Physiological and Transcriptomic Responses of Two Rhododendron L. Cultivars to Drought Stress: Insights into Drought Tolerance Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Drought Treatments
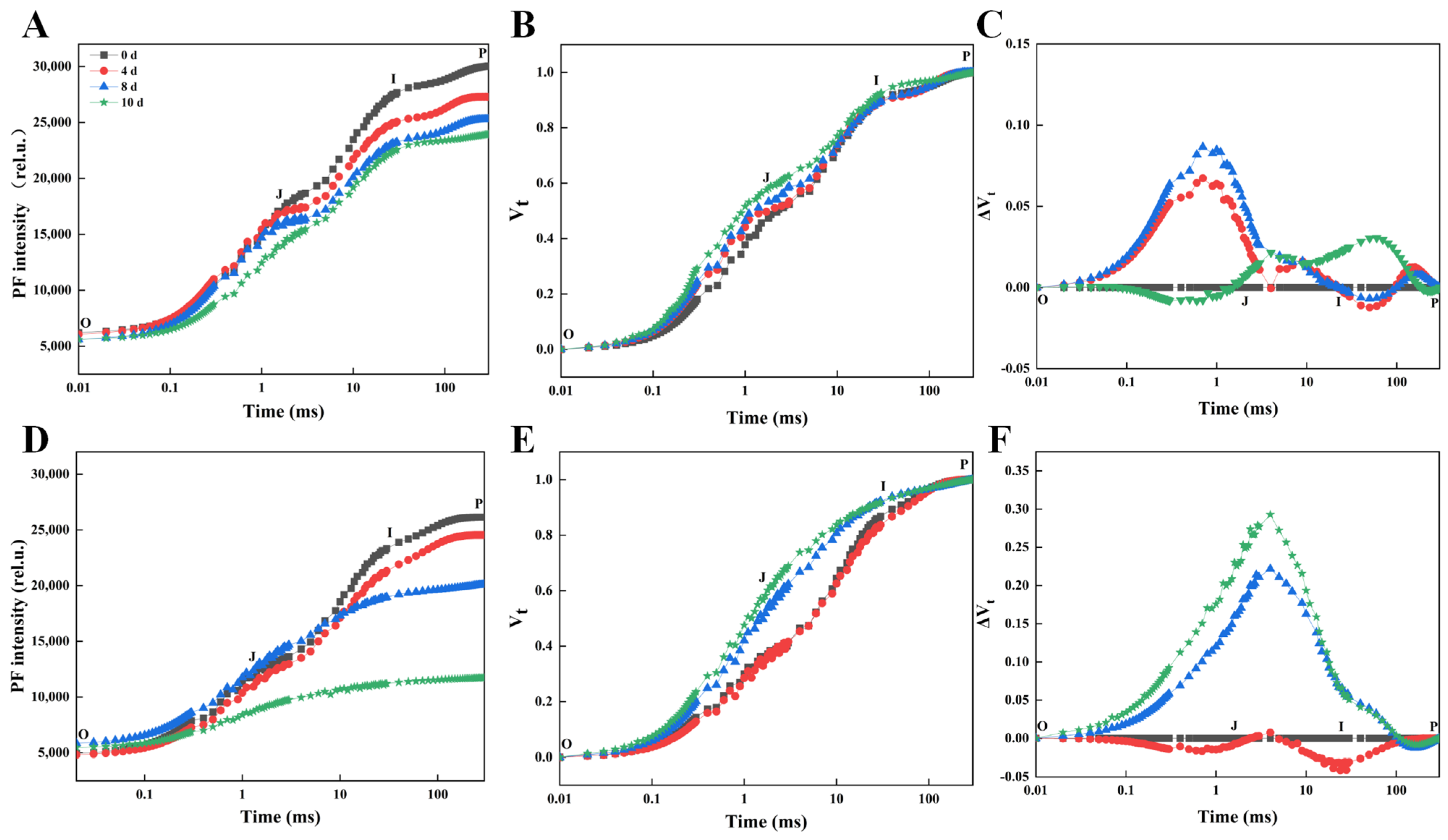
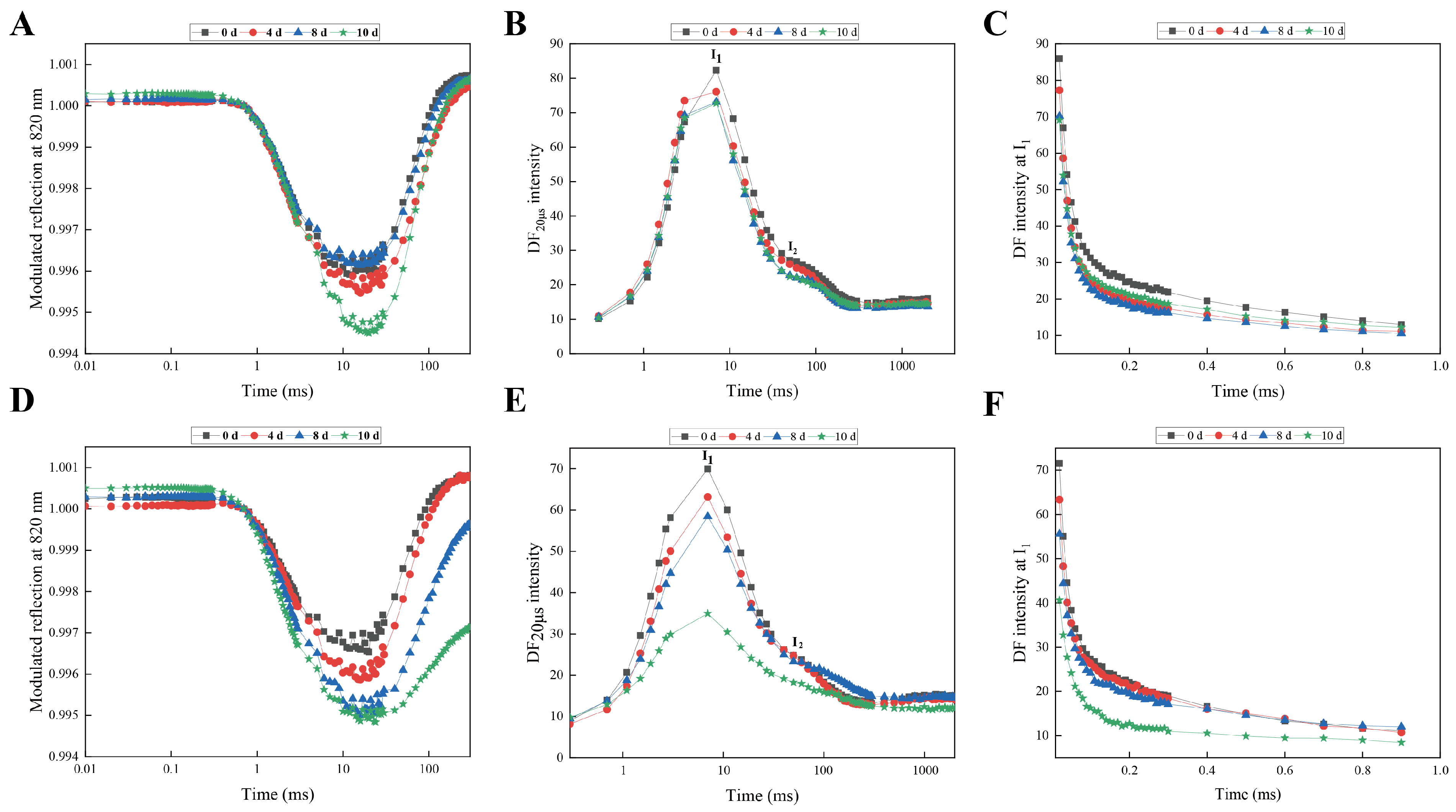
2.2. Simultaneous Measurement of the Kinetics of PF, DF, and MR
2.3. Total RNA Isolation and Transcriptome Sequencing
2.4. De Novo Transcriptome Assembly and Functional Annotation
2.5. Differential Gene Expression Analysis
2.6. Gene Validation of RNA-Seq by qRT-PCR Analysis
2.7. Statistical Analysis
3. Results
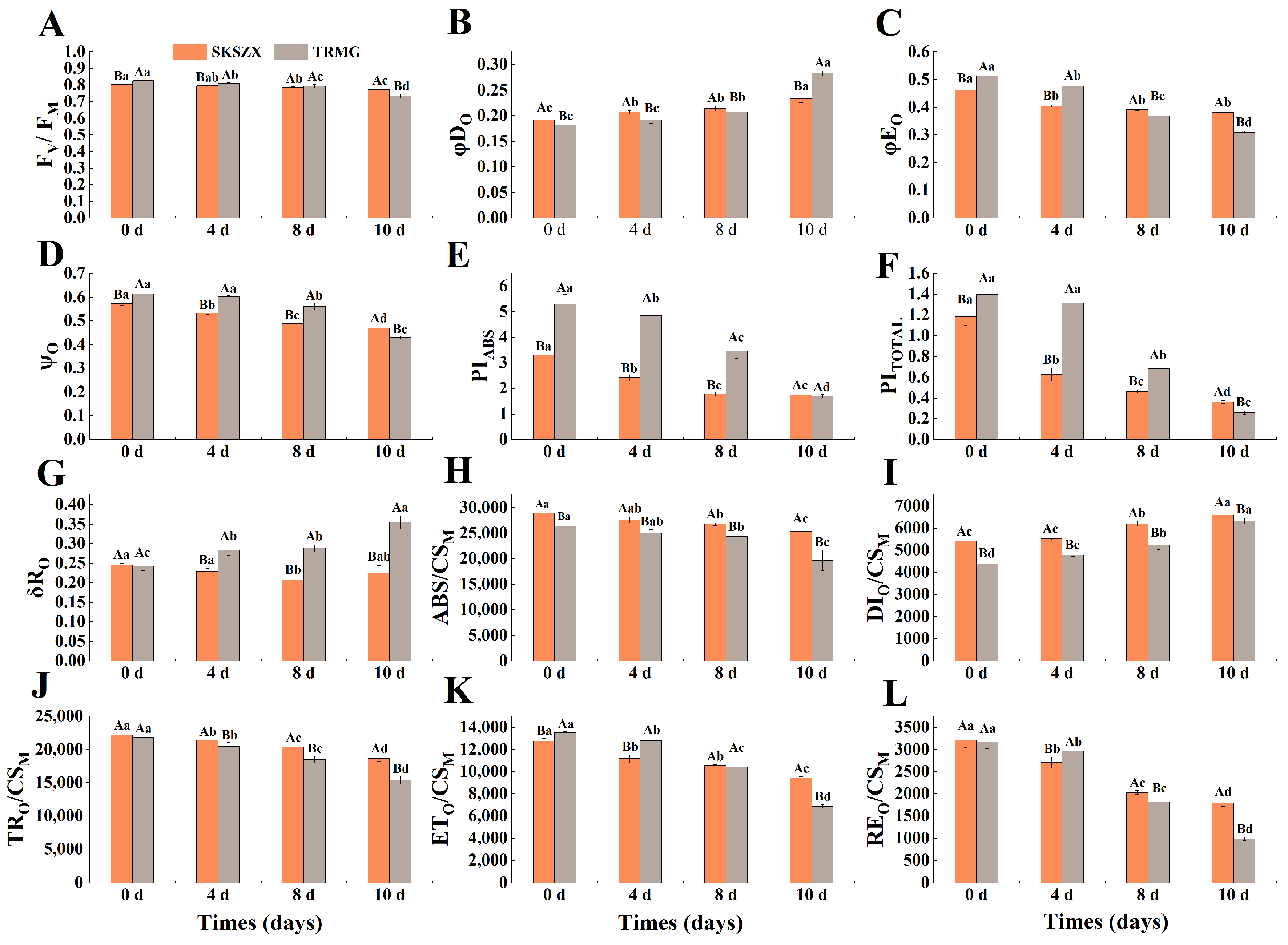
3.1. Comparative Analysis of Morphological and Chlorophyll Fluorescence of Responses of Rhododendron Cultivars Under Drought Stress
3.2. Illumina Sequencing and De Novo Assembly
3.3. Comparisons of DEGs Under the Different Drought Stress Stages
3.4. GO and KEGG Enrichments of DEGs
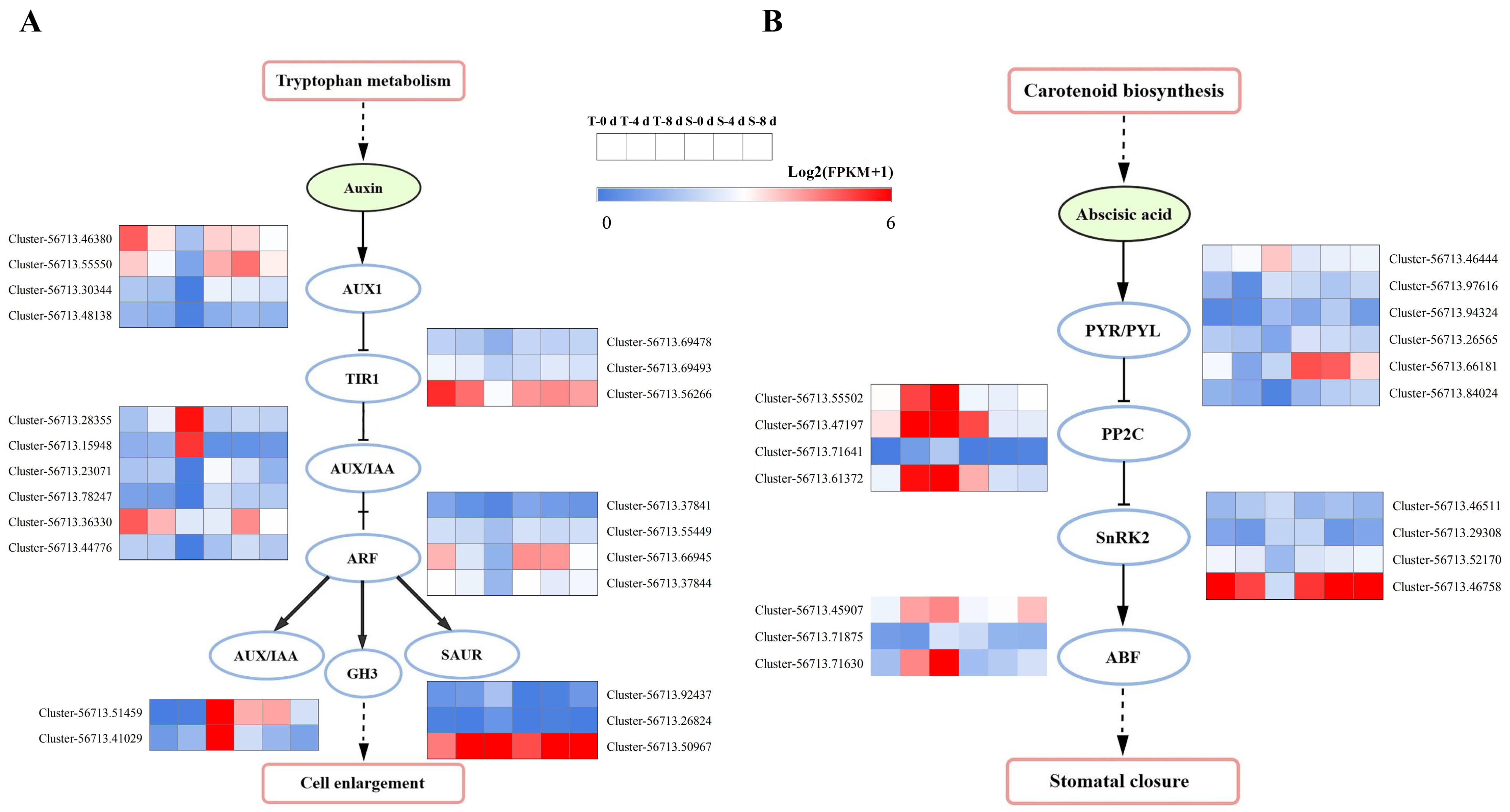
3.5. Identification of Plant Hormone and Signal-Transduction-Related DEGs
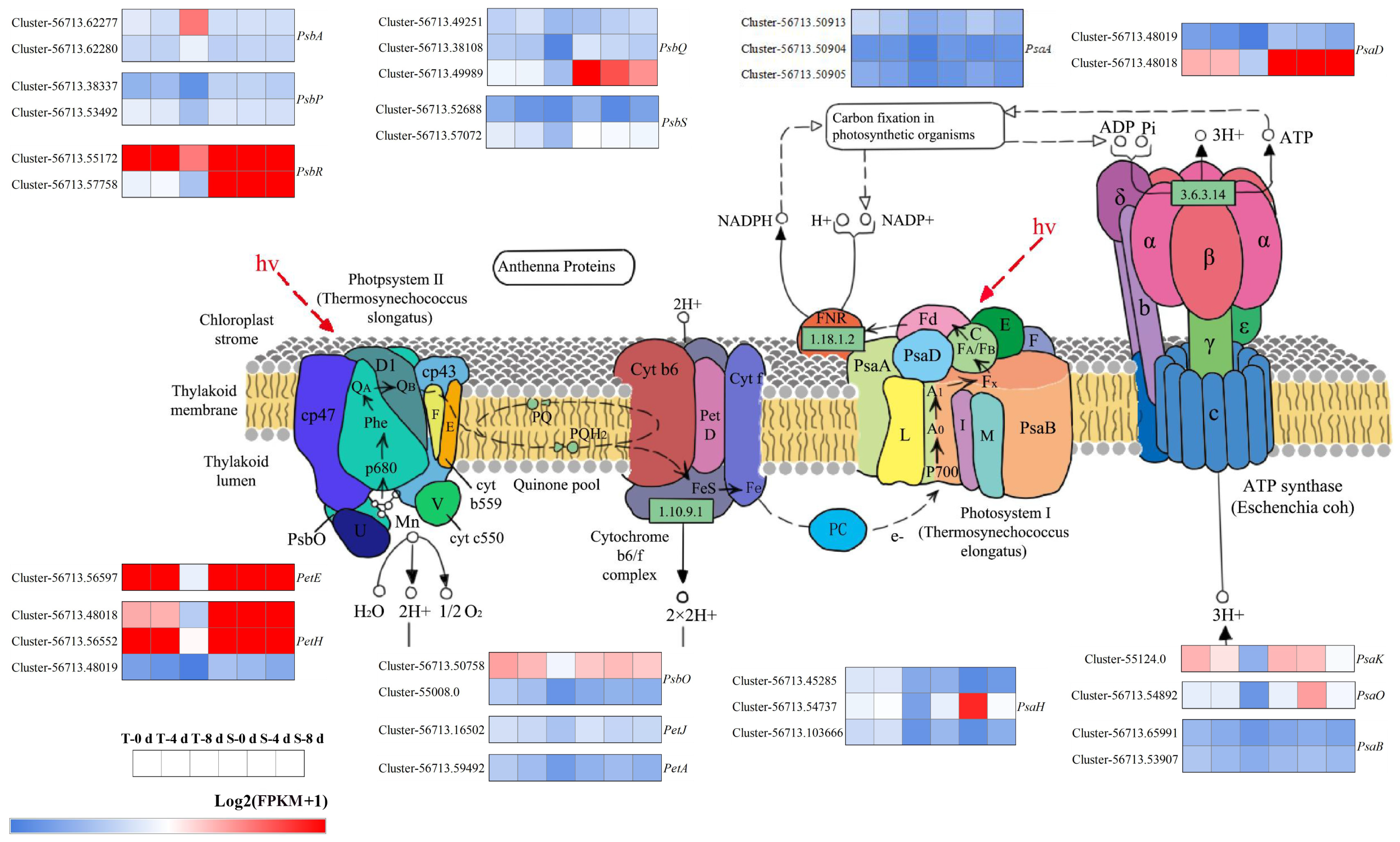
3.6. Photosynthesis-Related Genes Under Drought Stress
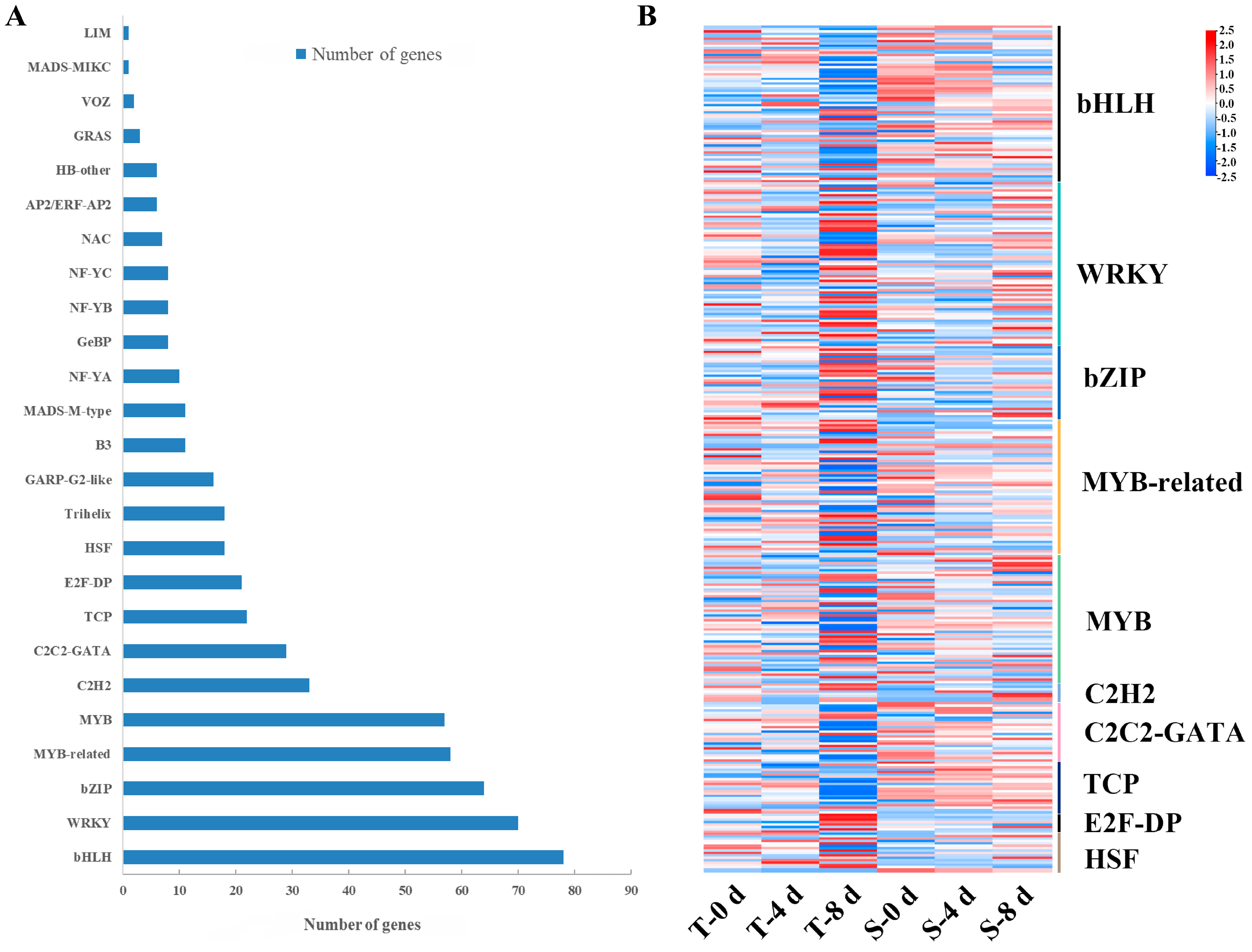
3.7. Transcription Factors Responding to Drought Stress
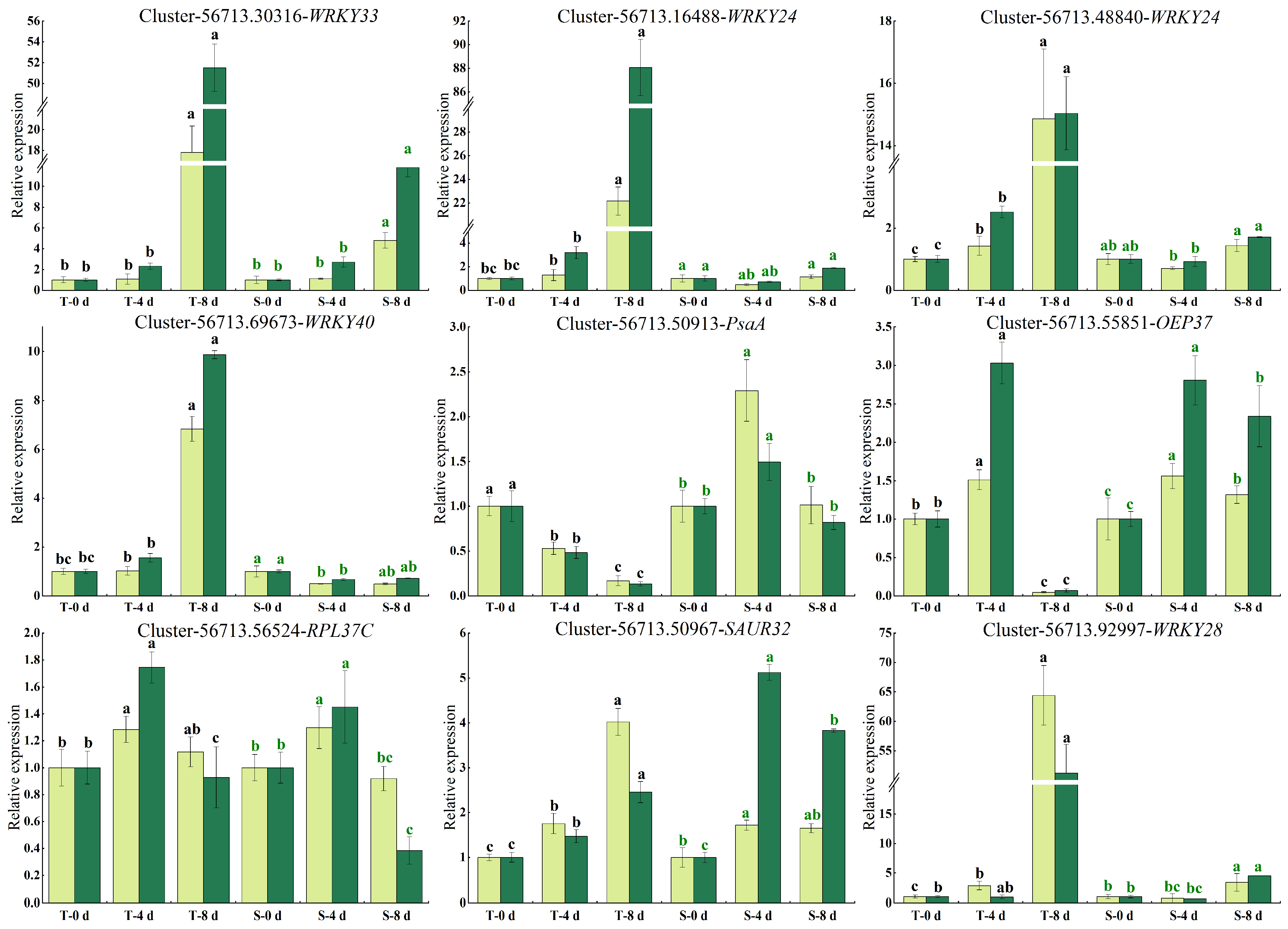
3.8. Validation of the DEGs by qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, M.B.; Mu, L.Q. Rhododendron plant resources and landscape application. Spec. Econ. Anim. Plants 2022, 25, 103–104. [Google Scholar]
- Scheiber, S.M.; Jarret, R.L.; Robacker, C.D.; Newman, M. Genetic relationships within Rhododendron L. section Pentanthera G. Don based on sequences of the internal transcribed spacer (ITS) region. Sci. Hortic. 2000, 85, 123–135. [Google Scholar] [CrossRef]
- Popescu, R.; Kopp, B. The genus Rhododendron: An ethnopharmacological and toxicological review. J. Ethnopharmacol. 2013, 147, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Gautam, V.; Kohli, S.K.; Kapoor, D.; Bakshi, P.; Ahmad, P. Stress Protective Effect of Rhododendron arboreum Leaves (MEL) on Chromium-Treated Vigna radiata Plants. J. Plant Growth Regul. 2021, 40, 423–435. [Google Scholar] [CrossRef]
- Wang, X.Q.; Huang, Y.; Long, C.L. Cross-amplification and characterization of microsatellite loci for the genus Rhododendron. Hortscience 2010, 45, 1394–1397. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Jin, W.; Fang, Y.; Yang, Q.; Xiang, J. Transcriptome analysis and identification of genes associated with flower development in Rhododendron pulchrum Sweet (Ericaceae). Gene 2018, 679, 108–118. [Google Scholar] [CrossRef] [PubMed]
- De Riek, J. Ornamental Crops; Springer: Cham, Switzerland, 2018; Volume 11, pp. 237–271. [Google Scholar]
- Xie, W.J. Thewell-established industrial system of Belgian Azaleas. China Flowers Hortic. 2015, 5, 56–57. [Google Scholar]
- Li, X.C. Greenhouse cultivation techniques of Belgian Azaleas. Chin. Hortic. Abstr. 2009, 25, 146–147. [Google Scholar]
- Wang, Z.C. The Landscape Construction of Rhododendron Special Garden in National Forest Park of Hunan Dawei Mountain. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2014. [Google Scholar]
- Zhu, C.Y.; Yu, J.L.; Lv, M.; Zhu, J.J. Flowering characteristics and cultivation adaptability of 28 Rhododendron cultivars. J. Agric. 2015, 5, 82–86. [Google Scholar]
- Zhou, L.L. Research on Plant Landscape of Rhododendron. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2013. [Google Scholar]
- Zhou, W.W. Development status of Rhododendron industry in China. China Flowers Hortic. 2010, 9, 12–14. [Google Scholar]
- Wu, H.; Yang, X.M.; Shao, H.M.; Wang, F. Germplasm resources base for Rhododendron horticulture: Status, problems and countermeasures. Biodivers. Sci. 2013, 21, 628–634. [Google Scholar]
- Ou, J.; Chen, X. Resources and landscape application prospects of Subgen. Hymenanthes in Guizhou Province. Jiangsu Agric. Sci. 2012, 40, 200–203. [Google Scholar]
- Sleumer, H. Ein system der gattung Rhododendron L. Bot. Jahrb. Syst. 1949, 74, 511–553. [Google Scholar]
- Todaka, D.; Zhao, Y.; Yoshida, T.; Kudo, M.; Kidokoro, S.; Mizoi, J.; Kodaira, K.S.; Takebayashi, Y.; Kojima, M.; Sakakibara, H.; et al. Temporal and spatial changes in gene expression, metabolite accumulation and phytohormone content in rice seedlings grown under drought stress conditions. Plant J. 2017, 90, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, Z.; Wang, Q.; Garrell, A.K.; Liu, M.; Guan, Y.; Zhou, W.; Liu, W. Comparative proteomic investigation of drought responses in foxtail millet. BMC Plant Biol. 2018, 18, 315. [Google Scholar] [CrossRef]
- Xu, K.; Chen, S.; Li, T.; Ma, X.; Liang, X.; Ding, X.; Liu, H.; Luo, L. OsGRAS23, a rice GRAS transcription factor gene, is involved in drought stress response through regulating expression of stress-responsive genes. BMC Plant Biol. 2015, 15, 141. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Wang, X.; Yang, Q.; Quan, X.; Zeng, J.; Dai, F.; Zeng, F.; Wu, F.; Zhang, G.; et al. Leaf epidermis transcriptome reveals drought-Induced hormonal signaling for stomatal regulation in wild barley. Plant Growth Regul. 2018, 87, 39–54. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, L.; Lai, J.; Zhao, H.; Song, W. Effects of drought stress and water recovery on physiological responses and gene expression in maize seedlings. BMC Plant Biol. 2018, 18, 68. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar]
- Zelitch, I. The close relationship between net photosynthesis and crop yield. Bioscience 1982, 32, 796–802. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Govindjee. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. Analysis of the chlorophyll a fluorescence transient. Chlorophyll fluorescence: A signature of photosynthesis. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Advances in Photosynthesis and Respiration Series; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 321–362. [Google Scholar]
- Schansker, G.; Tóth, S.Z.; Strasser, R.J. Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim. Biophys. Acta 1706, 2005, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A.; Govindjee. Chlorophyll a fluorescence induction: A personal perspective of the thermal phase, the J-I-P rise. Photosynth. Res. 2012, 113, 15–61. [Google Scholar] [CrossRef]
- Strehler, B.L.; Arnold, W. Light production by green plants. J. Gen. Physiol. 1951, 34, 809–820. [Google Scholar] [CrossRef]
- Schansker, G.; Srivastava, A.; Govindjee; Strasser, R.J. Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct. Plant Biol. 2003, 30, 785–796. [Google Scholar]
- Chen, L.; Yu, S.; Li, S.; Zhang, L.; Zou, C.; Yu, D. The role of WRKY transcription factors in plant abiotic stresses. Biochim. Biophys. Acta 2012, 1819, 120–128. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Emerich, D.W.; Sánchez-Díaz, M. Alfalfa leaf senescence induced by drought stress: Photosynthesis, hydrogen peroxide metabolism, lipid peroxidation and ethylene evolution. Physiol. Plant. 2010, 84, 67–72. [Google Scholar] [CrossRef]
- Shen, B.; Jensen, R.G.; Bohnert, H.J. Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiol. 1997, 113, 1177–1183. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene Expression and signal transduction in water-stress response. Plant Physiol. 1997, 115, 327–334. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Ou, H.; Gui, Y.; Wei, J.; Zhou, H.; Tan, H.; Li, Y. Comprehensive transcriptome analysis reveals genes in response to water deficit in the leaves of Saccharum narenga (Nees ex Steud.) hack. BMC Plant Biol. 2018, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Li, S.; Li, L.; Huang, M.; Wang, X.; Wei, M.; Shi, Q.; Li, Y.; Gong, B.; Yang, F. Cucumber Phospholipase D alpha gene overexpression in tobacco enhanced drought stress tolerance by regulating stomatal closure and lipid peroxidation. BMC Plant Biol. 2018, 18, 355. [Google Scholar] [CrossRef] [PubMed]
- Hrdlickova, R.; Toloue, M.; Tian, B. RNA-Seq methods for transcriptome analysis. Wiley Interdiscip. Rev. RNA 2017, 8, e1364. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, W.; Yang, Q.; Yin, B.; Zhang, Z.; Zhao, B.; Kuang, T.; Zhang, Y.; Zhang, D. The extremotolerant desert moss Syntrichia caninervis is a promising pioneer plant for colonizing extraterrestrial environments. Innovation 2024, 5, 100657. [Google Scholar] [CrossRef]
- Haas, B.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.; Bowden, J.; Couger, M.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Marchant, A.; Mougel, F.; Mendonça, V.; Quartier, M.; Jacquin-Joly, E.; Rosa, J.; Petit, E.; Harry, M. Comparing de novo and reference-based transcriptome assembly strategies by applying them to the blood-sucking bug Rhodnius prolixus. Insect Biochem. Mol. Biol. 2016, 69, 25–33. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta 2010, 1797, 1313–1326. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M. Experimental approach to estimate in vivo the balance of the mechanisms for performance of light reactions, dark reactions, energetic cooperativity, non-Q(A)-and non-Q(B)-reducing and non-oxygen-evolving PSII centers. Acta Physiol. Plant. 2004, 26, 94. [Google Scholar]
- Oukarroum, A.; Goltsev, V.; Strasser, R.J. Temperature effects on pea plants probed by simultaneous measurements of the kinetics of prompt fluorescence, delayed fluorescence and modulated 820 nm reflection. PLoS ONE 2013, 8, e59433. [Google Scholar] [CrossRef]
- Gao, J.; Li, P.; Ma, F.; Goltsev, V. Photosynthetic performance during leaf expansion in Malus micromalus probed by chlorophyll a fluorescence and modulated 820 nm reflection. J. Photochem. Photobiol. B 2014, 137, 144–150. [Google Scholar] [CrossRef]
- Dhriti, S.; Ashverya, L. Transcriptional regulation of drought response: A tortuous network of transcriptional factors. Front. Plant Sci. 2015, 6, 895. [Google Scholar]
- Lei, Y.T.; Xu, Y.X.; Hettenhausen, C.; Lu, C.K.; Shen, G.J.; Zhang, C.P.; Li, J.; Song, J.; Lin, H.H.; Wu, J.Q. Comparative analysis of alfalfa (Medicago sativa L.) leaf transcriptomes reveals genotype-specific salt tolerance mechanisms. BMC Plant Biol. 2018, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, T.C.; O’Toole, J.C.; Yambao, E.B.; Turner, N.C. Influence of osmotic adjustment on leaf rolling and tissue death in rice (Oryza sativa L.). Plant Physiol. 1984, 75, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, Q.X. Evalution of drought resistance of six Rhododendron varities. Jiangsu Agric. Sci. 2019, 47, 163–167. [Google Scholar]
- Wen, G.S.; Tian, H.T.; Zhang, M.R.; Jiang, W.W. Application of chlorophyll fluorescence Analysis in Forest Tree Cultivation. J. Appl. Ecol. 2006, 17, 1973–1977. [Google Scholar]
- Oukarroum, A.; Schansker, G.; Strasser, R.J. Drought stress effects on photosystem I content and photosystem II thermotolerance analyzed using Chl a fluorescence kinetics in barley varieties differing in their drought tolerance. Physiol. Plant. 2010, 137, 188–199. [Google Scholar] [CrossRef]
- Oukarroum, A.; Gharous, M.E.; Goltsev, V. Desiccation-induced changes of photosynthetic transport in Parmelina tiliacea, (Hoffm.) Ach. Analysed by simultaneous measurements of the kinetics of prompt fluorescence, delayed fluorescence and modulated 820 nm reflection. J. Lumin. 2018, 198, 302–308. [Google Scholar] [CrossRef]
- Zong, Y.Z.; Wang, W.F.; Xue, Q.W. Interactive effects of elevated CO2 and drought on photosynthetic capacity and PSII performance in maize. Photosynthetica 2014, 52, 63–70. [Google Scholar] [CrossRef]
- Oukarroum, A.; Bussotti, F.; Goltsev, V.; Kalaji, H.M. Correlation between reactive oxygen species production and photochemistry of photosystems I and II in Lemna gibba L. plants under salt stress. Environ. Exp. Bot. 2015, 109, 80–88. [Google Scholar] [CrossRef]
- Oukarroum, A.; Gharous, M.E.; Goltsev, V.; Strasser, R.J. Delayed fluorescence emission as a probe for the response of photosynthetic organisms to high temperature exposure: A comparative study. J. Lumin. 2016, 180, 321–327. [Google Scholar] [CrossRef]
- Paunov, M.; Koleva, L.; Vassilev, A.; Vangronsveld, J.; Goltsev, V. Effects of different metals on photosynthesis: Cadmium and zinc affect chlorophyll fluorescence in durum wheat. Int. J. Mol. Sci. 2018, 19, 787. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, W.; Wang, X. Comparative phytotoxicity of usnic acid, salicylic acid, cinnamic acid and benzoic acid on photosynthetic apparatus of Chlamydomonas reinhardtii. Plant Physiol. Biochem. 2018, 128, 1. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, P.; Baczewska, A.H.; Pawluśkiewicz, B. Prompt chlorophyll a fluorescence as a rapid tool for diagnostic changes in PSII structure inhibited by salt stress in Perennial ryegrass. J. Photochem. Photobiol. B 2016, 157, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.Z.; Gong, X.F.; Du, Y.L.; Han, Y.N.; Jin, S.H.; Wang, Z.X.; Qian, P.H.; Li, X.Q. Effects of simulated acid rain on photosynthesis in Pinus massoniana and Cunninghamia lanceolatain terms of prompt fluorescence, delayed fluorescence, and modulated reflection at 820 nm. Plants 2024, 13, 622. [Google Scholar] [CrossRef]
- Sheng, Q.; Chen, S.; Strasser, R.O.J.; Yang, J.; Zhang, M. Classifcation and characteristics of heat tolerance in Ageratina adenophora populations using fast chlorophyll a fuorescence rise O–J–I–P. Environ. Exp. Bot. 2016, 122, 126–140. [Google Scholar]
- Guo, Y.; Lu, Y.; Goltsev, V.; Strasser, R.J.; Kalaji, H.M.; Wang, H.; Wang, X.; Chen, S.; Qiang, S. Comparative efect of tenuazonic acid, diuron, bentazone, dibromothymoquinone and methyl viologen on the kinetics of Chl a fuorescence rise OJIP and the MR820 signal. Plant Physiol. Biochem. 2020, 156, 39–48. [Google Scholar] [CrossRef]
- Goltsev, V.; Chernev, P.; Zaharieva, I.; Lambrev, P.; Strasser, R.J. Kinetics of delayed chlorophyll a fluorescence registered in milliseconds time range. Photosynth. Res. 2005, 84, 209–215. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, M.; Gao, J. Thermotolerance of apple tree leaves probed by chlorophyll a fluorescence and modulated 820 nm reflection during seasonal shift. J. Photochem. Photobiol. B 2015, 152, 347–356. [Google Scholar] [CrossRef]
- Wit, M.; Galvão, V.; Fankhauser, C. Light−Mediated Hormonal Regulation of Plant Growth and Development. Annu. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef]
- Xuan, H.D.; Huang, Y.Z.; Zhou, L.; Deng, S.S.; Wang, C.C.; Xu, J.Y.; Wang, H.T.; Zhao, J.M.; Guo, N.; Xing, H. Key soybean seedlings drought-responsive genes and pathways revealed by comparative transcriptome analyses of two cultivars. Int. J. Mol. Sci. 2022, 23, 2893. [Google Scholar] [CrossRef]
- Lu, P.; Wu, Y.M.; Wu, Q.Q.; Li, X.Y. Genome-wide identification and bioinformatics analysis of Setaria italica bZIP transcription factor family. J. Shanxi Agric. Sci. 2020, 48, 1361–1370+1430. [Google Scholar]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Prasad, S.M.; Munné-Bosch, S.; Müller, M. Phytohormones and the regulation of stress tolerance in plants: Current status and future directions. Front. Plant Sci. 2017, 8, 1871. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Bai, Y.H.; Shen, C.J.; Wu, Y.R.; Zhang, S.N.; Jiang, D.A.; Guilfoyle, T.J.; Chen, M.; Qi, Y.H. Auxin-related gene families in abiotic stress response in Sorghum bicolor. Funct. Integr. Genom. 2010, 10, 533–546. [Google Scholar] [CrossRef]
- Jung, H.; Lee, D.K.; Do Choi, Y.; Kim, J.K. OsIAA6, a member of the rice Aux/IAA gene family, is involved in drought tolerance and tiller outgrowth. Plant Sci. 2015, 236, 304–312. [Google Scholar] [CrossRef]
- Kant, S.; Rothstein, S. Auxin-responsive SAUR39 gene modulates auxin level in rice. Plant Signal. Behav. 2009, 4, 1174–1175. [Google Scholar] [CrossRef]
- Yang, T.X.; Wang, Y.Y.; Teotia, S.; Wang, Z.H.; Shi, C.N.; Sun, H.W.; Gu, Y.Y.; Zhang, Z.H.; Tang, G.L. The interaction between miR160 and miR165/166 in the control of leaf development and drought tolerance in Arabidopsis. Sci. Rep. 2019, 9, 2832. [Google Scholar] [CrossRef]
- Chen, J.N.; Nolan, T.M.; Ye, H.X.; Zhang, M.C.; Tong, H.N.; Xin, P.Y.; Chu, J.F.; Chu, C.C.; Li, Z.H.; Yin, Y.H. Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. Plant Cell 2017, 29, 1425–1439. [Google Scholar] [CrossRef]
- Teng, K.; Li, J.; Liu, L.; Han, Y.; Du, Y.; Zhang, J.; Sun, H. Exogenous ABA induces drought tolerance in upland rice: The role of chloroplast and ABA biosynthesis-related gene expression on photosystem II during PEG stress. Acta Physiol. Plant. 2014, 36, 2219–2227. [Google Scholar] [CrossRef]
- Yuan, Y.; Qi, L.J.; Yang, J.; Wu, C.; Liu, Y.J.; Huang, L.Q. A Scutellaria baicalensis R2R3-MYB gene, SbMYB8, regulates flavonoid biosynthesis and improves drought stress tolerance in transgenic tobacco. Plant Cell Tissue Organ. Cult. 2015, 120, 961–972. [Google Scholar] [CrossRef]
- Liu, W.; Tai, H.; Li, S.; Gao, W.; Zhao, M.; Xie, C.; Li, W.-X. bHLH122 is important for drought and osmotic stress resistance in Arabidopsis and in the repression of ABA catabolism. New Phytol. 2014, 201, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.R.; Huang, Z.; Xiang, X.Y.; Xu, W.X.; Wang, J.T.; Chen, J.; Song, L.; Xiao, Y.; Li, X.; Ma, J.; et al. MfbHLH38, a Myrothamnus flabellifolia bHLH transcription factor, confers tolerance to drought and salinity stresses in Arabidopsis. BMC Plant Biol. 2020, 20, 542. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Wei, C.; Zhou, J.; Huang, H.; Chen, L.; Chen, H.; Xing, W.D. Basic leucine zipper transcription factor OsbZIP16 positively regulates drought resistance in rice. Plant Sci. 2012, 193/194, 8–17. [Google Scholar]
- Wu, J.D.; Jiang, Y.L.; Liang, Y.N.; Chen, L.; Chen, W.; Cheng, B. Expression of the maize MYB transcription factor ZmMYB3R enhances drought and salt stress tolerance in transgenic plants. Plant Physiol. Biochem. 2019, 137, 137179–137188. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Wang, X.Q.; Wang, H.Y.; Tang, X.; Liu, C.; Yin, H.; Ye, S.; Jiang, Y.; Duan, Y.; Luo, K. The poplar R2R3 MYB transcription factor PtrMYB94 coordinates with abscisic acid signaling to improve drought tolerance in plants. Tree Physiol. 2020, 40, 46–59. [Google Scholar] [CrossRef]
- Jiang, J.J.; Ma, S.H.; Ye, N.H.; Cao, J.S.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, J.; Sun, X.; Zhao, T.; Li, M.; Wang, Q.; Li, S.; Xin, H. Overexpression of VaWRKY14 increases drought tolerance in Arabidopsis by modulating the expression of stress-related genes. Plant Cell Rep. 2018, 37, 1159–1172. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, D.; Lv, K.; Zhang, X.; Jiang, T. Functional characterization of poplar WRKY75 in salt and osmotic tolerance. Plant Sci. 2019, 289, 110259. [Google Scholar] [CrossRef]


| Sample | Raw Reads | Raw Bases | Clean Reads | Clean Bases | Q20 | Q30 | GC% | Total Mapped |
|---|---|---|---|---|---|---|---|---|
| T0_1 | 21,348,835 | 6.40 G | 21,119,862 | 6.34 G | 97.36 | 92.78 | 46.50 | 15,489,054 (73.34%) |
| T0_2 | 20,665,066 | 6.20 G | 20,492,396 | 6.15 G | 97.13 | 92.22 | 47.08 | 15,222,784 (74.29%) |
| T0_3 | 21,496,496 | 6.45 G | 21,256,541 | 6.38 G | 97.32 | 92.66 | 47.00 | 15,611,864 (73.44%) |
| T4_1 | 21,410,582 | 6.42 G | 20,975,564 | 6.29 G | 97.46 | 93.01 | 46.70 | 15,471,085 (73.76%) |
| T4_2 | 22,322,178 | 6.70 G | 22,018,751 | 6.61 G | 97.30 | 92.56 | 47.06 | 16,301,578 (74.03%) |
| T4_3 | 20,940,896 | 6.28 G | 20,701,885 | 6.21 G | 97.10 | 92.16 | 46.54 | 15,441,596 (74.59%) |
| T8_1 | 21,688,245 | 6.51 G | 21,363,087 | 6.41 G | 96.84 | 91.62 | 46.56 | 15,646,965 (73.24%) |
| T8_2 | 22,029,407 | 6.61 G | 21,801,613 | 6.54 G | 96.97 | 91.9 | 46.19 | 15,731,135 (72.16%) |
| T8_3 | 22,015,159 | 6.60 G | 21,759,277 | 6.53 G | 97.16 | 92.35 | 46.25 | 15,839,005 (72.79%) |
| S0_1 | 22,043,155 | 6.61 G | 21,519,281 | 6.46 G | 97.12 | 92.19 | 46.33 | 15,635,255 (72.66%) |
| S0_2 | 19,892,516 | 5.97 G | 19,596,253 | 5.88 G | 96.99 | 92.02 | 46.54 | 14,005,860 (71.47%) |
| S0_3 | 21,969,798 | 6.59 G | 21,589,241 | 6.48 G | 96.70 | 91.30 | 46.56 | 15,376,810 (71.22%) |
| S4_1 | 21,774,077 | 6.53 G | 21,492,096 | 6.45 G | 97.38 | 92.78 | 46.82 | 15,554,786 (72.37%) |
| S4_2 | 20,938,283 | 6.28 G | 20,469,741 | 6.14 G | 97.23 | 92.47 | 47.41 | 14,817,884 (72.39%) |
| S4_3 | 21,065,427 | 6.32 G | 20,693,989 | 6.21 G | 97.19 | 92.36 | 47.29 | 15,136,408 (73.14%) |
| S8_1 | 21,221,783 | 6.37 G | 20,927,707 | 6.28 G | 97.10 | 92.24 | 46.75 | 15,142,624 (72.36%) |
| S8_2 | 20,830,406 | 6.25 G | 20,624,696 | 6.19 G | 97.14 | 92.25 | 46.69 | 15,037,787 (72.91%) |
| S8_3 | 20,394,267 | 6.12 G | 20,218,889 | 6.07 G | 97.15 | 92.29 | 46.89 | 14,725,314 (72.83%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zheng, X.; Wang, Y.; Jin, S.; Wan, Z. Physiological and Transcriptomic Responses of Two Rhododendron L. Cultivars to Drought Stress: Insights into Drought Tolerance Mechanisms. Agronomy 2025, 15, 1278. https://doi.org/10.3390/agronomy15061278
Li X, Zheng X, Wang Y, Jin S, Wan Z. Physiological and Transcriptomic Responses of Two Rhododendron L. Cultivars to Drought Stress: Insights into Drought Tolerance Mechanisms. Agronomy. 2025; 15(6):1278. https://doi.org/10.3390/agronomy15061278
Chicago/Turabian StyleLi, Xueqin, Xuguang Zheng, Yu Wang, Songheng Jin, and Ziyun Wan. 2025. "Physiological and Transcriptomic Responses of Two Rhododendron L. Cultivars to Drought Stress: Insights into Drought Tolerance Mechanisms" Agronomy 15, no. 6: 1278. https://doi.org/10.3390/agronomy15061278
APA StyleLi, X., Zheng, X., Wang, Y., Jin, S., & Wan, Z. (2025). Physiological and Transcriptomic Responses of Two Rhododendron L. Cultivars to Drought Stress: Insights into Drought Tolerance Mechanisms. Agronomy, 15(6), 1278. https://doi.org/10.3390/agronomy15061278






