Effects of Ammonium on Assimilate Translocation and Storage Root Growth in Sushu16 in Root-Swelling Stage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Morphological Measurement
2.3. Measurements of Photosynthetic Parameters and Photosynthetic Product Contents
2.4. Hormone Content Determination
2.5. Transcriptome Sequencing and Analysis
2.6. RT-qPCR Analysis
2.7. Statistics
3. Results
3.1. Morphological Traits of Sushu16 Seedlings in Root-Swelling Stage Under Different Ammonium Contents
3.2. Photosynthetic Parameters in Sushu16 Seedlings in Root-Swelling Stage Under Different Ammonium Contents
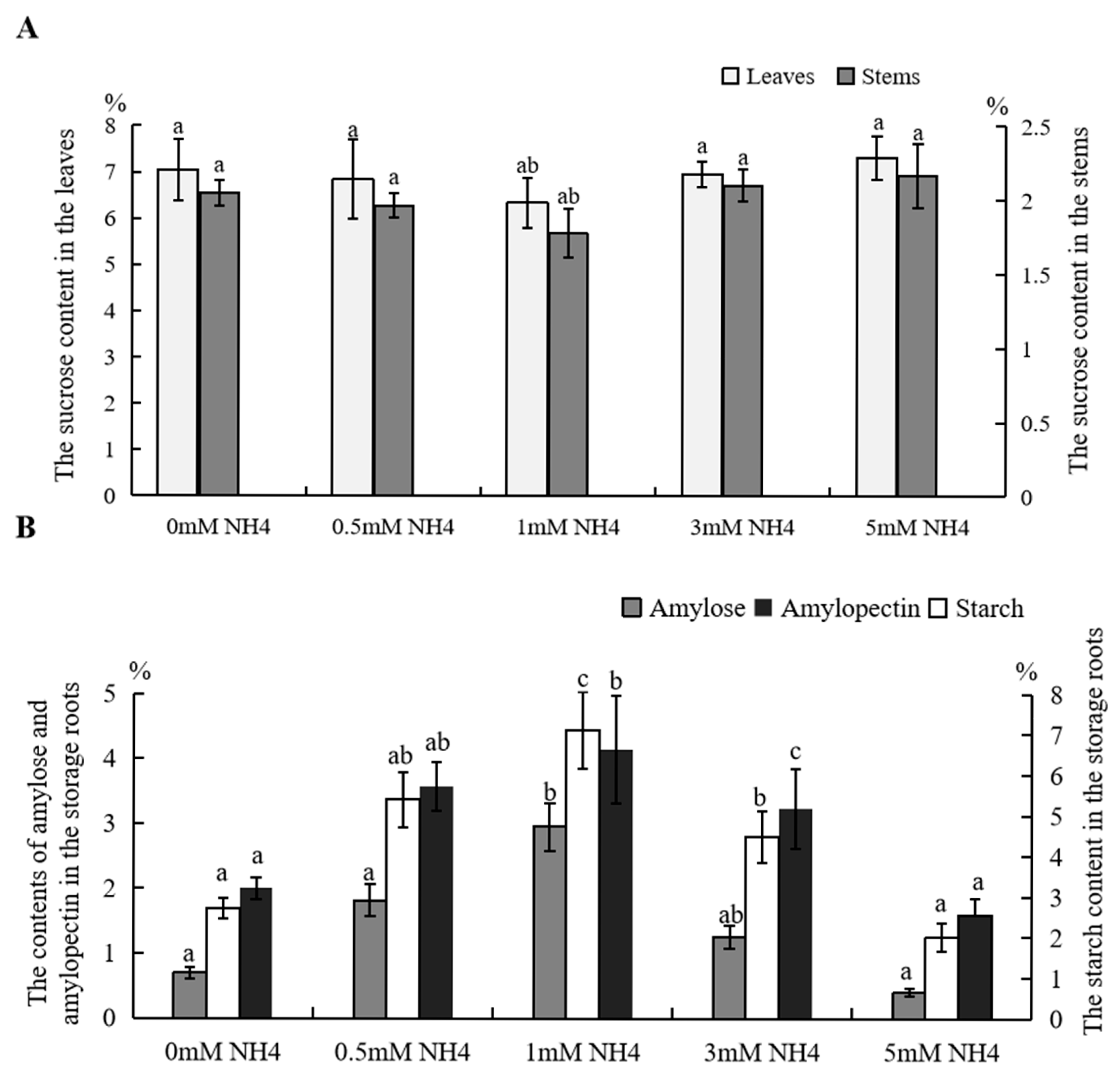
3.3. Photosynthetic Products in Sushu16 Seedlings in Root-Swelling Stage Under Different Ammonium Contents
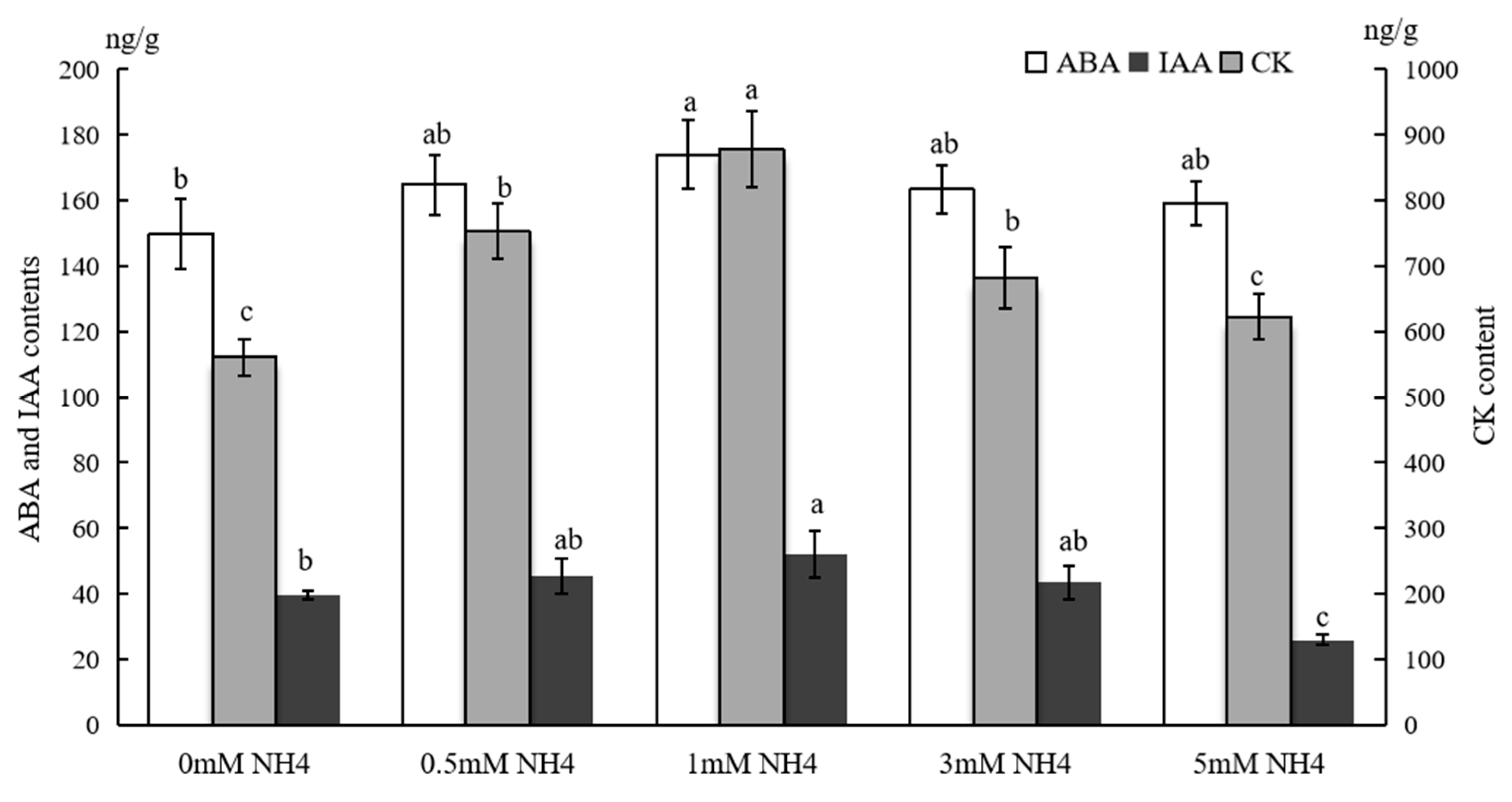
3.4. Hormone Contents in Sushu16 SRs in Root-Swelling Stage Under Different Ammonium Contents
3.5. Transcriptome Analysis of Sushu16 SRs in Root-Swelling Stage Under Different Ammonium Contents
3.5.1. The Screening of DEGs in Sushu16 SRs in the Root-Swelling Stage
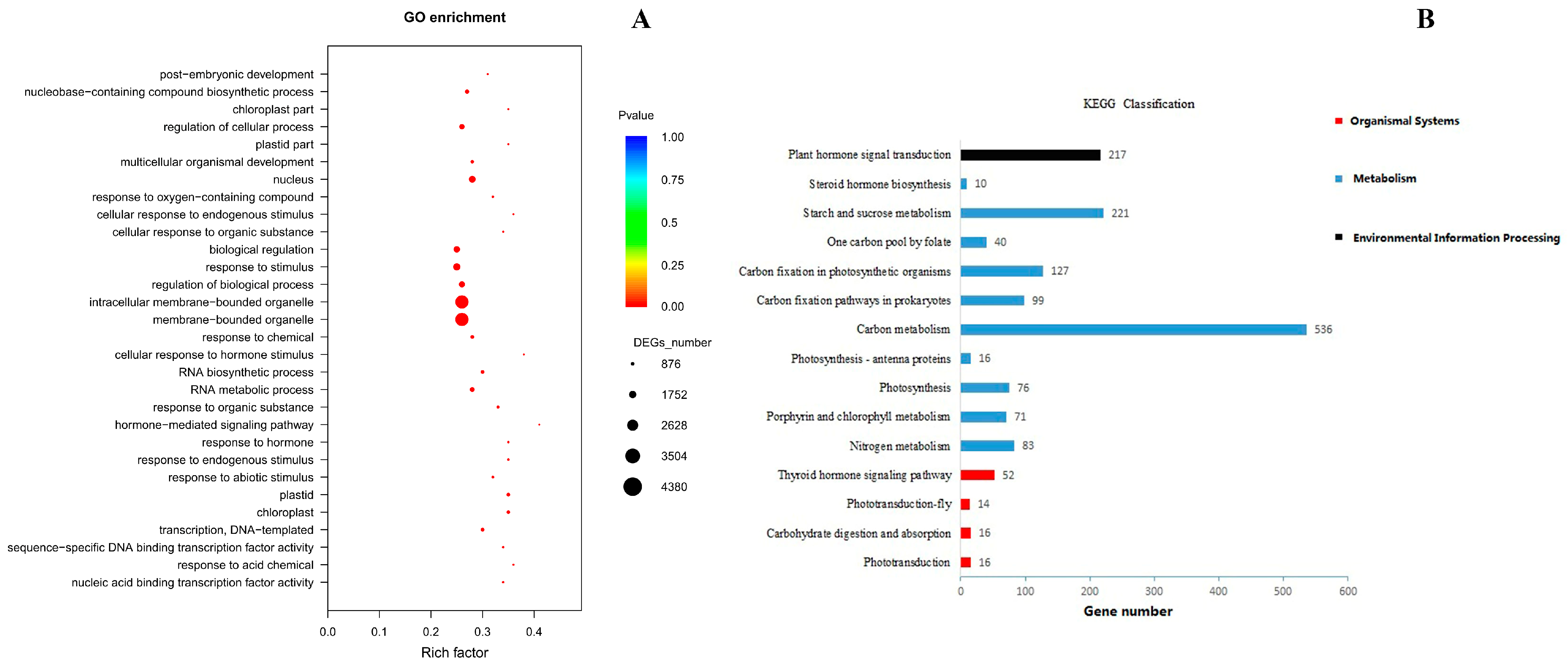
3.5.2. GO Enrichment and KEGG Classification of DEGs in Sushu16 SRs in Root-Swelling Stage
3.5.3. The Identification of Significant DEGs Involved in Photosynthesis, Starch Metabolism, and Hormone Signal Transduction in Sushu16 SRs in the Root-Swelling Stage
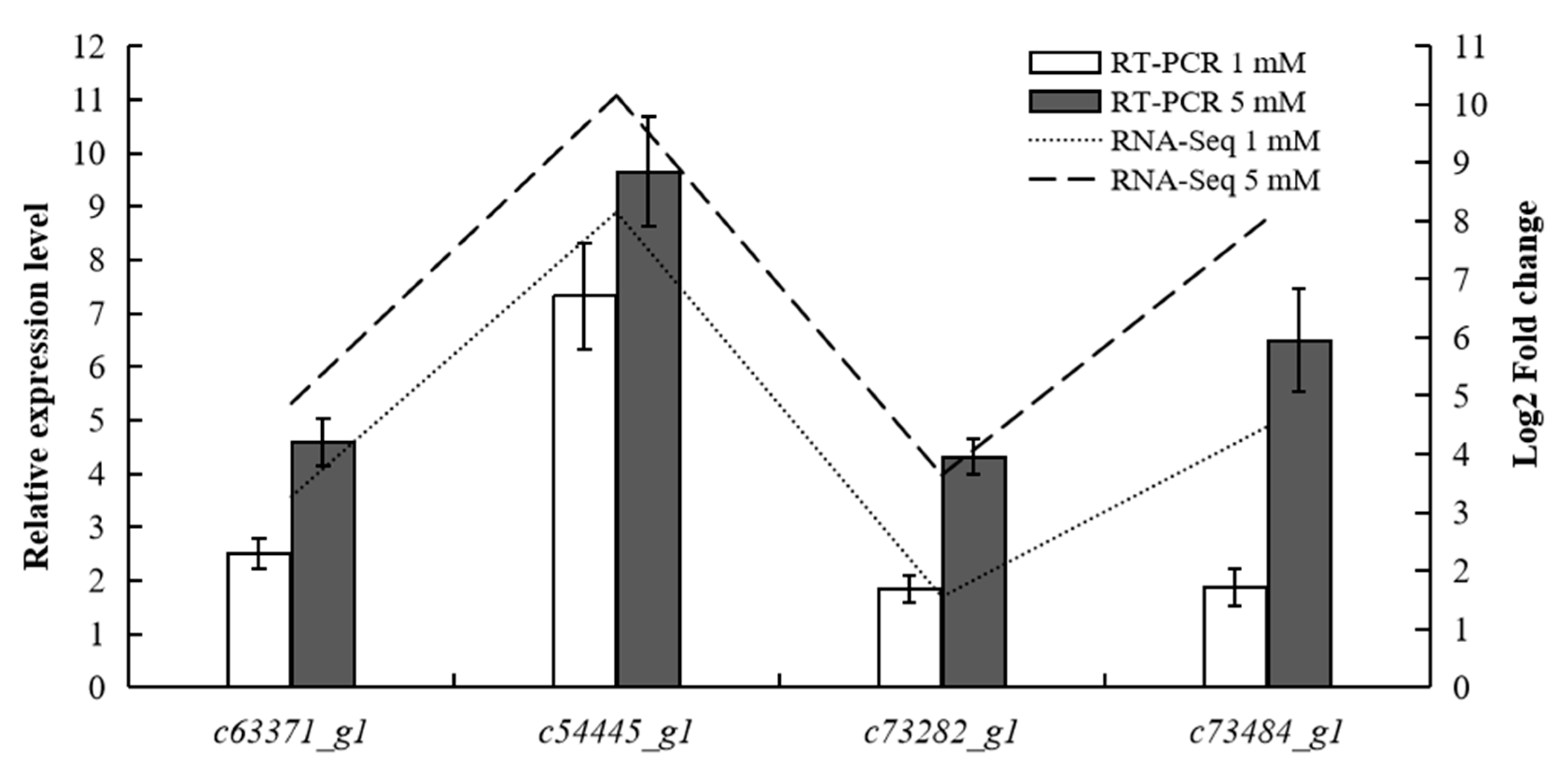
3.6. RT-qPCR Validation of Significant DEGs
4. Discussion
4.1. The Effects of Ammonium Application on Source–Sink Conversion and SR Production in Sweetpotato
4.2. The Effects of Ammonium Application on SR Hormone Contents in Sweetpotato
4.3. The Effects of Ammonium Application on Transcriptional Alterations in Sweetpotato
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanaka, M.; Ishiguro, K.; Oki, T.; Okuno, S. Functional components in sweetpotato and their genetic improvement. Breed. Sci. 2017, 67, 52–61. [Google Scholar] [CrossRef]
- Peng, J.; Shen, H.; Wang, G.; Zhang, Z.; Peng, B.; Xue, G.; Huang, S.; Zheng, W.; Hu, L. Experiment and analysis of physical properties of sweet potato varieties at different harvesting periods. Agriculture 2024, 14, 1641. [Google Scholar] [CrossRef]
- Lebot, V. Sweet potato. In Root and Tuber Crops; Springer: Berlin/Heidelberg, Germany, 2010; pp. 97–125. [Google Scholar]
- Gelaye, Y. Sweet potato: A versatile solution for nutritional challenges in Ethiopia. Technol. Agron. 2024, 4, e014. [Google Scholar] [CrossRef]
- Rukundo, P.; Shimelis, H.; Laing, M.; Gahakwa, D. Storage root formation, dry matter synthesis, accumulation and genetics in sweetpotato. Aust. J. Crop Sci. 2013, 7, 2054–2061. [Google Scholar]
- Ravi, V.; Naskar, S.; Makeshkumar, T.; Babu, B.; Krishnan, B.S.P. Molecular physiology of storage root formation and development in sweet potato (Ipomoea batatas (L.) Lam.). J. Root Crops 2009, 35, 1–27. [Google Scholar]
- Firon, N.; LaBonte, D.; Villordon, A.; Kfir, Y.; Solis, J.; Lapis, E.; Perlman, T.S.; Doron-Faigenboim, A.; Hetzroni, A.; Althan, L.; et al. Transcriptional profiling of sweetpotato (Ipomoea batatas) roots indicates down-regulation of lignin biosynthesis and up-regulation of starch biosynthesis at an early stage of storage root formation. BMC Genom. 2013, 14, 460. [Google Scholar] [CrossRef]
- Jiang, Z.; Wei, Z.; Zhang, J.; Zheng, C.; Zhu, H.; Zhai, H.; He, S.; Gao, S.; Zhao, N.; Zhang, H.; et al. Source-sink synergy is the key unlocking sweet potato starch yield potential. Nat. Commun. 2024, 15, 7260. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Sun, W.; Zhang, Q.; Diao, M.; Sun, J. Extreme soil salinity reduces N and P metabolism and related microbial network complexity and community immigration rate. Environ. Res. 2025, 264, 120361. [Google Scholar] [CrossRef]
- Loqué, D.; von Wirén, N. Regulatory levels for the transport of ammonium in plant roots. J. Exp. Bot. 2004, 55, 1293–1305. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, X.; Jin, M.; Bai, J.; Shu, X.; Deng, L.; Lv, Z. A new curve of critical leaf nitrogen concentration based on the maximum root dry matter for diagnosing nitrogen nutritional status of sweetpotato. Eur. J. Agron. 2024, 156, 127176. [Google Scholar] [CrossRef]
- Ning, Y.W.; Hong-Bo, M.A.; Zhang, H.; Wang, J.D.; Zhang, Y.C. Response of sweetpotato in source-sink relationship establishment, expanding, and balance to nitrogen application rates. Acta Agron. Sin. 2015, 41, 432–441. [Google Scholar] [CrossRef]
- Chen, X.G.; Ding, Y.F.; Tang, Z.H.; Wei, M.; Shi, X.M.; Zhang, A.J.; Li, H.M. Suitable nitrogen rate for storage root yield and quality of sweet potato. J. Plant Nutr. Fert. 2015, 21, 979–986. [Google Scholar]
- Guo, K.; Bian, X.; Jia, Z.; Zhang, L.; Wei, C. Effects of nitrogen level on structural and functional properties of starches from different colored-fleshed root tubers of sweet potato. Int. J. Biol. Macromol. 2020, 164, 3235–3242. [Google Scholar] [CrossRef]
- Hikosaka, K. Interspecific difference in the photosynthesis-nitrogen relationship: Patterns, physiological causes, and ecological importance. J. Plant Res. 2004, 117, 481–494. [Google Scholar] [CrossRef]
- Si, C.; Shi, C.; Liu, H.; Zhan, X.; Liu, Y.; Wang, D.; Meng, D.; Tang, L. Influence of two nitrogen forms on hormone metabolism in potential storage roots and storage root number of sweetpotato. Crop Sci. 2018, 58, 2558–2568. [Google Scholar] [CrossRef]
- Liu, Y.; von Wirén, N. Ammonium as a signal for physiological and morphological responses in plants. J. Exp. Bot. 2017, 68, 2581–2592. [Google Scholar] [CrossRef]
- Heuermann, D.; Hahn, H.; von Wirén, N. Seed yield and nitrogen efficiency in oilseed rape after ammonium nitrate or urea fertilization. Front. Plant Sci. 2021, 12, 608785. [Google Scholar] [CrossRef]
- Shilpha, J.; Song, J.; Jeong, B.R. Ammonium phytotoxicity and tolerance: An insight into ammonium nutrition to improve crop productivity. Agronomy 2023, 13, 1487. [Google Scholar] [CrossRef]
- Lima, J.E.; Kojima, S.; Takahashi, H.; von Wirén, N. Ammonium triggers lateral root branching in Arabidopsis in an AMMONIUM TRANSPORTER1;3-dependent manner. Plant Cell 2010, 22, 3621–3633. [Google Scholar] [CrossRef]
- Araya, T.; Kubo, T.; von Wirén, N.; Takahashi, H. Statistical modeling of nitrogen-dependent modulation of root system architecture in Arabidopsis thaliana. J. Integr. Plant Biol. 2016, 58, 254–265. [Google Scholar] [CrossRef]
- Shtratnikova, V.Y.; Kudryakova, N.V.; Kudoyarova, G.R.; Korobova, A.V.; Akhiyarova, G.R.; Danilova, M.N.; Kulaeva, O.N. Effects of nitrate and ammonium on growth of Arabidopsis thaliana plants transformed with the ARR5::GUS construct and a role for cytokinins in suppression of disturbances induced by the presence of ammonium. Russ. J. Plant Physiol. 2015, 62, 741–752. [Google Scholar] [CrossRef]
- Yang, H.; Von Der Fecht-Bartenbach, J.; Friml, J.; Lohmann, J.U.; Neuhäuser, B.; Ludewig, U. Auxin-modulated root growth inhibition in Arabidopsis thaliana seedlings with ammonium as the sole nitrogen source. Funct. Plant Biol. 2014, 42, 239–251. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water Culture Method for Growing Plants without Soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 39. [Google Scholar]
- Rady, A.M.; Guyer, D.E. Evaluation of sugar content in potatoes using NIR reflectance and wavelength selection techniques. Postharvest Biol. Technol. 2015, 103, 17–26. [Google Scholar] [CrossRef]
- Subroto, E.; Jeanette, G.; Meiyanasari, Y.; Luwinsky, I.; Baraddiaz, S. Review on the analysis methods of starch, amylose, amylopectin in food and agricultural products. Int. J. Emerg. Trends Eng. Res. 2020, 8, 3519–3524. [Google Scholar] [CrossRef]
- Qi, B.; Wu, C.; Liang, H.; Cui, K.; Fahad, S.; Wang, M.; Huang, J. Optimized high-performance liquid chromatography method for determining nine cytokinins, indole-3-acetic acid and abscisic acid. Sustainability 2021, 13, 6998. [Google Scholar] [CrossRef]
- McDermaid, A.; Monier, B.; Zhao, J.; Liu, B.; Ma, Q. Interpretation of differential gene expression results of RNA-seq data: Review and integration. Brief. Bioinf. 2019, 20, 2044–2054. [Google Scholar] [CrossRef]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, H.; Hu, B.; Chu, C. Nitrogen use efficiency in crops: Lessons from Arabidopsis and rice. J. Exp. Bot. 2017, 68, 2477–2488. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Z.; Pan, Q.; Sun, X.; Chen, H.; Chen, F.; Yuan, L.; Mi, G. Increased biomass accumulation in maize grown in mixed nitrogen supply is mediated by auxin synthesis. J. Exp. Bot. 2019, 70, 1859–1873. [Google Scholar] [CrossRef]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J. Exp. Bot. 2017, 68, 2501–2512. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef]
- Forde, B.G. Local and long-range signaling pathways regulating plant responses to nitrate. Annu. Rev. Plant Biol. 2002, 53, 203–224. [Google Scholar] [CrossRef]
- Krouk, G.; Ruffel, S.; Gutiérrez, R.A.; Gojon, A.; Crawford, N.M.; Coruzzi, G.M.; Lacombe, B. A framework integrating plant growth with hormones and nutrients. Trends Plant Sci. 2011, 16, 178–182. [Google Scholar] [CrossRef]
- Zhang, H.; Rong, H.; Pilbeam, D. Signalling mechanisms underlying the morphological responses of the root system to nitrogen in Arabidopsis thaliana. J. Exp. Bot. 2007, 58, 2329–2338. [Google Scholar] [CrossRef]
- Chen, T.; Li, G.; Islam, M.R.; Fu, W.; Feng, B.; Tao, L.; Fu, G. Abscisic acid synergizes with sucrose to enhance grain yield and quality of rice by improving the source-sink relationship. BMC Plant Biol. 2019, 19, 525. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Ravi, V.; Chakrabarti, S.K.; Makeshkumar, T.; Saravanan, R. Molecular regulation of storage root formation and development in sweet potato. Hortic. Rev. 2014, 42, 157–208. [Google Scholar]
- Yue, K.; Li, L.; Xie, J.; Liu, Y.; Xie, J.; Anwar, S.; Fudjoe, S.K. Nitrogen supply affects yield and grain filling of maize by regulating starch metabolizing enzyme activities and endogenous hormone contents. Front. Plant Sci. 2022, 12, 798119. [Google Scholar] [CrossRef]
- Yang, S.Y.; Hao, D.L.; Jin, M.; Li, Y.; Liu, Z.T.; Huang, Y.N.; Su, Y.H. Internal ammonium excess induces ROS-mediated reactions and causes carbon scarcity in rice. BMC Plant Biol. 2020, 20, 143. [Google Scholar] [CrossRef] [PubMed]
- Mitra, G.N. Regulation of Nutrient Uptake by Plants; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Yang, S.Y.; Hao, D.L.; Song, Z.Z.; Yang, G.Z.; Wang, L.; Su, Y.H. RNA-Seq analysis of differentially expressed genes in rice under varied nitrogen supplies. Gene 2015, 555, 305–317. [Google Scholar] [CrossRef]
- Sun, L.; Di, D.; Li, G.; Kronzucker, H.J.; Shi, W. Spatio-temporal dynamics in global rice gene expression (Oryza sativa L.) in response to high ammonium stress. J. Plant Physiol. 2017, 212, 94–104. [Google Scholar] [CrossRef]
- Kant, S.; Bi, Y.M.; Rothstein, S.J. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. Curr. Opin. Plant Biol. 2011, 14, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Konishi, M.; Yanagisawa, S. Molecular basis of the nitrogen response in plants. Soil Sci. Plant Nutr. 2017, 63, 329–341. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Wang, J.; Hu, Y.H.; Sun, Q.S.; Geng, R.; Ding, L.N. Antimicrobial Peptides: Classification, Mechanism, and Application in Plant Disease Resistance. Probiotics Antimicrob. Proteins 2025, 17, 1432–1446. [Google Scholar] [CrossRef] [PubMed]
- Si, C.; Shi, C.; Liu, H.; Zhan, X.; Liu, Y. Effects of nitrogen forms on carbohydrate metabolism and storage-root formation of sweet potato. J. Plant Nutr. Soil Sci. 2018, 181, 419–428. [Google Scholar] [CrossRef]
- Tian, C.E.; Muto, H.; Higuchi, K.; Matamura, T.; Tatematsu, K.; Koshiba, T.; Yamamoto, K.T. Disruption and overexpression of auxin response factor 8 gene of Arabidopsis affect hypocotyl elongation and root growth habit, indicating its possible involvement in auxin homeostasis in light condition. Plant J. 2004, 40, 333–343. [Google Scholar] [CrossRef]
- Gutierrez, L.; Bussell, J.D.; Pacurar, D.I.; Schwambach, J.; Pacurar, M.; Bellini, C. Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell 2009, 21, 3119–3132. [Google Scholar] [CrossRef]
- Goh, T.; Kasahara, H.; Mimura, T.; Kamiya, Y.; Fukaki, H. Multiple AUX/IAA-ARF modules regulate lateral root formation: The role of Arabidopsis SHY2/IAA3-mediated auxin signalling. Phil. Trans. R. Soc. B Biol. Sci. 2012, 367, 1461–1468. [Google Scholar] [CrossRef]

| Morphological Indices | 0 mM NH4 | 0.5 mM NH4 | 1 mM NH4 | 3 mM NH4 | 5 mM NH4 |
|---|---|---|---|---|---|
| Fresh weight (g) | 15.09 ± 2.44 c | 16.88 ± 1.34 c | 18.6 ± 2.34 bc | 22.64 ± 2.49 b | 32.15 ± 5.73 a |
| Dry weight (g) | 2.71 ± 0.44 c | 2.98 ± 0.24 c | 3.32 ± 0.42 bc | 3.96 ± 0.43 b | 5.54 ± 0.99 a |
| SR fresh weight (g) | 12.78 ± 2.23 b | 16.12 ± 2.86 ab | 22.55 ± 3.56 a | 15.77 ± 3.17 b | 6.17 ± 1.08 c |
| SR dry weight (g) | 3.21 ± 0.56 b | 4.07 ± 0.72 ab | 5.59 ± 0.88 a | 3.96 ± 0.79 b | 1.58 ± 0.27 c |
| SR volume (cm3) | 131.75 ± 16.64 b | 169.68 ± 19.55 ab | 219.89 ± 43.47 a | 162.57 ± 31.54 ab | 73.61 ± 11.46 c |
| SR number | 1.33 ± 0.47 ab | 1.66 ± 0.54 a | 1.85 ± 0.32 a | 1.63 ± 0.35 ab | 1 ± 0.12 b |
| Leaf area index | 2.84 ± 0.34 b | 3.14 ± 0.42 b | 3.67 ± 0.48 ab | 3.73 ± 0.53 ab | 4.25 ± 0.62 a |
| Photosynthetic Parameters | 0 mM NH4 | 0.5 mM NH4 | 1 mM NH4 | 3 mM NH4 | 5 mM NH4 |
|---|---|---|---|---|---|
| Chlorophyll content | 12.00 ± 1.54 b | 13.10 ± 2.62 ab | 15.63 ± 1.34 a | 12.40 ± 3.12 ab | 10.33 ± 2.99 b |
| Pn [μmol m−2 s −1] | 9.88 ± 0.25 b | 10.52 ± 0.48 ab | 11.75 ± 1.54 a | 10.11 ± 2.26 ab | 8.33 ± 1.32 b |
| Gs [μmol m−2 s −1] | 0.108 ± 0.011 b | 0.151 ± 0.025 ab | 0.200 ± 0.029 a | 0.143 ± 0.039 ab | 0.053 ± 0.006 c |
| Ci [μmol mol −1] | 212.5 ± 25.63 a | 229.0 ± 27.49 a | 234.5 ± 31.77 a | 213.0 ± 24.68 a | 108.5 ± 12.35 b |
| Tr [μmol m−2 s −1] | 4.33 ± 0.58 ab | 4.91 ± 0.31 ab | 5.50 ± 0.37 a | 4.57 ± 0.62 b | 2.11 ± 0.15 c |
| Gene ID | 1 mM vs. 5 mM (Up/Down) | 0 mM vs. 1 mM (Up/Down) | 0 mM vs. 5 mM (Up/Down) | Gene Annotation | Metabolic Pathways |
|---|---|---|---|---|---|
| c59492_g1 | Down(−1.81) | Up(4.40) | Up(2.58) | Phosphoenolpyruvate carboxylase 3 | Photosynthesis; carbon fixation in photosynthetic organisms |
| c63371_g1 | Up(1.61) | Up(3.27) | Up(4.87) | Chlorophyll a-b binding protein CP24 10A | Photosynthesis—antenna proteins |
| c67118_g1 | Up(1.61) | Up(2.09) | Up(3.70) | Solanesyl diphosphate synthase 2 | Photosynthesis |
| c67603_g1 | Down(−1.04) | Down(−2.85) | Down(−3.89) | Zinc finger protein ZAT10 | Photoprotection; photosynthesis |
| c67605_g1 | Up(1.25) | Up(1.76) | Up(3.01) | Serine/threonine-protein kinase STN7 | Regulation of photosynthesis; chloroplast thylakoid membrane |
| c67668_g1 | Up(1.54) | Up(3.18) | Up(4.13) | Chlorophyll a-b binding protein 40 | Photosynthesis—antenna proteins |
| c68701_g1 | Up(1.78) | Up(2.28) | Up(4.05) | Magnesium-protoporphyrin IX monomethyl ester [oxidative] cyclase | Photosynthesis; chlorophyll biosynthetic process |
| c73267_g1 | Up(2.34) | Up(2.28) | Up(4.63) | Chlorophyll a-b binding protein; ethylene-responsive elongation factor EF-Ts precursor | Photosynthesis—antenna proteins |
| c74975_g1 | Up(1.13) | Up(2.21) | Up(3.34) | Phosphoenolpyruvate carboxylase 4 | Photosynthesis; carbon fixation in photosynthetic organisms |
| c75565_g1 | Up(1.12) | Up(1.37) | Up(2.48) | Magnesium-chelatase subunit ChlI, chloroplastic | Chlorophyll biosynthetic process |
| c54445_g1 | Up(2.01) | Up(8.15) | Up(10.15) | Glucose-1-phosphate adenylyltransferase large subunit | Starch biosynthetic process; glycogen biosynthetic process |
| c55971_g1 | Up(1.32) | Up(2.26) | Up(3.58) | Pyruvate phosphate dikinase | Starch catabolic process; carbohydrate metabolic process |
| c62758_g1 | Down(−4.34) | Down(−2.30) | Down(−6.64) | Starch branching enzyme I | Starch biosynthetic process |
| c62840_g1 | Down(−2.75) | Down(−2.98) | Down(−5.73) | PfkB family carbohydrate kinase | Starch and sucrose metabolism |
| c67608_g1 | Up(1.45) | Up(1.96) | Up(3.41) | Granule-bound starch synthase 2 | Starch synthase activity; starch biosynthetic process |
| c68645_g4 | Up(1.12) | Up(2.10) | Up(3.22) | Phosphoglucan phosphatase DSP4 | Starch catabolic process; carbohydrate phosphatase activity |
| c70089_g2 | Up(1.18) | Up(4.16) | Up(5.34) | Isoamylase 2 | Starch biosynthetic process |
| c71691_g1 | Up(1.55) | Up(1.66) | Up(3.21) | Starch branching enzyme II | Starch and sucrose metabolism |
| c73282_g1 | Up(2.09) | Up(1.55) | Up(3.64) | Glucose-1-phosphate adenylyltransferase small subunit | Starch biosynthetic process; glycogen biosynthetic process |
| c73484_g1 | Up(3.57) | Up(4.47) | Up(8.04) | Pectinesterase/pectinesterase inhibitor | Starch and sucrose metabolism |
| c74637_g1 | Up(1.59) | Up(3.82) | Up(5.41) | Isoamylase 1 | Starch biosynthetic process |
| c74946_g4 | Up(2.41) | Up(1.99) | Up(4.40) | Phosphoribosyl transferase 2 | Starch and sucrose metabolism; Pentose and glucuronate interconversion |
| c74989_g1 | Up(2.27) | Up(2.03) | Up(3.35) | carbohydrate-binding module (CBM20) | Starch catabolic process; starch binding |
| c76971_g1 | Up(1.10) | Up(3.09) | Up(4.19) | 1,4-alpha-glucan branching enzyme/starch branching enzyme II | Carbohydrate transport and metabolism |
| c77775_g1 | Up(1.09) | Up(2.26) | Up(3.36) | 4-alpha-glucanotransferase | Starch catabolic process; starch binding |
| c78303_g2 | Up(1.29) | Up(1.19) | Up(2.18) | Isoamylase 3 | Carbohydrate metabolic process; starch catabolic process |
| c79207_g2 | Up(1.72) | Up(2.53) | Up(4.25) | Granule-bound starch synthase I | Starch biosynthetic process |
| c79256_g2 | Up(1.60) | Up(1.85) | Up(3.45) | Glucose-1-phosphate adenylyltransferase | Starch biosynthetic process; glycogen biosynthetic process |
| c79783_g1 | Up(1.28) | Up(1.36) | Up(2.64) | PPDK_N, Pyruvate phosphate dikinase | Starch catabolic process; carbohydrate kinase activity |
| c51798_g1 | Up(2.08) | Up(2.53) | Up(4.61) | Auxin-responsive protein, IAA16 | Plant hormone signal transduction |
| c66686_g1 | Up(1.26) | Up(2.98) | Up(4.24) | Auxin response factor, ARF8 | Auxin-mediated signaling pathway |
| c69629_g2 | Down(−2.08) | Down(−2.22) | Down(−4.30) | AUX/IAA transcriptional regulator, SHY2 | Auxin-mediated signaling pathway |
| c71847_g1 | Up(1.19) | Up(2.22) | Up(3.41) | Auxin response factor, ARF6 | Auxin-mediated signaling pathway |
| c78272_g2 | Up(1.31) | Up(1.21) | Up(2.52) | Rapid ALkalinization Factor 31 | Hormone activity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, W.; Zhou, R.; Tan, Q.; Zhuang, C.; Shao, W.; Chen, C.; Li, C. Effects of Ammonium on Assimilate Translocation and Storage Root Growth in Sushu16 in Root-Swelling Stage. Agronomy 2025, 15, 1272. https://doi.org/10.3390/agronomy15061272
Yao W, Zhou R, Tan Q, Zhuang C, Shao W, Chen C, Li C. Effects of Ammonium on Assimilate Translocation and Storage Root Growth in Sushu16 in Root-Swelling Stage. Agronomy. 2025; 15(6):1272. https://doi.org/10.3390/agronomy15061272
Chicago/Turabian StyleYao, Wenjing, Rui Zhou, Qin Tan, Chun Zhuang, Wenqi Shao, Chuan Chen, and Chuanzhe Li. 2025. "Effects of Ammonium on Assimilate Translocation and Storage Root Growth in Sushu16 in Root-Swelling Stage" Agronomy 15, no. 6: 1272. https://doi.org/10.3390/agronomy15061272
APA StyleYao, W., Zhou, R., Tan, Q., Zhuang, C., Shao, W., Chen, C., & Li, C. (2025). Effects of Ammonium on Assimilate Translocation and Storage Root Growth in Sushu16 in Root-Swelling Stage. Agronomy, 15(6), 1272. https://doi.org/10.3390/agronomy15061272







