Abstract
Streptomyces vinaceus strain SVFJ-07 is a biocontrol bacterium employed to control anthracnose disease caused by Colletotrichum gloeosporioides in Chinese orchids. This study investigated the mechanism of strain SVFJ-07-induced stomatal immunity-related closure in preventing the infection of anthracnose disease. After the foliar application of strain SVFJ-07, we analyzed the differential patterns of stomatal opening in Chinese orchids and measured the hormone levels of abscisic acid (ABA) and salicylic acid (SA). RNA sequencing (RNA-seq) was utilized to examine the differential expression of genes involved in SA and ABA signal transduction and disease resistance genes, which were induced by strain SVFJ-07. The results demonstrated that strain SVFJ-07 inhibited the infection of pathogens by inducing stomatal closure. Compared with the control group, the foliar application of strain SVFJ-07 significantly reduced stomatal length, width, and aperture. Furthermore, orchid plants treated with strain SVFJ-07 and infected with C. gloeosporioides exhibited elevated levels of endogenous ABA and SA, indicating that strain SVFJ-07 enhanced stomatal immunity and disease resistance in these plants. The transcriptome analysis revealed the upregulation of genes associated with stomatal immunity, particularly those involved in plant–pathogen interactions, peroxisome metabolism, plant hormone signaling, and mitogen-activated protein kinase (MAPK) signaling pathways. These findings confirmed that the induction of SVFJ-07 promoted stomatal closure to resist the infection of C. gloeosporioides and induced complex transcriptome-wide changes. Further investigation of the differentially expressed genes enhanced our understanding of the resistance mechanisms induced by S. vinaceus strain SVFJ-07.
1. Introduction
Anthracnose, caused by Colletotrichum gloeosporioides, represents the most prevalent and destructive disease affecting orchids around the world [1]. Since 2011, recurring outbreaks of C. gloeosporioides have significantly impacted Chinese orchid cultivation bases, resulting in substantial economic losses [2]. The pathogen not only affects common ornamental orchid varieties such as Jianlan (Cymbidium goeringii), Molan (Cymbidium kanran), and Chunlan (Cymbidium faberi) but also severely damages other orchid species including Huilan (Cymbidium sinense), Phalaenopsis, and Oncidium. The incidence of this disease has been reported to exceed 90% in Molan and Phalaenopsis [3,4]. The application of chemical fungicides is the primary means of controlling orchid anthracnose; however, C. gloeosporioides has simultaneously developed resistance to commonly used pesticides, including substitutive benzene fungicide [5,6]. This has resulted in a problematic cycle of increasing pesticide concentrations and the development of resistance [7], urgently necessitating the development of effective and sustainable biological control systems.
Fujian province, situated along the southeastern coast of China, harbors exceptionally diverse marine microbial resources. Recent isolation endeavors have resulted in the identification of over 400 strains from marine fish and intertidal soil, thereby establishing a biocontrol strain library predominantly comprising Streptomyces and Bacillus species. Notably, Streptomyces vinaceus SVFJ-07 (CGMCC No. 14020) has remarkable efficacy, achieving an 83.64% growth inhibition rate against C. gloeosporioides isolated from orchid. Both greenhouse and field trials have consistently demonstrated its control efficacy, with inhibition rates reaching 70.06% and 68.54%, respectively, after 30 days [8].
The plant innate immune system encompasses two primary responses: pathogen-associated molecular pattern-triggered immunity (PTI) and effector-triggered immunity (ETI). Stomatal immunity, a crucial component of PTI, plays a vital role in defending against pathogenic bacterial infection. Guard cells recognize pathogen-derived elicitors and respond through defense mechanisms, including stomatal closure [9].
Abscisic acid (ABA) and salicylic acid (SA) serve as key signaling molecules in regulating stomatal immunity. ABA modulates glutathione (GSH) level in defense cells, while GSH-deficient mutants enhance ABA-induced stomatal closure [9]. SA elevates hydrogen peroxide (H2O2) levels by regulating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity, and the subsequent increase in reactive oxygen species (ROS) enhances ABA accumulation. Conversely, ABA accumulation provides feedback regulation on ROS production in defense cells, thereby modulating stomatal closure [9]. Notably, different microorganisms employ distinct signaling mechanisms to mediate plant stomatal closure. In Arabidopsis thaliana, both ABA and SA signals mediate stomatal closure induced by Bacillus subtilis FB17, but the SA signaling pathway is not essential for this process [10]. In Lycopersicum esculentum, both ABA and SA synthesis and their mediated signaling pathways are involved in stomatal closure induced by Pseudomonas syringae [11]. Conversely, in Nicotiana benthamiana, only the SA-mediated signaling pathway is involved in the stomatal closure induced by B. amyloliquefaciens FZB42 [12]. Furthermore, H2O2 regulates ion channel activity on the plasma membranes of defense cells through ABA and SA pathways, leading to stomatal closure [13]. Additionally, H2O2 can also stimulate NO production to promote stomatal closure [14]. However, the molecular mechanism underlying S. vinaceus-induced stomatal closure and its potential mediation by ABA remains unexplored.
This study primarily investigated the mechanism underlying S. vinaceus strain SVFJ-07-induced stomatal immunity-related closure as a defense mechanism against the infection of C. gloeosporioides in Chinese orchids. We used scanning electron microscopy to evaluate the effects of the foliar application of strain SVFJ-07 on the ultrastructural changes of orchid leaf surfaces and the success rate of pathogen infection, in order to investigate the stomatal immunity-related closure induced by strain SVFJ-07. Furthermore, we conducted temporal measurements of changes in the SA and ABA hormone levels in orchid plants treated with strain SVFJ-07. RNA-seq analysis was then employed to investigate the differential expression patterns of genes involved in SA and ABA signal transduction, as well as disease-resistance related genes, at four time points following treatment with strain SVFJ-07. Overall, this study elucidated the molecular mechanism underlying SVFJ-07-induced stomatal immunity-related closure in preventing the infection of C. gloeosporioides.
2. Materials and Methods
2.1. Test Materials
The experimental strain, S. vinaceus (SVFJ-07, CGMCC No. 14020), and the pathogenic fungus, C. gloeosporioides (Accession number: KC010542), were provided by the Institute of Plant Protection, Fujian Academy of Agricultural Sciences.
Six-month-old healthy plants of C. ensifolium (Chinese orchid) were selected for this study. Each plant was individually cultivated in plastic pots (10 cm in diameter, with a 7 cm base diameter) containing a sterile substrate mixture of peat moss and bark (4:1, v/v). After transplantation and a recovery period, one plant per pot was maintained under standard cultivation conditions throughout the experimental period.
2.2. Sample Processing
The experiment was conducted in three main phases from August 2022 to May 2024. The first phase (August–September 2022) involved evaluating SVFJ-07-induced resistance to C. gloeosporioides in Chinese orchid leaves, assessing stomatal changes, and collecting leaf samples for transcriptomic analysis and ABA/SA detection. The second phase (September 2022–April 2023) focused on transcriptome data analysis, including quality control, differential expression analysis, and functional annotation. The third phase (April 2023–May 2024) aimed to validate key genes identified from the transcriptome analysis using qPCR.
Using a sterile puncher, five fungal plugs (5 mm in diameter) were excised from both activated cultures of S. vinaceus strain SVFJ-07 and C. gloeosporioides. The plugs were separately transferred into Potato Dextrose Broth (PDB) liquid medium and incubated at 28 °C with shaking at 4× g for 5 days to obtain the fermentation broth (3 × 108 cfu/mL) of strain SVFJ-07 and the spore suspension (5 × 108 cfu/mL) of C. gloeosporioides. Two experimental groups of healthy and undamaged Chinese orchid plants were established, each containing five pots of plants with three biological replicates per group.
The fermentation broth (3 × 108 cfu/mL) of strain SVFJ-07 was sprayed onto the fully expanded mature leaves of Chinese orchids, which were designated as the induction group (SC, Streptomyces vinaceus + Colletotrichum gloeosporioides). In contrast, sterile water was applied to the leaves of control group (WC, water + Colletotrichum gloeosporioides). After 5 days, all plants were inoculated with a spore suspension (5 × 108 cfu/mL) of C. gloeosporioides. Following inoculation, the plants were maintained at room temperature (28 °C). Inoculated leaves were collected at 24, 40, 60, and 96 h post-inoculation (hpi), and three sets of samples were collected from each time point for subsequent analyses.
2.3. Analysis of Strain SVFJ-07-Induced Resistance to C. gloeosporioides in Chinese Orchid Leaves
In accordance with Koch’s postulates, healthy and uninjured Chinese orchid plants were divided into two experimental groups: the SC (induced by strain SVFJ-07) and WC (control) groups. Five days after spraying the fermentation broth (3 × 108 cfu/mL) of strain SVFJ-07, the plants were inoculated with a spore suspension (5 × 108 cfu/mL) of C. gloeosporioides. The experiment was conducted in triplicate, with inoculated plants maintained at 28 °C.
Leaf samples were examined using a scanning electron microscope at 1, 3, 12, 24, 36, 48, and 96 hpi to monitor the progression of C. gloeosporioides infection. Disease development was assessed at 14 days post-inoculation (dpi) and 30 dpi to determine the inhibitory effect of SVFJ-07 on C. gloeosporioides and to calculate the disease index.
Disease severity was classified according to the following criteria:
Grade 0: no disease spots;
Grade 1: 1–3 leaf lesions or affected area < 5.0%;
Grade 2: 4–6 leaf lesions or affected area 5.1–10.0%;
Grade 3: 7–10 leaf spots or affected area 10.1–15.0%;
Grade 4: 11–20 leaf spots or affected area 15.1–25.0%;
Grade 5: >20 leaf lesions or affected area > 25.1%.
The disease index and control effect were calculated using the following formulas:
Disease index (%) = [∑(disease grade × number of plants in grade)/(highest disease grade × total number of plants)] × 100%
Control effect (%) = [(control disease index − treatment disease index)/control disease index] × 100%
2.4. Analysis of SVFJ-07-Induced Changes in Stomatal Aperture on the Chinese Orchid Epidermis
Stomatal aperture measurements were performed using the nail polish imprint method [15]. Three leaves of Chinese orchid were randomly selected from each time point specified in Section 2.2. A colorless transparent nail polish was applied to a 1 cm × 1 cm area located 1 cm away from the main vein on the abaxial leaf surface. After complete drying, the nail polish impression was peeled off and mounted onto a glass slide for microscopic examination. Observations and imaging were conducted using a Nikon E200 biological microscope (Nikon Corporation, Tokyo, Japan). A total of twenty impression samples were prepared for analysis, with five stomata being measured per sample using Oplenic imaging software (×64, 10.1.11908). The experiment was replicated three times to evaluate the effect of SVFJ-07 on stomatal closure in the leaves of Chinese orchids.
2.5. Analysis of SVFJ-07-Induced Endogenous ABA and SA Content in Chinese Orchid Plants
Chinese orchid plants at the five-leaf developmental stage with fully expanded mature leaves were treated with either fermentation liquid (3 × 108 cfu/mL) of strain SVFJ-07 or sterile water as blank control group. After a 5-day treatment period, the plants were inoculated with spore suspension (5 × 108 cfu/mL) of C. gloeosporioides and maintained at 28 °C, under a 14 L: 10 D photoperiod. Leaf samples were collected at 0, 3, 12, 24, 36, and 48 hpi for analysis of the ABA and SA.
For ABA extraction, 2.0 g of leaf tissue were flash-frozen in liquid nitrogen and homogenized into a fine powder. The homogenate was extracted with 20 mL of extraction solution containing 90% methanol, 20 mL/L acetic acid, and 10 mg/L butylated hydroxytoluene (Sigma-Aldrich Corporation, St. Louis, MO, USA). After incubation at 40 °C for 24 h, the mixture was centrifuged at 5000× g for 10 min at 4 °C. The supernatant was transferred to a 2 mL microcentrifuge tube for ABA quantification using a commercial kit (ZCIBIO Technology Co., Ltd., Shanghai, China).
For SA extraction, 2.0 g of leaf tissue was processed as described above for initial homogenization. The powder was then suspended in 20 mL of phosphate-buffered saline (PBS) buffer and centrifuged at 5000× g for 20 min at 4 °C. The supernatant was transferred to a 2 mL microcentrifuge tube for SA quantification using a commercial kit (ZCIBIO Technology Co., Ltd., Shanghai, China).
2.6. RNA Extraction, Library Construction and Sequencing
Leaf samples for transcriptome analysis were flash-frozen in liquid nitrogen and subsequently submitted to Applied Protein Technology (Jinhua, China) for sequencing. Samples from the SVFJ-07-induced group were designated as SC24, SC40, SC60, and SC96; meanwhile, those from the control group were labeled as WC24, WC40, WC60, and WC96, corresponding to their respective time points. Total RNA was extracted from leaves of Chinese orchid using TRIzol® Reagent (Invitrogen Corporation, Carlsbad, CA, USA), following the manufacturer’s protocol. Poly A-tailed mRNA was isolated using Oligo (dT) magnetic beads and was subsequently fragmented randomly using divalent cations in NEB Fragmentation Buffer (Invitrogen, USA). Following size selection and PCR amplification with AMPure XP beads, the final cDNA library was constructed. Sequencing was performed on an Illumina Novaseq 6000 platform (Illumina Inc, San Diego, CA, USA) using Synthesis technology. After quality control procedures, clean reads were obtained and assembled using Trinity software (v2.15.1) [16]. The longest transcript from each gene was selected as the representative Unigene for subsequent analyses.
2.7. Transcriptome Data Analysis
Clean data were used to perform de novo assembly with Trinity (http://trinityrnaseq.sourceforge.net/, accessed on 8 October 2023). The assembled transcriptome sequences were compared with BLASTx on five databases (NR, SwissProt, Pfam, GO and KEGG databases) to obtain the annotation information in each database. Clean reads were normalized, gene expression levels were filtered using a threshold of FPKM > 1, and subsequent differential expression analysis was performed using DESeq2 software (v1.40.2) [17]. Differentially expressed genes (DEGs) with |log2FC| > 1 and Padj < 0.05 were considered to be significantly differently expressed genes. The Benjamini–Hochberg (BH) method was employed to calculate the false discovery rate (FDR) [18,19], and p-values were computed using negative binomial distribution. Differentially expressed genes (DEGs) were defined based on the criteria of p-value < 0.05 [20]. Functional enrichment analysis of DEGs was conducted using GOseq software (v1.52.0) based on gene ontology (GO) annotations, with a threshold of FDR ≤ 0.05 [21]. Pathway enrichment analysis was performed using KOBAS (2.0), and metabolic pathway analysis of DEGs was based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, using a corrected p-value threshold of 0.05 [22].
2.8. Verification of DEGs by RT-qPCR
To validate the transcriptome sequencing results, six defense-related genes were selected for quantitative real-time PCR (qRT-PCR) analysis. Gene-specific primers were designed using Primer 5.0 software, with Actin being the internal reference gene. All primers were synthesized by Sangon Biotech (Shanghai, China). The qPCR reactions were performed using the LightCycler 96 Real-Time System (F. Hoffmann-La Roche AG, Basel, Switzerland). The thermal cycling conditions were as follows: initial denaturation at 94 °C for 30 s, followed by 45 cycles of denaturation at 94 °C for 5 s, annealing at 60 °C for 15 s, and extension at 72 °C for 15 s. All reactions were performed in triplicate, and the relative gene expression level was calculated using the 2−ΔΔCt method (Table S1) [23].
2.9. Statistical Analysis
Analysis of variance (ANOVA) on stomatal measurements, hormone quantification, and disease severity indices were executed by the IBM SPSS version 27 (IBM Corp., New York, NY, USA) software.
3. Results
3.1. Analysis of SVFJ-07-Induced Resistance Against Infection of C. gloeosporioides
To investigate whether strain SVFJ-07-induced disease resistance was associated with stomatal movement, we examined the effects of the application of fermentation broth of SVFJ-07 and infection with C. gloeosporioides on the orchid leaf surface ultrastructure, using scanning electron microscopy.
Stomatal closure was observed within 3 h of the application of fermentation broth of SVFJ-07. Notably, no successful infection of C. gloeosporioides was observed at 96 hpi, suggesting that strain SVFJ-07 conferred resistance to the infection of pathogen through induced stomatal closure (Figure 1a). After 14 days, the control group exhibited numerous anthracnose lesions following C. gloeosporioides inoculation (Figure 1b), while the SVFJ-07-treated group showed minimal disease symptoms (Figure 1c). The disease inhibition rates in the SVFJ-07-treated group reached 80.34% and 78.42% at 14 dpi and 30 dpi, respectively.

Figure 1.
Resistance of Cymbidium ensifolium leaves induced by SVFJ-07 to the infection of Colletotrichum gloeosporioides. (a) Observations of resistance to C. gloeosporioides in the stomatal closure of C. ensifolium leaves induced by SVFJ-07 after 3 h. (b) The control group for C. ensifolium leaves inoculated with C. gloeosporioides after 14 days. (c) SVFJ-07 induced group for C. ensifolium leaves inoculated with C. gloeosporioides after 14 days.
3.2. Analysis of SVFJ-07-Induced Plant Leaf Stomatal Closure
As illustrated in Figure 2, stomatal aperture progressively decreased with prolonged exposure of strain SVFJ-07. This response might be attributed to the plant immunity triggered by infection of C. gloeosporioides in Chinese orchid, manifesting as reduced stomatal aperture.
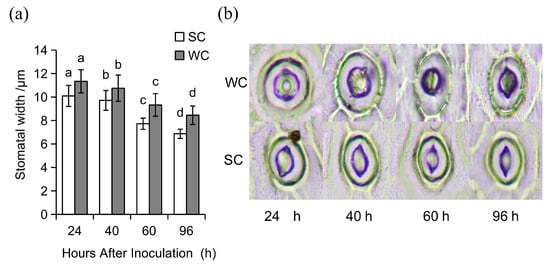
Figure 2.
Analysis of stomatal closure induced by SVFJ-07 in Cymbidium ensifolium leaves. (a) Analysis of stomatal closure of C. ensifolium leaves induced by SVFJ-07. (b) Microscopic view of stomatal closure of C. ensifolium leaves induced by SVFJ-07. Significant differences (p ≤ 0.05) between inoculation times are indicated by different letters above these bars.
After treatment with the fermentation broth (3 × 108 cfu/mL) of strain SVFJ-07, the stomatal apertures were reduced by 10.94%, 10.52%, 18.97%, and 20.98% at 24, 40, 60, and 96 h post-treatment (hpt), respectively, compared to the control group at the corresponding time points. These results demonstrated that SVFJ-07-treated orchid plants challenged with C. gloeosporioides exhibited significantly narrower stomatal apertures relative to untreated control plants.
3.3. Analysis of Endogenous ABA and SA Contents in SVFJ-07-Treated Chinese Orchid Plants
As illustrated in Figure 3, the endogenous levels of ABA and SA in the leaves of Chinese orchids showed distinct patterns of change following SVFJ-07 treatment and subsequent inoculation with C. gloeosporioides.
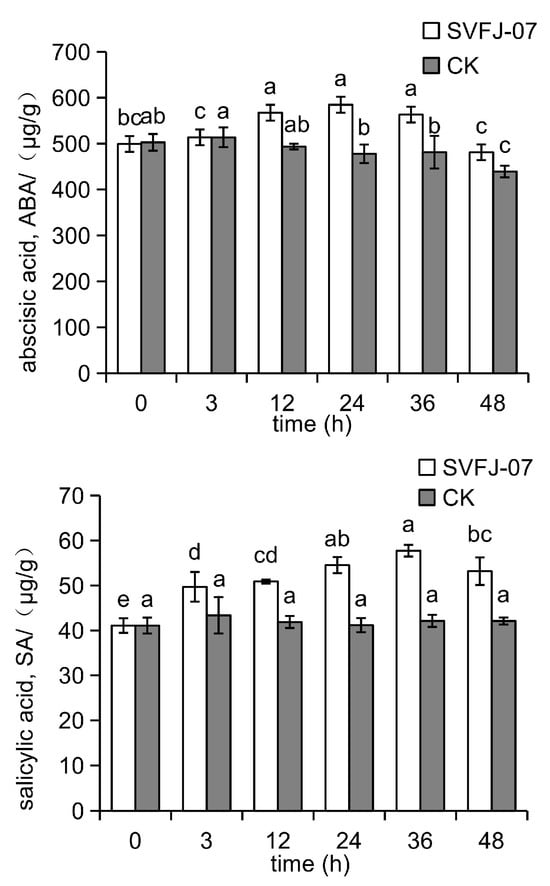
Figure 3.
The induction effect of SVFJ-07 on ABA and SA content in Cymbidium ensifolium leaves. Significant differences (p ≤ 0.05) between inoculation times are indicated by different letters above these bars.
In SVFJ-07-treated plants, the endogenous ABA levels remained relatively stable during the first 3 hpi, then increased significantly to peak at 584.602 ng/g at 24 hpi, followed by a gradual decline. The endogenous SA levels exhibited a continuous increase after inoculation, reaching a maximum concentration of 57.738 μg/g at 36 hpi. In contrast, the control plants showed only a slight increase in endogenous ABA levels within the first three hpi, followed by a continuous decrease, while the SA levels remained relatively constant.
Overall, SVFJ-07-treated orchids showed significantly elevated levels of both endogenous ABA and SA following inoculation with C. gloeosporioides, whereas untreated plants showed slight decreases in the levels of these two phytohormones.
3.4. Transcriptome Data Quality Analysis
To elucidate the molecular mechanisms underlying SVFJ-07-induced stomatal immunity against the infection of C. gloeosporioides, we performed the transcriptome sequencing on eight treatments groups, each with three biological replicates, resulting in a total of 24 samples. The sequencing yielded an average of 59,561,286 raw reads per sample (Table S2). Quality control processing generated 188 Gb of high-quality data, with Q30 score exceeding 93% and an error rate below 0.06. De novo assembly using Trinity software (v2.15.1) produced a reference transcriptome comprising 602,514 unigenes, with a total length of 348,713,386 bp, a mean length of 579 bp, and an N50 of 831 bp. Functional annotation was performed against five databases: namely NR, SwissProt, PFAM, GO, and KO, with 18.54% of unigenes receiving at least one database (Table S3).
3.5. Analysis of DEG Change Patterns
The temporal analysis of gene expression revealed distinct patterns between treatment and control groups. At 24 hpi, 6012 DEGs were identified, with 3713 upregulated and 2299 downregulated. At 40 hpi, the number of DEGs decreased to 4352 (2799 upregulated and 1553 downregulated). At 60 hpi, the number of DEGs increased to 5848 (3968 upregulated and 1880 downregulated). By 96 hpi, the pattern shifted towards downregulation, with 7800 DEGs being identified (3398 upregulated and 4402 downregulated) (Figure 4a,b).
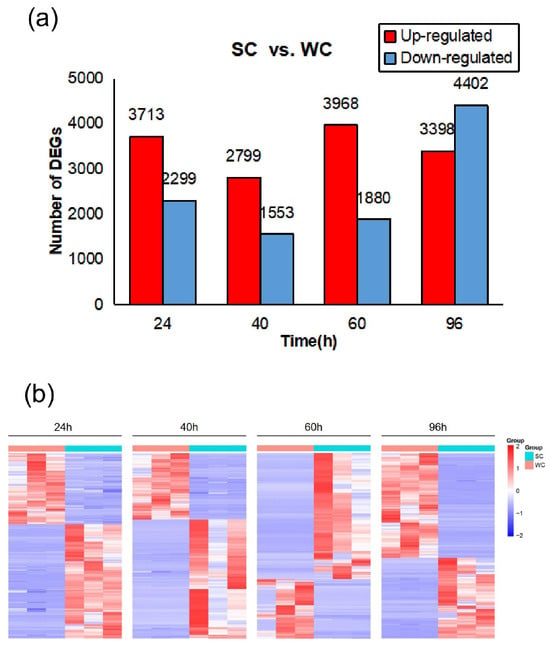
Figure 4.
Differential gene expression analysis in Cymbidium ensifolium leaves after foliar application of SVFJ-07 and sterile water at different time points. (a) Changes in differentially expressed genes over time in C. ensifolium leaves after treatment. (b) Volcano plot of differential expressed genes in C. ensifolium leaves after treatment at different time points.
3.6. GO Functional Analysis and Functional Enrichment of DEGs
To identify the main functions of DEGs in the SVFJ-07-induced response of Chinese orchids to the infection of C. gloeosporioides, GO analysis was performed to characterize the functional roles of DEGs within the transcriptome samples. The DEGs were categorized into three main GO domains: biological process (BP), cellular component (CC), and molecular function (MF) (Figure S1). Across all the temporal comparisons (SC24 vs. WC24, SC40 vs. WC40, SC60 vs. WC60, and SC96 vs. WC96), the distribution of GO terms remained relatively consistent, with variations predominantly being observed in expression levels rather than functional categories. The DEGs were mainly annotated to BPs (which were involved in interspecies interaction between organisms, biological regulation, cellular developmental processes, localization, metabolic processes, multicellular organismal processes, reproductive processes, response to stimuli, and signaling), CCs (such as cellular anatomical entities and genes related to general transcription factor activity), and MFs (such as binding, catalytic activity, structural molecule activity, transcription regulator activity, translation regulator activity, and transporter activity).
KEGG pathway enrichment analysis was conducted using hypergeometric testing to identify significantly enriched metabolic or signaling pathways among DEGs, which are shown in Figure S2. In the SC24 vs WC24 comparison group, 528 significant DEGs were annotated to 254 metabolic pathways, with 23 metabolic pathways being found to be significantly enriched (p ≤ 0.05). In the SC40 vs. WC40 comparison group, 347 significant DEGs were annotated to 227 metabolic pathways, revealing 28 significantly enriched metabolic pathways (p ≤ 0.05). In the SC60 vs. WC60 comparison group, 577 significant DEGs were annotated to 259 metabolic pathways, among which 20 metabolic pathways were significantly enriched (p ≤ 0.05). In the SC96 vs. WC96 comparison group, 633 significant DEGs were annotated to 263 metabolic pathways, showing 21 significantly enriched metabolic pathways (p ≤ 0.05).
3.7. KEGG Analysis of Plant Stomatal Immunity-Related DEGs
The KEGG pathway enrichment analysis revealed that DEGs in the SC group participated in a broader range of metabolic pathways across different time points following inoculation of C. gloeosporioides compared to the WC group. We focused on four pathways crucial to plant stomatal immune response, which were the plant–pathogen interaction pathway, the peroxisome metabolism pathway, the plant hormone signal transduction pathway, and the MAPK signaling pathway (Table S4). A hierarchical clustering analysis was subsequently performed on the DEGs associated with stomatal immunity (Figure 5).
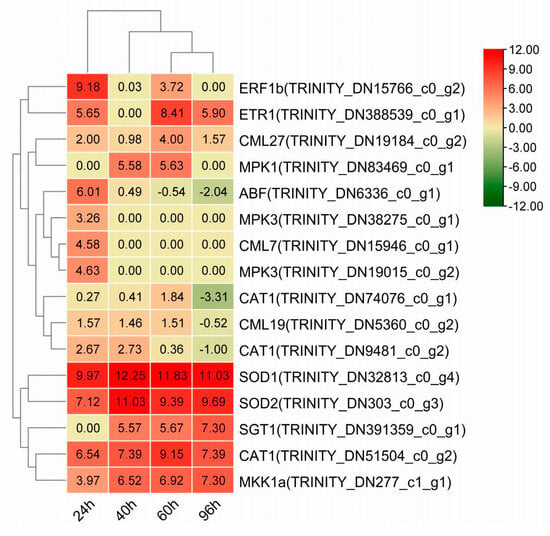
Figure 5.
Cluster heatmap of differentially expressed genes related to stomatal immunity.
3.8. DEG Analysis in Plant–Pathogen Interaction Pathways
The KEGG pathway enrichment analysis revealed that DEGs in the SC group participated in a broader range of metabolic pathways across different time points following inoculation with C. gloeosporioides compared to the WC group. We focused on four pathways crucial to plant stomatal immune response, which were the plant–pathogen interaction pathway, the peroxisome metabolism pathway, the plant hormone signal transduction pathway, and the MAPK signaling pathway (Table S4). A hierarchical clustering analysis was subsequently performed on the DEGs associated with stomatal immunity (Figure 5).
A comparative analysis between the SVFJ-07-treated and control groups revealed the temporal dynamics of gene expression in plant–pathogen interaction pathways following inoculation with C. gloeosporioides. Notable differential expression was observed in genes encoding calmodulin-like protein (CML) and important regulatory proteins involved in plant effector-triggered immunity (ETI), particularly SGT1. CML is an important Ca2+ sensor that plays crucial roles in regulating plant stress-response mechanisms. The CML gene family exhibited dynamic activation across different time points, with three, two, three, and three genes being expressed at 24, 40, 60, and 96 hpi, respectively. Specifically, the gene TRINITY_DN28360_c0_g1 was significantly upregulated at 60 and 96 hpi; the CML7 gene (TRINITY_DN15946_c0_g1) was only upregulated at 24 hpi; the CML27 gene (TRINITY_DN19184_c0_g2) was upregulated at 24, 40, 60, and 96 hpi; and the CML19 gene (TRINITY_DN5360_c0_g2) was significantly upregulated at 24, 40, and 60 hpi, but downregulated at 96 hpi. Additionally, the gene encoding SGT1 (TRINITY_DN391359_c0_g1), which is essential for ETI responses, was sustainably upregulated from 40 to 96 hpi.
3.9. Analysis of Differentially Expressed Genes in the Peroxisome Metabolism Pathway
H2O2 is an important signaling molecule in plants that can induce stomatal closure. Key enzymes involved in the peroxisome metabolism pathway, including copper-zinc superoxide dismutase (SOD1), manganese superoxide dismutase (SOD2), and catalase isozyme (CAT1), were activated; these are involved in H2O2 metabolism. The genes encoding SOD1 (TRINITY_DN32813_c0_g4) and SOD2 (TRINITY_DN303_c0_g3) were significantly upregulated throughout the observation period (24, 40, 60, and 96 hpi). Similarly, three distinct genes encoding CAT1 showed temporal activation patterns. Specifically, TRINITY_DN51504_c0_g2 maintained significant upregulation across all time points, while TRINITY_DN9481_c0_g2 and TRINITY_DN74076_c0_g1 were upregulated from 24 to 60 hpi but downregulated at 96 hpi. These findings suggest that the SVFJ-07 strain enhanced cellular antioxidant capacity by inducing the expression of SOD1 and SOD2, thereby facilitating the conversion of superoxide radicals into H2O2 and molecular oxygen and protecting cells against damage incited by anthracnose.
3.10. Analysis of DEGs in Plant Hormone Signal Transduction Pathways
The plant hormone signaling network exhibited the complex temporal regulation of multiple genes associated with stomatal immunity. The ethylene receptor gene ETR1 (TRINITY_DN388539_c0_g1) showed significant upregulation at 24, 60, and 96 hpi. The ethylene response factor gene ERF (TRINITY_DN15766_c0_g2) demonstrated strong upregulation at 24 hpi and sustainable elevation through 40 and 60 hpi. Notably, the ABA response element gene ABF (TRINITY_DN6336_c0_g1) displayed initial significant upregulation at 24 and 40 hpi, followed by downregulation at 60 and 96 hpi. These expression patterns suggested that SVFJ-07 modulated stomatal closure through the coordinated regulation of ethylene-receptor-related genes and their downstream effectors, including nitric oxide (NO) and H2O2 signaling molecules.
3.11. Analysis of DEGs in the MAPK Signaling Pathway
During the plant stomatal immunity response, the MKK1/3-MPK3/6 cascade functions downstream of ETR1 and H2O2 in the ethylene-induced stomatal closure signaling pathway [24,25]. Multiple components of this MAPK signaling cascade exhibited distinct temporal expression patterns. The MKK1a gene (TRINITY_DN277_c1_g1) maintained consistent upregulation throughout all time points (24, 40, 60, and 96 hpi). The MPK1 gene (TRINITY_DN83469_c0_g1) showed significant upregulation, specifically during the intermediate phase (40 and 60 hpi). Two MPK3 genes, TRINITY_DN38275_c0_g1 and TRINITY_DN19015_c0_g2, displayed distinct expression patterns; the former exhibited initial upregulation (at 24 and 40 hpi) followed by downregulation at 96 hpi, and the latter showed selective upregulation exclusively at 24 hpi. The MPK6 gene (TRINITY_DN46069_c0_g1) demonstrated remarkable upregulation during the early response phase (at 24 hpi).
3.12. Validation of Gene Expression by qRT-PCR
To validate the results obtained from transcriptome sequencing, six key genes involved in strain SVFJ-07-induced stomatal immunity in C. ensifolium were selected for qRT-PCR analysis at 24 hpi of infection with C. gloeosporioides. Using the ACT (muscle actin 2) gene as the internal reference gene for the normalization of gene expression, significant expression changes were observed for all the six selected genes. The expression profiles obtained using qRT-PCR closely matched those derived from transcriptome sequencing, thereby confirming the reliability of the transcriptomic analysis (Figure 6).
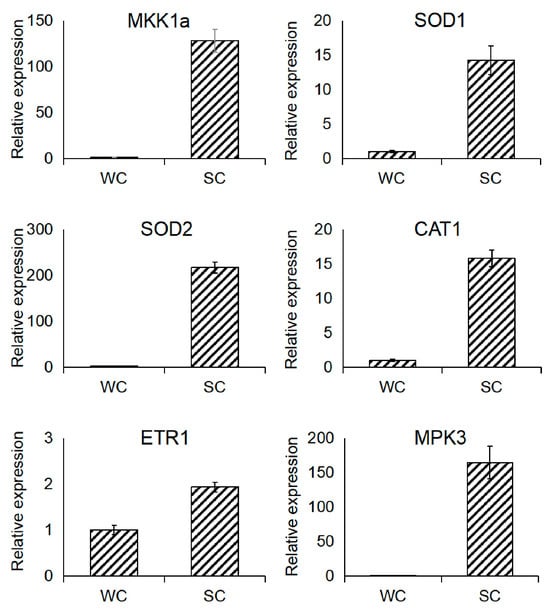
Figure 6.
Validation of selected candidate genes using qRT-PCR.
4. Discussion
Our results demonstrated that the SVFJ-07 strain significantly enhanced stomatal closure in C. ensifolium leaves during C. gloeosporioides infection, compared to untreated plants. Notably, SVFJ-07-treated plants exhibited minimal disease symptoms at 14 dpi. To elucidate the underlying mechanism of SVFJ-07-induced stomatal closure in orchid plants resisting the infection of C. gloeosporioides, we conducted transcriptome sequencing analysis. The transcriptomic data revealed significantly higher numbers of DEGs, upregulated genes, enriched GO terms, and metabolic pathways in SC compared to WC groups at 24, 40, and 60 hpi. These findings indicated that SVFJ-07-treated C. ensifolium plants rapidly initiated plant immune responses during early infection stage (24 hpi) and maintained this response throughout the middle infection stage (40 and 60 hpi). Further analyses of plant stomatal immune-related genes revealed differential expression patterns in several key pathways, including peroxisome metabolism pathways, plant hormone signal transduction pathways, and MAPK signaling pathways. These results suggested that SVFJ-07 enhanced plant stomatal immunity against the infection of C. gloeosporioides by modulating hormone synthesis and transport.
Plants employ active stomata closure as a defense mechanism against the infection of pathogens, a process known as stomatal immunity [26]. This process involves several key signaling molecules, including calciumions (Ca2+), H2O2, and ethylene, which function at multiple levels within the plant immune system [27]. CMLs play crucial roles in Ca2+ signal transduction and regulate various cellular functions by modulating target protein activities [28,29]. Our results showed that SVFJ-07 activated CML gene expression during the infection process of C. gloeosporioides, suggesting its involvement in Ca2+ regulation in orchid plants.
The SGT1 gene, which was activated during the late stages of infection in our study, constitutes a critical component of R-gene-mediated disease resistance in plants [30]. Previous research has shown that the overexpression of pumpkin CM-SGT1 mitigates the symptoms of powdery mildew infection in Arabidopsis leaves and concurrently enhances cell death and H2O2 accumulation [31]. In the present study, SVFJ-07 could activate DEGs within the plant–pathogen interaction pathway, promoting stomatal closure through the regulation of Ca2+ signal transduction and downstream of H2O2 accumulation. H2O2, a prevalent ROS, functions as a key signaling molecule in plant growth, development, stress resistance, and plant immune responses. ROS can interact with various plant hormone signaling pathways and is involved in the process of ABA-induced stomatal closure [32]. Our findings revealed significant upregulation of SOD1 and SOD2 genes in the peroxisome metabolism pathway at 24, 40, 60, and 96 hpi, suggesting that SVFJ-07 induced the expression of these genes and potentially increased the H2O2 content in plants. Additionally, CAT1 activation was observed in response to elevated H2O2 levels [33], indicating that SVFJ-07-treated orchid plants might more effectively regulate ROS production to facilitate stomatal closure and enhance resistance against the infection of pathogens.
The SVFJ-07-treated group exhibited the upregulation of genes involved in both the plant hormone signaling transduction pathway and the MPK signaling pathway. Specifically, SVFJ-07 activated the expression of ETR1 and ERF, which are crucial components of ethylene signaling. Given that H2O2-induced stomatal closure depends on ETR1 and its downstream elements, ethylene likely plays a role in SVFJ-07-induced stomatal immunity [34,35]. Furthermore, the MPK signaling pathway, particularly the MKK1/3-MPK3/6 cascade acting downstream of ETR1 and H2O2, contributed to ethylene-induced stomatal closure. The early upregulation (24 hpi) of MKK1a, MPK1, MPK3, and MPK6 in SVFJ-07-treated plants enhanced ethylene production during the resistance response to the infection of the pathogen.
Comparative transcriptome analysis between SC and WC orchid plants, supported by biological symptoms, demonstrated that the SC group exhibited a more robust response and enhanced stomatal closure. It was proposed that SVFJ-07 regulated Ca2+ signal transduction in orchid plants, promoting ROS accumulation and subsequent stomatal closure to resist the infection of pathogens. Similarly, Streptomyces kanasensis ZX01 induced Ca2+ rapidly in tobacco leaves and suspended cells, upregulated and enriched defense and immune reaction pathways, and improved the SA content [36]. These findings align with typical Streptomyces–host systems. Streptomyces sp. enhanced systemic resistance by inducing stomatal closure, strengthening plant secondary metabolites activities and regulating relative genes to form defensive barriers against pathogen invasion.
5. Conclusions
In this study, we demonstrated that SVFJ-07 inhibited infection with pathogens by inducing stomatal closure. The foliar application of this strain led to significant reductions in stomatal length, width, and aperture. Furthermore, orchid plants treated with SVFJ-07 exhibited enhanced stomatal immunity and disease resistance; moreover, levels of endogenous ABA and SA were elevated. Additionally, the transcriptome analysis revealed the upregulation of genes associated with stomatal immunity, particularly those involved in plant–pathogen interactions, peroxisome metabolism, plant hormone signaling, and mitogen-activated protein kinase (MAPK) signaling pathways. These findings confirmed that the induction of SVFJ-07 promoted stomatal closure to resist the infection of C. gloeosporioides and simultaneously induced complex transcriptome changes. Taken together, our findings provide insights into the mechanism underlying SVFJ-07-induced stomatal closure in orchid plants defending against the infection of C. gloeosporioides and advance our understanding of plant–pathogen–biocontrol agent interactions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15061282/s1, Figure S1: GO enrichment analysis of differentially expressed genes; Figure S2: KEGG enrichment analysis of differentially expressed genes; Table S1: Oligonucleotide primers used for gene-specific amplification in this study; Table S2: Quality analysis of transcriptome sequencing data treated at indicated post-inoculation days of Colletotrichum gloeosporioides; Table S3: Statistics of the unigene annotation; Table S4: DEGs related to C. Ensifolium stomatal immunity induced by S. vinaceus SVFJ-07.
Author Contributions
Conceptualization, J.Y. and P.H.; data curation, X.H.; formal analysis, J.Z.; funding acquisition, J.Y.; investigation, J.Z. and X.H.; methodology, X.H.; project administration, J.Y. and D.Y.; supervision, D.Y.; validation, J.Y., P.H. and J.Z.; writing—original draft preparation, P.H. and X.H.; writing—review and editing, J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fujian Provincial Department of Science and Technology (grant numbers 2023R1071, 2023R1022004, 2022R1024001, and 2020J011357), the Projects of Agricultural High-Quality Development Beyond the “5511” Collaborative Innovation (XTCXGC2021011 and XTCXGC2021017), and the Projects of the Fujian Academy of Agricultural Sciences (grant number CXTD2021016).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank the editor and anonymous reviewers for their comments, which helped to improve the manuscript. We also would like to acknowledge all the other individuals who contributed to this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ashwini, N.; Srividya, S. Potentiality of Bacillus subtilis as biocontrol agent for management of anthracnose disease of chili caused by Colletotrichum gloeosporioides OGC1. 3 Biotech 2014, 4, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.A.; Huang, P.; Yu, D.Y.; Lu, S.M.; Lin, L.P. Isolation and identification of anthracnose pathogen on Cymbidium ensifolium and its culture condition optimization. Chin. J. Trop. Crop. 2011, 32, 1940–1944. [Google Scholar]
- Yao, J.A.; Huang, P.; Yu, D.Y.; Lin, L.P.; Lu, S.M. Biological characteristics of the conidia of Colletotrichum gloeosporioides infected with Cymbidium ensifolium and the selection of fungicides against the disease. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2012, 41, 585–589. [Google Scholar]
- Gan, L.; Yao, J.A.; Chen, F.R. Research on the control technology of anthracnose of golden-sided tiger orchid. Fujian Sci. Technol. Trop. Crop. 2012, 2, 13–15. [Google Scholar]
- Yokosawa, S.; Eguchi, N.; Kondo, K.I.; Sato, T. Phylogenetic relationship and fungicide sensitivity of members of the Colletotrichum gloeosporioides species complex from apple. J. Gen. Plant Pathol. 2017, 83, 291–298. [Google Scholar] [CrossRef]
- Piccirillo, G.; Carrieri, R.; Polizzi, G.; Azzaro, A.; Lahoz, E.; Fernández-Ortuño, D.; Vitale, A. In vitro and in vivo activity of QoI fungicides against Colletotrichum gloeosporioides causing fruit anthracnose in Citrus sinensis. Sci. Hortic. 2018, 236, 90–95. [Google Scholar] [CrossRef]
- Zhang, D.D.; Qiu, J.K. Plant-pathogen interactions and synergistic evolution. Sci. Bull. 2017, 12, 1214–1220. [Google Scholar]
- Yao, J.A.; Huang, P.; Chen, F.; Huang, M.M.; Yu, D.Y. Screening and effect of antagonistic actinomycetes against anthracnose of Cymbidium ensifolium. Chin. J. Biol. Control 2019, 35, 805–812. [Google Scholar]
- Jean-Luc, M.; Heribert, H. New checkpoints in stomatal defense. Trends Plant Sci. 2013, 18, 295–297. [Google Scholar]
- Amutha, S.K.; Venkatachalam, L.; Caplan, J.L.; Deborah, P.; Czymmek, K.J.; Levia, D.F.; Bais, H.P. Rhizobacteria bacillus subtilis restricts foliar pathogen entry through stomata. Plant J. 2012, 72, 694–706. [Google Scholar]
- Sharma, A.; Pathak, A.; Sahgal, M.; Meyer, J.M.; Wray, V.; Johri, B.N. Molecular characterization of plant growth promoting rhizobacteria that enhance peroxidase and phenylalanine ammonia-lyase activities in chili (Capsicum annuum L.) and tomato (Lycopersicon esculentum Mill.). Arch. Microbiol. 2007, 188, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Huang, Z.; Li, X.; Ma, L.; Gu, Q.; Wu, H.; Liu, J.; Borriss, R.; Wu, Z.; Gao, X. Stomatal closure and SA-JA/ET-signaling pathways are essential for Bacillus amyloliquefaciens FZB42 to restrict leaf disease caused by Phytophthora nicotianae in Nicotiana benthamiana. Front. Microbiol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Khokon, A.R.; Okuma, E.; Hossain, M.A.; Munemasa, S.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 2011, 34, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Agurla, S.; Gayatri, G.; Raghavendra, A.S. Nitric oxide as a secondary messenger during stomatal closure as a part of plant immunity response against pathogens. Nitric Oxide 2014, 43, 89–96. [Google Scholar] [CrossRef]
- Bian, Z.; Zhang, X.; Wang, Y.; Lu, C.G. Improving drought tolerance by altering the photosynthetic rate and stomatal aperture via green light in tomato (Solanum lycopersicum L.) seedlings under drought conditions. Environ. Exp. Bot. 2019, 167, 103844. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotech. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A bioconduct or package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gudesblat, G.E.; Lusem, N.D.; Morris, P.C. Guard cell-specific inhibition of Arabidopsis MPK3 expression causes abnormal stomatal responses to abscisic acid and hydrogen peroxide. New Phytol. 2007, 173, 713–721. [Google Scholar] [CrossRef]
- Wang, H.; Ngwenyama, N.; Liu, Y.; Walker, J.C.; Zhang, S.Q. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 2007, 19, 63–73. [Google Scholar] [CrossRef]
- Melotto, M.; Underwood, W.; He, S.Y. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 2008, 46, 101–122. [Google Scholar] [CrossRef]
- Jiang, Y.X.; Ding, P.T. Calcium signaling in plant immunity: A spatiotemporally controlled symphony. Trends Plant Sci. 2023, 28, 74–89. [Google Scholar] [CrossRef]
- Thor, K.; Jiang, S.S.; Michard, E.; George, J.; Scherzer, S.; Huang, S.G.; Dindas, J.; Derbyshire, P.; Leitão, N.; DeFalco, T.A.; et al. The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity. Nature 2020, 585, 7826. [Google Scholar] [CrossRef]
- Leba, L.J.; Cécilia, C.; Inmaculada, O.M.; Benoit, R.; Carmen, R.B.; Jean-Philippe, G.; Didier, A. CML9, an Arabidopsis calmodulin-like protein, contributes to plant innate immunity through a flagellin-dependent signaling pathway. Plant J. 2012, 71, 976–989. [Google Scholar] [CrossRef]
- Austin, M.J.; Muskett, P.; Kahn, K.; Feys, B.J.; Jones, J.D.G.; Parker, J.E. Regulatory role of SGT1 in early R gene-mediated plant defenses. Science 2002, 295, 2077–2080. [Google Scholar] [CrossRef]
- Guo, W.L.; Chen, B.H.; Guo, Y.Y.; Yang, H.L.; Mu, J.Y.; Wang, Y.L.; Li, X.Z.; Zhou, J.G. Improved powdery mildew resistance of transgenic Nicotiana benthamiana overexpressing the Cucurbita moschata CmSGT1 gene. Front. Plant Sci. 2019, 10, 955. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.L.; Tan, R.; Kong, H.Y.; Bai, X.; Xiong, Y.C. Stomatal closure mediated by ABA-H2O2 in plants. J. Plant Physiol. 2012, 48, 739–746. [Google Scholar]
- Jannat, R.; Uraji, M.; Morofuji, M.; Islam, M.M.; Bloom, R.E.; Nakamura, Y.; McClung, C.R.; Schroeder, J.; Mori, I.C.; Murata, Y. Roles of intracellular hydrogen peroxide accumulation in abscisic acid signaling in Arabidopsis guard cells. J. Plant Physiol. 2011, 168, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.M.; Cai, H.L.; Lei, X.; Zhou, X.; Yue, M.; He, J.M. Heterotrimeric G protein mediates ethylene-induced stomatal closure via hydrogen peroxide synthesis in Arabidopsis. Plant J. 2015, 82, 138–150. [Google Scholar] [CrossRef]
- Ge, X.M.; Hu, X.; Zhang, J.; Huang, Q.M.; Gao, Y.; Li, Z.Q.; Li, S.; He, J.M. UV RESISTANCE LOCUS8 mediates ultraviolet-B-induced stomatal closure in an ethylene-dependent manner. Plant Sci. 2020, 301, 110672. [Google Scholar] [CrossRef]
- Han, L.; Sun, Y.; Zhou, X.; Hao, X.; Wu, M.; Zhang, X.; Feng, J. A novel glycoprotein from Streptomyces sp. triggers early responses of plant defense. Pestic. Biochem. Phys. 2021, 171, 104719. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).