Valorization of Vineyard By-Products Through Vermicomposting: A Comparative Pilot-Scale Study with Eisenia fetida and Eisenia andrei
Abstract
1. Introduction
2. Materials and Methods
2.1. Earthworms and Substrates
2.2. Physicochemical Analysis
2.3. Phytotoxicity Analysis
2.4. Microbial Activity
2.5. Extraction and Determination of Total Polyphenols
2.6. Statistical Analysis
3. Results
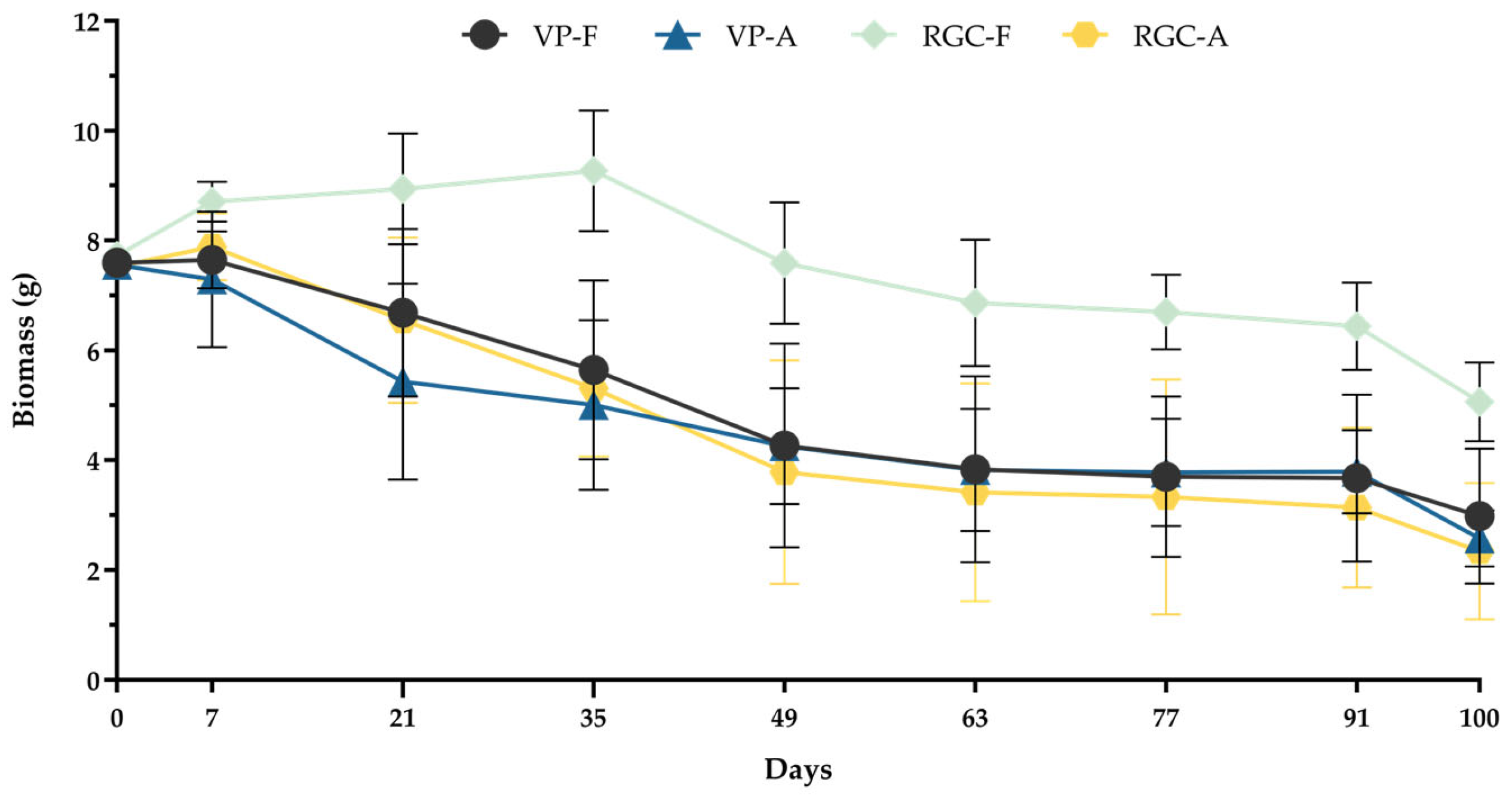
3.1. Evolution of Earthworm Population Dynamics
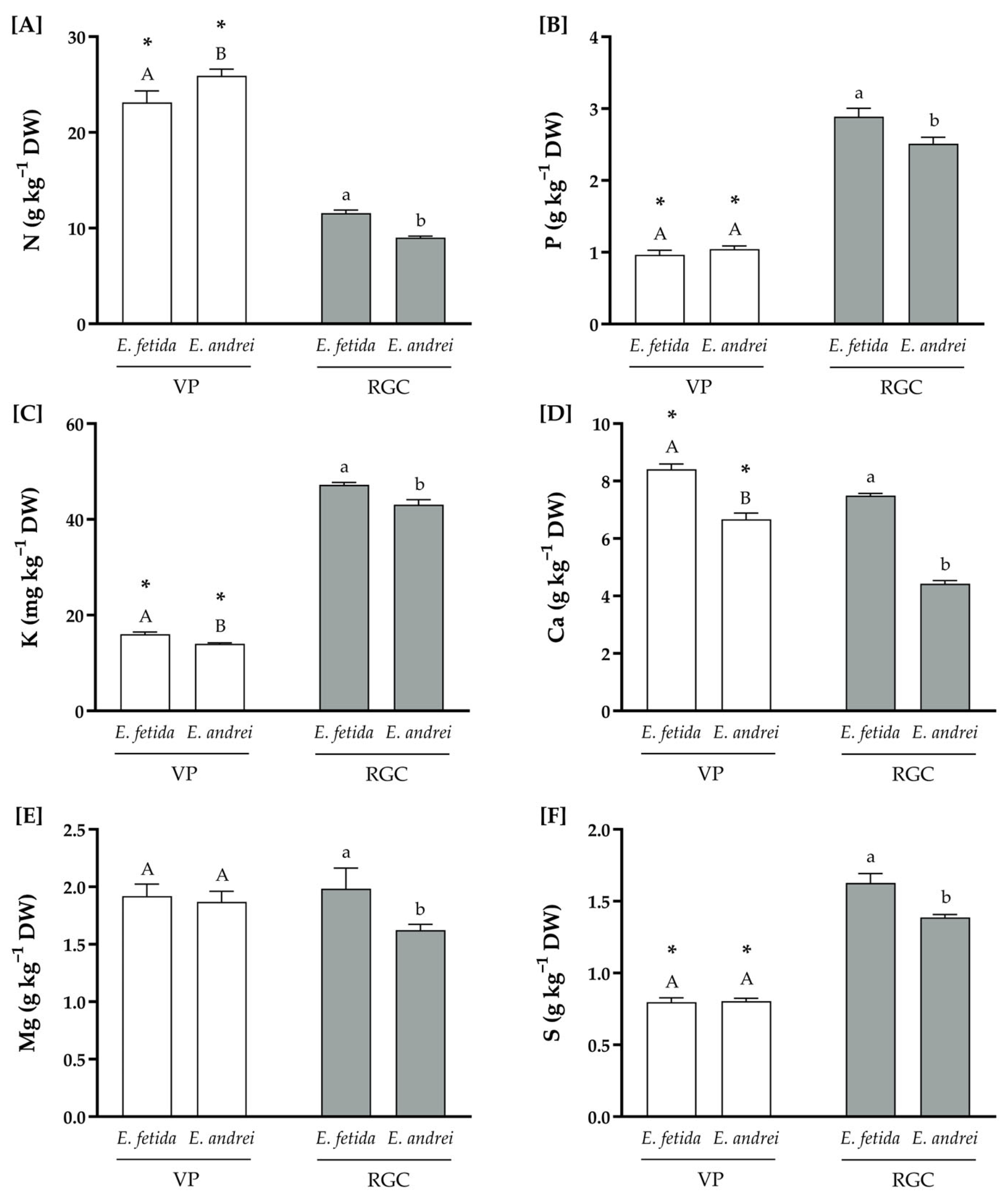
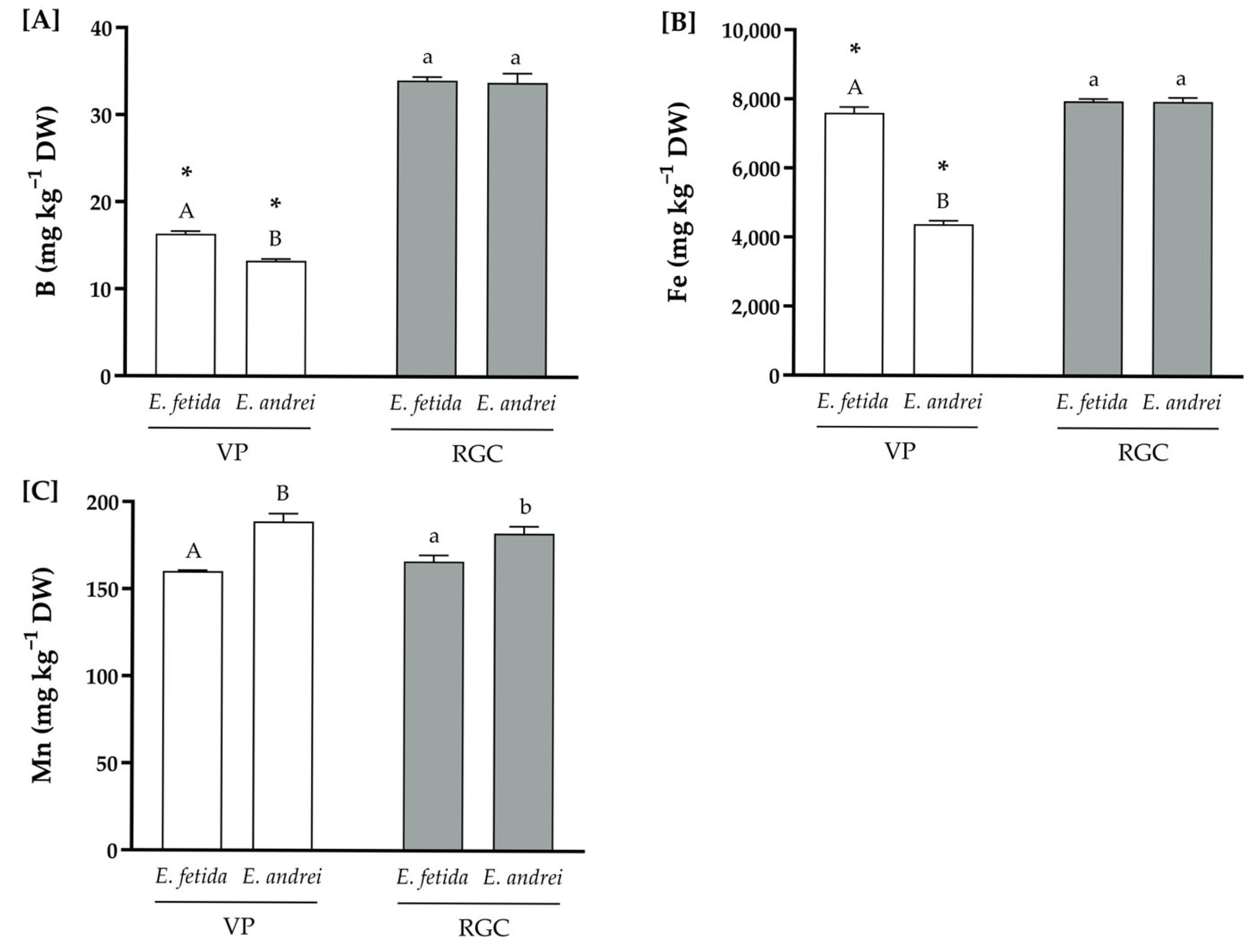
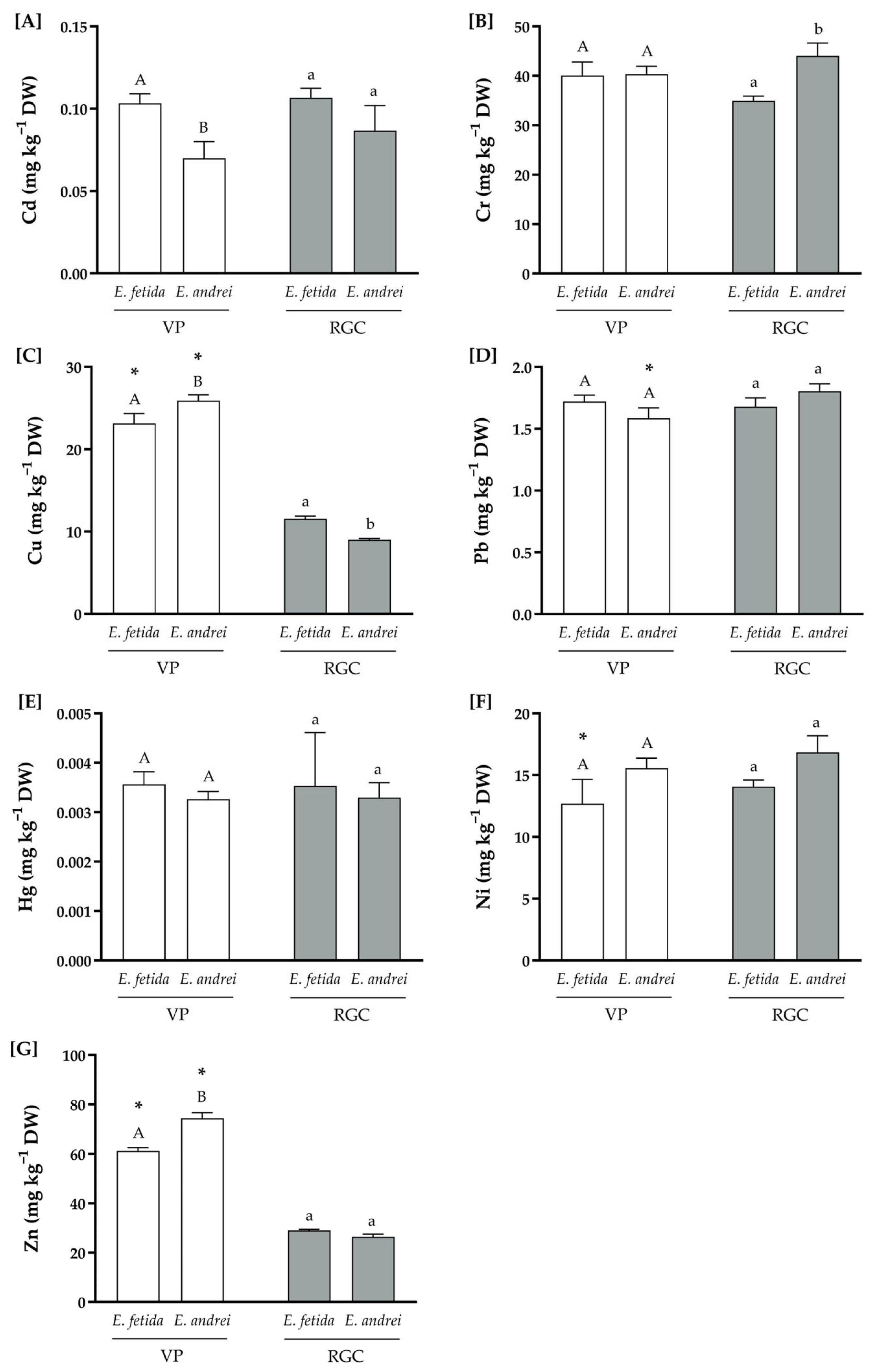
3.2. Physicochemical Compositions of the Substrates
3.3. Germination Index
3.4. Basal Respiration
3.5. Total Polyphenols
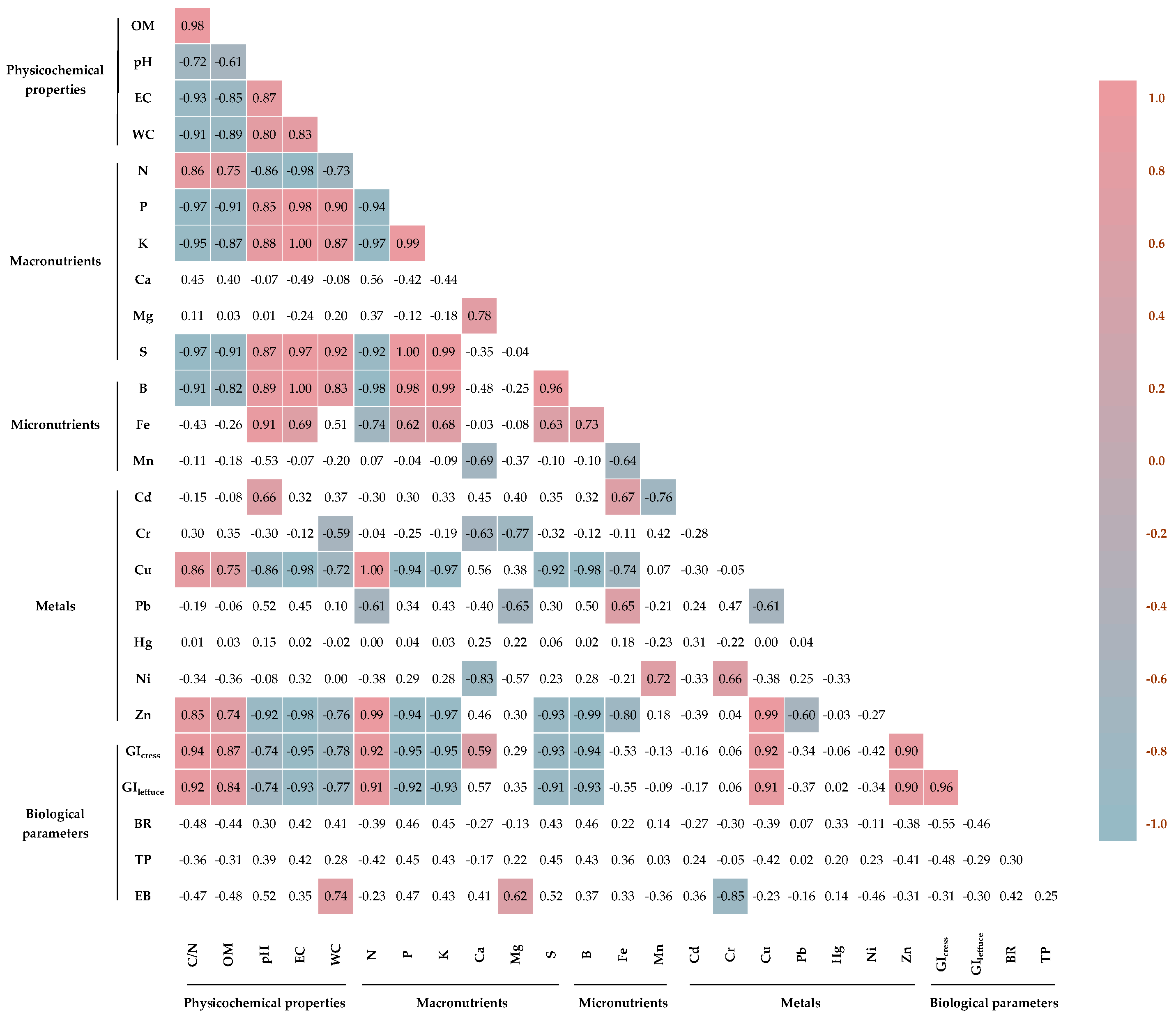
3.6. Correlation Analysis
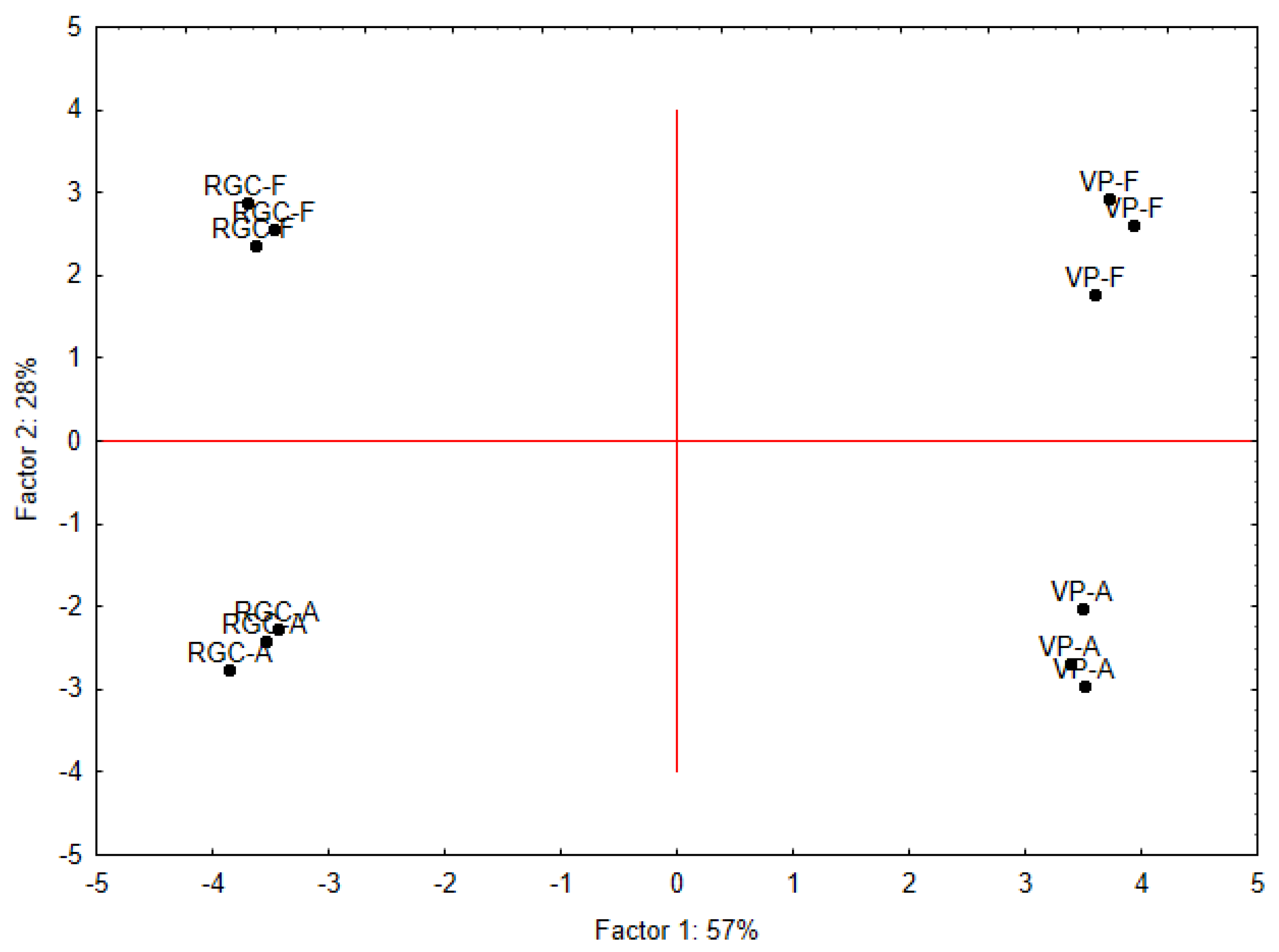
3.7. Principal Component Analysis
4. Discussion
4.1. General Effects of the Vermicomposting Process
4.2. Substrate-Specific Differences
4.3. Earthworm Species-Specific Influences
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DW | Dry weight |
| EC | Electrical conductivity |
| GAE | Gallic acid equivalent |
| MC | Moisture content |
| OM | Organic matter |
| RGC | Rotten grape cluster |
| SD | Standard deviation |
| VP | Vine pruning |
References
- Zhou, Y.; Xiao, R.; Klammsteiner, T.; Kong, X.; Yan, B.; Mihai, F.C.; Liu, T.; Zhang, Z.; Awasthi, M.K. Recent Trends and Advances in Composting and Vermicomposting Technologies: A Review. Bioresour. Technol. 2022, 360, 127591. [Google Scholar] [CrossRef] [PubMed]
- Carmona, I.; Aguirre, I.; Griffith, D.M.; García-Borrego, A. Towards a Circular Economy in Virgin Olive Oil Production: Valorization of the Olive Mill Waste (OMW) “Alpeorujo” through Polyphenol Recovery with Natural Deep Eutectic Solvents (NADESs) and Vermicomposting. Sci. Total Environ. 2023, 872, 162198. [Google Scholar] [CrossRef] [PubMed]
- Alshehrei, F.; Al-Enazi, N.M.; Ameen, F. Vermicomposting Amended with Microalgal Biomass and Biochar Produce Phytopathogen-Resistant Seedbeds for Vegetables. Biomass Convers. Biorefinery 2025, 15, 1795–1802. [Google Scholar] [CrossRef]
- Liu, N.; Graham, D.W.; Zhao, Y.; Yang, X.R.; Li, G.; Zhu, Y.G. Role of Earthworms and Their Excretion Products in Reducing Antimicrobial Resistance and Putative Pathogens during Vermicomposting. Chem. Eng. J. 2025, 512, 162765. [Google Scholar] [CrossRef]
- Nkurunziza, C.; Dukuzumuremyi, J.Y.; Tang, S.; Maimunah, M.A.; Kimani, S.M.; Sudo, S.; Tawaraya, K.; Horiguchi, K.I.; Wu, Y.; Cheng, W. Changes in Carbon and Nitrogen Contents and Greenhouse Gas Emissions during the Vermicomposting of Rice Straw Amended with Azolla. Soil Sci. Plant Nutr. 2025, 71, 323–332. [Google Scholar] [CrossRef]
- Manchal, R.; Venuste, T.; Verma, S.R. Vermicomposting, a Key to Sustainable Agriculture: A Review. Farming Manag. 2023, 8, 81–93. [Google Scholar] [CrossRef]
- Nogales, R.; Fernández-Gómez, M.J.; Delgado-Moreno, L.; Castillo-Díaz, J.M.; Romero, E. Eco-Friendly Vermitechnological Winery Waste Management: A Pilot-Scale Study. SN Appl. Sci. 2020, 2, 653. [Google Scholar] [CrossRef]
- Kolbe, A.R.; Aira, M.; Gómez-Brandón, M.; Pérez-Losada, M.; Domínguez, J. Bacterial Succession and Functional Diversity during Vermicomposting of the White Grape Marc Vitis vinifera v. Albariño. Sci. Rep. 2019, 9, 7472. [Google Scholar] [CrossRef]
- Thirunavukkarasu, A.; Sivashankar, R.; Nithya, R.; Sathya, A.B.; Priyadharshini, V.; Kumar, B.P.; Muthuveni, M.; Krishnamoorthy, S. Sustainable Organic Waste Management Using Vermicomposting: A Critical Review on the Prevailing Research Gaps and Opportunities. Environ. Sci. Process. Impacts 2023, 25, 364–381. [Google Scholar] [CrossRef]
- Dey Chowdhury, S.; Suhaib, K.H.; Bhunia, P.; Surampalli, R.Y. A Critical Review on the Vermicomposting of Organic Wastes as a Strategy in Circular Bioeconomy: Mechanism, Performance, and Future Perspectives. Environ. Technol. 2023, 1–38. [Google Scholar] [CrossRef]
- Teferedegn, G.D.; Ayele, C. Life Cycle Patterns of Epigeic Earthworm Species (Eisenia fetida, Eisenia andrei, and Dendrobaena veneta) in a Blend of Brewery Sludge and Cow Dung. Int. J. Zool. 2024, 2024, 6615245. [Google Scholar] [CrossRef]
- Suleiman, H.; Rorat, A.; Grobelak, A.; Grosser, A.; Milczarek, M.; Płytycz, B.; Kacprzak, M.; Vandenbulcke, F. Determination of the Performance of Vermicomposting Process Applied to Sewage Sludge by Monitoring of the Compost Quality and Immune Responses in Three Earthworm Species: Eisenia fetida, Eisenia andrei and Dendrobaena veneta. Bioresour. Technol. 2017, 241, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, J.; Velando, A.; Ferreiro, A. Are Eisenia fetida (Savigny, 1826) and Eisenia andrei (Oligochaeta, Lumbricidae) Different Biological Species? Pedobiologia 2005, 49, 81–87. [Google Scholar] [CrossRef]
- Lowe, C.N.; Butt, K.R. Earthworm Culture, Maintenance and Species Selection in Chronic Ecotoxicological Studies: A Critical Review. Eur. J. Soil Biol. 2007, 43, S281–S288. [Google Scholar] [CrossRef]
- Jaskulak, M.; Rorat, A.; Kurianska-Piatek, L.; Hofman, S.; Bigaj, J.; Vandenbulcke, F.; Plytycz, B. Species-Specific Cd-Detoxification Mechanisms in Lumbricid Earthworms Eisenia andrei, Eisenia fetida and Their Hybrids. Ecotoxicol. Environ. Saf. 2021, 208, 111425. [Google Scholar] [CrossRef]
- Plytycz, B.; Bigaj, J.; Osikowski, A.; Hofman, S.; Falniowski, A.; Panz, T.; Grzmil, P.; Vandenbulcke, F. The Existence of Fertile Hybrids of Closely Related Model Earthworm Species, Eisenia andrei and E. fetida. PLoS ONE 2018, 13, e0191711. [Google Scholar] [CrossRef]
- Rorat, A.; Suleiman, H.; Grobelak, A.; Grosser, A.; Kacprzak, M.; Płytycz, B.; Vandenbulcke, F. Interactions between Sewage Sludge-Amended Soil and Earthworms—Comparison between Eisenia fetida and Eisenia andrei Composting Species. Environ. Sci. Pollut. Res. 2016, 23, 3026–3035. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, D.; Zhang, Y.; Ke, J.; Chen, D.; Cai, M. Evaluation of Temperature on the Biological Activities and Fertility Potential during Vermicomposting of Pig Manure Employing Eisenia fetida. J. Clean. Prod. 2021, 302, 126804. [Google Scholar] [CrossRef]
- Gebrehana, Z.G.; Gebremikael, M.T.; Beyene, S.; Sleutel, S.; Wesemael, W.M.L.; De Neve, S. Organic Residue Valorization for Ethiopian Agriculture through Vermicomposting with Native (Eudrilus eugeniae) and Exotic (Eisenia fetida and Eisenia andrei) Earthworms. Eur. J. Soil Biol. 2023, 116, 103488. [Google Scholar] [CrossRef]
- Tajbakhsh, J.; Abdoli, M.A.; Mohammadi Goltapeh, E.; Alahdadi, I.; Malakouti, M.J. Recycling of Spent Mushroom Compost Using Earthworms Eisenia foetida and Eisenia andrei. Environmentalist 2008, 28, 476–482. [Google Scholar] [CrossRef]
- Brito, C.; Pereira, S.; Martins, S.; Monteiro, A.; Moutinho-Pereira, J.M.; Dinis, L. Strategies for Achieving the Sustainable Development Goals across the Wine Chain: A Review. Front. Sustain. Food Syst. 2024, 8, 1437872. [Google Scholar] [CrossRef]
- Nascimento-Gonçalves, E.; Azevedo, T.; Lopes, H.; Sousa, J.R.; Oliveira, P.A.; Roboredo, M.; Coimbra, A.M.; Morais, M.C. Vermicomposting as a Valorization Solution to the Winery Sector By-Products. Agronomy 2024, 14, 1111. [Google Scholar] [CrossRef]
- Nanni, A.; Parisi, M.; Colonna, M. Wine By-Products as Raw Materials for the Production of Biopolymers and of Natural Reinforcing Fillers: A Critical Review. Polymers 2021, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.P.; Gando-Ferreira, L.M.; Quina, M.J. Increasing Value of Winery Residues through Integrated Biorefinery Processes: A Review. Molecules 2022, 27, 4709. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M.D. Agrochemical Characterisation of the Solid By-Products and Residues from the Winery and Distillery Industry. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef]
- Geiger, C.A.; Daane, K.M. Seasonal Movement and Distribution of the Grape Mealybug (Homoptera: Pseudococcidae): Developing a Sampling Program for San Joaquin Valley Vineyards. J. Econ. Entomol. 2001, 94, 291–301. [Google Scholar] [CrossRef]
- Paradelo, R.; Moldes, A.B.; Barral, M.T. Carbon and Nitrogen Mineralization in a Vineyard Soil Amended with Grape Marc Vermicompost. Waste Manag. Res. 2011, 29, 1177–1184. [Google Scholar] [CrossRef]
- Gabur, G.D.; Teodosiu, C.; Fighir, D.; Cotea, V.V.; Gabur, I. From Waste to Value in Circular Economy: Valorizing Grape Pomace Waste through Vermicomposting. Agriculture 2024, 14, 1529. [Google Scholar] [CrossRef]
- Schröder, C.; Häfner, F.; Larsen, O.C.; Krause, A. Urban Organic Waste for Urban Farming: Growing Lettuce Using Vermicompost and Thermophilic Compost. Agronomy 2021, 11, 1175. [Google Scholar] [CrossRef]
- Koc-Jurczyk, J.; Jurczyk, Ł.; Podolak, A.; Pasek, A.; Dyka, M. Phytotoxicity of Products of Selected Technological Variants of Chemical Oxidation of Leachates from Municipal Waste Landfills. Ecohydrol. Hydrobiol. 2025, 100661. [Google Scholar] [CrossRef]
- Egler, S.G.; Roldão, T.M.; Santos, G.O.; Heidelmann, G.P.; Fraga, I.G.; Correia, F.V.; Saggioro, E.M. Phytotoxicity of Single and Mixed Rare Earth Element (La, Nd and Sm) Exposures on Lactuca sativa Seed Germination and Growth. Ecotoxicology 2024, 33, 1193–1209. [Google Scholar] [CrossRef] [PubMed]
- Hofstätter, K.; Schneider, S.I.; Altissimo, C.S.; Bauchspiess, K.; Medeiros, R.C.; Clasen, B.E.; Prestes, O.D.; Zanella, R.; Golombieski, J.I. Water Quality, Pesticides, and Phytotoxicity in Seeds of Lactuca sativa, Solanum lycopersicum, and Eruca sativa. Int. J. Environ. Sci. Technol. 2024, 21, 5971–5980. [Google Scholar] [CrossRef]
- Nogales, R.; Cifuentes, C.; Benítez, E. Vermicomposting of Winery Wastes: A Laboratory Study. J. Environ. Sci. Health Part B 2005, 40, 659–673. [Google Scholar] [CrossRef] [PubMed]
- Emino, E.R.; Warman, P.R. Biological Assay for Compost Quality. Compost Sci. Util. 2004, 12, 342–348. [Google Scholar] [CrossRef]
- Zucconi, F.; Forte, M.; Monaco, A.; De Bertoldi, M. Biological Evaluation of Compost Maturity. Biocycle 1981, 22, 27–29. [Google Scholar]
- Anderson, J.P.E. Soil Respiration. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1982; pp. 831–871. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Namlı, A.; Akça, H.; Akça, M.O. Vermicomposting of Agro-Industrial Waste by-Product of the Sugar Industry. Eurasian J. Soil Sci. 2020, 9, 292–297. [Google Scholar] [CrossRef]
- Pandit, L.; Sethi, D.; Pattanayak, S.K.; Nayak, Y. Bioconversion of Lignocellulosic Organic Wastes into Nutrient Rich Vermicompost by Eudrilus eugeniae. Bioresour. Technol. Rep. 2020, 12, 100580. [Google Scholar] [CrossRef]
- Vuković, A.; Velki, M.; Ečimović, S.; Vuković, R.; Štolfa Čamagajevac, I.; Lončarić, Z. Vermicomposting—Facts, Benefits and Knowledge Gaps. Agronomy 2021, 11, 1952. [Google Scholar] [CrossRef]
- Karapantzou, I.; Mitropoulou, G.; Prapa, I.; Papanikolaou, D.; Charovas, V.; Kourkoutas, Y. Physicochemical Changes and Microbiome Associations during Vermicomposting of Winery Waste. Sustainability 2023, 15, 7484. [Google Scholar] [CrossRef]
- Aira, M.; Monroy, F.; Domínguez, J. Earthworms Strongly Modify Microbial Biomass and Activity Triggering Enzymatic Activities during Vermicomposting Independently of the Application Rates of Pig Slurry. Sci. Total Environ. 2007, 385, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Q.; Zhang, Y.; Chen, J.; Zhang, D.; Tong, J. Changes in Fibrolytic Enzyme Activity during Vermicomposting of Maize Stover by an Anecic Earthworm Amynthas hupeiensis. Polym. Degrad. Stab. 2015, 120, 169–177. [Google Scholar] [CrossRef]
- Bhat, S.A.; Singh, J.; Vig, A.P. Instrumental Characterization of Organic Wastes for Evaluation of Vermicompost Maturity. J. Anal. Sci. Technol. 2017, 8, 2. [Google Scholar] [CrossRef]
- Shak, K.P.Y.; Wu, T.Y.; Lim, S.L.; Lee, C.A. Sustainable Reuse of Rice Residues as Feedstocks in Vermicomposting for Organic Fertilizer Production. Environ. Sci. Pollut. Res. 2014, 21, 1349–1359. [Google Scholar] [CrossRef]
- Devi, C.; Khwairakpam, M. Management of Lignocellulosic Green Waste Saccharum spontaenum through Vermicomposting with Cow Dung. Waste Manag. 2020, 113, 88–95. [Google Scholar] [CrossRef]
- Sharma, K.; Garg, V.K. Recycling of Lignocellulosic Waste as Vermicompost Using Earthworm Eisenia fetida. Environ. Sci. Pollut. Res. 2019, 26, 14024–14035. [Google Scholar] [CrossRef]
- Moral, R.; Paredes, C.; Bustamante, M.A.; Marhuenda-Egea, F.; Bernal, M.P. Utilisation of Manure Composts by High-Value Crops: Safety and Environmental Challenges. Bioresour. Technol. 2009, 100, 5454–5460. [Google Scholar] [CrossRef]
- Lv, B.; Zhang, D.; Cui, Y.; Yin, F. Effects of C/N Ratio and Earthworms on Greenhouse Gas Emissions during Vermicomposting of Sewage Sludge. Bioresour. Technol. 2018, 268, 408–414. [Google Scholar] [CrossRef]
- Lv, B.; Zhang, D.; Chen, Q.; Cui, Y. Effects of Earthworms on Nitrogen Transformation and the Correspond Genes (amoA and nirS) in Vermicomposting of Sewage Sludge and Rice Straw. Bioresour. Technol. 2019, 287, 121428. [Google Scholar] [CrossRef]
- Misra, D.; Dutta, W.; Jha, G.; Ray, P. Interactions and Regulatory Functions of Phenolics in Soil-Plant-Climate Nexus. Agronomy 2023, 13, 280. [Google Scholar] [CrossRef]
- Ganesh, P.S.; Gajalakshmi, S.; Abbasi, S.A. Vermicomposting of the Leaf Litter of Acacia (Acacia auriculiformis): Possible Roles of Reactor Geometry, Polyphenols, and Lignin. Bioresour. Technol. 2009, 100, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Brandón, M.; Lores, M.; Martínez-Cordeiro, H.; Domínguez, J. Effectiveness of Vermicomposting for Bioconversion of Grape Marc Derived from Red Winemaking into a Value-Added Product. Environ. Sci. Pollut. Res. 2020, 27, 33438–33445. [Google Scholar] [CrossRef] [PubMed]
- Gudeta, K.; Kumar, V.; Bhagat, A.; Julka, J.M.; Bhat, S.A.; Ameen, F.; Qadri, H.; Singh, S.; Amarowicz, R. Ecological Adaptation of Earthworms for Coping with Plant Polyphenols, Heavy Metals, and Microplastics in the Soil: A Review. Heliyon 2023, 9, e14572. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, M.; Taušnerová, H.; Hanč, A.; Tlustoš, P. Stabilization of Different Starting Materials through Vermicomposting in a Continuous-Feeding System: Changes in Chemical and Biological Parameters. Waste Manag. 2017, 62, 33–42. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Greer, D.H.; Hatfield, J.M.; Orchard, B.A.; Keller, M. Mineral Sinks within Ripening Grape Berries (Vitis vinifera L.). VITIS—J. Grapevine Res. 2006, 45, 115–123. [Google Scholar] [CrossRef]
- Bigard, A.; Romieu, C.; Sire, Y.; Veyret, M.; Ojéda, H.; Torregrosa, L. The Kinetics of Grape Ripening Revisited through Berry Density Sorting. OENO One 2019, 53, 709–724. [Google Scholar] [CrossRef]
- Hua, T.; Zhang, R.; Sun, H.; Liu, C. Alleviation of Boron Toxicity in Plants: Mechanisms and Approaches. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2975–3015. [Google Scholar] [CrossRef]
- Choudhary, S.; Zehra, A.; Mukarram, M.; Wani, K.I.; Naeem, M.; Khan, M.M.A.; Aftab, T. Salicylic Acid-Mediated Alleviation of Soil Boron Toxicity in Mentha arvensis and Cymbopogon flexuosus: Growth, Antioxidant Responses, Essential Oil Contents and Components. Chemosphere 2021, 276, 130153. [Google Scholar] [CrossRef]
- Behera, B.; Mrunalini, K.; Raza, M.B.; Shiv, A.; Mangal, V.; Rathod, G.; Altaf, M.A.; Kumar, A.; Aftab, T.; Kumar, R.; et al. Mechanistic Insight on Boron-Mediated Toxicity in Plant Vis-a-Vis Its Mitigation Strategies: A Review. Int. J. Phytoremediat. 2023, 25, 9–26. [Google Scholar] [CrossRef]
- Branham, S.E.; Daley, J.; Levi, A.; Hassell, R.; Wechter, W.P. QTL Mapping and Marker Development for Tolerance to Sulfur Phytotoxicity in Melon (Cucumis melo). Front. Plant Sci. 2020, 11, 1097. [Google Scholar] [CrossRef]
- Tu, Q.; Li, Y.; Ding, W.; Zhou, S.; Huang, Y.; Liu, S.; Min, X.; Zhang, J.; Li, J.; Yuan, C. Mixed Vineyard Prunings and Sheep Manure Compost: Creating Changes in Vineyard Soil Dominant Microbiota to Impact Fruit Quality. Appl. Soil Ecol. 2025, 208, 105973. [Google Scholar] [CrossRef]
- Rozas, A.; Aponte, H.; Maldonado, C.; Contreras-Soto, R.; Medina, J.; Rojas, C. Evaluation of Compost and Biochar as Partial Substitutes of Peat in Growing Media and Their Influence in Microbial Counts, Enzyme Activity and Lactuca sativa L. Seedling Growth. Horticulturae 2023, 9, 168. [Google Scholar] [CrossRef]
- Greco, C.; Comparetti, A.; Fascella, G.; Febo, P.; La Placa, G.; Saiano, F.; Mammano, M.M.; Orlando, S.; Laudicina, V.A. Effects of Vermicompost, Compost and Digestate as Commercial Alternative Peat-Based Substrates on Qualitative Parameters of Salvia officinalis. Agronomy 2021, 11, 98. [Google Scholar] [CrossRef]
- Cai, H.; Gao, D. Phytotoxicity of Salts in Composted Sewage Sludge and Correlation with Sodium Chloride, Calcium Nitrate, and Magnesium Nitrate. J. Plant Nutr. 2011, 34, 1788–1796. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of Salinity Stress on Plants and Its Tolerance Strategies: A Review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Turan, N.G. The Effects of Natural Zeolite on Salinity Level of Poultry Litter Compost. Bioresour. Technol. 2008, 99, 2097–2101. [Google Scholar] [CrossRef]
- Pace, R.; Benincasa, P. Effect of Salinity and Low Osmotic Potential on the Germination and Seedling Growth of Rapeseed Cultivars with Different Stress Tolerance. Ital. J. Agron. 2010, 5, 69–77. [Google Scholar] [CrossRef]
- Fukushima, M.; Yamamoto, K.; Ootsuka, K.; Komai, T.; Aramaki, T.; Ueda, S.; Horiya, S. Effects of the Maturity of Wood Waste Compost on the Structural Features of Humic Acids. Bioresour. Technol. 2009, 100, 791–797. [Google Scholar] [CrossRef]
- Spaccini, R.; Cozzolino, V.; Di Meo, V.; Savy, D.; Drosos, M.; Piccolo, A. Bioactivity of Humic Substances and Water Extracts from Compost Made by Ligno-Cellulose Wastes from Biorefinery. Sci. Total Environ. 2019, 646, 792–800. [Google Scholar] [CrossRef]
- Savy, D.; Cozzolino, V.; Nebbioso, A.; Drosos, M.; Nuzzo, A.; Mazzei, P.; Piccolo, A. Humic-like Bioactivity on Emergence and Early Growth of Maize (Zea mays L.) of Water-Soluble Lignins Isolated from Biomass for Energy. Plant Soil 2016, 402, 221–233. [Google Scholar] [CrossRef]
- Mendes, P.M.; Ribeiro, J.A.; Martins, G.A.; Lucia, T.; Araujo, T.R.; Fuentes-Guevara, M.D.; Corrêa, L.B.; Corrêa, É.K. Phytotoxicity Test in Check: Proposition of Methodology for Comparison of Different Method Adaptations Usually Used Worldwide. J. Environ. Manag. 2021, 291, 112698. [Google Scholar] [CrossRef] [PubMed]
- do Carmo, A.P.M.; Freitas, M.S.M.; Machado, L.C.; dos Santos Silva, L.; Petri, D.J.C.; Vimercati, J.C.; Matos, C.R.R.; Mathias, L.; Vieira, I.J.C.; de Carvalho, A.J.C. Electrical Conductivity of Nutrient Solutions Affects the Growth, Nutrient Levels, and Content and Composition of Essential Oils of Acmella oleracea (L.) R. K. Jansen from Southeastern Brazil. J. Agric. Food Res. 2024, 15, 100968. [Google Scholar] [CrossRef]
- Bignami, C.; Melegari, F.; Zaccardelli, M.; Pane, C.; Ronga, D. Composted Solid Digestate and Vineyard Winter Prunings Partially Replace Peat in Growing Substrates for Micropropagated Highbush Blueberry in the Nursery. Agronomy 2022, 12, 337. [Google Scholar] [CrossRef]
- Sokač Cvetnić, T.; Krog, K.; Lisak Jakopović, K.; Valinger, D.; Gajdoš Kljusurić, J.; Benković, M.; Jurina, T.; Jakovljević, T.; Radojčić Redovniković, I.; Jurinjak Tušek, A. Grape Skin Composting Process to Recycle Food Waste: Kinetics and Optimization. Foods 2024, 13, 824. [Google Scholar] [CrossRef]
- Baldacchino, F.; Lamaj, F. Application of Mealworm Frass in Organic Seedling Production of Allium cepa L., Beta vulgaris L., and Brassica rapa L. Seeds 2025, 4, 4. [Google Scholar] [CrossRef]
- Domínguez, J.; Martínez-Cordeiro, H.; Álvarez-Casas, M.; Lores, M. Vermicomposting Grape Marc Yields High Quality Organic Biofertiliser and Bioactive Polyphenols. Waste Manag. Res. J. Sustain. Circ. Econ. 2014, 32, 1235–1240. [Google Scholar] [CrossRef]
- Quintela-Sabarís, C.; Mendes, L.A.; Domínguez, J. Vermicomposting as a Sustainable Option for Managing Biomass of the Invasive Tree Acacia dealbata Link. Sustainability 2022, 14, 13828. [Google Scholar] [CrossRef]
- Rahman, M.; Hajam, Y.A. Selection and Evaluation of Optimal Medium for Eisenia fetida in Sustainable Waste Recycling. Discov. Anim. 2024, 1, 20. [Google Scholar] [CrossRef]
- Elvira, C.; Dominguez, J.; Briones, M.J.I. Growth and Reproduction of Eisenia andrei and E. fetida (Oligochaeta, Lumbricidae) in Different Organic Residues. Pedobiologia 1996, 40, 377–384. [Google Scholar] [CrossRef]
- Nigussie, A.; Kuyper, T.W.; Bruun, S.; de Neergaard, A. Vermicomposting as a Technology for Reducing Nitrogen Losses and Greenhouse Gas Emissions from Small-Scale Composting. J. Clean. Prod. 2016, 139, 429–439. [Google Scholar] [CrossRef]
- Pereira, M.M.A.; Moraes, L.C.; Mogollón, M.C.T.; Borja, C.J.F.; Duarte, M.; Buttrós, V.H.T.; Luz, J.M.Q.; Pasqual, M.; Dória, J. Cultivating Biodiversity to Harvest Sustainability: Vermicomposting and Inoculation of Microorganisms for Soil Preservation and Resilience. Agronomy 2023, 13, 103. [Google Scholar] [CrossRef]
- Gupta, S.; Kaushal, R.; Sood, G. Impact of Plant Growth–Promoting Rhizobacteria on Vegetable Crop Production. Int. J. Veg. Sci. 2018, 24, 289–300. [Google Scholar] [CrossRef]
- Devi, C.; Khwairakpam, M. Bioconversion of Lantana camara by Vermicomposting with Two Different Earthworm Species in Monoculture. Bioresour. Technol. 2020, 296, 122308. [Google Scholar] [CrossRef] [PubMed]
- Devi, J.; Pegu, R.; Mondal, H.; Roy, R.; Sundar Bhattacharya, S. Earthworm Stocking Density Regulates Microbial Community Structure and Fatty Acid Profiles during Vermicomposting of Lignocellulosic Waste: Unraveling the Microbe-Metal and Mineralization-Humification Interactions. Bioresour. Technol. 2023, 367, 128305. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Lores, M.; Domínguez, J. Species-Specific Effects of Epigeic Earthworms on Microbial Community Structure during First Stages of Decomposition of Organic Matter. PLoS ONE 2012, 7, e31895. [Google Scholar] [CrossRef]
- Das, D.; Abbhishek, K.; Banik, P.; Bhattacharya, P. A Valorisation Approach in Recycling of Organic Wastes Using Low-Grade Rock Minerals and Microbial Culture through Vermicomposting. Environ. Chall. 2021, 5, 100225. [Google Scholar] [CrossRef]
- Choukri, M.; Abouabdillah, A.; Bouabid, R.; Abd-Elkader, O.H.; Pacioglu, O.; Boufahja, F.; Bourioug, M. Zn Application through Seed Priming Improves Productivity and Grain Nutritional Quality of Silage Corn. Saudi J. Biol. Sci. 2022, 29, 103456. [Google Scholar] [CrossRef]
- Sullivan, B.K.; Keough, M.; Govers, L.L. Copper Sulphate Treatment Induces Heterozostera Seed Germination and Improves Seedling Growth Rates. Glob. Ecol. Conserv. 2022, 35, e02079. [Google Scholar] [CrossRef]
- Rather, B.A.; Mir, I.R.; Masood, A.; Anjum, N.A.; Khan, N.A. Nitric Oxide Pre-Treatment Advances Seed Germination and Alleviates Copper-Induced Photosynthetic Inhibition in Indian Mustard. Plants 2020, 9, 776. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, L.; Hu, K.; He, Y.; Wang, S.; Luo, J. Hydrogen Sulfide Promotes Wheat Seed Germination and Alleviates Oxidative Damage against Copper Stress. J. Integr. Plant Biol. 2008, 50, 1518–1529. [Google Scholar] [CrossRef]
- Wichuk, K.M.; McCartney, D. Compost Stability and Maturity Evaluation—A Literature Review. Can. J. Civ. Eng. 2010, 37, 1505–1523. [Google Scholar] [CrossRef]

| Laboratory Methods | Substrate | ||
|---|---|---|---|
| VP | RGC | ||
| Physical Properties | |||
| C/N | ISO 10694:1995 ISO 13878:1998 | 93.6 | 87.8 |
| OM (g kg−1 DW) | ISO 23400:2021 | 439 | 533 |
| Ph (H2O) | ISO 10390:2005 | 5.25 | 5.08 |
| EC (dS m−1) | ISO 11265:1994 | 1.45 | 1.53 |
| N-NH4+ (mg kg−1 FW) | n.d. | n.d. | |
| N-NO3− (mg kg−1 FW) | n.d. | n.d. | |
| WC (g kg−1 DW) | ISO 11465:1993 | 431.3 | 500.6 |
| Macronutrients (g kg−1 DW) | |||
| N | ISO 13878:1998 | 2.72 | 3.52 |
| P | ISO 11263:1994 | 0.37 | 0.74 |
| K | ISO 23470:2018 | 6.61 | 10.80 |
| Ca | ISO 11260:2018 | 2.53 | 1.68 |
| Mg | 1.05 | 0.93 | |
| S | 0.36 | 0.51 | |
| Micronutrients (mg kg−1 DW) | |||
| B | 12.4 | 16.9 | |
| Fe | ISO 11466:2005 | 9255 | 6671 |
| Mn | ISO 11466:1995 | 132.0 | 99.3 |
| Metals (mg kg−1 DW) | |||
| Cd | ISO 11466:1995 | 0.05 | 0.02 |
| Cr | 25.4 | 12.4 | |
| Cu | 13.69 | 7.07 | |
| Pb | 1.48 | 1.19 | |
| Hg | 0.012 | 0.015 | |
| Ni | 6.27 | 3.10 | |
| Zn | 31.7 | 12.0 | |
| Source of Variation | Physical Properties | |||||
|---|---|---|---|---|---|---|
| C/N | EC | OM | pH | N-NH4+ | WC | |
| Substrate | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Species | 0.288 | 0.040 | 0.004 | <0.001 | <0.001 | 0.004 |
| Substrate × Species | <0.001 | 0.325 | <0.001 | <0.001 | <0.001 | 0.001 |
| Source of Variation | Macronutrients | |||||
|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | |
| Substrate | <0.001 | <0.001 | <0.001 | <0.001 | 0.211 | <0.001 |
| Species | 0.811 | 0.017 | <0.001 | <0.001 | 0.016 | 0.001 |
| Substrate × Species | <0.001 | 0.001 | 0.018 | <0.001 | 0.050 | 0.001 |
| Source of Variation | Micronutrients | ||
|---|---|---|---|
| B | Fe | Mn | |
| Substrate | <0.001 | <0.001 | 0.907 |
| Species | 0.001 | <0.001 | <0.001 |
| Substrate × Species | 0.004 | <0.001 | 0.015 |
| Source of Variation | Metals | ||||||
|---|---|---|---|---|---|---|---|
| Cd | Cr | Cu | Pb | Hg | Ni | Zn | |
| Substrate | 0.122 | 0.582 | <0.001 | 0.054 | 1.000 | 0.110 | <0.001 |
| Species | 0.002 | 0.005 | 0.811 | 0.902 | 0.448 | 0.005 | <0.001 |
| Substrate × Species | 0.282 | 0.007 | <0.001 | 0.011 | 0.923 | 0.944 | <0.001 |
| GI (%) | Basal Respiration (mg CO2 g−1 OM d−1) | Total Polyphenols (mg GAE g−1 DW) | ||
|---|---|---|---|---|
| Garden Cress | Lettuce | |||
| Vine Prunings (VPs) | ||||
| E. Fetida (VP-F) | 250.04 ± 30.43 * | 125.54 ± 26.78 * | 4.930 ± 3.360 | 0.85 ± 0.09 |
| E. Andrei (VP-A) | 212.06 ± 39.78 * | 112.06 ± 6.22 * | 6.020 ± 0.095 | 0.81 ± 0.16 |
| Rotten Grape Clusters (RGCs) | ||||
| E. Fetida (RGC-F) | 48.52 ± 18.53 | 34.05 ± 8.01 | 7.863 ± 1.438 | 0.95 ± 0.24 |
| E. Andrei (RGC-A) | 39.82 ± 13.83 | 29.80 ± 16.59 | 8.093 ± 4.953 | 0.96 ± 0.15 |
| Two-Way ANOVA (p Values) | ||||
| Substrate | <0.001 | <0.001 | 0.197 | 0.235 |
| Species | 0.181 | 0.381 | 0.720 | 0.851 |
| Substrate × Species | 0.385 | 0.642 | 0.815 | 0.773 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, T.; Nascimento-Gonçalves, E.; Lopes, H.; Medeiros, C.; Falco, V.; Sousa, J.R.; Coimbra, A.M.; Roboredo, M.; Oliveira, P.A.; Morais, M.C. Valorization of Vineyard By-Products Through Vermicomposting: A Comparative Pilot-Scale Study with Eisenia fetida and Eisenia andrei. Agronomy 2025, 15, 1340. https://doi.org/10.3390/agronomy15061340
Azevedo T, Nascimento-Gonçalves E, Lopes H, Medeiros C, Falco V, Sousa JR, Coimbra AM, Roboredo M, Oliveira PA, Morais MC. Valorization of Vineyard By-Products Through Vermicomposting: A Comparative Pilot-Scale Study with Eisenia fetida and Eisenia andrei. Agronomy. 2025; 15(6):1340. https://doi.org/10.3390/agronomy15061340
Chicago/Turabian StyleAzevedo, Tiago, Elisabete Nascimento-Gonçalves, Henda Lopes, Catarina Medeiros, Virgílio Falco, João R. Sousa, Ana M. Coimbra, Marta Roboredo, Paula A. Oliveira, and Maria C. Morais. 2025. "Valorization of Vineyard By-Products Through Vermicomposting: A Comparative Pilot-Scale Study with Eisenia fetida and Eisenia andrei" Agronomy 15, no. 6: 1340. https://doi.org/10.3390/agronomy15061340
APA StyleAzevedo, T., Nascimento-Gonçalves, E., Lopes, H., Medeiros, C., Falco, V., Sousa, J. R., Coimbra, A. M., Roboredo, M., Oliveira, P. A., & Morais, M. C. (2025). Valorization of Vineyard By-Products Through Vermicomposting: A Comparative Pilot-Scale Study with Eisenia fetida and Eisenia andrei. Agronomy, 15(6), 1340. https://doi.org/10.3390/agronomy15061340










