The Identification, Environmental Factors, and Fungicide Sensitivity of Colletotrichum siamense Causing Leaf Disease of Oil Palm (Elaeis guineensis) in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Disease Assessment
2.2. PCR Amplification and Sequence Analysis
2.3. Phylogenetic Analyses
2.4. Morphological Observation
2.5. Pathogenicity Test
2.6. Effects of pH and Temperature on Mycelial Growth In Vitro
2.7. Reponses to Chemical Compounds In Vitro
2.8. Statistical Analysis
3. Results
3.1. Disease Symptoms
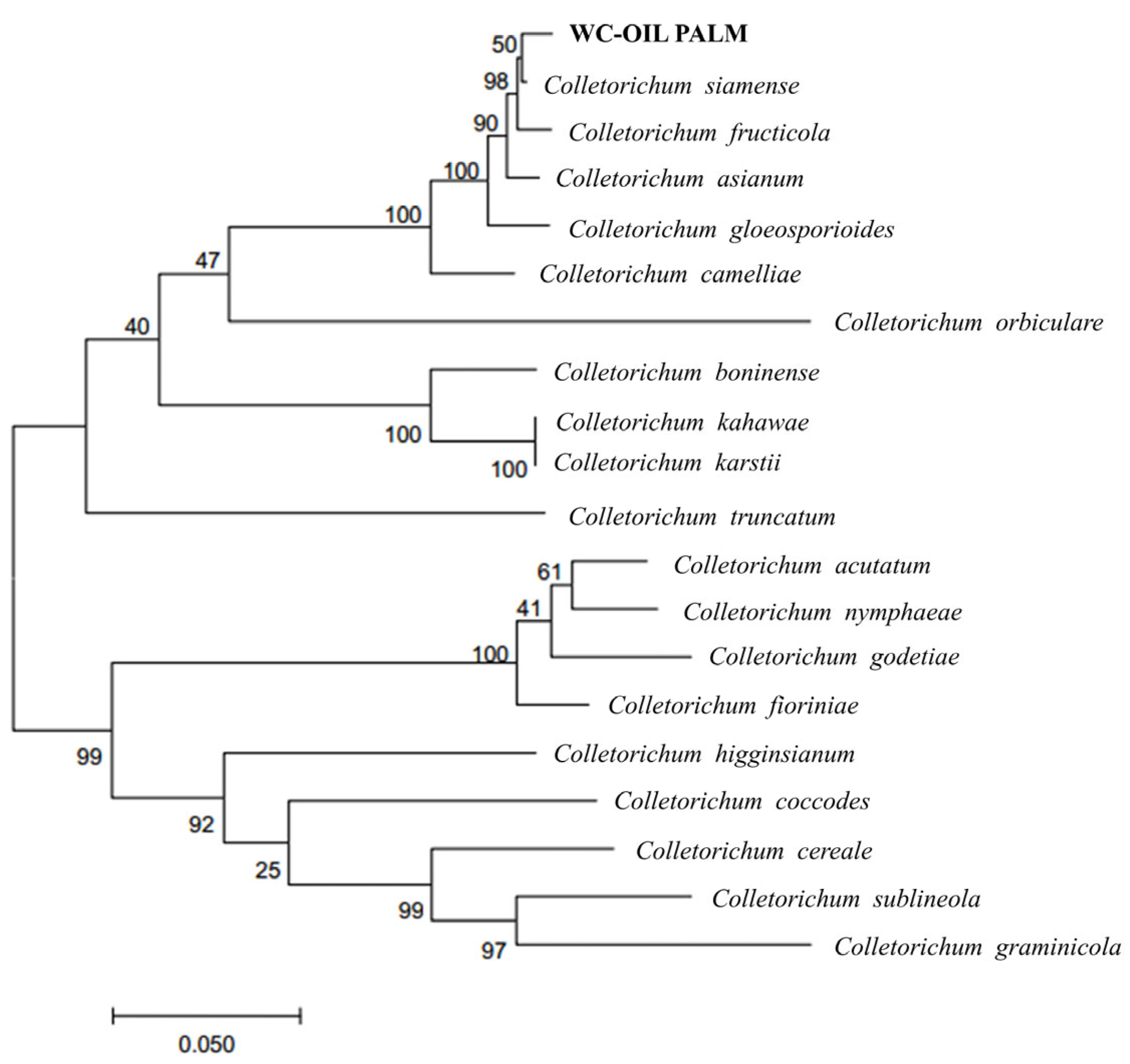
3.2. Species Identification and Phylogenic Analysis Within the Genus Colletotrichum
3.3. Morphology Characterization
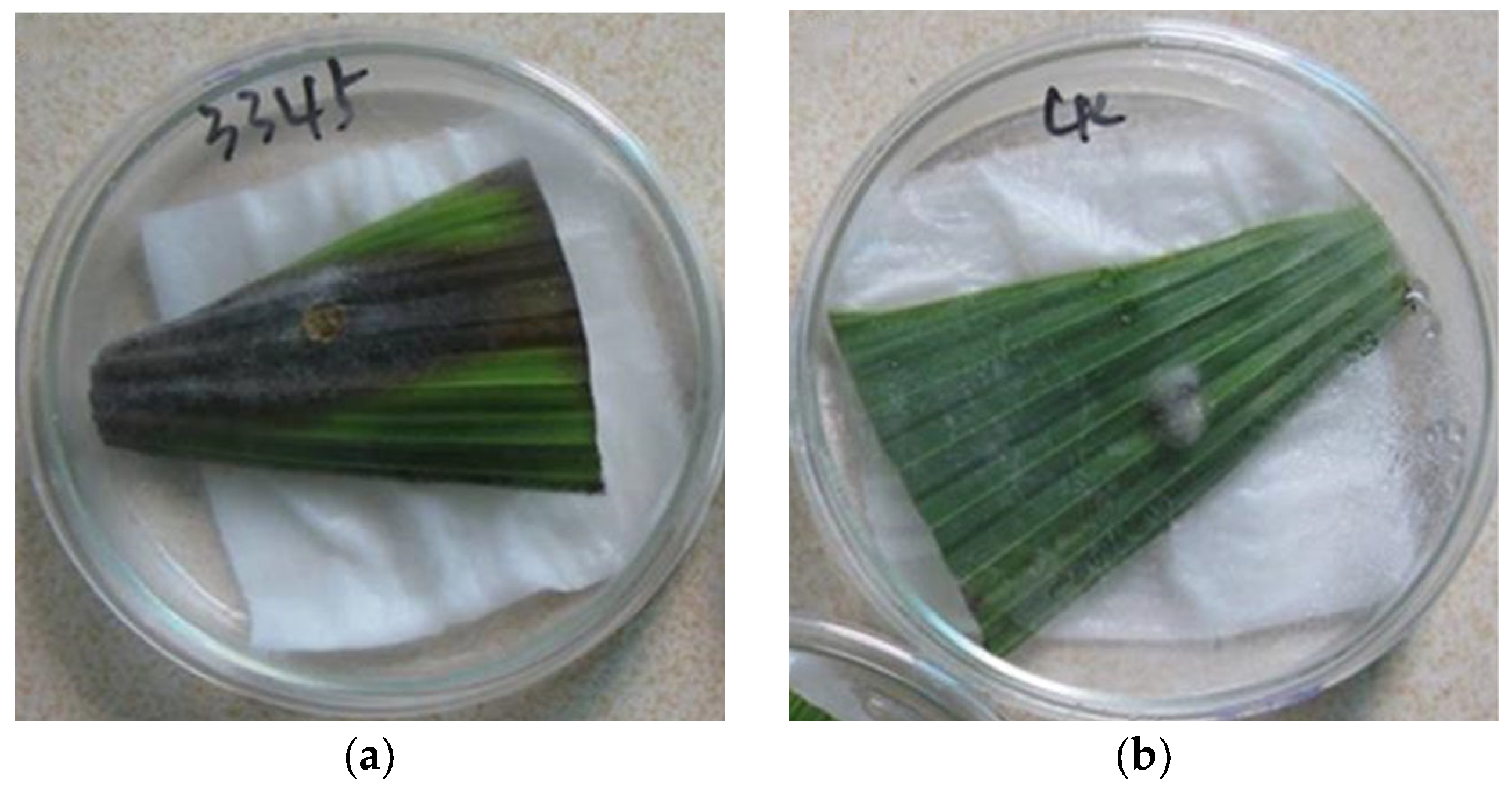
3.4. The Result of Pathogenicity Test
3.5. Effect of Various Environmental Conditions on the Mycelial Growth of C. siamense
3.6. Reponses of C. siamense to Chemical Compounds In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EC50 | Effective concertation, the minimum concentration of a chemical substance required to induce 50% inhibition of the growth of C. siamense after 3 days of in vitro exposure |
| LC90 | Lethal concentration, the minimum concentration of a chemical substance required to induce 90% mortality of C. siamense after 3 days of in vitro exposure |
| 95%CI | 95% confidence interval for the LC90 (EC50) |
Appendix A
| Gene Name | Gene Function | Primer Name | Primer Sequence |
|---|---|---|---|
| ITS | Taxonomic identification and phylogenetic reconstruction | ITS1 | TCCGTAGGTGAACCTGCGG |
| ITS4 | TCCTCCGCTTATTGATATGC | ||
| GAPDH | Housekeeping gene | GDF | GCCGTCAACGACCCCTTCATTGA |
| GDR | GGGTGGAGTCGTACTTGAGCATGT | ||
| CHS-1 | Mediating chitin biosynthesis | CHS-79F | TGGGGCAAGGATGCTTGGAAGAAG |
| CHS-345R | TCGAAGAACCATCTGTGAGAGTTG | ||
| ACT | Common reference gene | ACT-512F | ATGTGCAAGGCCGTTTCGC |
| ACT-783R | TACGAGTCCTTCTGGCCCAT | ||
| CAL | Modulating Ca2+-dependent signaling cascades | CL1C | GATTCAAGGAGGCCTTCTC |
| CL2C | CTTCTGCATCATGAGCTGGAC | ||
| TUB2 | Builds microtubules for cell division and structure | Bt2a | GGTAACCAAATCGGTGCTGCTTTC |
| Bt2b | ACCCTCAGTGTAGTGACCCTTGGC |
| Gene Name | Gene Function | Primer Name | Primer Sequence |
|---|---|---|---|
| CAL | Modulating Ca2+-dependent signaling cascades | CALDF1 | AGCAAGTCTCCGAGTTCAAGG |
| CALDR1 | CTTCTGCATCATCAYCTGGACG | ||
| TEF-1α | Housekeeping gene | EF-1Ha | ATGGGTAAGGAAGACAAGAC |
| EF-2Tb | GGAAGTACCAGTGATCATGTT | ||
| EFdF | AAGGAYGNCARACYCGNGARCAYGC | ||
| EF1-2218R | ATGACACCRACRGCRACRGTYTG |
References
- Wei, L.; John Martin, J.J.; Zhang, H.; Zhang, R.; Cao, H. Problems and prospects of improving abiotic stress tolerance and pathogen resistance of oil palm. Plants 2021, 10, 2622. [Google Scholar] [CrossRef] [PubMed]
- Nurrochmat, D.R.; Boer, R.; Ardiansyah, M.; Immanuel, G.; Purwawangsa, H. Policy forum: Reconciling palm oil targets and reduced deforestation: Landswap and agrarian reform in Indonesia. Forest Policy Econ. 2020, 119, 102291. [Google Scholar] [CrossRef]
- Bentivoglio, D.; Finco, A.; Bucci, G. Factors affecting the indonesian palm oil market in food and fuel industry: Evidence from a time series analysis. Int. J. Energy Econ. Policy 2018, 8, 49–57. [Google Scholar]
- Workman, D. Palm Oil Exports by Country. Available online: https://www.worldstopexports.com/palm-oil-exports-by-country/ (accessed on 23 August 2024).
- Wang, R.; Lee, K.E.; Mokhtar, M.; Goh, T.L. The challenges of palm oil sustainable consumption and production in China: An institutional theory perspective. Sustainability 2022, 14, 4856. [Google Scholar] [CrossRef]
- Zeng, X.H.; Pan, D.L.; Liu, Z.; Chen, J.M.; Lin, W.F. Evaluation of cold tolerant high yielding oil palm germplasm in Guangdong Province of south China, a northern tropical region. J. Oil Palm Res. 2016, 28, 266–280. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, L.; Li, X.; Xu, Y.; Du, Z.; Kanniah, K.; Li, C.; Cai, W.; Lin, H.; Peng, D.; et al. The expansion and remaining suitable areas of global oil palm plantations. Glob. Sustain. 2024, 7, e9. [Google Scholar] [CrossRef]
- Shen, H.; Zheng, L.; Zhang, J.; Lin, B.; Xiaoming, P.; Qin, X.; Li, J.; Xie, C. First Report of Pestalotiopsis microspora Causing Leaf Spot of Oil Palm (Elaeis guineensis) in China. Plant Dis. 2014, 98, 140722152922009. [Google Scholar] [CrossRef]
- Zheng, L.; Xi, P.; Tu, J.-J.; Chen, X.; Li, J.; Qin, X.; Shen, H.; Xie, C.-P. First report of Phoma herbarum causing leaf spot of oil palm (Elaeis guineensis) in China. Plant Dis. 2016, 101, 629. [Google Scholar] [CrossRef]
- Escalante, M.G.; Damas, D.; Márquez, D.; Gelvez, W.; Chacón, H.; Díaz, A.; Moreno, B. Diagnosis and evaluation of pestalotiopsis, and insect vectors, in an oil palm plantation at the south of Maracaibo Lake, Venezuela. Bioagro 2010, 22, 211–216. [Google Scholar]
- Penet, L.; Barthe, E.; Alleyne, A.; Blazy, J.M. Disease risk perception and diversity of management strategies by farmers: The case of anthracnose caused by Colletotrichum gloeosporioides on water yams (Dioscorea alata) in Guadeloupe. Crop Prot. 2016, 88, 7–17. [Google Scholar] [CrossRef]
- Ji, Y.; Li, X.; Gao, Q.-H.; Geng, C.; Duan, K. Colletotrichum species pathogenic to strawberry: Discovery history, global diversity, prevalence in China, and the host range of top two species. Phytopathol. Res. 2022, 4, 42. [Google Scholar] [CrossRef]
- Chang, L.; Li, Y.; Gao, Z.; Bonello, P.; Cleary, M.; Munck, I.A.; Santini, A.; Sun, H. Novel pathogen–plant host interaction: Colletotrichum jiangxiense and Fraxinus americana L. (white ash) in a sentinel garden in China. Plants 2023, 12, 4001. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Luo, C.X.; Wu, H.J.; Peng, B.; Kang, B.S.; Liu, L.M.; Zhang, M.; Gu, Q.S. Colletotrichum species associated with anthracnose disease of watermelon (Citrullus lanatus) in China. J. Fungi 2022, 8, 790. [Google Scholar] [CrossRef]
- Dowling, M.; Peres, N.; Villani, S.; Schnabel, G. Managing Colletotrichum on fruit crops: A “complex” challenge. Plant Dis. 2020, 104, 2301–2316. [Google Scholar] [CrossRef]
- Montri, P.; Taylor, P.W.J.; Mongkolporn, O. Pathotypes of Colletotrichum capsici, the causal agent of Chili anthracnose, in Thailand. Plant Dis. 2009, 93, 17–20. [Google Scholar] [CrossRef]
- Lin, Z.; Wei, J.; Zhang, M.; Xu, S.; Powell, C.A. Identification and characterization of a new fungal pathogen causing twisted leaf disease of sugarcane in China. Plant Dis. 2015, 99, 325–332. [Google Scholar] [CrossRef]
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum–current status and future directions. Stud. in Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef]
- Chen, J.; Cong, L.; Zhou, R.; Li, Z.; Piao, J.; Hao, N. Identification and characterization of Sclerotium delphinii causing southern blight on Aconitum kusnezoffii in northeast China. Plant Dis. 2022, 8, 106. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Nagadi, S.A.; Abo-Elyousr, K.A.M.; El-Fawy, M.M. Mitigating helminthosporium leaf spot disease in sesame: Evaluating the efficacy of castor essential oil and sodium bicarbonate on disease management and crop yield enhancement. J. Plant Pathol. 2024, 106, 683–694. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mishra, M.; Bohra, A.; Naik SJ, S.; Saabale, P.; Kumar, K.; Patil, P.G.; Srivastava, D.; Singh, N.P. First report of Fusarium equiseti causing wilt of Cajanus scarabaeoides, a wild relative of Pigeonpea, in India. Plant Dis. 2021, 105, 2735. [Google Scholar] [CrossRef]
- Innis, M.A.; Gelfand, D.H.; Sninsky, J.J.; White, T.J. PCR protocols: A guide to methods and applications. Trends Biotechnol. 1990, 8, 335. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microb. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.R.; Crandall, K.A.; Sing, C.F. A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. cladogram estimation. Genetics 1992, 132, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef]
- Moral, J.; Agustí-Brisach, C.; Raya, M.C.; Jurado-Bello, J.; López-Moral, A.; Roca, L.F.; Chattaoui, M.; Rhouma, A.; Nigro, F.; Sergeeva, V.; et al. Diversity of Colletotrichum species associated with olive anthracnose worldwide. J. Fungi 2021, 7, 741. [Google Scholar] [CrossRef]
- Zhang, M.; Forte-Perri, V.; Sun, W.; Tang, L.; Huang, S.; Guo, T.; Chen, X.; Li, Q. Identification and observation of infection processes of Colletotrichum species associated with persimmon anthracnose in Guangxi, China. Plant Dis. 2023, 107, 1670–1679. [Google Scholar] [CrossRef]
- Yağmur, A.; Demir, S.; Canpolat, S.; Rezaee Danesh, Y.; Farda, B.; Djebaili, R.; Pace, L.; Pellegrini, M.J.P. Onion Fusarium basal rot disease control by arbuscular mycorrhizal fungi and Trichoderma harzianum. Plants 2024, 13, 386. [Google Scholar] [CrossRef]
- Hall, B.G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evolution. 2013, 30, 1229–1235. [Google Scholar] [CrossRef]
- Senanayake, I.; Rathnayaka, A.; Sandamali, D.; Calabon, M.; Gentekaki, E.; Lee, H.; Pem, D.; Dissanayake, L.; Wijesinghe, S.; Bundhun, D.; et al. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere 2020, 11, 2678–2754. [Google Scholar] [CrossRef]
- Dhaver, P.; Pletschke, B.; Sithole, B.; Govinden, R. Isolation, screening, preliminary optimisation and characterisation of thermostable xylanase production under submerged fermentation by fungi in Durban, South Africa. Mycology 2022, 13, 271–292. [Google Scholar] [CrossRef]
- Liu, F.; Weir, B.S.; Damm, U.; Crous, P.W.; Wang, Y.; Liu, B.; Meng, Z.; Cai, L. Unravelling Colletotrichum species associated with Camellia: Employing ApMat and GS loci to resolve species in the C. gloeosporioides complex. Persoonia Mol. Phylogeny Evol. Fungi 2015, 35, 63–86. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Zhao, G.; Li, Q.; Solangi, G.S.; Tang, L.; Guo, T.; Huang, S.; Hsiang, T. Identification and characterization of Colletotrichum species associated with mango anthracnose in Guangxi, China. Plant Dis. 2018, 102, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kawy, M.A.; Michel, C.G.; Kirollos, F.N.; Hussien, R.A.A.; Al-Mahallawi, A.M.; Sedeek, M.S. Chemical composition and potentiation of insecticidal and fungicidal activities of Citrus trifoliata L. fruits essential oil against Spodoptera littoralis, Fusarium oxysporum and Fusarium solani via nano-cubosomes. Nat. Prod. Res. 2021, 35, 2438–2443. [Google Scholar] [CrossRef]
- Riolo, M.; Aloi, F.; Pane, A.; Cara, M.; Cacciola, S.O. Twig and shoot dieback of citrus, a new disease caused by Colletotrichum species. Cells 2021, 10, 449. [Google Scholar] [CrossRef]
- Pandey, A.K.; Samota, M.K.; Tanti, A.J.; Babu, A. Trichoderma reesei induces defense-related biochemical markers associated with resistance to Fusarium dieback in tea crop. Biol. Control 2023, 180, 105200. [Google Scholar] [CrossRef]
- Lee, D.M.; Hassan, O.; Chang, T. Identification, characterization, and pathogenicity of Colletotrichum species causing anthracnose of peach in Korea. Mycobiology 2020, 48, 210–218. [Google Scholar] [CrossRef]
- Hassan, O.; Kim, J.S.; Romain, B.B.N.D.; Chang, T. An account of Colletotrichum species associated with anthracnose of Atractylodes ovata in South Korea based on morphology and molecular data. PLoS ONE 2022, 17, e0263084. [Google Scholar] [CrossRef]
- Wang, Y.R.; Hu, Z.; Zhong, J.; Chen, Y.; Zhu, J.Z. First report of Colletotrichum cliviicola causing leaf spot on tobacco (Nicotiana tabacum) in Hunan Province of China. Plant Dis. 2022, 106, 316. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, W.; Li, Y.; Ji, S.; Li, X.; Hyde, K.D.; Zhang, K.; Phillips, A.J.L.; Manawasinghe, I.S.; Yan, J. Identification and characterization of Colletotrichum species associated with cherry leaf spot disease in China. Plant Dis. 2023, 107, 500–513. [Google Scholar] [CrossRef]
- Li, P.; Zhu, J.Z.; Li, X.G.; Zhong, J. Identification and characterization of Colletotrichum fructicola and Colletotrichum siamense causing anthracnose on luffa sponge gourd in China. Plant Dis. 2022, 11, 1537. [Google Scholar] [CrossRef]
- Anuradha; Sharma, A.; Sood, S.; Badiyal, A.; Sood, T. Fruit rot of Capsicum spp.: Implications and management strategies. J. Hortic. Sci. Biotech. 2023, 98, 715–731. [Google Scholar] [CrossRef]
- Zou, H.Y.; Li, T.W.; Zhang, J.; Shao, H.J.; Kageyama, K.; Feng, W.Z. Rapid detection of Colletotrichum siamense from infected tea plants using filter-disc DNA extraction and loop-mediated isothermal amplification. Plant Dis. 2024, 108, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, L.; Li, X.; Wang, Y.; Feng, Y.; Huang, G.J.A. The diseases and pests of rubber tree and their natural control potential: A bibliometric analysis. Agronomy 2023, 13, 1965. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, D.; Tu, W.Y.; Ma, F.W.; Liu, C.H. Overexpression of auxin/indole-3-acetic acid gene MdIAA24 enhances Glomerella leaf spot resistance in apple (Malus domestica). Hortic. Plant J. 2024, 10, 15–24. [Google Scholar] [CrossRef]
- Fang, Y.; Zhao, P.; Wang, S.; Cui, X.; Zhang, T.; Ye, R.; Zeng, Y.; Zhang, Y. Colletotrichum siamense causing leaf spot on Parthenocissus semicordata in China. Crop Prot. 2024, 185, 106878. [Google Scholar] [CrossRef]
- Cao, X.; Xu, X.; Che, H.; West, J.S.; Luo, D. Eight Colletotrichum species, including a novel species, are associated with areca palm anthracnose in Hainan, China. Plant Dis. 2020, 104, 1369–1377. [Google Scholar] [CrossRef]
- Kennedy, A.H.; Schoch, C.L.; Marrero, G.; Brover, V.; Robbertse, B. Publicly available and validated DNA Reference sequences are critical to fungal identification and global plant protection efforts: A use-case in Colletotrichum. Plant Dis. 2022, 106, 1573–1596. [Google Scholar] [CrossRef]
- Gadd, G.M.; Fomina, M.; Pinzari, F. Fungal biodeterioration and preservation of cultural heritage, artwork, and historical artifacts: Extremophily and adaptation. Microbiol. Mol. Bio Rev. 2024, 88, e00200-00222. [Google Scholar] [CrossRef]
- Sandoval-Contreras, T.; Iñiguez-Moreno, M.; Garrido-Sánchez, L.; Ragazzo-Sánchez, J.A.; Narváez-Zapata, J.A.; Ascencio, F.; Calderón-Santoyo, M. Predictive model for the effect of environmental conditions on the postharvest development of Colletotrichum gloeosporioides strains isolated from papaya (Carica papaya L.). J. Food Protect 2020, 83, 1495–1504. [Google Scholar] [CrossRef]
- Lin, S.-R.; Lin, Y.-H.; Ariyawansa, H.A.; Chang, Y.-C.; Yu, S.-Y.; Tsai, I.; Chung, C.-L.; Hung, T.-H. Analysis of the pathogenicity and phylogeny of Colletotrichum species associated with brown blight of tea (Camellia sinensis) in Taiwan. Plant Dis. 2023, 107, 97–106. [Google Scholar] [CrossRef]
- Brugneti, F.; Rossini, L.; Drais, M.I.; Turco, S.; Mazzaglia, A. Effect of temperature on in vitro germination and growth of Colletotrichum fioriniae, a new emerging pathogen of olive fruits. Env. Microbiol. Rep. 2024, 16, e13275. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liao, Y.-C.-Z.; Wan, Y.; Li, D.-W.; Zhu, L.-H. Colletotrichum siamense, a novel causal agent of Viburnum odoratissimum leaf blotch and its sensitivity to fungicides. J. Fungi 2023, 9, 882. [Google Scholar] [CrossRef] [PubMed]
- Iqra; ul Haq, I.; Waseem Khan Qadri, R.; Amrao, L.; Ijaz, S. Effect of environmental conditions (temperature and precipitation) on severity of guava die-back caused by Colletotrichum spp. under climatic conditions of Pakistan. J. Plant Pathol. 2022, 104, 179–190. [Google Scholar] [CrossRef]
- Tao, R.; Yang, B.; Lin, L.; Munir, S.; Li, Y.; Wang, X.; Huang, M. Biological characterization of emerging fungal pathogen Colletotrichum associated with mango (Mangifera indica L.) post-harvest anthracnose from Vietnam. Mol. Biol. Rep. 2024, 51, 557. [Google Scholar] [CrossRef]
- Wei, R.; Wang, R.; Li, Y.; Yue, M.; Ding, W. Identification, biological characteristics and fungicide sensitivity of Colletotrichum species that cause anthracnose on Anemarrhena asphodeloides in China. J. Phytopathol. 2022, 170, 373–381. [Google Scholar] [CrossRef]
- Delna, R.; Sharma, G.; Rawat, S. Impact of rice-husk biochar on Colletotrichum falcatum, the pathogen of sugarcane red rot disease. Indian Phytopathol. 2022, 75, 325–329. [Google Scholar] [CrossRef]
- Ishii, H.; Watanabe, H.; Yamaoka, Y.; Schnabel, G. Sensitivity to fungicides in isolates of Colletotrichum gloeosporioides and C. acutatum species complexes and efficacy against anthracnose diseases. Pestic. Biochem. Phys. 2022, 182, 105049. [Google Scholar] [CrossRef]
- Usman, H.M.; Tan, Q.; Karim, M.M.; Adnan, M.; Yin, W.-X.; Zhu, F.-X.; Luo, C.-X. Sensitivity of Colletotrichum fructicola and Colletotrichum siamense of peach in China to multiple classes of fungicides and characterization of pyraclostrobin-resistant isolates. Plant Dis. 2021, 105, 3459–3465. [Google Scholar] [CrossRef]
- Nawaz, H.H.; Manzoor, A.; Iqbal, M.Z.; Ansar, M.R.; Ali, M.; Muhammad Kakar, K.; Ali Awan, A.; Weiguo, M. Colletotrichum acutatum: Causal agent of olive anthracnose isolation, characterization, and fungicide susceptibility screening in Punjab, Pakistan. Plant Dis. 2023, 107, 1329–1342. [Google Scholar] [CrossRef]
- Karim, M.M.; Usman, H.M.; Tan, Q.; Hu, J.-J.; Fan, F.; Hussain, R.; Luo, C.-X. Fungicide resistance in Colletotrichum fructicola and Colletotrichum siamense causing peach anthracnose in China. Pestic. Biochem. Phys. 2024, 203, 106006. [Google Scholar] [CrossRef]
- Gelain, J.; Lykins, S.; Rosa, P.F.; Soares, A.T.; Dowling, M.; Schnabel, G.; May De Mio, L.L. Identification and fungicide sensitivity of Colletotrichum spp. from apple flowers and fruitlets in Brazil. Plant Dis. 2023, 107, 1183–1191. [Google Scholar] [CrossRef]



| Species | Origin | GenBank Accession Number | |||
|---|---|---|---|---|---|
| ITS | GAPDH | ACT | TUB2 | ||
| C. siamense | China | MG830351 | MG830377 | MG830429 | MG830326 |
| C. boninense | Japan | JQ005153 | JQ005240 | JQ005501 | JQ005588 |
| C. asianum | India | JQ894679 | JQ894623 | JQ894545 | JQ894601 |
| C. gloeosporioides | Italy | JX010152 | JX010056 | JX009531 | JX010445 |
| C. camelliae | China | KJ955094 | KJ954795 | KJ954376 | KJ955243 |
| C. fructicola | China | MH370509 | MH370516 | MH370530 | MH370551 |
| C. kahawae | Colombia | JQ005215 | JQ005302 | JQ005563 | JQ005649 |
| C. sublineola | China | MF405438 | KY038879 | KY038877 | KY038881 |
| C. graminicola | Brazil | MF803827 | MF803828 | MF803829 | MF803830 |
| C. cereale | China | JX625161 | KC843518 | KC843535 | JX625188 |
| C. karstii | Colombia | JQ005215 | JQ005302 | JQ005563 | JQ005649 |
| C. truncatum | Brazil | MG543285 | MG543284 | MG543286 | MG543283 |
| C. coccodes | The Netherlands | HM171679 | HM171673 | HM171667 | JX546873 |
| C. higginsianum | China | MF033888 | MF033889 | MF033892 | MF033895 |
| C. orbiculare | India | KP898982 | KP898936 | KP899005 | KP899052 |
| C. godetiae | Italy | KY293406 | KY293404 | KY293402 | KY293407 |
| C. fioriniae | UK | JQ948344 | JQ948674 | JQ949665 | JQ949995 |
| C. acutatum | Australia | JQ005776 | JQ948677 | JQ005839 | JQ005860 |
| C. nymphaeae | Republic of Korea | LC428839 | LC428842 | LC428840 | LC428841 |
| Compound | EC50 (95%CI) | r2 * | LC90 (95%CI) | r # |
|---|---|---|---|---|
| Polyoxin B | 12.74 (4.91, 33.98) | 0.9777 | 5857.70 (1629.00, 45075.08) | 0.9955 |
| Pyraclostrobin | 0.35 (0.20, 0.63) | 0.9751 | 109.56 (57.12, 246.85) | 0.9962 |
| Tebuconazole | 0.10 (0.06, 0.18) | 0.9785 | 1.24 (1.02, 1.53) | 0.9532 |
| Thiram | 15.54 (9.80, 24.88) | 0.9938 | 309.95 (171.19, 708.54) | 0.9911 |
| Prochloraz | 0.02 (0.01, 0.04) | 0.9683 | 0.19 (0.15, 0.23) | 0.9554 |
| Mancozeb | 8.75 (5.03, 15.03) | 0.9909 | 64.58 (44.06, 107.28) | 0.9902 |
| Chlorothalonil | 10.10 (2.97, 33.61) | 0.9557 | 402.70 (193.63, 1200.55) | 0.9906 |
| Carbendazim | 0.1381 (0.11, 0.18) | 0.9938 | 0.91 (0.72, 1.14) | 0.9496 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Pang, Q.; Wang, Z.; Jiang, C.; Sun, X.; Liu, Z.; Zhou, M.; Chen, Y.; Bian, Q. The Identification, Environmental Factors, and Fungicide Sensitivity of Colletotrichum siamense Causing Leaf Disease of Oil Palm (Elaeis guineensis) in China. Agronomy 2025, 15, 1331. https://doi.org/10.3390/agronomy15061331
Li H, Pang Q, Wang Z, Jiang C, Sun X, Liu Z, Zhou M, Chen Y, Bian Q. The Identification, Environmental Factors, and Fungicide Sensitivity of Colletotrichum siamense Causing Leaf Disease of Oil Palm (Elaeis guineensis) in China. Agronomy. 2025; 15(6):1331. https://doi.org/10.3390/agronomy15061331
Chicago/Turabian StyleLi, Haipeng, Qiangqiang Pang, Zhuoying Wang, Changchang Jiang, Xiaodong Sun, Zhenghui Liu, Man Zhou, Yisong Chen, and Qiang Bian. 2025. "The Identification, Environmental Factors, and Fungicide Sensitivity of Colletotrichum siamense Causing Leaf Disease of Oil Palm (Elaeis guineensis) in China" Agronomy 15, no. 6: 1331. https://doi.org/10.3390/agronomy15061331
APA StyleLi, H., Pang, Q., Wang, Z., Jiang, C., Sun, X., Liu, Z., Zhou, M., Chen, Y., & Bian, Q. (2025). The Identification, Environmental Factors, and Fungicide Sensitivity of Colletotrichum siamense Causing Leaf Disease of Oil Palm (Elaeis guineensis) in China. Agronomy, 15(6), 1331. https://doi.org/10.3390/agronomy15061331





