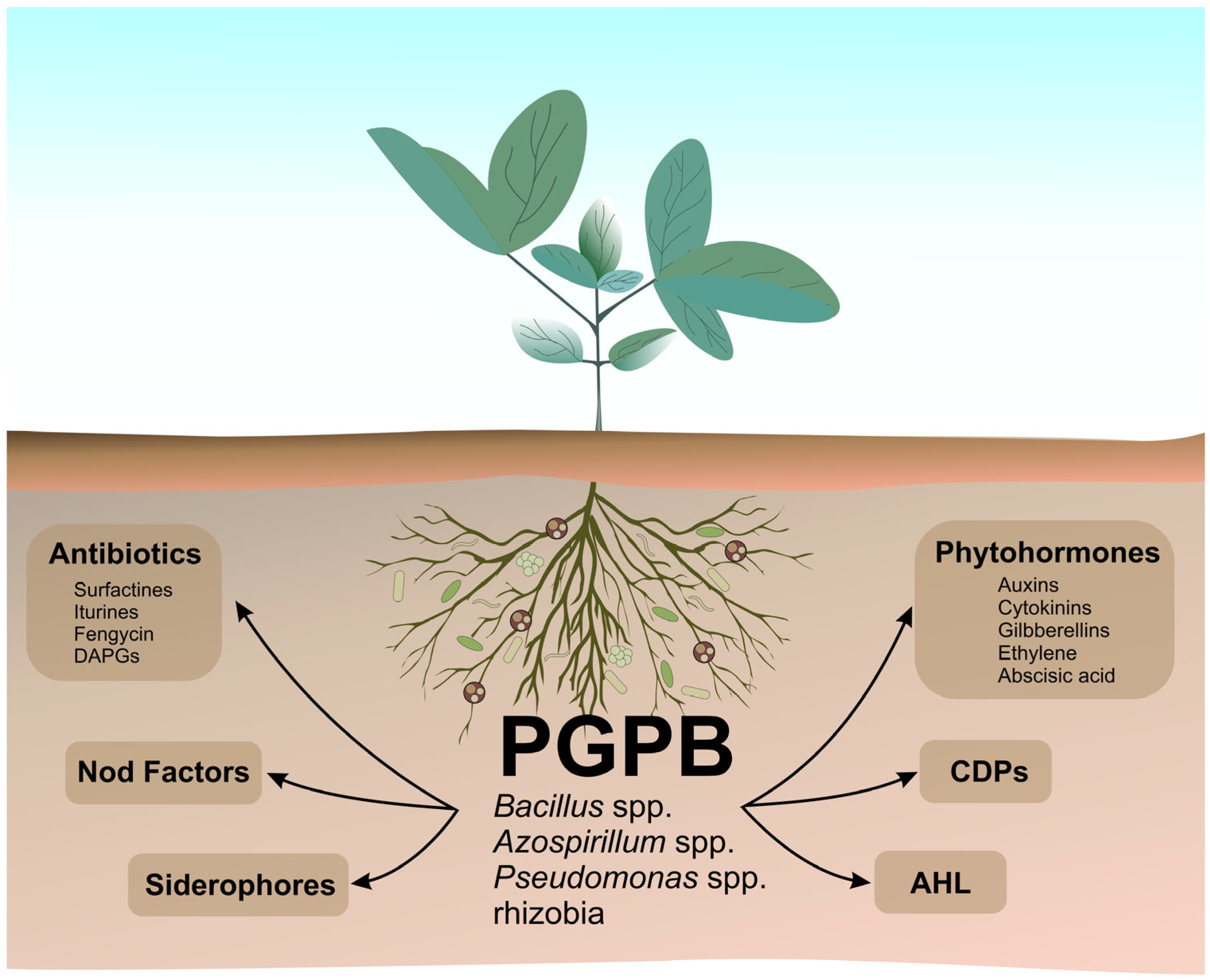
2. Microbial Secondary Metabolites (MSMs)
- Promoting defense against competitors or pathogens;
- Increasing adaptability to adverse environmental conditions;
- Modulating the interaction and symbiosis with other organisms;
- Inducing signaling and communication among other participants in the ecosystem where the microorganism is inserted.
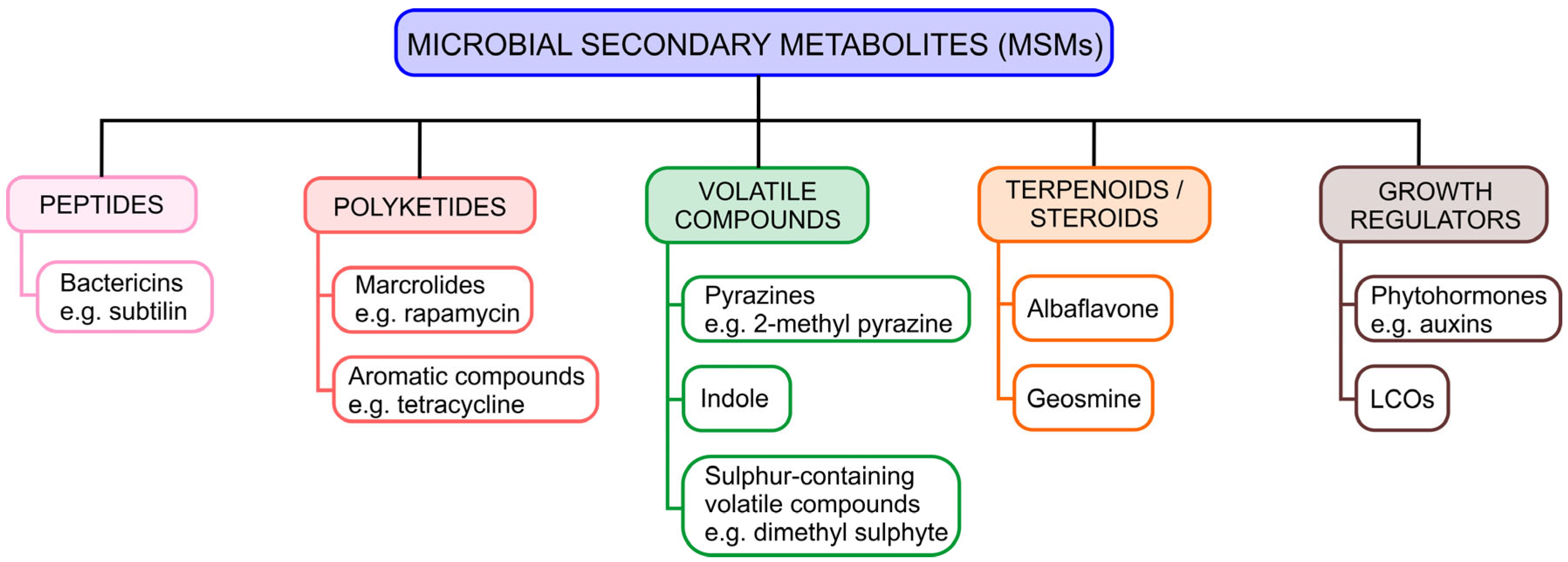
2.1. Classification of MSMs
2.1.1. Peptides

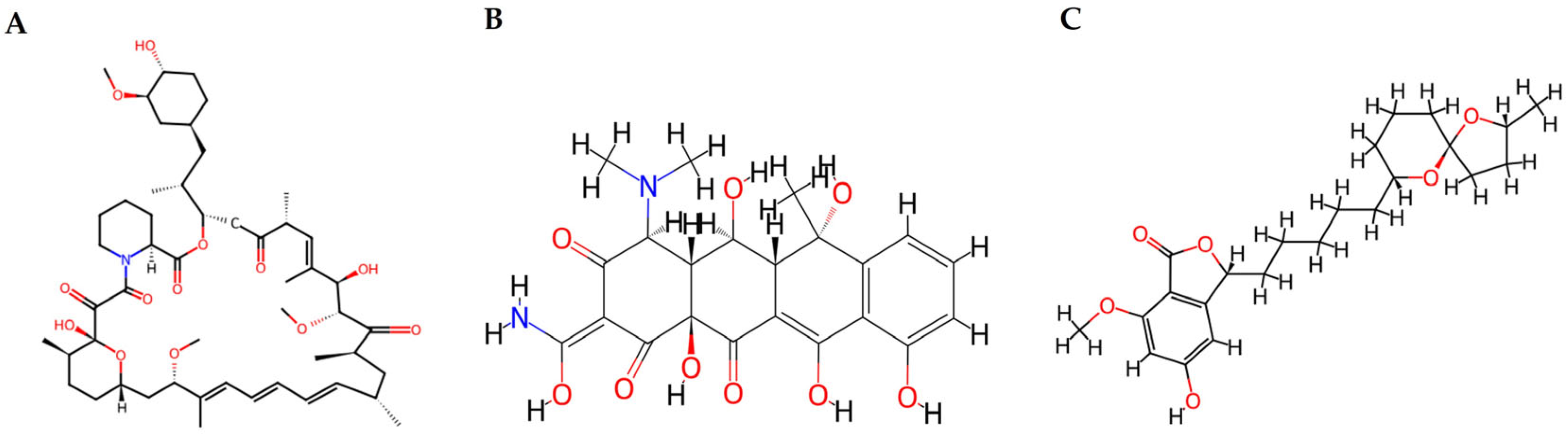
2.1.2. Polyketides
2.1.3. Volatile Compounds
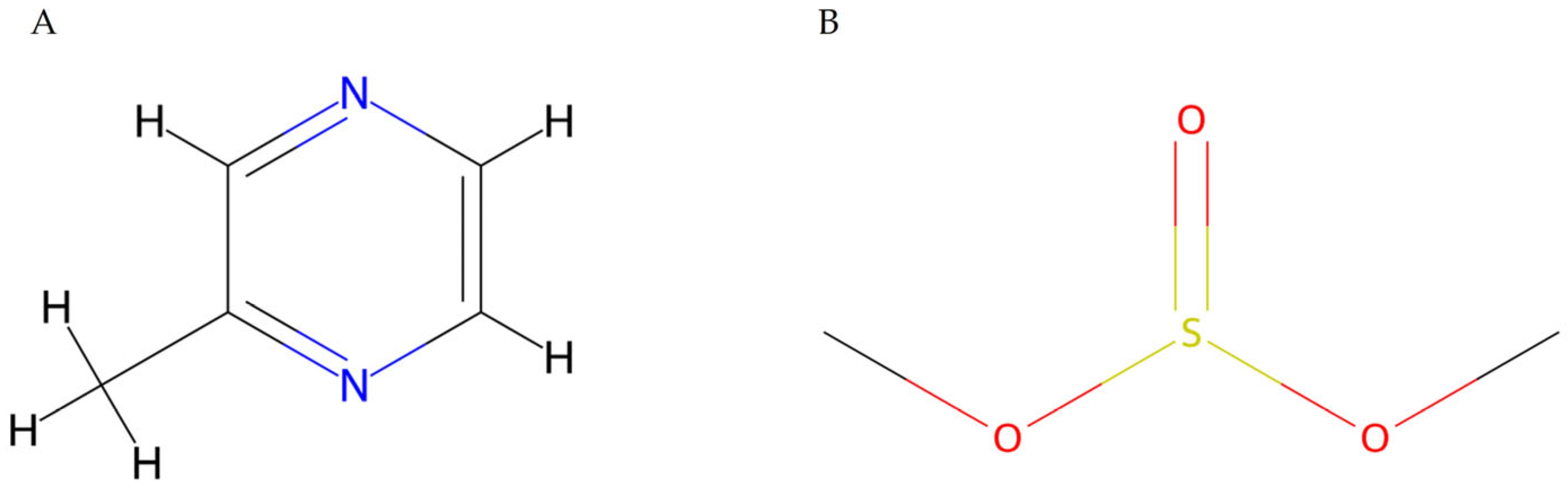
- Pyrazines: Well-studied secondary metabolites known for their antimicrobial properties. Typically produced by Bacillus spp. and Pseudomonas spp. [45], such as 2-methylpyrazine (Figure 5A), produced by Pseudomonas putida, a molecule with antifungal action against Magnaporthe oryzae, a pathogen of rice (Oriza sativa) [46].
- Sulfur-containing volatile compounds: Dimethyl sulfite (Figure 5B), dimethyl bisulfite, and dimethyl trisulfide metabolites that contain sulfur in their composition. They are known for their action as fungal growth inhibitors and as key components in microorganism–plant interactions [49]. Furthermore, dimethyl sulfite produced by Serratia odorifera was described to cause negative effects on the growth of Arabidopsis thaliana [50].
2.1.4. Terpenoids/Steroids
2.1.5. Growth Regulators
- LCOs (lipochitooligosaccharides)
2.2. Extraction, Purification, Quantification, and Characterization of MSMs
2.2.1. Extraction
2.2.2. Thin-Layer Chromatography
2.2.3. High-Performance Liquid Chromatography (HPLC)
2.2.4. Gas Chromatography–Mass Spectrometry (GC-MS)
2.2.5. Other Techniques for the Identification and Characterization of MSMs
2.2.6. Metabolomics
3. Actual and Potential Applications of MSMs in Agriculture
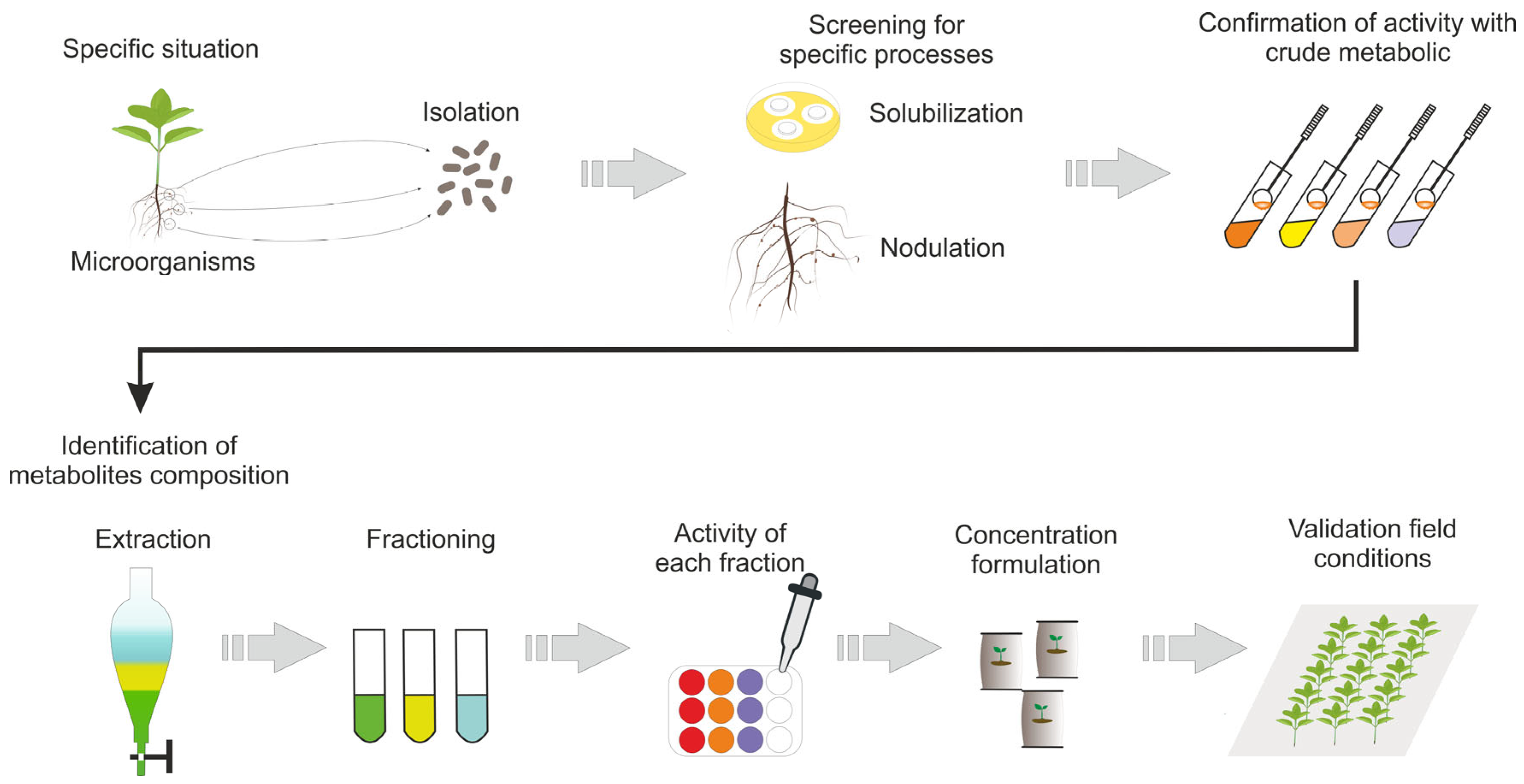
Field Applications of MSMs
4. Industrial Production of MSMs for Agriculture Use
4.1. First Steps
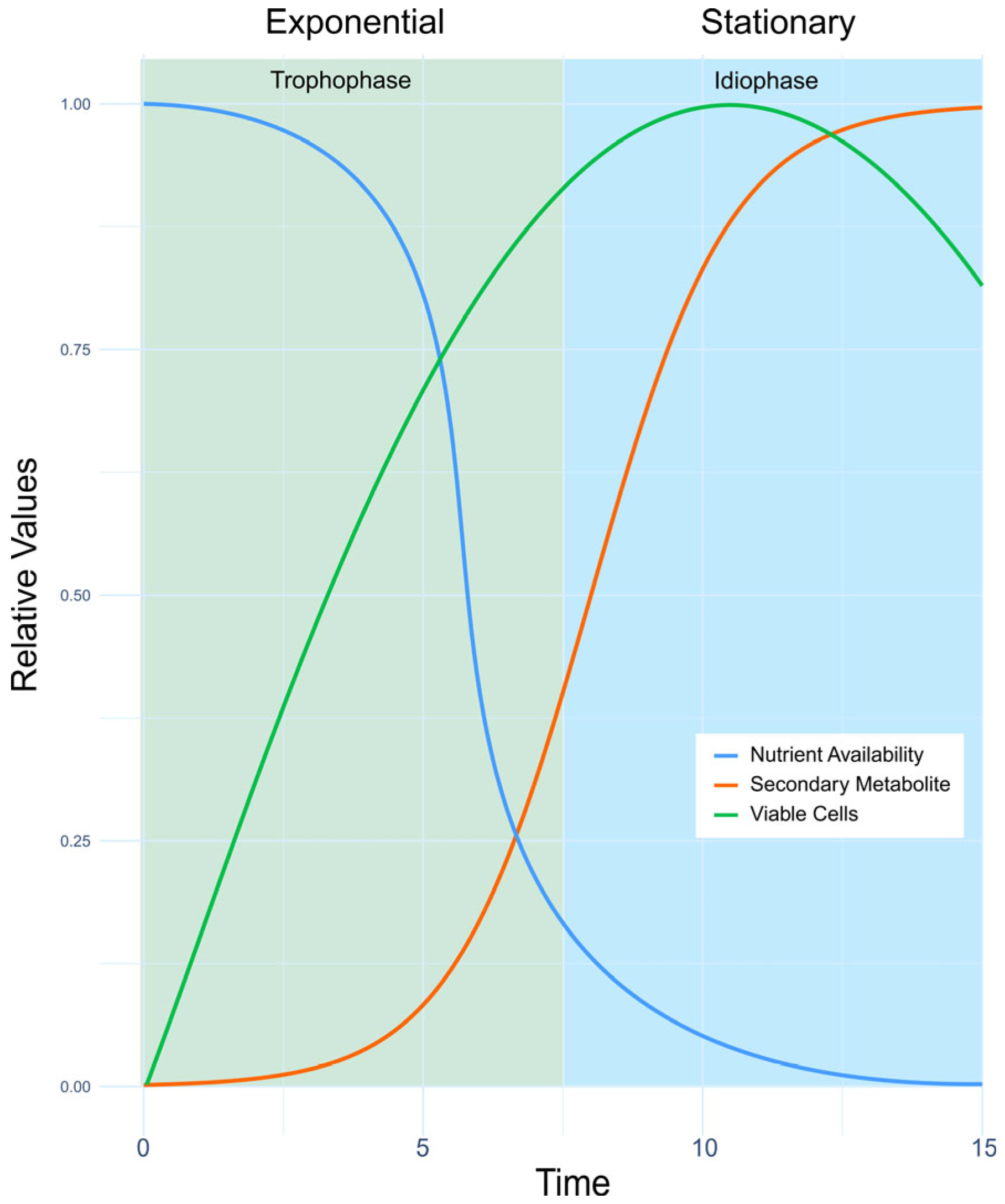
4.2. Liquid (Submerged) Fermentation
4.3. Solid-State Fermentation (SSF)
4.4. Challenges in the Industrial Production of MSMs
4.4.1. Upstream Processes
- Scalability
- 2.
- Production Levels
4.4.2. Downstream Processes
- Extraction and isolation
- 2.
- Residue generation
4.5. Solutions and Alternatives
4.5.1. Basic Research
4.5.2. Optimization of Parameters
4.5.3. Strain Improvement and the Use of Biofactories
4.5.4. Design of Efficient Bioreactors for Solid-State Fermentation
4.6. Circular Economy Applied to Industrial MSM Production
4.7. Industrial Production of Agricultural Interest MSMs: Challenges and Future Perspectives
5. Concluding Remarks About the Greatest Challenge in Agriculture in the Next Decade
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziska, L.; Crimmins, A.; Auclair, A.; Degrasse, S.; Garofalo, J.F.; Khan, A.S.; Loladze, I.; Pérez De León, A.A.; Showler, A.; Thurston, J.; et al. Food safety, nutrition, and distribution. In The Impacts of Climate Change on Human Health in the United States: A Scientific Assessment; U.S. Global Change Research Program: Washington, DC, USA, 2016; pp. 189–216. [Google Scholar]
- Reeves, T.G.; Thomas, G.; Ramsay, G. Save and Grow in Practice: Maize, Rice, Wheat. A Guide to Sustainable Cereal Production; FAO: Rome, Italy, 2016. [Google Scholar]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World. Repurposing Food and Agricultural Policies to Make Healthy Diets More Affordable; FAO: Rome, Italy, 2022. [Google Scholar]
- Arora, N.K. Impact of climate change on agriculture production and its sustainable solutions. Environ. Sustain. 2019, 2, 95–96. [Google Scholar] [CrossRef]
- Barros, M.V.; Salvador, R.; De Francisco, A.C.; Piekarski, C.M. Mapping of research lines on circular economy practices in agriculture: From waste to energy. Renewab. Sustain. Energy Rev. 2020, 131, 109958. [Google Scholar] [CrossRef]
- Mahmud, A.A.; Upadhyay, S.K.; Srivastava, A.K.; Bhojiya, A.A. Biofertilizers: A nexus between soil fertility and crop productivity under abiotic stress. Curr. Res. Environ. Sustain. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Rajput, V.D.; Kumari, A.; Espinosa-Saiz, D.; Menendez, E.; Minkina, T.; Dwivedi, P.; Mandzhieva, S. Plant growth-promoting rhizobacteria: A potential bio-asset for restoration of degraded soil and crop productivity with sustainable emerging techniques. Environ. Geochem. Health 2022, 45, 9321–9344. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Wright, G. An ecological perspective of microbial secondary metabolism. Curr. Opin. Biotechnol. 2011, 22, 552–558. [Google Scholar] [CrossRef]
- Demain, A.L. Microbial secondary metabolism: A new theoretical frontier for academia, a new opportunity for industry. Ciba Found. Symp. 1992, 171, 3–16. [Google Scholar]
- Awan, A.R.; Shaw, W.M.; Ellis, T. Biosynthesis of therapeutic natural products using synthetic biology. Adv. Drug Deliv. Rev. 2016, 105, 96–106. [Google Scholar] [CrossRef]
- Li, G.-H.; Zhang, K.-Q. Nematode-toxic fungi and their nematicidal metabolites. In Nematode-Trapping Fungi; Fungal Diversity Research Series; Zhang, K.-Q., Hyde, K., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 23. [Google Scholar]
- Lioussanne, L.; Jolicoeur, M.; St-Arnaud, M. Role of the modification in root exudation induced by arbuscular mycorrhizal colonization on the intraradical growth of Phytophthora nicotianae in tomato. Mycorrhiza 2009, 19, 443–448. [Google Scholar] [CrossRef]
- Malatesti, N.; Munitic, I.; Jurak, I. Porphyrin-based cationic amphiphilic photosensitisers as potential anticancer, antimicrobial, and immunosuppressive agents. Biophys. Rev. 2017, 9, 149–168. [Google Scholar] [CrossRef]
- Fukami, J.; Ollero, F.J.; Megías, M.; Hungria, M. Phytohormones and induction of plant-stress tolerance and defense genes by seed and foliar inoculation with Azospirillum brasilense cells and metabolites promote maize growth. AMB Express 2017, 7, 153. [Google Scholar] [CrossRef]
- Bentley, R. Microbial secondary metabolites play important roles in medicine, prospects for discovery of new drugs. Perspect. Biol. Med. 1997, 40, 364–394. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Osborne, J.W. Biodegradation and biosorption of Reactive Red 120 dye by immobilized Pseudomonas guariconensis: Kinetic and toxicity study. Water Environ. Res. 2020, 92, 1230–1241. [Google Scholar] [CrossRef]
- Radzki, W.; Mañero, F.J.G.; Algar, E.; García, J.A.L.; García-Villaraco, A.; Solano, B.R. Bacterial siderophores efficiently provide iron to iron-starved tomato plants in hydroponics culture. Antonie Van Leeuwenhoek 2013, 104, 321–330. [Google Scholar] [CrossRef]
- Jiang, J.; Zu, Y.; Li, X.; Meng, Q.; Long, X. Recent progress towards industrial rhamnolipids fermentation: Process optimization and foam control. Bioresour. Technol. 2020, 298, 122394. [Google Scholar] [CrossRef]
- Thirumurugan, D.; Cholarajan, A.; Raja, S.S.S.; Vijayakumar, R. An introductory chapter: Secondary metabolites. In Secondary Metabolites—Sources and Applications; Vijayakumar, R., Raja, S.S.S., Eds.; InTech: Houston, TX, USA, 2018. [Google Scholar]
- Reddy, S.; Sinha, A.; Osborne, J.W. Chapter 1—Microbial secondary metabolites: Recent developments and technological challenges. In Volatiles and Metabolites of Microbes; Kumar, A., Singh, J., Samuel, J., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 1–22. [Google Scholar]
- Verpoorte, R.; Van Der Heijden, R.; Memelink, J. Engineering the plant cell factory for secondary metabolite production. Transgenic Res. 2000, 9, 323–343. [Google Scholar] [CrossRef]
- Yeoman, M.M.; Yeoman, C.L. Manipulating secondary metabolism in cultured plant cells. New Phytol. 1996, 134, 553–569. [Google Scholar] [CrossRef]
- Lopes, F.C. Produção e Análise de Metabólitos Secundários de Fungos Filamentosos. Master’s Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brasil, 2011. [Google Scholar]
- Collin, H.A. Secondary product formation in plant tissue cultures. Plant Growth Regul. 2001, 34, 119–134. [Google Scholar] [CrossRef]
- Vining, L.C. Roles of secondary metabolites from microbes. In Secondary Metabolites: Their Function and Evolution; CIBA Foundation Symposium; Chadwick, D.J., Whelan, J., Eds.; Wiley: New York, NY, USA, 2007; Volume 171. [Google Scholar]
- Kumar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent developments on solid-state fermentation for production of microbial secondary metabolites, challenges and solutions. Bioresour. Technol. 2021, 323, 124566. [Google Scholar] [CrossRef]
- Singh, B.P.; Rateb, M.E.; Rodriguez-Couto, S.; Polizeli, M.L.T.M.; Li, W.-J. Microbial secondary metabolites: Recent developments and technological challenges. Front. Microbiol. 2019, 10, 914. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Janjua, H.T. Microbial secondary metabolites and defense of plant stress. In Microbial Services in Restoration Ecology; Singh, J.S., Vimal, S.R., Eds.; Elsevier: New York, NY, USA, 2020; pp. 37–46. [Google Scholar]
- Bhadra, S.; Chettri, D.; Kumar, V. Biosurfactants: Secondary metabolites involved in the process of bioremediation and biofilm removal. Appl. Biochem. Biotechnol. 2023, 195, 5541–5567. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Ahlawat, Y.K.; Sharma, A.J.; Chamoli, N.; Thakur, M.; Sharma, A.; Mehmood, S.; Malik, A.; Ahmed, M.; Punia, H.; et al. A comprehensive review on microbial production and significant applications of multifunctional biomolecules: Biosurfactants. Biodegradation 2025, 36, 26. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-X.; Zhang, C.-X.; Hu, Y.; Zhang, M.-H.; Wang, Y.-N.; Qian, Y.-X.; Yang, J.; Yang, W.-Z.; Jiang, M.-M.; Guo, D.-A. Application of multiple chemical and biological approaches for quality assessment of Carthamus tinctorius L. (safflower) by determining both the primary and secondary metabolites. Phytomed 2019, 58, 152826. [Google Scholar]
- Sanchez, S.; Demain, A.L. Bioactive products from fungi. In Food Bioactives; Puri, M., Ed.; Springer: Cham, Switzerland, 2017; pp. 59–87. [Google Scholar]
- Abriouel, H.; Franz, C.M.A.P.; Omar, N.B.; Gálvez, A. Diversity and applications of Bacillus bacteriocions. FEMS Microb. Rev. 2010, 35, 201–232. [Google Scholar] [CrossRef]
- Stone, M.J.; Williams, D.H. On the evolution of functional secondary metabolites (natural products). Mol. Microbiol. 1992, 6, 29–34. [Google Scholar] [CrossRef]
- ChemSpider. Available online: https://www.chemspider.com/Chemical-Structure.17286529.html (accessed on 20 May 2025).
- Risdian, C.; Mozef, T.; Wink, J. Byosynthesis of polyketides in Streptomyces. Microorganisms 2019, 7, 124. [Google Scholar] [CrossRef]
- Pickens, L.B.; Tang, Y. Oxytetracycline biosynthesis. J. Biolog. Chem. 2010, 285, 27509–27515. [Google Scholar] [CrossRef]
- Navarro-Muñoz, J.C.; Collemare, J. Evolutionary histories of Type III polyketide synthases in Fungi. Front. Microbiol. 2020, 10, 318. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Zhang, S.; Yu, D.; Qin, Y.; Huang, H.; Wang, W.; Zhan, J. Identification of a type III polyketide synthase involved in the biosynthesis of spirolaxine. Appl. Microbiol. Biotechnol. 2016, 100, 7103–7113. [Google Scholar] [CrossRef]
- ChemSpider. Available online: https://www.chemspider.com/Chemical-Structure.10482078.html (accessed on 20 May 2025).
- ChemSpider. Available online: https://www.chemspider.com/Chemical-Structure.4444458.html (accessed on 20 May 2025).
- ChemSpider. Available online: https://www.chemspider.com/Chemical-Structure.9363979.html (accessed on 20 May 2025).
- Piechulla, B.; Lemfack, M.C.; Kai, M. Effects of discrete bioactive microbial volatiles on plants and fungi. Plant Cell Environ. 2017, 40, 2042–2067. [Google Scholar] [CrossRef]
- Rajini, K.S.; Aparna, P.; Sasikala, C.; Ramana, C.V. Microbial metabolism of pyrazines. Crit. Rev. Microbiol. 2011, 37, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Kumar, A.; Sheoran, N.; Kumar, M.; Sahu, K.P.; Ganeshan, P.; Ashajyothi, M.; Gopalakrishnan, S.; Gogoi, R. Antifungal and defense elicitor activities of pyrazines identified in endophytic Pseudomonas putida BP25 against fungal blast incited by Magnaporthe oryzae in rice. J. Plant Dis. Prot. 2020, 128, 261–272. [Google Scholar] [CrossRef]
- Vimal, S.; Patel, V.K.; Singh, J.S. Plant growth-promoting Curtobacterium albidum strain SRV4: An agriculturally important microbe to alleviate salinity stress in paddy plants. Ecol. Indic. 2019, 105, 553–562. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Garladinne, M.; Lee, Y.H. Volatile indole produced by rhizobacterium Proteus vulgaris JBLS202 stimulates growth of Arabidopsis thaliana through auxin, cytokinin, and brassinosteroid pathways. J. Plant Growth Regul. 2015, 34, 158–168. [Google Scholar] [CrossRef]
- Garbeva, P.; Hordijk, C.; Gerards, S.; De Boer, W. Volatiles produced by the mycophagous soil bacterium Collimonas. FEMS Microbiol. Ecol. 2014, 87, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Crespo, E.; Cristescu, S.M.; Harren, F.J.M.; Francke, W.; Piechulla, B. Serratia odorifera: Analysis of volatile emission and biological impact of volatile compounds on Arabidopsis thaliana. Appl. Microbiol. Biotechnol. 2010, 88, 965–976. [Google Scholar] [CrossRef]
- ChemSpider. Available online: https://www.chemspider.com/Chemical-Structure.7688.html (accessed on 20 May 2025).
- ChemSpider. Available online: https://www.chemspider.com/Chemical-Structure.62436.html (accessed on 20 May 2025).
- Lemfack, M.C.; Gohlke, B.O.; Toguem, S.M.T.; Preissner, S.; Piechulla, B.; Preissner, R. mVOC 2.0: A database of microbial volatiles. Nucleic Acids Res. 2018, 46, D1261–D1265. [Google Scholar] [CrossRef] [PubMed]
- Misztal, P.K.; Lymperopoulou, D.S.; Adams, R.I.; Scott, R.A.; Lindow, S.E.; Bruns, T.; Taylor, J.W.; Uehling, J.; Bonito, G.; Vilgalys, R.; et al. Emission factors of microbial volatile organic compounds from environmental bacteria and fungi. Environ. Sci. Technol. 2018, 52, 8272–8282. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, E.V.; Luchnikiva, N.A.; Grishko, V.V.; Ivshina, I.B. Actinomycetes as producers of biologically active terpenoids: Current trend and patents. Pharmac 2023, 16, 872. [Google Scholar] [CrossRef]
- Moody, S.C.; Zhao, B.; Lei, L.; Nelson, D.R.; Mullins, J.G.; Waterman, M.R.; Kelly, S.L.; Lamb, D.C. Investigating conservation of the albaflavenone biosynthetic pathway and CYP170 bifunctionality in Streptomycetes. FEBS J. 2012, 279, 1640–1649. [Google Scholar] [CrossRef]
- Song, C.; Schmidt, R.; De Jager, V.; Krzyzanowska, D.; Jongedijk, E.; Cankar, K.; Beekwilder, J.; Van Veen, A.; De Boer, W.; Van Veen, J.A.; et al. Exploring the genomic traits of fungus-feeding bacterial genus Collimonas. BMC Genom. 2015, 16, 1103. [Google Scholar] [CrossRef]
- ChemSpider. Available online: https://www.chemspider.com/Chemical-Structure.24846735.html (accessed on 20 May 2025).
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Inoculation of Brachiaria spp. with the plant growth-promoting bacterium Azospirillum brasilense: An environment-friendly component in the reclamation of degraded pastures in the tropics. Agric. Ecosyst. Environ. 2016, 221, 125–131. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.; Martinoia, E.; Geisler, M. Plant hormone transporters: What we know and what we would like to know. BMC Biol. 2017, 15, 93. [Google Scholar] [CrossRef]
- Vimal, S.R.; Singh, J.S. Salt tolerant PGPR and FYM application in saline soil paddy agriculture sustainability. Clim. Change Environ. Sustain. 2019, 7, 61–71. [Google Scholar] [CrossRef]
- Piotrowska, A.; Czerpak, R. Cellular response of light/dark-grown green alga Chlorella vulgaris Beijerinck (Chlorophyceae) to exogenous adenine-and phenylurea-type cytokinins. Acta Physiol. Plant. 2009, 31, 573–585. [Google Scholar] [CrossRef]
- Vimal, S.R.; Singh, J.S.; Arora, N.K.; Singh, S. Soil-plant-microbe interactions in stressed agriculture management: A review. Pedosphere 2017, 27, 177–192. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.-M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Helene, L.C.F.; Klepa, M.S.; Hungria, M. New insights into the taxonomy of bacteria in the genomic era and a case study with rhizobia. Int. J. Microbiol. 2022, 2022, 4623713. [Google Scholar]
- Prithiviraj, B.; Zhou, X.; Souleimanov, A.; Khan, W.M.; Smith, D.L. A host-specific bacteria-to-plant signal molecule (Nod factor) enhances germination and early growth of diverse crop plants. Planta 2003, 216, 437–445. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A. Nitrogen fixation. In Marschner’s Mineral Nutrition of Plants, 4th ed.; Rengel, Z., Cakmak, I., White, P.J., Eds.; Elsevier: London, UK, 2023; pp. 615–650. [Google Scholar]
- Hungria, M.; Stacey, G. Molecular signals exchanged between host plants and rhizobia: Basic aspects and potential application in agriculture. Soil Biol. Biochem. 1997, 29, 819–830. [Google Scholar] [CrossRef]
- D’Haeze, W.; Holsters, M. Nod factor structures, responses, and perception during initiation of nodule development. Glycobiology 2002, 12, 79R–105R. [Google Scholar] [CrossRef] [PubMed]
- del Cerro, P.; Rolla-Santos, A.A.P.; Gomes, D.F.; Marks, B.B.; del Rosario Espuny, M.; Rodríguez-Carvajal, M.Á.; Soria-Díaz, M.E.; Nakatani, A.S.; Hungria, M.; Hungria, F.J.; et al. Opening the “black box” of nodD3, nodD4 and nodD5 genes of Rhizobium tropici strain CIAT899. BMC Genom. 2015, 16, 2033. [Google Scholar] [CrossRef]
- del Cerro, P.; Rolla-Santos, A.A.P.; Gomes, D.F.; Marks, B.B.; del Rosario Espuny, M.; Rodríguez-Carvajal, M.Á.; Soria-Díaz, M.E.; Nakatani, A.S.; Hungria, M.; Hungria, F.J.; et al. Regulatory nodD1 and nodD2 genes of Rhizobium tropici strain CIAT899 and their roles in the early stages of molecular signaling and host-legume nodulation. BMC Genom. 2015, 16, 1458. [Google Scholar] [CrossRef] [PubMed]
- Perret, X.; Staehelin, C.H.; Broughton, W.J. Molecular basis of symbiotic promiscuity. Microbiol. Mol. Biol. Rev. 2000, 64, 180–201. [Google Scholar] [CrossRef]
- Lhuissier, F.G.P.; De Ruijter, N.C.A.; Sieberer, B.J.; Esseling, J.J.; Emons, A.M.C. Time course of cell biological events evoked in legume root hairs by Rhizobium Nod factors: State of the art. Ann. Bot. 2001, 87, 289–302. [Google Scholar] [CrossRef]
- Horvath, B.; Heidstra, R.; Lados, M.; Moerman, M.; Spaink, H.P.; Promé, J.C.; van Kammen, A.; Bisseling, T. Lipo-chitooligosaccharide of Rhizobium induces infection-related early nodulin gene expression in pea root hairs. Plant J. 1993, 4, 727–733. [Google Scholar] [CrossRef]
- Schlaman, H.R.; Gisel, A.A.; Quaedvlieg, N.E.; Bloemberg, G.V.; Lugtenberg, B.J.; Kijne, J.W.; Potrykus, I.; Spaink, H.P.; Sautter, C. Chitin oligosaccharides can induce cortical cell division in roots of Vicia sativa when delivered by ballistic microtargeting. Development 1997, 124, 4887–4895. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Abarca, F.; Herrera-Cervera, J.A.; Bueno, P.; Sanjuan, J.; Bisseling, T.; Olivares, J. Involvement of salicylic acid in the establishment of the Rhizobium meliloti–alfalfa symbiosis. Mol. Plant-Microbe Interact. 1998, 11, 153–155. [Google Scholar] [CrossRef]
- Dénarié, J.; Cullimore, J. Lipo-oligosaccharide nodulation factors: A new class of signalling molecules mediating recognition and morphogenesis. Cell 1993, 74, 951–954. [Google Scholar] [CrossRef]
- Maillet, F.; Poinsot, V.; André, O.; Puech-Pagès, V.; Haouy, A.; Gueunier, M.; Cromer, L.; Giraudet, D.; Formey, D.; Niebel, A.; et al. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 2011, 469, 58–64. [Google Scholar] [CrossRef]
- Zipfel, C.; Oldroyd, G.E.D. Plant signalling in symbiosis and immunity. Nature 2017, 543, 328–336. [Google Scholar] [CrossRef]
- Rush, T.A.; Puech-Pagès, V.; Bascaules, A.; Jargeat, P.; Maillet, F.; Haouy, A.; Maës, A.Q.; Carriel, C.C.; Khokhani, D.; Keller-Pearson, M.; et al. Lipo-chitooligosaccharides as regulatory signals of fungal growth and development. Nat. Commun. 2020, 11, 3897. [Google Scholar] [CrossRef]
- Bonhomme, M.; Bensmihen, S.; André, O.; Amblard, E.; Garcia, M.; Maillet, F.; Puech-Pagès, V.; Gough, C.; Fort, S.; Cottaz, S.; et al. Distinct genetic bases for plant roots responses to lipo-chitooligosaccharide signal molecules from distinct microbial origins. J. Exp. Bot. 2020, 72, 3821–3834. [Google Scholar] [CrossRef] [PubMed]
- Girardin, A.; Wang, T.; Ding, Y.; Keller, J.; Buendia, L.; Gaston, M.; Ribeyre, C.; Gasciolli, V.; Auriac, M.C.; Vernié, T.; et al. LCO receptors involved in arbuscular mycorrhiza are functional for rhizobia perception in legumes. Curr. Biol. 2019, 29, 4249–4259. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Nod factor [Nod Bj V (C18:1, MeFuc)] and lumichrome enhance photosynthesis and growth of corn and soybean. J. Plant Physiol. 2008, 165, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Cao, Y.; Tanaka, K.; Thibivilliers, S.; Wan, J.; Choi, J.; Kang, C.H.; Qiu, J.; Stacey, G. Non-legumes respond to rhizobial Nod factors by suppressing the innate immune response. Science 2013, 341, 1384–1387. [Google Scholar] [CrossRef]
- Singh, V.K.; Kumar, A. Secondary metabolites from endophytic fungi: Production, methods of analysis, and diverse pharmaceutical potential. Symbiosis 2023, 90, 111–125. [Google Scholar] [CrossRef]
- Brader, G.; Compant, S.; Mitter, B.; Trognitz, F.; Sessitsch, A. Metabolic potential of endophytic bacteria. Curr. Opin. Biotechnol. 2014, 27, 30–37. [Google Scholar] [CrossRef]
- Marks, B.B.; Megías, M.; Nogueira, M.A.; Hungria, M. Biotechnological potential of rhizobial metabolites to enhance the performance of Bradyrhizobium spp. and Azospirillum brasilense inoculants with soybean and maize. AMB Express 2013, 3, 21. [Google Scholar] [CrossRef]
- Dirar, A.I.; Alsaadi, D.H.M.; Wada, M.; Mohamed, M.A.; Watanabe, T.; Devkota, H.P. Effects of extraction solvents on total phenolic and flavonoids contents and biological activities of extracts from Sudanese medicinal plants. S. Afr. J. Bot. 2019, 120, 261–267. [Google Scholar] [CrossRef]
- Ngo, T.N.; Scarlett, C.J.; Bowyer, M.C.; Ngo, P.D.; Vuong, Q.V. Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. J. Food Qual. 2017, 2017, 9305047. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Sharma, D.; Jadon, N.; Agrawal, P.K. Antimicrobial and phytochemical screening of endophytic fungi isolated from spikes of Pinus roxburghii. Arch. Clin. Microbiol. 2015, 6. [Google Scholar]
- Jin, G.; Yang, F.; Hu, C.; Shen, H.; Zhao, Z.-K. Enzyme-assisted extraction of lipids directly from the culture of the oleaginous yeast Rhodosporidium toruloides. Biores. Tech. 2012, 111, 378–382. [Google Scholar] [CrossRef]
- Esquivel-Hernández, D.A.; Rodríguez-Rodríguez, J.; Rostro-Alanis, M.; Cuéllar-Bermúdez, S.P.; Mancera-Andrade, E.I.; Núñez-Echevarría, J.E.; García-Pérez, J.S.; Chandra, R.; Parra-Saldívar, R. Advancement of green process through microwave-assisted extraction of bioactive metabolites from Arthrospira platensis and bioactivity evaluation. Bior. Tech. 2017, 224, 618–629. [Google Scholar] [CrossRef]
- Behzadnia, M.; Moosavi-Nasab, M.; Ojha, S.; Tiwari, B.K. Exploitation of ultrasound technique for enhancement of microbial metabolites production. Molecules 2020, 25, 5473. [Google Scholar] [CrossRef] [PubMed]
- Smirnou, D.; Knotek, P.; Nesporova, K.; Smejkalova, D.; Pavlik, V.; Franke, L.; Velebny, V. Ultrasound-assisted production of highly-purified β-glucan schizophyllan and characterization of its immune properties. Process Biochem. 2017, 58, 313–319. [Google Scholar] [CrossRef]
- Alkhulaifi, M.M.; Awaad, A.S.; Al-Mudhayyif, H.A.; Alothman, M.R.; Alqasoumi, S.I.; Zain, S.M. Evaluation of antimicrobial activity of secondary metabolites of fungi isolated from Sultanate Oman soil. Saudi Pharm. J. 2019, 27, 401–405. [Google Scholar] [CrossRef]
- Kumar, A.; Patil, D.; Rajamohanan, P.R.; Ahmad, A. Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS ONE 2013, 8, e71805. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Jia, X.; Lu, W. Isolation of secondary metabolites with antimicrobial activities from Bacillus amylo-liquefaciens LWYZ003. Trans. Tianjin Univ. 2019, 25, 38–44. [Google Scholar] [CrossRef]
- Ramadhana, E.R.; Herwin, H.; Nuryanti, S. Isolation of endophytic fungi from Aloe vera against bacteria that cause digestive tract infections by TLC-Bioautography and agar diffusion. J. Microbiol. Sci. 2024, 4, 1. [Google Scholar] [CrossRef]
- Parera-Valadez, Y.; Yam-Puc, A.; López-Aguiar, L.K.; Borges-Argáez, R.; Figueroa-Saldivar, M.A.; Cáceres-Farfán, M.; Márquez-Velázquez, N.A.; Prieto-Davó, A. Ecological strategies behind the selection of cultivable Actinomycete strains from the Yucatan Peninsula for the discovery of secondary metabolites with antibiotic activity. Microb. Ecol. 2019, 77, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E. Bioassays | Effects-Detection in Chromatography. In Encyclopedia of Analytical Science; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 261–270. [Google Scholar]
- Jamshidi-Aidji, M.; Dimkic, I.; Ristivojevic, P.; Stankovic, S.; Morlock, G.E. Effect-directed screening of Bacillus lipopeptide extracts via hyphenated high-performance thin-layer chromatography. J. Chromatogr. A 2019, 1605, 460366. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Prakash, H.S.; Nalini, M.S. Antioxidative properties of phenolic compounds isolated from the fungal endophytes of Zingiber nimmonii (J. Graham) Dalzell. Front. Biol. 2017, 12, 151–162. [Google Scholar] [CrossRef]
- Enyi, E.O.; Ekpunobi, N.F. Secondary metabolites from endophytic fungi of Moringa oleifera: Antimicrobial and antioxidant properties. J. Microbiol. Experim. 2022, 10, 5. [Google Scholar]
- Wang, F.; Zhao, Z.; Han, Y.; Li, S.; Bi, X.; Ren, S.; Pan, Y.; Wang, D.; Liu, X. The bacterial and fungal compositions in the rhizosphere of Asarum heterotropoides Fr. Schmidt var. mandshuricum (Maxim.) Kitag. in a typical planting region. Microorganisms 2024, 12, 692. [Google Scholar]
- El-Sayed, A.S.A.; Shindia, A.; Ammar, H.; Seadawy, M.G.; Khashana, S.A. Bioprocessing of Epothilone B from Aspergillus fumigatus under solid state fermentation: Antiproliferative activity, tubulin polymerization and cell cycle analysis. BMC Microbiol. 2024, 24, 43. [Google Scholar] [CrossRef]
- Tan, Z.Q.; Leow, H.Y.; Weerasingam, D.C.; Karisnan, K.; Song, A.A.L.; Mai, C.W.; Yap, W.S.; Lim, S.H.E.; Lai, K.S. Co-culture systems for the production of secondary metabolites: Current and future prospects. Open Biotech. J. 2019, 13, 18–26. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kour, D.; Rana, K.L.; Yadav, N.; Singh, B.; Chauhan, V.S.; Rastegari, A.A.; Hesham, A.E.; Gupta, V.K. Metabolic Engineering to Synthetic Biology of Secondary Metabolites Production. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Pandey, A., Eds.; Elsevier: New York, NY, USA, 2019; pp. 279–320. [Google Scholar]
- Kende, A.; Portwood, D.; Senior, A.; Earll, M.; Bolygo, E.; Seymour, M. Target list building for volatile metabolite profiling of fruit. J. Chromatogr. A 2010, 1217, 6718–6723. [Google Scholar] [CrossRef]
- Nas, F.; Aissaoui, N.; Mahjoubi, M.; Mosbah, A.; Arab, M.; Abdelwahed, S.; Khrouf, R.; Masmoudi, A.; Cherif, A.; Klouche-Khelil, N. A comparative GC-MS analysis of bioactive secondary metabolites produced by halotolerant Bacillus spp. isolated from the Great Sebkha of Oran. Intern. Microbiol. 2021, 24, 455–470. [Google Scholar] [CrossRef]
- Chakraborty, B.; Kumar, R.S.; Almansour, A.I.; Gunasekaran, P.; Nayaka, S. Bioprospection and secondary metabolites profiling of marine Streptomyces levis strain KS46. Saud. J. Biol. Sci. 2022, 29, 667–679. [Google Scholar] [CrossRef]
- Maulidia, V.; Soesanto, L.; Amsuddin, S.; Khairan, K.; Hamaguchi, T.; Hasegawa, K.; Sriwati, R. Secondary metabolites produced by endophytic bacteria against the root-knot nematode (Meloidogyne sp.). Biodiversitas 2020, 21, 5270–5275. [Google Scholar] [CrossRef]
- Madhusudhan, M.; Bharathi, T.; Prakash, H. Isolation and purification of bioactive metabolites from fungal endophytes—A review. Curr. Biochem. Eng. 2015, 2, 111–117. [Google Scholar] [CrossRef]
- Guo, Z.; Zou, Z. Discovery of new secondary metabolites by epigenetic regulation and NMR comparison from the plant endophytic fungus Monosporascus eutypoides. Molecules 2020, 25, 4192. [Google Scholar] [CrossRef] [PubMed]
- Orfali, R.; Perveen, S. Secondary metabolites from the Aspergillus sp. in the rhizosphere soil of Phoenix dactylifera (palm tree). BMC Chem. 2019, 13, 103. [Google Scholar] [CrossRef]
- Padhi, L.; Mohanta, Y.K.; Panda, S.K. Endophytic fungi with great promises: A review. J. Adv. Pharm. Educ. Res. 2013, 3, 152–171. [Google Scholar]
- Chen, Y.; Wang, H.; Ke, X.; Sang, Z.; Kuang, M.; Peng, W.; Tan, J.; Zheng, Y.; Zou, Z.; Tan, H. Five new secondary metabolites from an endophytic fungus Phomopsis sp. SZSJ-7B. Front. Plant Sci. 2022, 13, 1049015. [Google Scholar] [CrossRef]
- Song, Z.; Sun, Y.J.; Xu, S.; Li, G.; Yuan, C.; Zhou, K. Secondary metabolites from the endophytic fungi Fusarium decem-cellulare F25 and their antifungal activities. Front. Microbiol. 2023, 14, 1127971. [Google Scholar]
- Sivarajan, A.; Shanmugasundaram, T.; Sangeetha, M.; Radhakrishnan, M.; Balagurunathan, R. Screening, production, and characterization of biologically active secondary metabolite(s) from marine Streptomyces sp. PA9 for antimicrobial, antioxidant, and mosquito larvicidal activity. Ind. J. Geo Mar. Sci. 2019, 48, 1319–1326. [Google Scholar]
- Montoya-Escobar, N.; Ospina-Acero, D.; Velásquez-Cock, J.A.; Gómez-Hoyos, C.; Serpa Guerra, A.; Gañan Rojo, P.F.; Vélez Acosta, L.M.; Escobar, J.P.; Correa-Hincapié, N.; Triana-Chávez, O.; et al. Use of Fourier series in X-ray diffraction (XRD) analysis and Fourier-Transform Infrared Spectroscopy (FTIR) for estimation of crystallinity in cellulose from different sources. Polymers 2022, 14, 5199. [Google Scholar] [CrossRef]
- Covington, B.C.; McLean, J.A.; Bachmann, B.O. Comparative mass spectrometry-based metabolomics strategies for the investigation of microbial secondary metabolites. Nat. Prod. Rep. 2017, 34, 6–24. [Google Scholar] [CrossRef]
- Scherlach, K.; Hertweck, C. Triggering cryptic natural product biosynthesis in microorganisms. Org. Biomol. Chem. 2009, 7, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; Fischbach, M.A. Natural products version 2.0: Connecting genes to molecules. J. Am. Chem. Soc. 2010, 132, 2469–2493. [Google Scholar] [CrossRef] [PubMed]
- Hautbergue, T.; Jamin, E.L.; Debrauwer, L.; Puel, O.; Oswald, I.P. From genomics to metabolomics, moving toward an integrated strategy for the discovery of fungal secondary metabolites. Nat. Prod. Rep. 2018, 35, 147–173. [Google Scholar] [CrossRef] [PubMed]
- Breitling, R.; Ceniceros, A.; Jankevics, A.; Takano, E. Metabolomics for secondary metabolite research. Metabolites 2013, 3, 1076–1083. [Google Scholar] [CrossRef]
- Shuikan, A.M.; Hozzein, W.N.; Alzharani, M.M.; Sandouka, M.N.; Al Yousef, S.A.; Alharbi, S.A.; Damra, E. Enhancement and identification of microbial secondary metabolites. In Extremophilic Microbes and Metabolites—Diversity, Bioprospecting and Biotechnological Applications; Najjari, A., Cherif, A., Sghaier, H., Ouzari, H.I., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Sun, Y.; Liu, W.; Shi, X.; Zheng, H.; Zheng, Z.; Lu, X.; Xing, Y.; Ji, K.; Liu, M.; Dong, Y. Inducing secondary metabolite production of Aspergillus sydowii through microbial co-culture with Bacillus subtilis. Microb. Cell Fact. 2021, 20, 42. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Masamba, P.; Kappo, A.P. Metabolomics and chemoinformatics in agricultural biotechnology research: Complementary probes in unravelling new metabolites for crop improvement. Biology 2022, 11, 1156. [Google Scholar] [CrossRef]
- Sinha, R.; Sharma, B.; Dangi, A.K.; Shukla, P. Recent metabolomics and gene editing approaches for synthesis of microbial secondary metabolites for drug discovery. World J. Microbiol. Biotechnol. 2019, 35, 166. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Bever, J.D.; Labbé, J.; Yang, X.-H.; Yin, H.-F. Mitigating climate change through managing constructed-microbial communities in agriculture. Agric. Ecosyst. Environ. 2016, 216, 304–308. [Google Scholar] [CrossRef]
- Rosier, A.; Medeiros, F.H.; Bais, H.P. Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil 2018, 428, 35–55. [Google Scholar] [CrossRef]
- Gosal, S.K.; Kaur, J.; Kaur, J. Plant growth-promoting rhizobacteria: A probiotic for plant health and productivity. In Probiotics and Plant Health; Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer: Singapore, 2017; pp. 589–600. [Google Scholar]
- Maheshwari, D.K.; Saraf, M.; Dheeman, S. Plant growth-promoting rhizobacteria (PGPR) as protagonists of ever-sustained agriculture: An introduction. In Field Crops: Sustainable Management by PGPR; Maheshwari, D.K., Dheeman, S., Eds.; Springer: Cham, Switzerland, 2019; pp. 1–10. [Google Scholar]
- Ercole, T.G.; Kava, V.M.; Petters-Vandresen, D.A.L.; Gomes, M.E.N.; Aluizio, R.; Ribeiro, R.A.; Hungria, M.; Galli, L.V. Unlocking the growth-promoting and antagonistic power: A comprehensive whole genome study on Bacillus velezensis strains. Gene 2024, 927, 148669. [Google Scholar] [CrossRef]
- Vasques, N.C.; Nogueira, M.A.; Hungria, M. Increasing application of multifunctional Bacillus for biocontrol of pests and diseases and plant growth promotion: Lessons from Brazil. Agronomy 2024, 14, 1654. [Google Scholar] [CrossRef]
- Miljakovic, D.; Marinkovic, J.; Balesevic-Tubic, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Mnif, I.; Ghribi, D. Potential of bacterial derived biopesticides in pest management. Crop Prot. 2015, 77, 52–64. [Google Scholar] [CrossRef]
- Nihorimbere, V.; Cawoy, H.; Seyer, A.; Brunelle, A.; Thonart, P.; Ongena, M. Impact of rhizosphere factors on cyclic lipopeptide signature from the plant beneficial strain Bacillus amyloliquefaciens S499. FEMS Microbiol. Ecol. 2012, 79, 176–191. [Google Scholar] [CrossRef]
- Ongena, M.; Jourdan, E.; Adam, A.; Paquot, M.; Brans, A.; Joris, B.; Arpigny, J.L.; Thonart, P. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol. 2007, 9, 1084–1090. [Google Scholar] [CrossRef]
- Kuan, K.B.; Othman, R.; Rahim, K.A.; Shamsuddin, Z.H. Plant growth-promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilisation of maize under greenhouse conditions. PLoS ONE 2016, 11, e0152478. [Google Scholar] [CrossRef]
- Saeid, A.; Prochownik, E.; Dobrowolska-Iwanek, J. Phosphorus solubilization by Bacillus species. Molecules 2018, 23, 2897. [Google Scholar] [CrossRef] [PubMed]
- Idris, E.E.; Iglesias, D.J.; Talon, M.; Borriss, R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant-Microbe Interact. 2007, 20, 619–626. [Google Scholar] [CrossRef]
- González, O.; Ortíz-Castro, R.; Díaz-Pérez, C.; Díaz-Pérez, A.L.; Magaña-Dueñas, V.; López-Bucio, J.; Campos-García, J. Non-ribosomal peptide synthases from Pseudomonas aeruginosa play a role in cyclodipeptide biosynthesis, quorum-sensing regulation, and root development in a plant host. Microb. Ecol. 2017, 73, 616–629. [Google Scholar] [CrossRef]
- Liu, F.; Bian, Z.; Jia, Z.; Zhao, Q.; Song, S. The GCR1 and GPA1 participate in promotion of Arabidopsis primary root elongation induced by N-acyl-homoserine lactones, the bacterial quorum-sensing signals. Mol. Plant-Microbe Interact. 2012, 25, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Yu, K.O.; Ramzi, A.B.; Choe, S.H.; Kim, S.W.; Han, S.O. Improvement of surfactin production in Bacillus subtilis using synthetic wastewater by overexpression of specific extracellular signaling peptides, comX and phrC. Biotechnol. Bioeng. 2012, 109, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Weller, D.M.; Landa, B.B.; Mavrodi, O.V.; Schroeder, K.L.; De La Fuente, L.; Blouin Bankhead, S.; Allende Molar, R.; Bonsall, R.F.; Mavrodi, D.V.; Thomashow, L.S. Role of 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp. in the defense of plant roots. Plant Biol. 2007, 9, 4–20. [Google Scholar] [CrossRef]
- Bonsall, R.F.; Weller, D.M.; Thomashow, L.S. Quantification of 2,4-diacetylphloroglucinol produced by fluorescent Pseudomonas spp. in vitro and in the rhizosphere of wheat. Appl. Environ. Microbiol. 1997, 63, 951–955. [Google Scholar] [CrossRef]
- Brazelton, J.N.; Pfeufer, E.E.; Sweat, T.A.; Gardener, B.B.M.; Coenen, C. 2,4-diacetylphloroglucinol alters plant root development. Mol. Plant-Microbe Interact. 2008, 21, 1349–1358. [Google Scholar] [CrossRef]
- Trapet, P.; Avoscan, L.; Klinguer, A.; Pateyron, S.; Citerne, S.; Chervin, C.; Mazurier, S.; Lemanceau, P.; Wendehenne, D.; Besson-Bard, A. The Pseudomonas fluorescens siderophore pyoverdine weakens Arabidopsis thaliana defense in favor of growth in iron-deficient conditions. Plant Physiol. 2016, 171, 675–693. [Google Scholar] [CrossRef]
- Meziane, H.; Van Der Sluis, I.; Van Loon, L.C.; Höfte, M.; Bakker, P.A.H.M. Determinants of Pseudomonas putida WCS358 involved in inducing systemic resistance in plants. Mol. Plant Pathol. 2005, 6, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits that go far beyond biological nitrogen fixation. AMB Express 2018, 8, 87. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J. Auxin signaling in Azospirillum brasilense: A proteome analysis. In Biological Nitrogen Fixation; De Brujin, F.J., Ed.; Wiley: Hoboken, NJ, USA, 2015; p. 91. [Google Scholar]
- Sahoo, R.K.; Ansari, M.W.; Pradhan, M.; Dangar, T.K.; Mohanty, S.; Tuteja, N. Phenotypic and molecular characterization of native Azospirillum strains from rice fields to improve crop productivity. Protoplasma 2014, 251, 943–953. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Marques, A.C.R.; Oliveira, L.B.; Nicoloso, F.T.; Jacques, J.S.; Giacomini, S.J.; Quadros, F.L.F. Biological nitrogen fixation in C4 grasses of different growth strategies of South America natural grasslands. Appl. Soil Ecol. 2017, 113, 54–61. [Google Scholar] [CrossRef]
- Rodriguez, H.; Gonzalez, T.; Goire, I.; Bashan, Y. Gluconic acid production and phosphate solubilization by the plant growth-promoting bacterium Azospirillum spp. Naturwissenschaften 2004, 91, 552–555. [Google Scholar] [CrossRef]
- Moretti, L.G.; Crusciol, C.A.C.; Bossolani, J.W.; Calonego, J.C.; Moreira, A.; Garcia, A.; Momesso, L.; Kuramae, E.E.; Hungria, M. Beneficial microbial species and metabolites alleviate soybean oxidative damage and increase grain yield during short dry spells. Eur. J. Agron. 2021, 127, 126293. [Google Scholar] [CrossRef]
- Hungria, M.; Phillips, D.A. Effects of a seed color mutation on rhizobial nod-gene-inducing flavonoids and nodulation in common bean. Mol. Plant-Microbe Interact. 1993, 6, 418–422. [Google Scholar] [CrossRef]
- Liang, Y.; Tóth, K.; Cao, Y.; Tanaka, K.; Espinoza, C.; Stacey, G. Lipochitooligosaccharide recognition: An ancient history. New Phytol. 2014, 204, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, C.A.; Coelho, L.G.F.; Mendes, I.C.; Vale, H.M.M.; Ollero, F.J.; Megías, M.; Junior, F.B.R. Secondary metabolites of Rhizobium tropici CIAT 899 added to Bradyrhizobium spp. inoculant promote soybean growth and increase yield. J. Soil Sci. Plant Nutr. 2021, 21, 3354–3366. [Google Scholar] [CrossRef]
- Marks, B.B.; Megías, M.; Ollero, F.J.; Nogueira, M.A.; Araujo, R.S.; Hungria, M. Maize growth promotion by inoculation with Azospirillum brasilense and metabolites of Rhizobium tropici enriched on lipo-chitooligosaccharides (LCOs). AMB Express 2015, 5, 71. [Google Scholar] [CrossRef]
- De Jong, A.J.; Heidstra, R.; Spaink, H.P.; Hartog, M.V.; Hendriks, T.; Schafio, F.L.; Terzi, M.; Bisseling, T.; van Kammen, A.; de Vries, S.C. Rhizobium lipooligosaccharides rescue a carrot somatic embryo mutant. Plant Cell 1993, 5, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Egertsdotter, U.; van Arnold, S. Development of somatic embryos in Norway spruce. J. Exp. Bot. 1998, 49, 155–162. [Google Scholar] [CrossRef]
- Khan, W.; Costa, C.; Souleimanov, A.; Prithiviraj, B.; Smith, D.L. Response of Arabidopsis thaliana roots to lipochitooligosaccharide from Bradyrhizobium japonicum and other chitin-like compounds. Plant Growth Regul. 2011, 63, 243–249. [Google Scholar] [CrossRef]
- Tanaka, K.; Cho, S.-H.; Lee, H.; Pham, A.Q.; Batek, J.M.; Cui, S.; Qiu, J.; Khan, S.M.; Joshi, T.; Zhang, Z.J.; et al. Effect of lipo-chitooligosaccharide on early growth of C4 grass seedlings. J. Exp. Bot. 2015, 66, 5727–5738. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.F.; Long, S.R. Rhizobium–plant signal exchange. Nature 1992, 357, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; McIver, J.; Yang, Y.; Bai, Y.; Schultz, B.; McIver, A. Foliar application of lipo-oligosaccharides (Nod factors) to tomato (Lycopersicum esculentum) enhances flowering and fruit production. Can. J. Plant Sci. 2005, 87, 365–372. [Google Scholar]
- Rey, T.; Nars, A.; Bonhomme, M.; Bottin, A.; Huguet, S.; Balzergue, S.; Jardinaud, M.F.; Bono, J.J.; Cullimore, J.; Dumas, B.; et al. NFP, a LysM protein controlling Nod factor perception, also intervenes in Medicago truncatula resistance to pathogens. New Phytol. 2013, 198, 875–886. [Google Scholar] [CrossRef]
- Lucas, J.A.; García-Villaraco, A.; Ramos-Solano, B.; Akdi, K.; Gutierrez-Mañero, F.J. Lipo-chitooligosaccharides (LCOs) as elicitors of the enzymatic activities related to ROS scavenging to alleviate oxidative stress generated in tomato plants under stress by UV-B radiation. Plants 2022, 11, 1246. [Google Scholar] [CrossRef]
- Nandhini, D.U.; Somasundaram, E.; Amanullah, M.M. Effect of rhizobial nod factors (lipo-chitooligosaccharide) on seedling growth of blackgram under salt stress. Legume Res. Int. J. 2018, 41, 159–162. [Google Scholar]
- Atti, S.; Bonnell, R.; Prasher, S.; Smith, D.L. Response of soybean (Glycine max (L.) Merr.) under chronic water deficit to LCO application during flowering and pod filling. Irrig. Drain. 2005, 54, 15–30. [Google Scholar] [CrossRef]
- Duzan, H.M.; Mabood, F.; Zhou, X.; Souleimanov, A.; Smith, D.L. Nod factor induces soybean resistance to powdery mildew. Plant Physiol. Biochem. 2005, 43, 1022–1030. [Google Scholar] [CrossRef]
- Miransari, M.; Balakrishnan, P.; Smith, D.; Mackenzie, A.F.; Bahrami, H.A.; Malakouti, M.J.; Rejali, F. Overcoming the stressful effect of low pH on soybean root hair curling using lipochitooligosaccharides. Commun. Soil Sci. Plant Anal. 2006, 37, 1103–1110. [Google Scholar] [CrossRef]
- Le Strange, K.K.; Bender, G.L.; Djordjevic, M.A.; Rolfe, B.G.; Redmond, J.W. The Rhizobium strain NGR234 nodD1 gene product responds to activation by the simple phenolic compounds vanillin and isovanillin present in wheat seedling extracts. Mol. Plant-Microbe Interact. 1990, 3, 214–220. [Google Scholar] [CrossRef]
- Moretti, L.G.; Crusciol, C.A.C.; Kuramae, E.E.; Bossolani, J.W.; Moreira, A.; Costa, N.R.; Alves, C.J.; Pascoaloto, I.M.; Rondina, A.B.L.; Hungria, M. Effects of growth-promoting bacteria on soybean root activity, plant development and yield. Agron. J. 2019, 112, 418–428. [Google Scholar] [CrossRef]
- Moretti, L.G.; Crusciol, C.A.C.; Bossolani, J.W.; Momesso, L.; Garcia, A.; Kuramae, E.E.; Hungria, M. Bacterial consortium and microbial metabolites increase grain quality and soybean yield. J. Soil Sci. Plant Nutr. 2020, 20, 1923–1934. [Google Scholar] [CrossRef]
- Jacob, S.; Sajjalaguddam, R.R.; Kumar, K.V.K.; Varshney, R.; Sudini, H.K. Assessing the prospects of Streptomyces sp. RP1A-12 in managing groundnut stem rot disease caused by Sclerotium rolfsii Sacc. J. Gen. Plant Pathol. 2016, 82, 96–104. [Google Scholar] [CrossRef]
- Fatima, S.; Anjum, T. Identification of a potential ISR determinant from Pseudomonas aeruginosa PM12 against Fusarium wilt in tomato. Front. Plant Sci. 2017, 8, 848. [Google Scholar] [CrossRef]
- Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Improved heat stress tolerance of wheat seedlings by bacterial seed treatment. Plant Soil 2014, 379, 337–350. [Google Scholar] [CrossRef]
- Ashraf, A.; Bano, A.; Ali, S.A. Characterisation of plant growth-promoting rhizobacteria from rhizosphere soil of heat-stressed and unstressed wheat and their use as bio-inoculant. Plant Biol. J. 2019, 21, 762–769. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, Á.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef]
- Fukami, J.; Ollero, F.J.; De La Osa, C.; Valderrama-Fernández, R.; Nogueira, M.A.; Megías, M.; Hungria, M. Antioxidant activity and induction of mechanisms of resistance to stresses related to the inoculation with Azospirillum brasilense. Arch. Microbiol. 2018, 200, 191–1203. [Google Scholar] [CrossRef]
- Kang, S.; Khan, A.L.; Waqas, M.; You, Y.; Hamayun, M.; Joo, G.; Shahzad, R.; Choi, K.; Lee, I. Gibberellin-producing Serratia nematodiphila PEJ1011 ameliorates low temperature stress in Capsicum annuum L. Europ. J. Soil Biol. 2015, 68, 85–93. [Google Scholar] [CrossRef]
- Korhonen, J.; Nuur, C.; Feldmann, A.; Birkie, S.E. Circular economy as an essentially contested concept. J. Clean. Prod. 2018, 175, 544–552. [Google Scholar] [CrossRef]
- Arana-Landin, G.; Sigüenza, W.; Landeta-Manzano, B.; Laskurain-Iturbe, I. Circular economy: On the road to ISO 59000 family of standards. Corp. Soc. Responsib. Environ. Manag. 2023, 31, 1977–2009. [Google Scholar] [CrossRef]
- Rizobacter. Inoculante Signum Bioindutor. Signum Inoculante Rizobacter. Available online: https://rizobacter.com.ar/es/productos/argentina/signum (accessed on 19 March 2024).
- Novozymes. NodPro LCO. Available online: https://www.novozymes.com/en/products/bioag/soybeans/nodpro-lco (accessed on 30 April 2024).
- Shahzad, R.; Waqas, M.; Khan, A.L.; Asaf, S.; Khan, M.A.; Kang, S.M.; Yun, B.W.; Lee, I.J. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol. Biochem. 2016, 106, 236–243. [Google Scholar] [CrossRef]
- Cohen, A.C.; Bottini, R.; Piccoli, P. Role of abscisic acid producing PGPR in sustainable agriculture. In Bacterial Metabolites in Sustainable Agroecosystem, 2nd ed.; Maheshwari, D., Ed.; Springer: Cham, Switzerland, 2015; Volume 12, pp. 259–282. [Google Scholar]
- Damodaran, T.; Sah, V.; Rai, R.B.; Sharma, D.K.; Mishra, V.K.; Jha, S.; Kannan, R. Isolation of salt tolerant endophytic and rhizospheric bacteria by natural selection and screening for promising plant growth-promoting rhizobacteria (PGPR) and growth vigour in tomato under sodic environment. Afric. J. Microbiol. Res. 2013, 7, 5082–5089. [Google Scholar]
- Rocha, T.M.; Marcelino, P.R.F.; Da Costa, R.A.M.; Rubio-Ribeaux, D.; Barbosa, F.G.; Silva, S.S. Agricultural bioinputs obtained by solid-state fermentation: From production in biorefineries to sustainable agriculture. Sustainability 2024, 16, 1076. [Google Scholar] [CrossRef]
- Botelho, G.R.; Mendonça-Hagler, L.C. Fluorescent Pseudomonad associated with the rhizosphere of crops—An over-view. Braz. J. Microbiol. 2006, 37, 401–416. [Google Scholar] [CrossRef]
- Fouillaud, M.; Dufossé, L. Microbial secondary metabolism and biotechnology. Microorganisms 2021, 10, 123. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Hungria, M.; Johnston, A.W.B.; Phillips, D.A. Effects of flavonoids released naturally from bean (Phaseolus vulgaris) on nodD-regulated gene transcription in Rhizobium leguminosarum bv. phaseoli. Mol. Plant-Microbe Interact. 1992, 5, 199–203. [Google Scholar] [CrossRef]
- Phillips, D.A. Biosynthesis and release of rhizobial nodulation gene inducers by legumes. In Prokaryotic Nitrogen Fixation: A Model System for the Analysis of a Biological Process; Triplett, E.W., Ed.; Horizon Scientific Press: Wymondham, UK, 2000; pp. 349–364. [Google Scholar]
- Iwashita, K. Recent studies of protein secretion by filamentous fungi. J. Biosc. Bioeng. 2002, 94, 530–535. [Google Scholar] [CrossRef]
- Demain, A.L. Pharmaceutically active secondary metabolites of microorganisms. Appl. Microbiol. Biotech. 1999, 52, 455–463. [Google Scholar] [CrossRef]
- Mekala, N.K.; Singhania, R.R.; Sukumaran, R.K.; Pandey, A. Cellulase production under solid-state fermentation by Trichoderma reesei RUTC30: Statistical optimization of process parameters. Appl. Biochem. Biotechnol. 2008, 151, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Singhania, R.R.; Saini, R.; Adsul, M.; Saini, J.K.; Mathur, A.; Tuli, D.K. An integrative process for bio-ethanol production employing SSF produced cellulase without extraction. Biochem. Engin. J. 2015, 102, 45–48. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, F.; Wei, Z.; Ran, W.; Shen, Q. The usage of rice straw as a major substrate for the production of Beri-Kashvili, V.; Sokhadze, K.; Kachlishvili, E.; Elisashvili, V.; Chikindas, M.L. Bacillus amyloliquefaciens spore production under solid-state fermentation of lignocellulosic residues. Probiotics Antimicrob. Proteins 2010, 10, 755–761. [Google Scholar]
- El-Bendary, M.A. Production of Mosquitocidal Bacillus sphaericus by solid-state fermentation using agricultural wastes. World J. Microbiol. Biotechnol. 2010, 26, 153–159. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Li, Z. Growth of Rhizobium leguminosarum in a periodic pressure oscillating, solid-state fermentation of wheat straw. Biotechnol. Lett. 2001, 23, 827–829. [Google Scholar] [CrossRef]
- Agarwal, B.; Ahluwalia, V.; Pandey, A.; Sangwan, R.S.; Elumalai, S. Sustainable production of chemicals and energy fuel precursors from lignocellulosic fractions. In Biofuels; Agarwal, A., Agarwal, R., Gupta, T., Gurjar, B., Eds.; Springer: Singapore, 2017; Volume 3, pp. 154–196. [Google Scholar]
- Krishania, M.; Sindhu, R.; Binod, P.; Ahluwalia, V.; Kumar, V.; Sangwan, R.S.; Pandey, A. Design of bioreactors in solid-state fermentation. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Larroche, C., Soccol, C.R., Eds.; Elsevier: New York, NY, USA, 2018; Chapter 5; pp. 83–96. [Google Scholar]
- Salgado-Bautista, D.; Volke-Sepúlveda, T.; Figueroa-Martínez, F.; Carrasco-Navarro, U.; Chagolla-López, A.; Favela-Torres, E. Solid-state fermentation increases secretome complexity in Aspergillus brasiliensis. Fungal Biol. 2020, 124, 723–734. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Ramteke, P.; Mishra, P. Chapter 23—Solid-state fermentation strategy for microbial metabolites production: An overview. In New and Future Developments in Microbial Technology and Bioengineering; Gupta, V.K., Pandey, A., Eds.; Elsevier: New York, NY, USA, 2019; pp. 345–354. [Google Scholar]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Thomas, L.; Larroche, C.; Pandey, A. Current developments in solid-state fermentation. Biochem. Eng. J. 2013, 81, 146–161. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Gottumukkala, L.D.; Rajasree, K.; Soccol, C.R.; Pandey, A. Solid-state fermentation: Current trends and future prospects. In Fermentation Microbiology and Biotechnology; El-Mansi, E.M.T., Nielsen, J., Mousdale, D., Allman, T., Carlson, R., Eds.; CRC Pres: Boca Raton, FL, USA, 2019; Chapter 13; pp. 243–254. [Google Scholar]
- Ashok, A.; Doriya, K.; Rao, D.R.M.; Kumar, D.S. Design of solid-state bioreactor for industrial applications: An overview to conventional bioreactors. Biocat. Agri. Biotechnol. 2017, 9, 11–18. [Google Scholar] [CrossRef]
- Kaaniche, F.; Hamed, A.; Elleuch, L.; Chakchouk-Mtibaa, A.; Smaoui, S.; Karray-Rebai, I.; Koubaa, I.; Arcile, G.; Allouche, N.; Mellouli, L. Purification and characterization of seven bioactive compounds from the newly isolated Streptomyces cavourensis TN638 strain via solid-state fermentation. Microb. Pathog. 2020, 142, 104–106. [Google Scholar] [CrossRef]
- Nigam, P.S. Production of bioactive secondary metabolites. In Biotechnology for Agro-Industrial Residues Utilisation; Nigam, P.S., Pandey, A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 129–145. [Google Scholar]
- Rodríguez-Fernandéz, D.E.; Léon, J.A.R.; Carvalho, J.C.; Karp, S.G.; Parada, J.L.; Soccol, C.R. Process development to recover pectinases produced by solid-state fermentation. J. Bioprocess. Biotech. 2012, 2, 4. [Google Scholar]
- Finkel, O.M.; Castrillo, G.; Herrera Paredes, S.; Salas González, I.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef]
- Wakefield, J.; Hassan, H.M.; Jaspars, M.; Ebel, R.; Rateb, M.E. Dual induction of new microbial secondary metabolites by fungal bacterial co-cultivation. Front. Microbiol. 2017, 8, 1284. [Google Scholar] [CrossRef]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J. Metabolite induction via microorganism co-culture: A potential way to enhance chemical diversity for drug discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef]
- Baral, B.; Akhgari, A.; Metsä-Ketelä, M. Activation of microbial secondary metabolic pathways: Avenues and challenges. Synth. Syst. Biotechnol. 2018, 3, 163–178. [Google Scholar] [CrossRef]
- Paranagama, P.A.; Wijeratne, E.M.K.; Gunatilaka, A.A.L. Uncovering biosynthetic potential of plant-associated fungi: Effect of culture conditions on metabolite production by Paraphaeosphaeria quadriseptata and Chaetomium chiversii. J. Nat. Prod. 2007, 70, 1939–1945. [Google Scholar] [CrossRef]
- Liao, Y.; Wei, Z.-H.; Bai, L.; Deng, Z.; Zhong, J.-J. Effect of fermentation temperature on validamycin A production by Streptomyces hygroscopicus 5008. J. Biotechnol. 2009, 142, 271–274. [Google Scholar] [CrossRef]
- Hayes, A.; Hobbs, G.; Smith, C.P.; Oliver, S.G.; Butler, P.R. Environmental signals triggering methylenomycin production by Streptomyces coelicolor A3(2). J. Bacteriol. 1997, 179, 5511–5515. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Ahmed, A.S.; El-Sayed, E.R. Optimization of submerged fermentation conditions for immunosuppressant mycophenolic acid production by Penicillium roqueforti isolated from blue-molded cheeses: Enhanced production by ultraviolet and gamma irradiation. World J. Microbiol. Biotechnol. 2014, 30, 2625–2638. [Google Scholar] [CrossRef]
- El-Sayed, E.R.; Abdelhakim, H.K.; Ahmed, A.S. Solid-state fermentation for enhanced production of selenium nanoparticles by gamma-irradiated Monascus purpureus and their biological evaluation and photocatalytic activities. Bioproc. Biosyst. Eng. 2020, 43, 797–809. [Google Scholar] [CrossRef]
- Baltz, R. Genetic manipulation of secondary metabolite biosynthesis for improved production in Streptomyces and other actinomycetes. J. Ind. Microbiol. Biotechnol. 2016, 43, 343–370. [Google Scholar] [CrossRef]
- Hu, F.; Liu, Y.; Li, S. Rational strain improvement for surfactin production: Enhancing the yield and generating novel structures. Microb. Cell Fact. 2019, 18, 42. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.; Zhang, D.; Li, X.; Yu, H.; Shen, Z. Overexpression of specific proton motive force-dependent transporters facilitate the export of surfactin in Bacillus subtilis. J. Ind. Microbiol. Biotechnol. 2015, 42, 93–103. [Google Scholar] [CrossRef]
- Zhang, M.M.; Wang, Y.; Ang, E.L.; Zhao, H. Engineering microbial hosts for production of bacterial natural products. Nat. Prod. Rep. 2016, 33, 963–987. [Google Scholar] [CrossRef]
- Baltz, R.H. Combinatorial biosynthesis of cyclic lipopeptide antibiotics: A model for synthetic biology to accelerate the evolution of secondary metabolite biosynthetic pathways. Synth. Biol. 2012, 3, 748–758. [Google Scholar] [CrossRef]
- Milshteyn, A.; Schneider, J.S.; Brady, S.F. Mining the metabiome: Identifying novel natural products from microbial communities. Chem. Biol. 2014, 21, 1211–1223. [Google Scholar] [CrossRef]
- Pickens, L.B.; Tang, Y.; Chooi, Y.H. Metabolic engineering for the production of natural products. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 211–236. [Google Scholar] [CrossRef]
- Bhalkar, B.N.; Bedekar, P.A.; Kshirsagar, A.D.; Govindwar, S.P. Solid-state fermentation of soybean waste and an up-flow column bioreactor for continuous production of camptothecine by an endophytic fungus Fusarium oxysporum. RSC Adv. 2016, 6, 56527–56536. [Google Scholar] [CrossRef]
- Carboué, Q.; Rébufa, C.; Dupuy, N.; Roussos, S.; Bombarda, I. Solid-state fermentation pilot-scaled plug flow bioreactor, using partial least square regression to predict the residence time in a semicontinuous process. Biochem. Eng. J. 2019, 149, 107248. [Google Scholar] [CrossRef]
- IPCC. AR5 Climate Change 2014: Mitigation of Climate Change. Available online: https://www.ipcc.ch/report/ar5/wg3/ (accessed on 19 March 2024).
- Shahzad, A.; Ullah, S.; Dar, A.A. Nexus on climate change: Agriculture and possible solution to cope future climate change stresses. Environ. Sci. Pollut. Res. 2021, 28, 14211–14232. [Google Scholar] [CrossRef]
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A review on circular economy: The expected transition to a balanced interplay of environmental and economic systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Pinheiro, U.V.; Wancura, J.H.; Brondani, M.; da Silva, C.M.; Mainardi, M.A.; Gai, R.M.; Jahn, S.L. Production of gibberellic acid by solid-state fermentation using wastes from rice processing and brewing industry. Appl. Biochem. Biotechnol. 2023, 196, 1493–1508. [Google Scholar] [CrossRef] [PubMed]
- Slivinski, C.T.; Mallmann, E.; de Araújo, J.M.; Mitchell, D.A.; Krieger, N. Production of surfactin by Bacillus pumilus UFPEDA 448 in solid-state fermentation using a medium based on okara with sugarcane bagasse as a bulking agent. Process Biochem. 2012, 47, 1848–1855. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, F.; Wei, Z.; Ran, W.; Shen, Q. The usage of rice straw as a major substrate for the production of surfactin by Bacillus amyloliquefaciens XZ-173 in solid-state fermentation. J. Environ. Manag. 2013, 127, 96–102. [Google Scholar] [CrossRef]
- Ghribi, D.; Abdelkefi-Mesrati, L.; Mnif, I.; Kammoun, R.; Ayadi, I.; Saadaoui, I.; Maktouf, S.; Chaabouni-Ellouze, S. Investigation of antimicrobial activity and statistical optimization of Bacillus subtilis SPB1 biosurfactant production in solid-state fermentation. Biomed. Res. Int. 2012, 2012, 373682. [Google Scholar]
- Mejías, N.; Orozco, E.; Galán, N. Aprovechamiento de los residuos agroindustriales y su contribución al desarrollo sostenible de México. Rev. Cienc. Ambie. Rec. Nat. 2016, 2, 27–41. [Google Scholar]
- Donia, E.; Mineo, A.M.; Sgroi, F. A methodological approach for assessing business investments in renewable resources from a circular economy perspective. Land Use Pol. 2018, 76, 823–8277. [Google Scholar] [CrossRef]
- Bérdy, J. Bioactive microbial metabolites. J. Antib. 2005, 58, 1–26. [Google Scholar] [CrossRef]
- Watve, M.G.; Tickoo, R.; Jog, M.M.; Bhole, B.D. How many antibiotics are produced by the genus Streptomyces? Arch. Microbiol. 2001, 176, 386–390. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Kumar, J.; Rana, V.S.; Sati, O.P.; Walia, S. Comparative evaluation of two Trichoderma harzianum strains for major secondary metabolite production and antifungal activity. Nat. Prod. Res. 2015, 29, 914–920. [Google Scholar] [CrossRef]
- Dong, J.; Cai, L.; Li, X.; Duan, R.; Shu, Y.; Chen, F.; Wang, J.; Zhou, H.; Ding, Z. Production of a new tetracyclic triterpene sulfate metabolite sambacide by solid-state cultivated Fusarium sambucinum B10.2 using potato as substrate. Biores. Technol. 2016, 218, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, V.; Walia, S.; Sati, O.P.; Kumar, J.; Kundu, A.; Shankar, J.; Paul, Y.S. Isolation, characterisation of major secondary metabolites of the Himalayan Trichoderma koningii and their antifungal activity. Arch. Phyt. Plant Prot. 2014, 47, 1063–1071. [Google Scholar]
- Wen, A.; Havens, K.L.; Bloch, S.E.; Shah, N.; Higgins, D.A.; Davis-Richardson, A.G.; Sharon, J.; Sharon, F.; Mohiti-Asli, M.; Johnson, A.; et al. Enabling biological nitrogen fixation for cereal crops in fertilizes fields. Synthet. Biol. 2021, 10, 3264–3277. [Google Scholar] [CrossRef] [PubMed]



| PGPB | MSM | Benefit | Culture | Reference | Readiness for Commercialization |
|---|---|---|---|---|---|
| Curtobacterium albidum | ACC-deaminase/Indole-3-acetic-acid | Salinity resistance | Rice (Oriza sativa) | [47] | No |
| Rhizobium tropici | LCOs | Increased productivity | Maize (Zea mays), Soybean (Glycine max) | [87,161] | Yes [184,185] |
| Azospirillum brasilense | Salicylic acid Abscisic acid | Salinity resistance | Maize | [14,182] | No |
| Rhizobium tropici; Bradyrhizobium diazoefficiens | LCOs | Increased grain quality and yield Increased root activity | Soybean | [157,176] | Yes [186,187] |
| Bacillus amyloliquefaciens | Auxins Abscisic acid Gibberellins | Salinity resistance | Rice | [188] | No |
| Serratia nematodiphila | Gibberellins | Cold resistance | Pepper (Capsicum annuum) | [183] | No |
| Azospirillum brasilense | Salicylic acid | Drought resistance | Arabidopsis thaliana | [189] | No |
| Bacillus pumillus; Bacillus subtillis | Indole-3-acetic-acid | Salinity resistance Drought resistance | Tomato (Solanum lycopersicum) | [190] | No |
| Bradyrhizobium japonicum | LCOs | Increased seed germination rate | Maize Rice Soybean Common bean (Phaseolus vulgaris) | [66] | Yes [186,187] |
| Streptomyces sp. | Fungal cell wall-degrading enzymes | Increased resistance to phytopathogenic fungi | Peanut (Arachis hypogaea) | [177] | Yes [191] |
| Arbuscular mycorrhizae | Strigolactones | Drought resistance | Tomato, Lettuce (Lactuca sativa) | [181] | No |
| Rhizobium tropici CIAT 899 | LCOs | Increased productivity Plant growth promotion | Soybean | [160] | Yes [186,187] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marks, B.B.; Nogueira, M.A.; Hungria, M. Microbial Secondary Metabolites and Their Use in Achieving Sustainable Agriculture: Present Achievements and Future Challenges. Agronomy 2025, 15, 1350. https://doi.org/10.3390/agronomy15061350
Marks BB, Nogueira MA, Hungria M. Microbial Secondary Metabolites and Their Use in Achieving Sustainable Agriculture: Present Achievements and Future Challenges. Agronomy. 2025; 15(6):1350. https://doi.org/10.3390/agronomy15061350
Chicago/Turabian StyleMarks, Bettina Berquó, Marco Antonio Nogueira, and Mariangela Hungria. 2025. "Microbial Secondary Metabolites and Their Use in Achieving Sustainable Agriculture: Present Achievements and Future Challenges" Agronomy 15, no. 6: 1350. https://doi.org/10.3390/agronomy15061350
APA StyleMarks, B. B., Nogueira, M. A., & Hungria, M. (2025). Microbial Secondary Metabolites and Their Use in Achieving Sustainable Agriculture: Present Achievements and Future Challenges. Agronomy, 15(6), 1350. https://doi.org/10.3390/agronomy15061350