Simple Summary
Cotton bollworm (Helicoverpa armigera) is a polyphagous pest that can produce high levels of damage to maize, soybean, and cotton, with significant impacts worldwide, except on the North American continent. Up until ten years ago, it was considered a minor pest in Romania. We monitored the population of this pest in southeast Romania using pheromonal traps from 2020 to 2024, and we studied the behavior of nine maize hybrids from three maturity groups in response to a cotton bollworm attack in 2024. We found an increase in corn earworm captures in the traps starting from 2022, more than 12 times higher compared to 2020–2021. There was a high population in 2024. In most of the years, we found two flight peaks in August and September; however, in 2022 and 2024, a peak occurred in July too. In September 2024, regarding all the maize hybrids in this study, the pest attack incidence on cobs was 100%. We analyzed the maize hybrids’ yield for total aflatoxin (B1 + B2 + G1 + G2) levels. Because of the high number of pest attacks in 2024, the aflatoxin levels were higher than the maximum limits (Reg. EC 1881/2006) for all hybrids in this study.
Abstract
This paper presents a five-year study monitoring cotton bollworm (Helicoverpa armigera) flight dynamics using pheromonal traps and a study relating to the behavior of nine maize hybrids from two maturity groups in response to a pest attack in 2024. The monitoring and field assessments were conducted in Southeast Romania, Călărași County, at the NARDI Fundulea. During the monitoring period, from 2020 to 2024, air temperature was higher than average in the summer months while rainfall was below average, except in June 2021. The total number of moths captured in the traps was 246 in 2020, 406 in 2021, 5064 in 2022, 1024 in 2023, and 4145 in 2024. In the middle of July 2022, the average captured moths per trap was 483.3; in the middle of September, it was 589.0 catches. In 2024, in the last 10 days of July, the average captured moths per trap was 311.7; in the last 10 days of August, it was 358.0, while in the middle of September, it was 362.3. In 2024, at the beginning of August, the attack incidence of corn earworm on maize hybrids ranged from 43.75 to 53.75%, and on 13 September, it was 100% for all hybrids. This is the first report from southeast Romania that mentions a higher population of cotton bollworm in the late summer and beginning of autumn and the first report to mention a large number of pest attacks on maize cobs in September.
1. Introduction
Cotton bollworm (CBW) (Hepicoverpa armigera Hübner, 1809, Lepidoptera: Noctuidae) is one of the most dangerous pests for agricultural and horticultural crops worldwide, except in the United States [1,2,3,4,5]. It is invasive in South America and was first reported in Brazil in 2013 [6]. Many authors considered this species a pest from the Old World [7,8]. In the USA, the CBW is a quarantine pest, detected only in Puerto Rico in 2014 [9,10]. However, in this country, it is Helicoverpa zea, a species closely related to H. armigera [11]. In Europe, it is considered a native pest, widespread in the central and southern parts of the continent, which occasionally have outbreaks [3,12,13,14]. Data from the literature reveal that CBW is a polyphagous pest with more than 100 plant species from different botanical families [15,16,17,18]. Recent studies have shown that CBWs have more than 200 host plants [4,19,20]. The most important crops for this pest are cotton, maize, sorghum, soybean, pigeon pea, chickpea, cowpea, and tomato [1]. In recent years, global economic losses caused by CBW were over USD 3 billion [4,21]. In a short period after the invasion of CBW in Brazil, financial losses caused by this pest were over USD 2 billion [22]. However, most of the economic losses caused by CBW are in tropical and subtropical areas and are less impactful in countries with temperate climates [7,23,24]. In the EU, this pest was introduced to the A2 list [25]. CBW has up to 12 generations per year in countries with tropical climates and from 3 to 5 generations per year in countries in subtropical and Mediterranean areas [1,25]. In regions with a temperate climate, this pest has 1 to 3 generations per year and can overwinter [26,27,28]. CBW is characterized by a high degree of prolificity; a single female can lay more than 1000 eggs during her life span, with a maximum of 2700 eggs [1]. Although early reports indicate that CBW does not exhibit migratory behavior [5], recent papers demonstrate that moths can fly more than 10 km, especially during egg laying, specifically between 10 and 500 km [13,29]. Other papers mentioned that CBW moths can migrate up to 2000 km, using wind currents [30]. Research conducted in China, using an entomological radar, revealed that the moths migrate up to 2000 m in altitude, when winds are favorable [31]. The same research shows that the moths’ speed reaches 30–33 km/hour during this migration. In Europe, CBW was considered a facultative migratory pest [24,32]. According to Pedgley [33], CBW moths can migrate long distances, borne by wind, from Southern Europe to the British Isles. Other studies have shown evidence of the migration of this pest from the northern regions of Europe to the south and the Mediterranean basin in autumn [1]. There have been many reports concerning CBW’s resistance to insecticides from different chemical classes [34,35,36,37,38,39,40], including newer active ingredients from the diamide class [41,42,43]. CBW develops resistance mechanisms to transgenic (Bt) cotton, soybean, and maize in countries where these crops are cultivated on a large scale [44,45,46,47,48,49]. Its high adaptability to different climates, overwintering capacity, high prolificity, and high resistance to insecticides and Bt crops make it very challenging to control this pest. The grain lesions caused by feeding CBW larvae on the maize cobs can be infected with fungi from the Aspergillus and Fusarium genera [50,51]. Mycotoxins are metabolic products of the fungus and can accumulate in the infected maize grains [52,53,54]. The aflatoxins were produced by the Aspergillus flavus and A. parasiticus fungi [55,56,57]. There are several types of aflatoxins, but the most dangerous for human consumption and animal feeding is aflatoxin B1, which can cause liver cancer [52,57,58,59]. The same authors mentioned that people exposed to aflatoxins and chronic infection with the hepatitis B virus have an increased risk of hepatocellular carcinoma. This danger occurs when infected maize grains are directly consumed [60,61]. If the livestock consumes the infected maize grains, the B and G type aflatoxins are metabolized into M type aflatoxins [62], found in milk and dairy products [63,64]. Drought and high temperatures favor Aspergillus flavus activity and can increase aflatoxin levels in the maize grains attacked by CBW larvae [52,65,66]. Because of climate change, the aflatoxins are not a problem only in tropical and subtropical areas, but there are also increasing cases in some European countries, including Hungary [67]. According to EU Commission Regulation (EC) No 1881/2006, the maximum level of total aflatoxins (B1 + B2 + G1 + G2) in maize grains is 10.00 μg/kg, while for B1 aflatoxins it is 5.00 μg/kg [68]. Farmers cannot use maize for livestock and human consumption if the maximum aflatoxin level exceeds this limit [69,70]. This fact can negatively affect local and national agriculture, with severe economic losses for the farmers and even bankruptcy [71,72]. In Romania, maize is one of the most important crops. Data from the Ministry of Agriculture and Rural Development show that in this country, from 2015 to 2023, the area cultivated with maize constituted over 2.5 million hectares in 2015, 2016, 2019, 2020, and 2021 [73]. Also, Romania has a higher area cultivated with maize compared to other EU27 countries [74]. However, in Romania, total production ranged from 8.037.134 tons in 2022 to 18.663.939 tons in 2018 [73]. In some years, maize production decreases due to drought, weeds, or pests [75,76,77]. The most dangerous pests for maize crops in Romania are maize leaf weevil (Tanymecus dilaticollis), wireworms (Agriotes spp.), European corn borer (Ostrinia nubilallis), and western corn rootworm (Diabrotica virgifera virgifera) [78,79,80,81]. Until the first decade of this century, CBW was considered a minor pest for the maize crops in Romania [82]. In 2009, a Romanian paper presented data confirming yield damages CBW produced to maize crops [83]. The author mentioned that in the counties south of Romania, the percentage of the attacked cobs by CBW larvae ranged from 0 to 34%, while maize yield losses ranged from 4.4 to 6.6%. This is the first reference from the Romanian literature, after the 2000s, about maize yield losses caused by this pest. More recent data reveal a higher CBW population in Central Moldavia (East Romania) from 2019 to 2024 [84]. In an article published in a journal for farmers, Cotună [85] presents evidence regarding a high CBW population in western Romania in 2022 and high aflatoxin levels in the maize grains in this area, exceeding the limit of 10.00 μg/kg. In recent years, information concerning CBW dynamics and attacks on maize cobs has been missing in southeast Romania. Also, there is no recent information concerning aflatoxin levels in maize grains. The last data from this region are from Smeu and Casian [86]. The authors mentioned that in 2019, only one sample of maize registered aflatoxin levels higher than the 10.00 μg/kg limit set by the Commission Regulation (EC) No 1881/2006. In southeast Romania, there are many areas cultivated with maize [87], and an increase in CBW attacks combined with high aflatoxin content can threaten farmers from this region, with economic consequences to national agriculture.
This study aimed to evaluate CBW flight dynamics, assess CBW attacks on maize cobs, and analyze maize grains for aflatoxin content in a maize field in southeast Romania.
2. Materials and Methods
2.1. Experimental Site
CBW flight was monitored from 2019 to 2024. Field assessments were conducted in 2024 at the maize field site at the Plant Protection Collective, Agricultural Engineering Laboratory at the National Agricultural Research and Development Institute (NARDI) Fundulea, Călărași County, southeast Romania (latitude: 44°46′ N; longitude: 26°32′ E, 68 m a.s.l). The normal climate for this area is temperate and continental, with a yearly average temperature of 10 °C and an average annual rainfall of 571 mm [88,89]. At the field site, the terrain is flat, and the soil type is clay loam (33% clay and 35% loam) with medium texture, a humus content of 2.8–3.2%, and a pH of 6.4–6.8 [88]. Table 1 presents average temperatures and rainfall amounts at the field site.

Table 1.
Temperature and rainfall multiyear averages at NARDI Fundulea field site from April to November.
During the CBW flight monitoring period, from 2020 to 2024, the average monthly temperatures were higher than the 50-year average in most cases (Figure 1). In all summer months, the temperatures were higher than average. In September, except in 2021, the temperature exceeded the 50-year average. In June 2024, there was a positive deviation of 5.3 °C from the 50-year average, while in July 2024, there was a positive deviation of 5.0 °C from the 50-year average. In August 2024, there was a positive deviation of 4.1 °C from the 50-year average. The Supplementary Materials (Figure S2-1) present the yearly temperatures registered at the field site from 1960 to 2024. In the last two decades, the average annual temperatures registered at the station exceeded the average. In 2023 and 2024, the average temperature was higher than 14 °C. Data from the Romanian literature show that 2024 was the warmest year in Romania since the start of the meteorological recordings [90]. Meteorological data from the NARDI Fundulea field site reveal that during the CBW flight period, from 2020 to 2024, the rainfall amounts were lower (Figure 2) in most months.
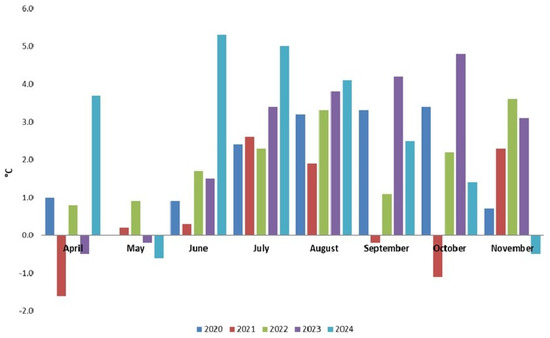
Figure 1.
Temperature deviation from 50-year average at NARDI Fundulea from 2020 to 2024.
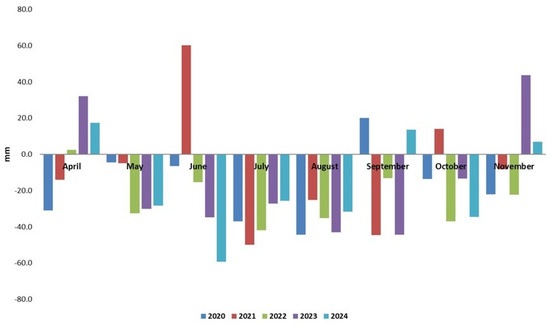
Figure 2.
Rainfall deviation from 50-year average at NARDI Fundulea from 2020 to 2024.
In the summer months, except June 2021, the rainfall amounts were lower than the 50-year average. The lowest deviation from the average was in June 2024 (−59.3 mm). In Romania, June and July were the year’s wettest months [90]. However, in recent years, the amount of rain registered in the first two months of summer has been lower than the average. The Supplementary Materials (Figure S2-2) present the yearly rainfall registered at the field site from 1960 to 2024 at NARDI Fundulea. In recent years, the rainfall was close to or below average, with a minimum of 285.2 mm in 2022. The Supplementary Materials (S1) present daily meteorological data from 2020 to 2024 at NARDI Fundulea. This data reveal that in the years with total rainfall amounting close to the average, the rain distribution throughout the year was unequal. Overall, weather conditions during this study at the field site in southeast Romania were characterized by temperatures higher than the 50-year average and rainfall below the average.
2.2. CBW Flight Monitoring
From 2020 to 2024, the flight pattern of CBW was monitored at NARDI Fundulea. Three pheromonal funnel traps (VRAL+ type) from Csalomon were used [91]. The traps were placed in a maize field in a triangle system, 100 m from each other and 15 m from the field margins, covering an area of 2 hectares planted with maize.
The traps were placed in the field at the beginning of April, before the maize sowing, to register a possible early CBW flight, and maintained until the end of November to register a possible late flight of this pest. The traps were kept in the field after the harvest, which usually occurs in late September or October. The traps were checked twice weekly, and all CBW moths were counted. The pheromone was changed one time every 6 weeks in the spring and autumn months and one time every 4 weeks in the summer months when the maximum air temperature exceeded 30 °C. Table 2 shows maize sowing, plant emergence, and harvest data during the 5-year CBW flight monitoring period. In 2020 and 2021, the monitored field site used the F423 maize hybrid; in 2022 and 2023, it used the Iezer hybrid; and in 2024, it used the Felix hybrid. These hybrids were created at NARDI Fundulea and were from the same FAO 401–500 group [92,93]. In the experimental field, soil work, fertilization, and herbicide application were following standard technology for maize crops.

Table 2.
Data on maize sowing, emergence, and harvest at the field site where Helicoverpa armigera were monitored.
2.3. Field Trial
In 2024, we organized an experiment at the NARDI Fundulea field site to assess the attack of CBW larvae on maize cobs. For this study, it has nine maize hybrids from three maturity groups. These hybrids are presented in Table 3. The experiment was organized according to a randomized block scheme. Each variant (maize hybrid) was replicated four times. Each plot had a length of 10 m, a width of 4.2 m, and a total area of 42 square meters. The maize was sown with a manual planter on 15 May. The distance between rows was 0.7 m, and the density was 65,000 plants/ha. Maize emergence was registered on 24 May.

Table 3.
Experimental variants used in this study at NARDI Fundulea.
The assessments were conducted on 18 July, 23 July, and 2 August after the appearance of cobs and the grain-filling stage. Also, one evaluation was conducted on 13 September at the maturity stage, and the last on 4 October, before the harvest. At each plot, 20 maize plants were chosen randomly, and the cobs were analyzed. We recorded the CBW attack incidence (the number of attacked plants from the number of analyzed plants) and the number of CBW larvae/plant.
2.4. Aflatoxin Analysis
On 26 September, we sampled maize cobs from each plot. After being detached from the plants, they were taken to the laboratory, and the grains were separated from the cobs. From each plot, 250 g of grains were harvested, resulting in 1000 g per variant. The grains were deposited in paper bags and, on 3 October, sent to the Sanitary Veterinary and Food Safety Laboratory in Bucharest, part of the National Sanitary Veterinary and Food Safety Authority in Romania. The liquid chromatography-mass spectrometry method LC-MS/MS (PS-L-RM-15, Ed4, Rev0) determined the aflatoxin level from the maize grains. This method combines liquid chromatography’s physical separation capabilities with mass spectrometry’s mass analysis capabilities [94].
2.5. Statistical Analysis
Data were statistically analyzed using Tukey’s honestly significant difference test (HSD) at a significance level of p ≤ 0.05. For statistical analysis, the ARM 2022 software [95] was used. The results of CBW flight monitoring were presented as the total number of moths captured in all traps during one maize growing season and the mean values of the CBW moths captured per trap, per 10 days. The chart showing CBW flight dynamics at NARDI Fundulea, from 2020 to 2024, was made with Microsoft Excel 2007. The results of the field trial were presented as mean values for the CBW larva attack incidence (AI%), the CBW larva average number per plant, the standard deviation (SD) from the average values, and the coefficient of variation (CV).
3. Results
3.1. CBW Flight Dynamics
In 2020, at the maize field site at NARDI Fundulea, the total number of captured CBW moths in the traps was 206. The number of captures slightly increased in 2021. In 2022, the traps captured 5064 moths, more than 12 times the number of insects compared with the previous year. In 2023, 1024 CBW moths were registered in the traps, while 4145 CBW moths were captured one year later (Figure 3). This study reveals a high increase in the CBW population in a maize field in southeast Romania.
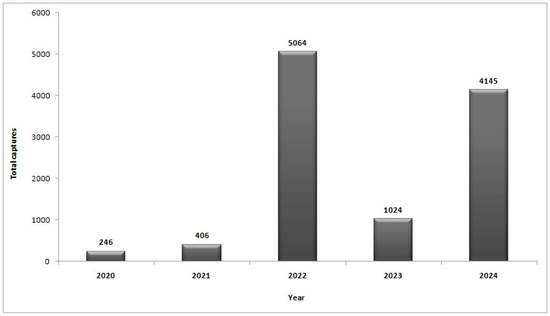
Figure 3.
The total number of cotton bollworm captures from the traps during one year in the maize field site, NARDI Fundulea.
Table 4 and Table 5 and Figure 4 present the results concerning CBW flight dynamics at the maize field site at NARDI Fundulea, southeast Romania.

Table 4.
Cotton bollworm flight dynamics at field site at NARDI Fundulea, between 2020 and 2024.

Table 5.
Data of first and last captures of cotton bollworm in traps at field site at NARDI Fundulea, from 2020 to 2024.
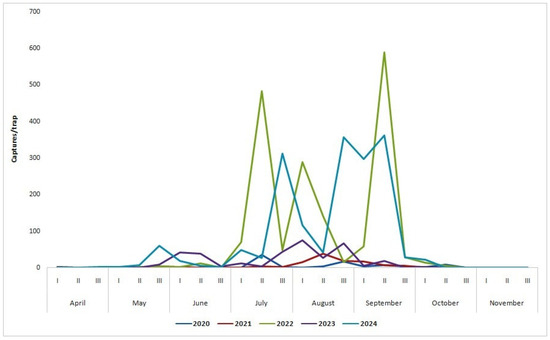
Figure 4.
Cotton bollworm flight dynamics at field site at NARDI Fundulea, between 2020 and 2024.
In 2020, there was reduced CBW flight; the first month’s moths were captured in the traps on 1 June. We registered two flight peaks in the middle of July (36.0 moths/trap) and the last days of August (17.0 moths/trap). The flight of CBW continued in September, while the last insects were captured in the traps on 13 October.
In 2021, the CBW flight dynamics were similar to those of the previous year. The first captured moth was on 26 May. We registered a flight peak in the middle of August. In the last 10 days of this month, the average captures/trap was 18.7. CBW was captured in the traps until 21 September.
In 2022, it was ascertained that there was high CBW migration in the maize field site at NARDI Fundulea. The first moths were captured on 7 April. In the middle of July, there was the first flight peak (483.3 moths/trap). The second flight peak was registered in the middle of September, with 589.9 moths/trap. It was the first time we registered high CBW activity in the maize field in September. Maize was harvested on 4 October. In the first 20 days of August, we registered many captured moths in the traps (288 and 140.3 moths/trap). The flight of this pest continues until the middle of October. However, on 13 November, two CBW moths were found in the traps. It was the first time we confirmed a CBW flight in November, at NARDI Fundulea.
In 2023, CBW activity was reduced compared with the previous year but higher than in 2020 and 2021. The flight started on 6 April. The first flight peak was registered in the first 10 days of August (76.0 moths/trap), while the second peak was in the last 10 days of the same month (68.0 moths/trap). The flight was continuous in the first 20 days of September, but the number of captured months was lower than the previous year. There were no CBW moths captured in the last 10 days of September, while this pest appeared in the traps in the first 20 days of October, but with low numbers. In the last 10 days of October, no CBW moths were captured, but one moth was found in the traps on 10 November.
In 2024, we captured more than 4100 moths in the traps, the second-highest number of CBW captures from the study period after 2022. The first adults were in the traps on 27 April. In May, CBW activity was reduced. The first flight peak was in the last 10 days of July (311.7 moths/trap). The second flight peak was registered in the last 10 days of August (358.0 moths/trap). In the first 10 days of September, a high number of CBW moths were caught in the traps, while the third flight peak was registered in the next 10 days of this month (362.3 moths/trap). This year, there was a continued and high CBW flight in the last 10 days of August into the first 20 days of September. CBW continued to fly in the maize field site in the next 20 days, with a few moths captured in the traps. The last moth was captured on 22 October.
3.2. CBW Attack Incidence
Data from Table 6 reveal that in the NARDI Fundulea maize field site trial, CBW larva attacks on maize cobs were not registered in the assessments conducted on 18 and 23 July. On 2 August, the maize hybrids from this trial had an attack incidence that ranged from 43.75% to 50.00%. In the following assessment, conducted on 13 September, when maize was at maturity, the attack incidence was at its maximum for all maize hybrids in this study.

Table 6.
Attack incidence of cotton bollworm larvae on maize cobs, NARDI Fundulea field site.
There were no significant statistical differences among the maize hybrids in this study concerning CBW attack incidence or between the maize hybrids’ maturity groups. A possible explanation is higher pest activity in 2024, especially in August and September.
3.3. CBW Number of Larvae per Plant
Data from Table 7 reveal that the first two assessments conducted on 18 and 23 July did not reveal CBW larvae on the maize plants in this field trial. In the third assessment on 2 August, the number of CBW larvae varied from 0.34 to 0.39 larvae/plant. On 13 September, there was a higher number of larvae per plant. However, the variation among the maize hybrids from this trial was slight, from 0.93 to 1.01 CBW larvae/plant. In the last assessment, conducted on 4 October, there were no CBW larvae found in the maize plots, even though moths were captured in the traps in the last 10 days of September.

Table 7.
The number of cotton bollworm larvae on maize cobs, NARDI Fundulea field site.
There were no significant statistical differences among the maize hybrids concerning the number of CBW larvae or their maturity group. This is the first study to reveal a high number of CBW larvae per plant close to the middle of September.
3.4. Aflatoxin Level in the Maize Grains
Table 8 and Table 9 present the total aflatoxin (B1 + B2 + G1 + G2) level analysis results for the nine maize hybrid grains in this field study.

Table 8.
The level of aflatoxins in the maize yield from this experiment.

Table 9.
The level of total aflatoxins in the maize yield from this experiment.
In this field trial, the B1 and total aflatoxin levels in all maize hybrids from the three maturity groups exceeded the limits established by EC Regulation 1881/2006. Regarding B1 aflatoxin, however, it was ascertained that there were significant differences among variants. Lower aflatoxin content was found in the KWS Kashmir and KWS Inteligens hybrids, while higher B1 aflatoxin content was found in the Magnus hybrids. At the same time, G2 aflatoxin was not detected in the maize hybrids from this trial. Regarding the level of total aflatoxins (B1 + B2 + G1 + G2) in this trial, it was observed that there were significant differences among the maize hybrids in this trial. However, all variants were over the allowed limit of 10 µ/kg. High temperatures registered in August and September and drought combined with high pest pressure on the maize favored the Aspergillus spp. fungus and contributed to higher levels of aflatoxins in the maize grains in all variants from this field trial, located in southeast Romania.
4. Discussion
OVER five years of monitoring, at the NARDI Fundulea maize field site in Călărași County, southeast Romania, we found a high CBW population in 2022 and 2024 compared with 2020 and 2021. The population of this pest increased by more than 12 times compared with the first two years of this study. In September 2024, we found high pest pressure in all maize hybrids from the field trial. The analysis of aflatoxin levels using LC-MS/MS (PS-L-RM-15, Ed4, Rev0) reveals higher total and B1 aflatoxin levels in the maize grains of all hybrids from this field trial. Compared with a previous study conducted in 2008 in the south of Romania [82], we found an intense CBW flight in August and September and a 100% CBW larva attack incidence in September. In 2019, Smeu and Casian [86] reported that in only one maize sample did aflatoxin levels exceed the limits of 10 µ/kg in south Romania, while in our study, conducted in southeast Romania in 2024, the aflatoxin levels were higher than the limits in all nine samples. In the west of Romania, in 2022, Cotună [82] found high CBW attack incidence in eight maize hybrids, ranging from 56 to 100%. The same author showed high total aflatoxin content, exceeding the 10 µ/kg limit. In a 30-year monitoring study with a light trap, at ARDS Secuieni, located in Central Moldavia (East Romania), Pintilie et al. [84] showed an increasing CBW population from 2019 to 2024 compared with the past. Between 1994 and 1998, at ARDS Secuieni, there were only 9 CBW captures in the light trap, between 1999 and 2003, only 2 captures, between 2004 and 2008, 38 captures, and between 2009 and 2013, 4 captures. The number of CBW captures in the light trap increased to 544 between 2014 and 2018, while in the next five years it increased to 4520 moths. The results from NARDI Fundulea, southeast Romania, reported in this paper, the results reported at ARDS Secuieni, by Pintilie et al. [84], and the results reported by Cotună [82], in 2022, in the west of Romania, suggest a CBW outbreak in Romania in recent years. Possible reasons for this are climate change and global warming, which can affect crop pest populations and intensify their attacks [96,97]. Liu et al., 2024 [98] mentioned that when the temperature is in the optimal range, from 32 to 35 °C, the development of CBW accelerates, their life cycle shortens, and female prolificity increases. However, if temperatures exceed 35 °C, mortality increases and female prolificity decreases. This can be a possible explanation for the higher CBW captures in the trap, in our study, at the end of summer and beginning of autumn, when temperatures are optimal for this pest, and lower captures in the summer period when temperatures exceeded 35 °C. The increase in temperature in Romania in recent years, because of global warming, has led to the rise in the CBW population in areas where it had been a minor pest. Global warming can create a shift in lepidopteran pest migrations to northern latitudes [99]. At the same time, high temperatures and drought represent favorable conditions for Aspergillus spp. Fungus, which can consequently increase aflatoxin levels in maize grains [65]. Increasing European temperatures and drought can increase the pest attacks on maize and their aflatoxin content [67]. Research conducted in different parts of the world concluded that CBW is a facultative migratory pest [13,29], but in some cases can be highly migrant, up to 2000 km [30] using wind currents [33]. A European study reveals that in some conditions, CBW second-generation moths migrate from northern regions to southern areas of the Mediterranean basin [1]. We hypothesized that a possible explanation for the higher number of CBW registered in the traps in Romania, in recent years, is due to pest migration in the second part of the summer. Further studies are necessary to elucidate this phenomenon. Huang and Hao [100] analyzed the effects of climate change on the CBW population dynamics in south Xinjiang, China. The authors of this study revealed that in recent years, the CBW abundance in the first and second generation decreased while in the third generation it increased. A similar trend was observed during our 5-year monitoring of this pest at NARDI Fundulea, in the southeast of Romania.
This paper is the first report from the Romanian literature about high CBW activity in southeast Romania in September. It is also the first report of a significant CBW larva attack on maize cobs close to the middle of September. The high CBW pressure in this period makes protecting maize very challenging.
5. Conclusions
During the five years of monitoring, the average monthly temperatures at NARDI Fundulea, in the southeast of Romania, were generally higher than the 50-year average, while rainfall was below average.
The five-year monitoring study revealed a high increase in CBW population in the field site in southeast Romania starting in 2022.
In 2022 and 2024, during the maize growing season, the CBW maximum flight peak was in mid-September.
In mid-September 2024, the incidence of CBW larva attacks was 100% in all maize hybrids from the field trial.
In 2024, the total aflatoxin level of the maize grains belonging to nine hybrids from this study was higher than the limit of 10 µ/kg.
Under global warming conditions, the CBW pest can threaten the maize crops in southeastern Romania.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15061306/s1, S1. Daily meteorological data (temperature, precipitation, RH) recorded at the NARDI Fundulea field site, from 2020 to 2024. S2. Charts with yearly temperatures and rainfall registered at NARDI Fundulea between 1960 and 2024. S3. Pictures from the maize field site, NARDI Fundulea.
Author Contributions
Conceptualization, E.G. and M.T.; methodology, E.G.; software, E.G. and L.C.; validation, C.R., A.C. and L.R.; formal analysis, C.C.; investigation, E.G.; resources, E.G. and I.S.B.; data curation, P.-L.P.; writing—original draft preparation, E.G.; writing—review and editing, M.T. and I.S.B.; visualization, R.-G.A.; supervision, H.D.; project administration, L.C.; funding acquisition, I.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Agriculture and Rural Development, Romania, through the ADER Program 2023–2026 within the project ADER 2.1.5 “Fly dynamics of the Ostrinia nubilalis and Helicoverpa armigera based on classical and automated traps in the South-East area of Romania and influence of those pests’ concerning contamination with mycotoxins” (contract nr. 2.1.5./4.04.2024).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors especially thank the field technicians Loghinescu Ioana, Stoian Silvia, Radu Daniela, Cenea Daniela, and Vasilescu Adrian, laboratory technician Putineanu Felicia, and tractor driver Gunică Daniel. They all work at the Plant Protection Collective, Agricultural Engineering Laboratory from the National Agricultural Research and Development Institute Fundulea, Romania.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Helicoverpa armigera (Cotton Bollworm). CABI Compendium. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.26757 (accessed on 15 April 2025).
- Pomari-Fernandes, A.; de Freitas Bueno, A.; Sosa-Gómez, D.R. Helicoverpa armigera: Current status and future perspectives in Brazil. Current Agric. Sci. Technol. 2015, 21, 1–7. [Google Scholar] [CrossRef]
- Anderson, C.J.; Tay, W.T.; McGaughran, A.; Gordon, K.; Walsh, T.K. Population structure and gene flow in the global pest, Helicoverpa armigera. Mol. Ecol. 2016, 25, 5296–5311. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Johnson, J.B.; Ahmad, M.; Fitt, G.P.; Naiker, M. A review on biological interactions and management of the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Appl. Entomol. 2021, 145, 467–498. [Google Scholar] [CrossRef]
- Yadav, S.P.S.; Lahutiya, V.; Paudel, P. A review on the biology, ecology, and management tactics of Helicoverpa armigera (Lepidoptera: Noctuidae). Turk. J. Agric. Food Sci. Technol. 2022, 10, 2467–2476. [Google Scholar] [CrossRef]
- Czepak, C.; Albernaz, K.C.; Vivan, L.M.; Guimarães, H.O.; Carvalhais, T. First reported occurrence of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Brazil. Pesqui. Agric. Trop. 2013, 43, 110–113. [Google Scholar] [CrossRef]
- Tay, W.T.; Soria, M.F.; Walsh, T.; Thomazoni, D.; Silvie, P.; Behere, G.T.; Anderson, C.; Downes, S. A brave new world for an Old World pest: Helicoverpa armigera (Lepidoptera: Noctuidae) in Brazil. PLoS ONE 2013, 8, e80134. [Google Scholar] [CrossRef]
- de Freitas Bueno, A.; Sosa-Gómez, D.R. The old world bollworm in the Neotropical region: The experience of Brazilian growers with Helicoverpa armigera. Outlooks Pest Manag. 2014, 25, 261–264. [Google Scholar] [CrossRef]
- Tembrock, L.R.; Timm, A.E.; Zink, F.A.; Gilligan, T.M. Phylogeography of the recent expansion of Helicoverpa armigera (Lepidoptera: Noctuidae) in South America and the Caribbean Basin. An. Entomol. Soc. Am. 2019, 112, 388–401. [Google Scholar] [CrossRef]
- Gilligan, T.M.; Goldstein, P.Z.; Timm, A.E.; Farris, R.; Ledezma, L.; Cunningham, A.P. Identification of Heliothine (Lepidoptera: Noctuidae) larvae intercepted at US ports of entry from the New World. J. Econ. Entomol. 2019, 112, 603–615. [Google Scholar] [CrossRef]
- Reisig, D. Helicoverpa zea (bollworm); CABI Compendium: Wallingford, UK, 2022; p. 26776. [Google Scholar] [CrossRef]
- Keszthelyi, S.; Nowinszky, L.; Puskás, J. The growing abundance of Helicoverpa armigera in Hungary and its areal shift estimation. Cent. Eur. J. Biol. 2013, 8, 756–764. [Google Scholar] [CrossRef]
- Jones, C.M.; Parry, H.; Tay, W.T.; Reynolds, D.R.; Chapman, J.W. Movement ecology of pest Helicoverpa: Implications for ongoing spread. An. Rev. Entomol. 2019, 64, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Karakantza, E.; Rumbos, C.I.; Cavalaris, C.; Athanassiou, C.G. Different Trap Types Depict Dissimilar Spatio-Temporal Distribution of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Cotton Fields. Agronomy 2023, 13, 1256. [Google Scholar] [CrossRef]
- Firempong, S.; Zalucki, M.P. Host plant preferences of populations of Helicoverpa-armigera (Hubner) (Lepidoptera, Noctuidae) from different geographic locations. Aust. J. Zool. 1989, 37, 665–673. [Google Scholar] [CrossRef]
- Liu, Z.; Li, D.; Gong, P.; Wu, K. Life table studies of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae), on different host plants. Environ. Entomol. 2004, 33, 1570–1576. [Google Scholar] [CrossRef]
- Talekar, N.S.; Opena, R.T.; Hanson, P. Helicoverpa armigera management: A review of AVRDC’s research on host plant resistance in tomato. Crop Prot. 2006, 25, 461–467. [Google Scholar] [CrossRef]
- Hemati, S.A.; Naseri, B.; Nouri Ganbalani, G.; Rafiee Dastjerdi, H.; Golizadeh, A. Effect of different host plants on nutritional indices of the pod borer, Helicoverpa armigera. J. Insect Sci. 2012, 12, 55. [Google Scholar] [CrossRef]
- Dourado, P.M.; Pantoja-Gomez, L.M.; Horikoshi, R.J.; Carvalho, R.A.; Omoto, C.; Corrêa, A.S.; Kim, H.J.; Martinelli, S.; Head, G.P. Host plant use of Helicoverpa spp. (Lepidoptera: Noctuidae) in the Brazilian agricultural landscape. Pest Manag. Sci. 2021, 77, 780–794. [Google Scholar] [CrossRef]
- Yang, L.; Li, M.; Liu, J.; Zeng, J.; Lu, Y. Long-term expansion of cereal crops promotes regional population increase of polyphagous Helicoverpa armigera. J. Pest Sci. 2024, 98, 131–144. [Google Scholar] [CrossRef]
- Shanker, R.; Prajapati, M.R.; Singh, R.P.; Singh, R.; Singh, J.; Kumar, P. Isolation, molecular characterization of indigenous Metarhizium anisopliae (Metchnikoff) isolate, using ITS-5.8s rDNA region, and its efficacy against the Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Contr. 2023, 33, 23. [Google Scholar] [CrossRef]
- Lopes-da-Silva, M.; Sanches, M.; Stancioli, A.; Alves, G.; Sugayama, R. The Role of Natural and Human-Mediated Pathways for Invasive Agricultural Pests: A Historical Analysis of Cases from Brazil. Agric. Sci. 2014, 5, 634–646. [Google Scholar] [CrossRef][Green Version]
- Specht, A.; Sosa-Gomez, D.R.; Rios, D.A.M.; Claudino, V.C.M.; Paula-Moraes, S.V.; Malaquias, J.V.; Silva, F.A.M.; Roque-Specht, V.F. Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Brazil: The big outbreak monitored by light traps. Neotrop. Entomol. 2021, 50, 53–67. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Plant Health (PLH). Scientific Opinion on the pest categorisation of Helicoverpa armigera (Hübner). EFSA J. 2014, 12, 3833. [Google Scholar] [CrossRef]
- Helicoverpa armigera (HELIAR). EPPO Global Database. Available online: https://gd.eppo.int/taxon/HELIAR/datasheet (accessed on 17 April 2025).
- Shimizu, K.; Shimizu, K.; Fujisaki, K. Timing of diapause induction and overwintering success in the cotton bollworm Helicoverpa armigera (Hb.) (Lepidoptera: Noctuidae) under outdoor conditions in temperate Japan. Appl. Entomol. Zool. 2006, 41, 151–159. [Google Scholar] [CrossRef]
- Mironidis, G.K.; Stamopoulos, D.C.; Savopoulou-Soultani, M. Overwintering survival and spring emergence of Helicoverpa armigera (Lepidoptera: Noctuidae) in Northern Greece. Environ. Entomol. 2010, 39, 1068–1084. [Google Scholar] [CrossRef]
- Huang, J.; Li, J. Effects of climate change on overwintering pupae of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Int. J. Biometeorol. 2015, 59, 863–876. [Google Scholar] [CrossRef]
- Jyothi, P.; Aralimarad, P.; Wali, V.; Dave, S.; Bheemanna, M.; Ashoka, J.; Shivayogiyappa, P.; Lim, K.S.; Chapman, J.W.; Sane, S.P. Evidence for facultative migratory flight behavior in Helicoverpa armigera (Noctuidae: Lepidoptera) in India. PLoS ONE 2021, 16, e0245665. [Google Scholar] [CrossRef]
- Pinto, F.A.; Mattos, M.V.V.; Silva, F.W.S.; Rocha, S.L.; Elliot, S.L. The Spread of Helicoverpa armigera (Lepidoptera: Noctuidae) and Coexistence with Helicoverpa zea in Southeastern Brazil. Insects 2017, 8, 87. [Google Scholar] [CrossRef]
- Feng, H.Q.; Wu, K.M.; Ni, Y.X.; Cheng, D.F.; Guo, Y.Y. Return migration of Helicoverpa armigera (Lepidoptera: Noctuidae) during autumn in northern China. Bull. Entomol. Res. 2005, 95, 361–370. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Ota, N.; Hutchison, W.D.; Beddow, J.; Walsh, T.; Tay, W.T.; Borchert, D.M.; Paula-Moreas, S.V.; Czepak, C.; Zalucki, M.P. The potential distribution of invading Helicoverpa armigera in North America: Is it just a matter of time? PLoS ONE 2015, 10, e0119618. [Google Scholar] [CrossRef]
- Pedgley, D.E. Windborne migration of Heliothis armigera (Hübner) (Lepidoptera: Noctuidae) to the British Isles. Entomol. Gaz. 1985, 36, 15–20. [Google Scholar]
- Torres-Vila, L.M.; Rodrıguez-Molina, M.C.; Lacasa-Plasencia, A.; Bielza-Lino, P. Insecticide resistance of Helicoverpa armigera to endosulfan, carbamates and organophosphates: The Spanish case. Crop Prot. 2002, 21, 1003–1013. [Google Scholar] [CrossRef]
- Srinivas, R.; Udikeri, S.S.; Jayalakshmi, S.K.; Sreeramulu, K. Identification of factors responsible for insecticide resistance in Helicoverpa armigera. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 137, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Bues, R.; Bouvier, J.C.; Boudinhon, L. Insecticide resistance and mechanisms of resistance to selected strains of Helicoverpa armigera (Lepidoptera: Noctuidae) in the south of France. Crop Prot. 2005, 24, 814–820. [Google Scholar] [CrossRef]
- Avilla, C.; González-Zamora, J.E. Monitoring resistance of Helicoverpa armigera to different insecticides used in cotton in Spain. Crop Prot. 2010, 29, 100–103. [Google Scholar] [CrossRef]
- Joußen, N.; Heckel, D.G. Resistance mechanisms of Helicoverpa armigera. In Advances in Insect Control and Resistance Management; Springer: Berlin/Heidelberg, Germany, 2016; pp. 241–261. [Google Scholar] [CrossRef]
- Wang, Q.; Rui, C.; Wang, L.; Nahiyoon, S.A.; Huang, W.; Zhu, J.; Ji, X.; Yang, Q.; Yuan, H.; Cui, L. Field-evolved resistance to 11 insecticides and the mechanisms involved in Helicoverpa armigera (Lepidoptera: Noctuidae). Pest Manag. Sci. 2021, 77, 5086–5095. [Google Scholar] [CrossRef]
- Stavrakaki, M.; Ilias, A.; Simoglou, K.B.; Mironidis, G.K.; Zimmer, C.T.; Souza, D.; Roditakis, E. Revision of Helicoverpa armigera insecticide resistance status in Greece. Crop Prot. 2024, 175, 106446. [Google Scholar] [CrossRef]
- Richardson, E.B.; Troczka, B.J.; Gutbrod, O.; Davies, T.E.; Nauen, R. Diamide resistance: 10 years of lessons from lepidopteran pests. J. Pest Sci. 2020, 93, 911–928. [Google Scholar] [CrossRef]
- Abbade-Neto, D.; Amado, D.; Pereira, R.M.; Basso, M.; Spineli-Silva, S.; Gonçalves, T.M.; Corrêa, A.S.; Omoto, C. First Report of Helicoverpa armigera (Lepidoptera: Noctuidae) Resistance to Flubendiamide in Brazil: Genetic Basis and Mechanisms of the Resistance. Agronomy 2022, 12, 1664. [Google Scholar] [CrossRef]
- Amado, D.; Koch, E.L.; Cordeiro, E.M.; Araújo, W.A.; Garcia, A.A.; Heckel, D.G.; Montejo-Kovacevich, G.; North, H.L.; Corrêa, A.S.; Jiggins, C.D.; et al. The genetic architecture of resistance to flubendiamide insecticide in Helicoverpa armigera (Hübner). PLoS ONE 2025, 20, e0318154. [Google Scholar] [CrossRef]
- Wu, K. Monitoring and management strategy for Helicoverpa armigera resistance to Bt cotton in China. J. Invertebr. Pathol. 2007, 95, 220–223. [Google Scholar] [CrossRef]
- Downes, S.; Mahon, R. Successes and challenges of managing resistance in Helicoverpa armigera to Bt cotton in Australia. GM Crops Food 2012, 3, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Downes, S.; Kriticos, D.; Parry, H.; Paull, C.; Schellhorn, N.; Zalucki, M.P. A perspective on management of Helicoverpa armigera: Transgenic Bt cotton, IPM, and landscapes. Pest Manag. Sci. 2017, 73, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Windus, L.C.; Jones, A.M.; Downes, S.; Walsh, T.; Knight, K.; Kinkema, M. Hear NPV susceptibility in Helicoverpa armigera and Helicoverpa punctigera strains resistant to Bt toxins Cry1Ac, Cry2Ab, and Vip3Aa. J. Invertebr. Pathol. 2021, 183, 107598. [Google Scholar] [CrossRef]
- Shahid, M.R.; Farooq, M.; Shakeel, M.; Ashraf, M.; Zia, Z.U.; Ahmad, S.; Mahmood, A. Need for growing non-Bt cotton refugia to overcome Bt resistance problem in targeted larvae of the cotton bollworms, Helicoverpa armigera and Pectinophora gossypiella. Egypt. J. Biol. Pest Contr. 2021, 31, 1–8. [Google Scholar] [CrossRef]
- Tang, J.; Lu, J.; Zhang, C.; Yu, S.; Ding, Z.; Soe, E.T.; Liang, G. The evaluation of resistance risk to Cry2Ab and cross-resistance to other insecticides in Helicoverpa armigera. J. Pest Sci. 2024, 97, 173–184. [Google Scholar] [CrossRef]
- Darvas, B.; Bánáti, H.; Takács, E.; Lauber, É.; Szécsi, Á.; Székács, A. Relationships of Helicoverpa armigera, Ostrinia nubilalis and Fusarium verticillioides on MON 810 maize. Insects 2011, 2, 1–11. [Google Scholar] [CrossRef]
- Niculina, N.G.; Cotună, O.; Sărățeanu, V.; Durău, V.; Claudia, C.; Suba, T. Research regarding the relationship among the pests Ostrinia nubilalis, Helicoverpa armigera and the fungi Fusarium verticillioides, Aspergillus flavus in corn in the climatic conditions from Lovrin (Timiș County). Res. J. Agric. Sci. 2019, 51, 282–291. [Google Scholar]
- Miller, J.D. Mycotoxins in small grains and maize: Old problems, new challenges. Food Addit. Contam. 2008, 25, 219–230. [Google Scholar] [CrossRef]
- de Galarreta, J.I.R.; Butrón, A.; Ortiz-Barredo, A.; Malvar, R.A.; Ordás, A.; Landa, A.; Revilla, P. Mycotoxins in maize grains grown in organic and conventional agriculture. Food Contr. 2015, 52, 98–102. [Google Scholar] [CrossRef]
- Palumbo, R.; Gonçalves, A.; Gkrillas, A.; Logrieco, A.; Dorne, J.L.; Dall’Asta, C.; Venâncio, A.; Battilani, P. Mycotoxins in maize. Phytopat. Med. 2020, 59, 5–28. [Google Scholar] [CrossRef]
- Payne, G.A.; Widstrom, N.W. Aflatoxin in maize. Crit. Rev. Plant Sci. 1992, 10, 423–440. [Google Scholar] [CrossRef]
- Kos, J.; Mastilović, J.; Hajnal, E.J.; Šarić, B. Natural occurrence of aflatoxins in maize harvested in Serbia during 2009–2012. Food Contr. 2013, 34, 31–34. [Google Scholar] [CrossRef]
- Wu, F.; Stacy, S.L.; Kensler, T.W. Global risk assessment of aflatoxins in maize and peanuts: Are regulatory standards adequately protective? Toxicol. Sci. 2013, 135, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Okun, D.O.; Khamis, F.M.; Muluvi, G.M.; Ngeranwa, J.J.; Ombura, F.O.; Yongo, M.O.; Kenya, E.U. Distribution of indigenous strains of atoxigenic and toxigenic Aspergillus flavus and Aspergillus parasiticus in maize and peanuts agro-ecological zones of Kenya. Agric. Food Secur. 2015, 4, 1–10. [Google Scholar] [CrossRef]
- Wu, F. Global impacts of aflatoxin in maize: Trade and human health. World Mycotoxin J. 2015, 8, 137–142. [Google Scholar] [CrossRef]
- Kilonzo, R.M.; Imungi, J.K.; Muiru, W.M.; Lamuka, P.O.; Njage, P.M.K. Household dietary exposure to aflatoxins from maize and maize products in Kenya. Food Addit. Contam. Part A 2014, 31, 2055–2062. [Google Scholar] [CrossRef]
- Kibwana, M.; Kimbokota, F.; Christopher, R.; Mmongoyo, J.A. Aflatoxins in stored maize, maize flours, and stiff porridge consumed in schools: A case study of Dodoma region, Tanzania. Food Contr. 2023, 146, 109519. [Google Scholar] [CrossRef]
- Garrido, N.S.; Iha, M.H.; Santos Ortolani, M.R.; Duarte Fávaro, R.M. Occurrence of aflatoxins M1 and M2 in milk commercialized in Ribeirão Preto-SP, Brazil. Food Addit. Contam. 2003, 20, 70–73. [Google Scholar] [CrossRef]
- Prandini, A.; Tansini, G.; Sigolo, S.; Filippi, L.; Laporta, M.; Piva, G. On the occurrence of aflatoxin M1 in milk and dairy products. Food Chem. Toxicol. 2009, 47, 984–991. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Jinap, S.; Pirouz, A.A.; Faizal, A.A. Aflatoxin M1 in milk and dairy products, occurrence and recent challenges: A review. Trends Food Sci. Technol. 2015, 46, 110–119. [Google Scholar] [CrossRef]
- Fountain, J.C.; Scully, B.T.; Ni, X.; Kemerait, R.C.; Lee, R.D.; Chen, Z.Y.; Guo, B. Environmental influences on maize-Aspergillus flavus interactions and aflatoxin production. Front. Microbiol. 2014, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Milićević, D.; Petronijević, R.; Petrović, Z.; Đjinović-Stojanović, J.; Jovanović, J.; Baltić, T.; Janković, S. (2019). Impact of climate change on aflatoxin M1 contamination of raw milk with special focus on climate conditions in Serbia. J. Sci. Food Agric. 2019, 99, 5202–5210. [Google Scholar] [CrossRef] [PubMed]
- Molnár, K.; Rácz, C.; Dövényi-Nagy, T.; Bakó, K.; Pusztahelyi, T.; Kovács, S.; Adácsi, C.; Pócsi, I.; Dobos, A. The Effect of Environmental Factors on Mould Counts and AFB1 Toxin Production by Aspergillus flavus in Maize. Toxins 2023, 15, 227. [Google Scholar] [CrossRef]
- EUR-Lex. Document 02006R1881-20140701: Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Available online: https://eur-lex.europa.eu/eli/reg/2006/1881/2014-07-01 (accessed on 22 April 2025).
- Marete, G.N.; Kanja, L.W.; Mbaria, J.M.; Okumu, M.O.; Ateku, P.A.; Korhonen, H.; Joutsjoki, V. Effects of the Use of Good Agricultural Practices on Aflatoxin Levels in Maize Grown in Nandi County, Kenya. Sci 2020, 2, 85. [Google Scholar] [CrossRef]
- Xu, F.; Baker, R.C.; Whitaker, T.B.; Luo, H.; Zhao, Y.; Stevenson, A.; Boesch, C.J.; Zhang, G. Review of good agricultural practices for smallholder maize farmers to minimise aflatoxin contamination. World Mycotoxin J. 2022, 15, 171–186. [Google Scholar] [CrossRef]
- Robens, J.; Cardwell, K. The costs of mycotoxin management to the USA: Management of aflatoxins in the United States. J. Toxicol. Toxin Rev. 2003, 22, 139–152. [Google Scholar] [CrossRef]
- Umar, A.; Bhatti, H.S.; Honey, S.F. A call for aflatoxin control in asia. CABI Agric. Biosci. 2023, 4, 27. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Development Romania. Available online: https://madr.ro/culturi-de-camp/cereale/porumb.html (accessed on 23 April 2025).
- Eurostat Database. Available online: https://ec.europa.eu/eurostat/data/database (accessed on 23 April 2025).
- Dinca, V.M.; Trocinescu, B.; Stamule, S.; Bunea, M.; Dinu, V. Opportunities and challenges for managers within the East-European agriculture sector: Case study on Romania. E&M Ec. Manag. 2024, 27, 121–134. [Google Scholar] [CrossRef]
- Toader, M.; Georgescu, E.; Ion, V.; Cionga, C.; Radu, C.; Epure, L.I.; Bășa, A.G. Pests of maize crops and integrated control strategy in Romania. Sci. Pap. Ser. A Agric. 2024, 67, 717–724. [Google Scholar]
- Partal, E.; Contescu, L.E.; Paraschivu, M.; Sălceanu, C.; Oltenacu, C.V. Impact of crop rotation and soil management practices on weeding and soil water dynamics in maize crop in southern Romania. Ann. Univ. Craiova Agric. Mont. Cadastre Ser. 2024, 54, 228–234. [Google Scholar] [CrossRef]
- Georgescu, E.; Toader, M.; Canǎ, L.; Horhocea, D.; Manole, T.; Zaharia, R.; Rîşnoveanu, L. Researches concerning the effectiveness of the maize foliar treatment compared with seeds treatment for chemical control of the maize leaf weevil (Tanymecus dilaticollis Gyll) in the South-East of Romania. Rom. Agric. Res. 2021, 38, 357–369. [Google Scholar] [CrossRef]
- Traşcă, F.; Traşcă, G.; Podea, M.M.; Ghiorghe, C.; Dinuță, C.I.; Gheorhe, R.M.; Georgescu, E.I. Damages and Integrated Control Possibilities of Wire Worms in Corn Crops, in the Area of Subcarpatic Hills; Institutul National de Cercetare-Dezvoltare Agricola Fundulea: Recas, Romania, 2021; Volume 89, pp. 1–10, (In Romanian). Available online: https://www.incda-fundulea.ro/anale/anale89.html (accessed on 24 April 2025).
- Pintilie, P.L.; Trotuș, E.; Tălmaciu, N.; Irimia, L.M.; Herea, M.; Mocanu, I.; Amarghioalei, R.G.; Popa, L.D.; Tălmaciu, M. European Corn Borer (Ostrinia nubilalis Hbn.) Bioecology in Eastern Romania. Insects 2023, 14, 738. [Google Scholar] [CrossRef] [PubMed]
- Amarghioalei, R.-G.; Tălmaciu, N.; Herea, M.; Mocanu, I.; Pintilie, P.-L.; Pintilie, A.-S.; Trotuș, E.; Tălmaciu, M. Chemical Control of Western Corn Rootworm (Diabrotica virgifera virgifera Le Conte, Coleoptera: Chrysomelidae) in Eastern Romania. Insects 2025, 16, 293. [Google Scholar] [CrossRef]
- Popov, C.; Bărbulescu, A. 50 Years of Scientific Activity in Field Protection Domain Against Diseases and Pests; Institutul National de Cercetare-Dezvoltare Agricola Fundulea: Recas, Romania, 2007; Volume 75, pp. 371–404. Available online: https://www.incda-fundulea.ro/anale/anale75.html (accessed on 24 April 2025).
- Roşca, I. Research regarding interaction of Mon810 biotech corn on the Helicoverpa armigera in Romania. Sci. Pap. UASVM Bucharest Ser. A 2009, 53, 403–411. [Google Scholar]
- Pintilie, P.L.; Amarghioalei, R.G.; Trotuş, E.; Buburuz, A.A.; Isticioaia, S.F.; Leonte, A.; Popa, L.D. Helicoverpa armigera Hbn. (Lepidoptera: Noctuidae) a pest of agricultural crops in eastern Romania (Helicoverpa armigera Hbn. (Lepidoptera: Noctuidae) un dăunător al culturilor agricole din estul României). In Proceedings of the Scientific Problems in Field Crops Area-Realisations and Perspectives (Probleme Științifice în Domeniul Culturilor de Câmp-Realizări și Perspective), Balti, Moldova, 13–14 June 2024; pp. 300–313. [Google Scholar]
- Cotună, O. Aflatoxins or “Cocktail” of Mycotoxins from Maize Grains (Aflatoxinele Sau “Cocktailul” de Micotoxine din Boabele de Porumb). Farmer Journal (Revista Fermierului). 2023. Available online: https://www.revistafermierului.ro/din-revista/protectia-plantelor/item/5589-aflatoxinele-sau-cocktailul-de-micotoxine-din-boabele-de-porumb.html (accessed on 25 April 2025).
- Smeu, I.; Casian, H. Food safety perspectives and management on total aflatoxin levels in 2019 Romanian maize (Zea mays L.) samples. In Proceedings of the ISB-INMA TEH’ 2020, Agricultural and Mechanical Engineering, Bucharest, Romania, 30 October 2020; pp. 176–180. [Google Scholar]
- Dragomir, V.; Ioan Sebastian, B.; Alina, B.; Victor, P.; Tanasă, L.; Horhocea, D. An overview of global maize market compared to Romanian production. Rom. Agric. Res. 2022, 39, 535–544. [Google Scholar] [CrossRef]
- National Agricultural Research and Development Institute, General Information. Available online: https://www.incda-fundulea.ro/informatii_en.htm (accessed on 26 April 2025).
- Busuioc, A.; Giorgi, F.; Bi, X.; Ionita, M. Comparison of regional climate model and statistical downscaling simulations of different winter precipitation change scenarios over Romania. Theor. Appl. Climatol. 2006, 86, 101–123. [Google Scholar] [CrossRef]
- Ionita, M.; Nagavciuc, V. The year with too much summer in the eastern part of Europe. Weather 2025. Early View-Online Version. [Google Scholar] [CrossRef]
- Csalomon, Cotton Bollworm—Helicoverpa (heliothis) armigera Hbn. Available online: https://www.csalomontraps.com/4listbylatinname/helicoverpaarmigera.htm (accessed on 27 April 2025).
- Horhocea, D.; Martura, T.; Iordan, H.L.; Bãduț, C.; Ciocãzanu, I. Felix, a New Semi-Late Maize Hybrid Released by the NARDI Fundulea; Institutul National de Cercetare-Dezvoltare Agricola Fundulea: Recas, Romania, 2019; Volume 87, pp. 57–80. Available online: https://www.incda-fundulea.ro/anale/anale87.html (accessed on 27 April 2025).
- Horhocea, D.; Petcu, E.; Iordan, H.; Ciontu, C. Evaluation of New Maize Genotypes for Seed Yield Potential and Stability. Rom. Agric. Res. 2024, 41, 489–496. [Google Scholar] [CrossRef]
- Spanjer, M.C.; Rensen, P.M.; Scholten, J.M. LC–MS/MS multi-method for mycotoxins after single extraction, with validation data for peanut, pistachio, wheat, maize, cornflakes, raisins and figs. Food Addit. Contam. 2008, 25, 472–489. [Google Scholar] [CrossRef]
- Gylling Data Management Inc. ARM 2022® GDM Software, Revision 9.2022.5 25 October 2022 (B = 28627); Gylling Data Management Inc.: Brookings, SD, USA, 2022. [Google Scholar] [CrossRef]
- Shrestha, S. Effects of climate change on agricultural insect pests. Acta Sci. Agric. 2019, 3, 74–80. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The Impact of Climate Change on Agricultural Insect Pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, B.; Yu, H.; Zhang, H.; He, Z.; Zhuo, Z. The Effects of Global Climate Warming on the Developmental Parameters of Helicoverpa armigera (Hübner, 1808) (Lepidoptera: Noctuidae). Insects 2024, 15, 888. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Liu, Y.; Zhang, H.; Liu, J.; Jiang, Y.; Wyckhuys, K.A.; Wu, K. Global warming modifies long-distance migration of an agricultural insect pest. J. Pest Sci. 2020, 93, 569–581. [Google Scholar] [CrossRef]
- Huang, J.; Hao, H. Effects of climate change and crop planting structure on the abundance of cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Ecol. Evol. 2020, 10, 1324–1338. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).