Overexpression of PagLAR3 in Populus alba × P. glandulosa Promotes Resistance to Hyphantria cunea
Abstract
1. Introduction
2. Materials and Methods
2.1. Gene Cloning and Agrobacterium-Mediated Transformation
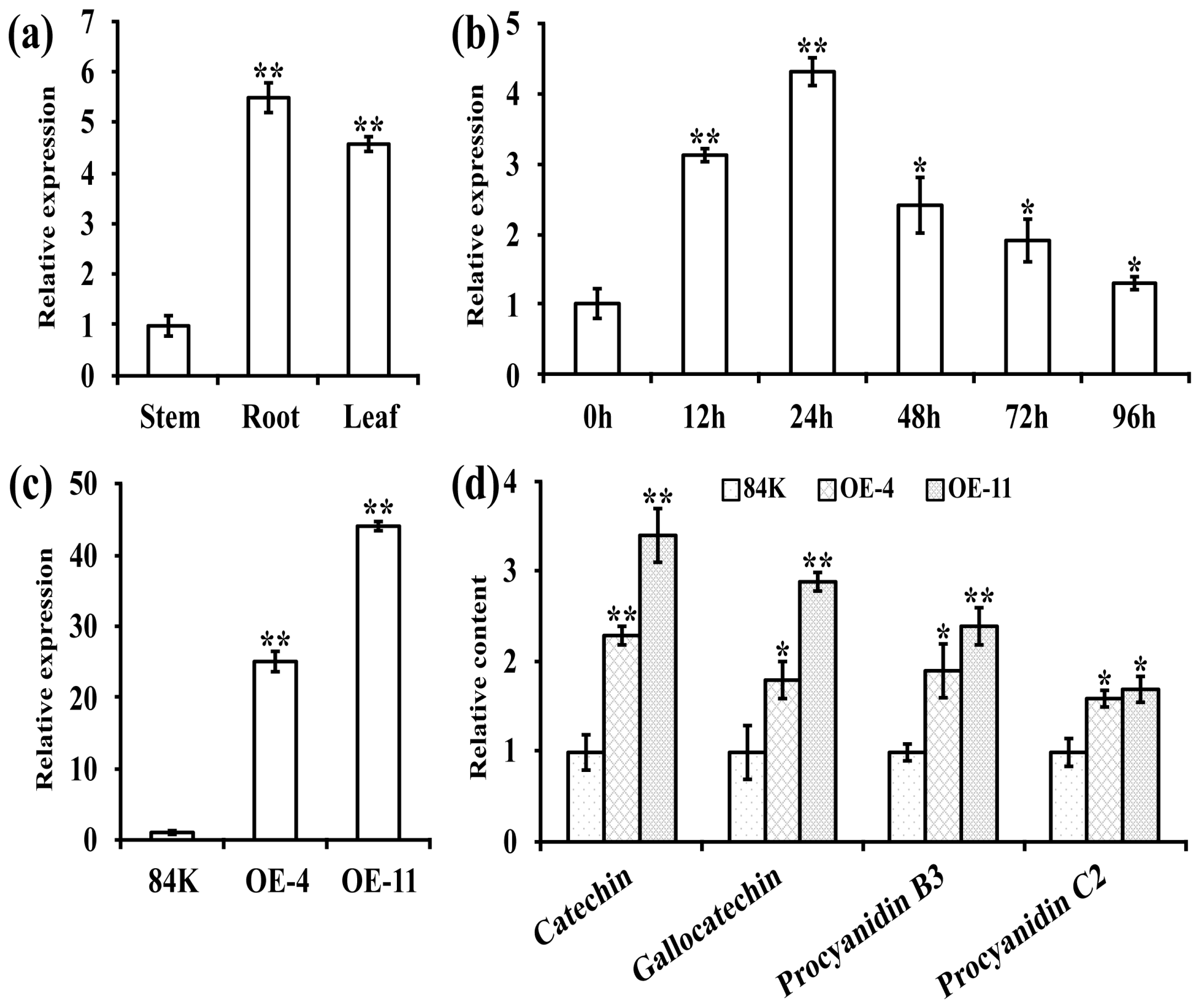
2.2. Transgenic Plant Verification and Cultivation Conditions
2.3. Gene Expression Analysis
2.4. Relative Quantification of CTs in Leaves
2.5. Evaluation of H. cunea Resistance
2.6. Statistical Analyses
3. Results
3.1. Confirmation of Transgenic Poplars Overexpressing PagLAR3
3.2. Transgenic Poplar Trees Overexpressing PagLAR3 Inhibited Larval Development
3.3. Overexpression of PagLAR3 in Poplar Decreased the Success of Adult Emergence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.M.; Jia, G.D.; Liu, Z.Q.; Wang, D.D.; Yu, X.X. Populus simonii Carr. Reduces Wind Erosion and Improves Soil Properties in Northern China. Forests 2019, 10, 315. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, W.D.; Peng, Y.Y.; Wan, M.; Farooq, T.H.; Fan, W.; Lei, J.J.; Yuan, C.L.; Wang, W.C.; Qi, Y.Q.; et al. Biomass Production and Carbon Stocks in Poplar-Crop Agroforestry Chronosequence in Subtropical Central China. Plants 2023, 12, 2451. [Google Scholar] [CrossRef]
- He, M.J.; Zhang, J.; Li, Z.; Li, M.L. Production and Mechanical Performance of Scrimber Composite Manufactured from Poplar Wood for Structural Applications. J. Wood Sci. 2016, 62, 429–440. [Google Scholar] [CrossRef]
- Liu, B.; Yan, J.Y.; Wang, D.; Wang, Y.; Zhou, Y.T.; Chen, Y.F. Occurrence of Major Forest Pests in China in 2023 and Prediction for Trend in 2024. For. Pest. Dis. 2024, 43, 41–45. (In Chinese) [Google Scholar] [CrossRef]
- Ning, J.; Lu, P.F.; Fan, J.T.; Ren, L.L.; Zhao, L.L. American Fall Webworm in China: A New Case of Global Biological Invasions. Innovation 2022, 3, 100201. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.Y.; Hao, D.J.; Zhao, X.D.; Geng, Y.S.; Hu, T.Y.; Xie, C.X. Effects of Extreme Climate on the Distribution and Potential Habitat of Hyphantria cunea in China. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2024, 48, 197–203. (In Chinese) [Google Scholar] [CrossRef]
- Sharan, S.; Chakraborty, A.; Roy, A.; Singh, I.K.; Singh, A. Transgenic Poplar for Resistance against Pest and Pathogen Attack in Forests: An Overview. Front. For. Glob. Change 2024, 7, 1490562. [Google Scholar] [CrossRef]
- Ding, L.P.; Chen, Y.J.; Wei, X.L.; Ni, M.; Zhang, J.W.; Wang, H.Z.; Zhu, Z.; Wei, J.H. Laboratory Evaluation of Transgenic Populus davidiana × Populus bolleana Expressing Cry1Ac + SCK, Cry1Ah3, and Cry9Aa3 Genes against Gypsy Moth and Fall Webworm. PLoS ONE 2017, 12, e0178754. [Google Scholar] [CrossRef]
- Tian, Y.C.; Li, T.Y.; Mang, K.Q.; Han, Y.F.; Li, L.; Wang, X.P.; Lu, M.Z.; Dai, L.Y.; Han, Y.N.; Yan, J.J.; et al. Insect Tolerance of Transgenic Populus nigra Plants Transformed with Bacillus thuringiensis Toxin Gene. Chin. J. Biotechnol. 1993, 9, 291–297. (In Chinese) [Google Scholar] [CrossRef]
- Xu, C.; Wei, H.; Wang, L.K.; Yin, T.M.; Zhuge, Q. Optimization of the cry1Ah1 Sequence Enhances the Hyper-Resistance of Transgenic Poplars to Hyphantria cunea. Front. Plant Sci. 2019, 10, 335. [Google Scholar] [CrossRef]
- Zhu, G.Q.; Fang, D.; Li, X.F.; Sun, W.B.; Huang, M.R.; Wang, M.X. Transformation of Populus × Euramericana Cv. ‘Nanlin895’ Sing Bt and CpTI Insect-Resistant Genes. Mol. Plant Breed. 2006, 4, 819–824. (In Chinese) [Google Scholar]
- Li, K.Y.; Fan, J.F.; Zhao, Z.; Li, L.; Zhu, H.L. Transformation of CryIAc and API Two Insect-Resistant Genes to Poplar (Populus alba × P. glandulosa). For. Res. 2007, 20, 699–704. (In Chinese) [Google Scholar]
- Zuo, L.L.; Wang, Z.Y.; Liang, C.; Xie, S.P. Transformation of Populus davidiana × P. bollena with Bt+ Spider Toxin Gene. J. Northeast. For. Univ. 2009, 37, 112–114. (In Chinese) [Google Scholar] [CrossRef]
- Domingo, J.L.; Bordonaba, J.G. A Literature Review on the Safety Assessment of Genetically Modified Plants. Environ. Int. 2011, 37, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Negin, B.; Jander, G. Convergent and Divergent Evolution of Plant Chemical Defenses. Curr. Opin. Plant Biol. 2023, 73, 102368. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.S.; Qu, L.J.; Fan, Z.B.; Hou, L.X.; Hu, J.J.; Wang, L.J. Dynamic Metabolic Responses of Resistant and Susceptible Poplar Clones Induced by Hyphantria cunea Feeding. Biology 2024, 13, 723. [Google Scholar] [CrossRef]
- Ding, Y.T.; Shen, J.J.; Li, H.X.; Sun, Y.; Jiang, T.B.; Kong, X.B.; Han, R.; Zhao, C.L.; Zhang, X.X.; Zhao, X.Y. Physiological and Molecular Mechanism of Populus pseudocathayana × Populus deltoides Response to Hyphantria cunea. Pestic. Biochem. Phys. 2024, 202, 105969. [Google Scholar] [CrossRef]
- Liu, X.X.; Jiang, D.; Meng, Z.J.; Yan, S.C. Effects of Secondary Substances on Food Utilization by Hyphantria cunea Larvae. J. Northeast. For. Univ. 2020, 48, 99–103. (In Chinese) [Google Scholar] [CrossRef]
- Wang, M.; Jiang, D.; Meng, Z.J.; Yan, S.C. Adaptability of Larval Growth and Development in Hyphantria cunea to Different Host Plant Secondary Metabolites. J. Northeast. For. Univ. 2020, 48, 100–104. (In Chinese) [Google Scholar] [CrossRef]
- Tanner, G.J.; Francki, K.T.; Abrahams, S.; Watson, J.M.; Larkin, P.J.; Ashton, A.R. Proanthocyanidin Biosynthesis in Plants: Purification of Legume Leucoanthocyanidin Reductase and Molecular Cloning of Its cDNA. J. Biol. Chem. 2003, 278, 31647–31656. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, L.J.; Han, Z.J.; Jiang, Y.Z.; Zhao, L.L.; Liu, H.; Yang, L.; Luo, K.M. Molecular Cloning and Characterization of PtrLAR3, a Gene Encoding Leucoanthocyanidin Reductase from Populus trichocarpa, and Its Constitutive Expression Enhances Fungal Resistance in Transgenic Plants. J. Exp. Bot. 2012, 63, 2513–2524. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.S.; Ge, X.L.; Wang, R.; Yang, H.-F.; Bai, Y.E.; Guo, Y.H.; Zhang, J.; Lu, M.Z.; Zhao, S.T.; Wang, L.Q. An Efficient Agrobacterium-Mediated Transformation Method for Hybrid Poplar 84K (Populus alba × P. glandulosa) Using Calli as Explants. Int. J. Mol. Sci. 2022, 23, 2216. [Google Scholar] [CrossRef]
- Xin, Y.C.; Meng, S.; Ma, B.; He, W.M.; He, N.J. Mulberry Genes MnANR and MnLAR Confer Transgenic Plants with Resistance to Botrytis cinerea. Plant Sci. 2020, 296, 110473. [Google Scholar] [CrossRef]
- Wen, O.Y.; Ning, J.M.; Zhu, X.Z.; Jiang, Y.W.; Wang, J.J.; Yuan, H.B.; Hua, J.J. UPLC-ESI-MS/MS Analysis Revealed the Dynamic Changes and Conversion Mechanism of Non-Volatile Metabolites during Green Tea Fixation. LWT Food Sci. Technol. 2024, 198, 116010. [Google Scholar] [CrossRef]
- Li, W.; Wen, L.C.; Chen, Z.T.; Zhang, Z.L.; Pang, X.L.; Deng, Z.C.; Liu, T.; Guo, Y.F. Study on Metabolic Variation in Whole Grains of Four Proso Millet Varieties Reveals Metabolites Important for Antioxidant Properties and Quality Traits. Food Chem. 2021, 357, 129791. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Qu, L.J.; Hu, J.J.; Zhang, L.W.; Tang, F.; Lu, M.Z. Metabolomics Reveals Constitutive Metabolites That Contribute Resistance to Fall Webworm (Hyphantria cunea) in Populus deltoides. Environ. Exp. Bot. 2017, 136, 31–40. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.; Cardinali, A. Role of Phenolics in the Resistance Mechanisms of Plants against Fungal Pathogens and Insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of Plant Defense against Insect Herbivores. Plant Signal Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Salminen, J.P.; Karonen, M. Chemical Ecology of Tannins and Other Phenolics: We Need a Change in Approach. Funct. Ecol. 2011, 25, 325–338. [Google Scholar] [CrossRef]
- Pang, Y.Z.; Peel, G.J.; Wright, E.; Wang, Z.Y.; Dixon, R.A. Early Steps in Proanthocyanidin Biosynthesis in the Model Legume Medicago truncatula. Plant Physiol. 2007, 145, 601–615. [Google Scholar] [CrossRef]
- Schweitzer, J.A.; Madritch, M.D.; Bailey, J.K.; LeRoy, C.J.; Fischer, D.G.; Rehill, B.J.; Lindroth, R.L.; Hagerman, A.E.; Wooley, S.C.; Hart, S.C.; et al. From Genes to Ecosystems: The Genetic Basis of Condensed Tannins and Their Role in Nutrient Regulation in a Populus Model System. Ecosystems 2008, 11, 1005–1020. [Google Scholar] [CrossRef]
- Ruttanaphan, T.; Songoen, W.; Pluempanupat, W.; Bullangpoti, V. Potential Insecticidal Extracts from Artocarpus lacucha against Spodoptera litura (Lepidoptera: Noctuidae) Larvae. J. Econ. Entomol. 2023, 116, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Leppä, M.M.; Laitila, J.E.; Salminen, J.-P. Distribution of Protein Precipitation Capacity within Variable Proanthocyanidin Fingerprints. Molecules 2020, 25, 5002. [Google Scholar] [CrossRef] [PubMed]
- Barbehenn, R.V.; Constabel, C.P. Tannins in Plant–Herbivore Interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Lindroth, R.L. Clonal Variation in Foliar Chemistry of Aspen: Effects on Gypsy Moths and Forest Tent Caterpillars. Oecologia 1997, 111, 99–108. [Google Scholar] [CrossRef]
- Osier, T.L.; Hwang, S.Y.; Lindroth, R.L. Effects of Phytochemical Variation in Quaking Aspen Populus Tremuloides Clones on Gypsy Moth Lymantria Dispar Performance in the Field and Laboratory. Ecol. Entomol. 2000, 25, 197–207. [Google Scholar] [CrossRef]
- Forkner, R.E.; Marquis, R.J.; Lill, J.T. Feeny Revisited: Condensed Tannins as Anti-herbivore Defences in Leaf-Chewing Herbivore Communities of Quercus. Ecol. Entomol. 2004, 29, 174–187. [Google Scholar] [CrossRef]
- Tikkanen, O.P.; Julkunen-Tiitto, R. Phenological Variation as Protection against Defoliating Insects: The Case of Quercus robur and Operophtera brumata. Oecologia 2003, 136, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Thitz, P.; Mehtätalo, L.; Välimäki, P.; Randriamanana, T.; Lännenpää, M.; Hagerman, A.E.; Andersson, T.; Julkunen-Tiitto, R.; Nyman, T. Phytochemical Shift from Condensed Tannins to Flavonoids in Transgenic Betula pendula Decreases Consumption and Growth but Improves Growth Efficiency of Epirrita autumnata Larvae. J. Chem. Ecol. 2020, 46, 217–231. [Google Scholar] [CrossRef]
- Keathley, C.P.; Potter, D.A. Quantitative Resistance Traits and Suitability of Woody Plant Species for a Polyphagous Scarab, Popillia japonica Newman. Environ. Entomol. 2008, 37, 1548–1557. [Google Scholar] [CrossRef]
- Ayres, M.P.; Clausen, T.P.; Stephen, F.M.J.; Redman, A.M.; Reichardt, P.B. Diversity of Structure and Antiherbivore Activity in Condensed Tannins. Ecology 1997, 78, 1696–1712. [Google Scholar] [CrossRef]
- Villanueva, X.; Zhen, L.L.; Ares, J.N.; Vackier, T.; Lange, H.; Crestini, C.; Steenackers, H.P. Effect of Chemical Modifications of Tannins on Their Antimicrobial and Antibiofilm Effect against Gram-Negative and Gram-Positive Bacteria. Front. Microbiol. 2023, 13, 987164. [Google Scholar] [CrossRef] [PubMed]
- Winder, R.S.; Lamarche, J.; Constabel, C.P.; Hamelin, R. The Effects of High-Tannin Leaf Litter from Transgenic Poplars on Microbial Communities in Microcosm Soils. Front. Microbiol. 2013, 4, 290. [Google Scholar] [CrossRef] [PubMed]
- Mallampalli, N.; Barbosa, P.; Weinges, K. Effects of Condensed Tannins and Catalpol on Growth and Development of Compsilura concinnata (Diptera: Tachinidae) Reared in Gypsy Moth (Lepidoptera: Lymantriidae). J. Entomol. Sci. 1996, 31, 289–300. [Google Scholar] [CrossRef]
- Zhang, Y.; Bartlow, A.W.; Wang, Z.; Yi, X. Effects of Tannins on Population Dynamics of Sympatric Seed-Eating Rodents: The Potential Role of Gut Tannin-Degrading Bacteria. Oecologia 2018, 187, 667–678. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Z.; Hou, L.; Wang, Z.; Wang, L. Overexpression of PagLAR3 in Populus alba × P. glandulosa Promotes Resistance to Hyphantria cunea. Agronomy 2025, 15, 1347. https://doi.org/10.3390/agronomy15061347
Fan Z, Hou L, Wang Z, Wang L. Overexpression of PagLAR3 in Populus alba × P. glandulosa Promotes Resistance to Hyphantria cunea. Agronomy. 2025; 15(6):1347. https://doi.org/10.3390/agronomy15061347
Chicago/Turabian StyleFan, Zhibin, Luxuan Hou, Zheshu Wang, and Lijuan Wang. 2025. "Overexpression of PagLAR3 in Populus alba × P. glandulosa Promotes Resistance to Hyphantria cunea" Agronomy 15, no. 6: 1347. https://doi.org/10.3390/agronomy15061347
APA StyleFan, Z., Hou, L., Wang, Z., & Wang, L. (2025). Overexpression of PagLAR3 in Populus alba × P. glandulosa Promotes Resistance to Hyphantria cunea. Agronomy, 15(6), 1347. https://doi.org/10.3390/agronomy15061347





