Abstract
The study’s objectives were three-fold. Firstly, the impacts of three advanced harvest times (10 days, 5 days, 0 days) on the nutrient composition of peanut vine (PV) were investigated to determine the optimal harvesting time for peanut vine; secondly, the chemical composition and fermentation characteristics of silage produced by combining peanut vine with either wheat bran (PV-WB silage) or corn meal (PV-CM silage) with adding different inoculants (named as TTX, DBN, and JNK) were evaluated; finally, the post-ensilage microbial community was analyzed to assess the effects of inoculants and silage substrates on the bacterial and fungal communities, as well as their interactions. The results indicated that harvesting peanut vine 5 days early significantly enhanced the retention of crude protein and reduced the content of acid detergent fiber and acid detergent lignin compared to harvesting at 0 days (p < 0.05). PV-WB silage exhibited higher crude protein content than PV-CM silage, irrespective of the inoculant used (p < 0.05). The application of the TTX inoculant notably reduced the acid detergent lignin content and enhanced the potential biodegradation, while also increasing the concentrations of acetic acid and lactic acid (p < 0.05). The bacterial community was predominantly composed of the genera Lactobacillus, Pediococcus, and Weissella; however, a greater abundance of Enterobacter and Enterococcus was obtained in the control (CON) treatment. The use of the JNK inoculant resulted in a Saccharomyces abundance exceeding 89%, which led to elevated levels of ammonia-N and higher pH values. In summary, the advanced 5 days to harvest peanut vines retained more crude protein content and decreased the acid detergent fiber and acid detergent lignin content. Fresh peanut vines co-silage with WB including TTX inoculant improved the peanut value nutrient preservation and fermentation parameters.
1. Introduction
With the continuous enhancement of living standards, there is a growing tendency among individuals to consume livestock products. To meet the market’s demands for both the quantity and quality of these products, the intensification of animal husbandry has evolved. However, the expansion of intensive animal husbandry is heavily reliant on grain production, prompting the exploration of alternative energy and protein feed resources as a significant area of research [1]. China, with its robust agricultural foundation, possesses an abundant supply of agro-residue resources that hold substantial developmental potential. Among these, peanuts are a prominent oilseed and cash crop, extensively cultivated worldwide. According to FAO statistics, global peanut production in 2023 was approximately 54.27 million tons, with China contributing 19.23 million tons, representing over 35% of the global output [2]. The primary by-product of peanut cultivation is peanut vine (PV). Based on Bi’s reported straw/grain ratio of peanuts being 1.5, the quantity of PV can be calculated accordingly [3]. Consequently, in 2023, China possessed a PV resource of 28.85 million tons. Peanut vine is recognized for its substantial nutritional value, as documented by Zhang et al. [4], containing approximately 7.70% crude protein (CP) and 2.11% ether extract (EE), alongside a moderate fiber content relative to other straws, such as corn stalk, rice straw, and wheat straw.
To effectively develop and utilize these resources, it is imperative to adopt advanced peanut harvesting technologies, with a particular emphasis on enhancing mechanized harvesting techniques. Currently, the primary mechanical methods for peanut harvesting are the one-stage and two-stage approaches. Notably, 38.95% of China’s domestic peanut supply is processed using the two-stage harvesting method [5]. However, this method presents several challenges. Primarily, the peanut harvest season frequently coincides with the rainy season, complicating the air-drying process of peanuts in the field [6]. In addition, during the drying phase, peanut leaves, which possess higher nutrient content, become detached from the stalk and are challenging to recollect in subsequent processing stages. Furthermore, the separation of peanut stalks and pods generates a substantial amount of dust, posing environmental and health risks to practitioners [7]. To mitigate the pollution caused by plastic film in mulched plantings, Wang et al. [5] proposed a three-stage harvesting process comprising peanut vine cutting, peanut pod digging and drying, and pod pickup and collection. This method can be adapted for peanut plants grown without plastic mulching film, thereby preserving more peanut leaves and enhancing the nutritional and feed value of peanut vines.
However, rapid deterioration occurs in fresh peanut vine due to its high moisture content. Although drying methods can address this issue, they inevitably increase production costs, and effectively drying the large volume of peanut vine produced during peak seasons within a limited timeframe remains a significant challenge [8]. Silage is considered an appropriate technique for storing these seasonal agricultural residues, ensuring a stable long-term supply of peanut vine. Similarly to other legume pastures, peanut vines exhibit limited water-soluble carbohydrate (WSC) content, making direct and exclusive ensiling of peanut vines inadvisable [9]. The addition of lactic acid bacteria (LAB) has proven to be an effective strategy for rapidly establishing LAB dominance during the anaerobic fermentation phase, thereby enhancing fermentation quality and maximizing nutrient preservation. Research by He et al. [10] demonstrated that LAB inoculation improved both the fermentation indicators and bacterial community composition in silage composed of peanut vines and sweet potato vines. The practice of combining high-moisture agricultural waste with dry substrates to perform wet–dry coupled silage is widely recognized as an effective method for overcoming the challenges associated with high-moisture silage [11]. Specifically, wheat bran has been identified as an effective water-holding substrate for co-ensiling with high-moisture agricultural waste [11,12]. Ultimately, the quality of silage is highly contingent upon the microbial communities involved, their dynamic succession, and the resultant fermentation-derived metabolites [13]. Therefore, understanding the interaction between bacteria and fungi during the silage process, particularly through the application of microbial sequencing technology, is of paramount importance. However, there is a paucity of studies addressing the changes in microbiological flora within peanut silage [9,10].
This study aims to (1) investigate the variations in the nutrient content of peanut vines concerning different pre-harvest timings to identify the optimal timing that maximizes nutrient retention; (2) evaluate the effect of various LAB inoculants and substrates (wheat bran and corn meal) on the nutrient components and fermentation indicators of peanut vines silage; and (3) utilize high-throughput sequencing technology to analyze 16S ribosomal RNA and the internal transcribed spacer regions, thereby evaluating the effects of inoculants and substrates on the bacterial and fungal communities, as well as their interactions.
2. Materials and Methods
2.1. Raw Materials
Six fresh peanut vine samples were collected from Zhengyang County, Henan Province, in September 2023. Specifically, four peanut vine samples were harvested from the fields of Xintiandi Grass Industry Company (latitude 32°39′50″ N, longitude 114°31′40″ E). The remaining two samples were obtained from local agricultural fields, specifically Field C (located at 32°40′31″ N, 114°33′47″ E) and Field D (located at 32°41′24″ N, 114°33′15″ E). Sample collection for peanut vine was conducted at three distinct time points: 10 days prior to the anticipated harvest, 5 days prior, and on the day of normal harvest. At each time point, 50 peanut plants were harvested in situ using scissors, and the plant material was subsequently divided into leaves and stalks. The peanut plants, along with their leaves and stalks, were sectioned into small segments measuring 1–2 cm in length and placed into fine-mesh nylon bags for sun-drying. Fresh peanut vine from Field D was selected for silage preparation. Corn meal (CM) and wheat bran (WB), intended for co-ensiling with the peanut vine, were procured from the local feed market in Yuanzhai Township, Zhengyang County. The chemical compositions of peanut vine, wheat bran, and corn meal for preparation of the silage are shown in Supplementary Table S1.
2.2. Co-Ensiling of Corn Meal and Wheat Bran with Peanut Vine
The moisture content of peanut vine, corn meal, and wheat bran was determined using microwave oven drying, yielding values of 73.07%, 12.27%, and 12.94%, respectively. The fresh peanut vines were chopped into segments of 1–2 cm and mixed with corn meal and wheat bran. The moisture content for the co-ensiling process was standardized to 70%, and the mixing ratios of peanut vine with wheat bran or peanut vine with corn meal were calculated accordingly. The moisture content of the co-ensiling process was standardized at 70%. Subsequently, the mixing ratios of peanut vine and wheat bran (PV-WB silage), as well as peanut vine and corn meal (PV-CM silage), were determined based on the moisture content of each component using the crossover method. As a result, the mixing ratios for peanut vine and wheat bran and peanut vine and corn meal were established as 237.24 g:12.76 g and 237.38 g:12.62 g, respectively. The mixture was then randomly divided into 12 sub-samples, each weighing 250 g. These sub-samples were randomly assigned to a control treatment group (CON) and three groups receiving exogenous inoculant treatments. Two of these inoculants were Lactobacillus plantarum strains obtained from Tiantianxiang Company (TTX) and Dabeinong Company (DBN). An additional exogenous probiotic, provided by the Jilin Academy of Agricultural Sciences, consisted of Lactobacillus plantarum and yeasts, as described by the provider. The populations of LAB and yeasts were enumerated separately on Man Rogosa Sharpe (MRS) agar and Bengal Red Medium following cultivation within an anaerobic environment at 30 °C for 48 h. The counts of LAB and yeasts per gram of inoculant are presented in Supplementary Table S2. In 100 mL of distilled water, one gram of exogenous inoculant was dissolved and applied separately by spraying at a weight of 1.0% relative to the weight of the fresh silage. In the CON group, pure water equivalent to 1.0% of the fresh weight was sprayed to reduce the influence of moisture. Following thorough mixing, the bags containing the co-ensilage materials were tightly sealed using a wrapping machine.
2.3. Analysis of Chemical Compositions and Fermentation Characteristics for Silage
The silage bags were then stored at room temperature (20–26 °C). After a 70-day ensiling period, the bags were opened to evaluate the ensiling characteristics and chemical composition. The evaluation procedure is summarized as follows: Initially, samples underwent sensory evaluation, which included assessments of smell, texture, and color, following the organoleptic scoring criteria for silage quality established by the German Agricultural Society [14]. Subsequently, a 180 mL of distilled water was used to homogenize a 20 g fresh sub-sample for 2 min (S10, Kemai Instruments Co., Ltd., Ningbo, China). The resulting mixture was filtered through four layers of sterile gauze and centrifuged at 15,000 g for 10 min. The supernatant was then recovered via gravity filtration using Whatman #1 filter paper (11 μm pore size, Whatman Internation Ltd., Maidstone, UK). The filtrates were collected for pH measurement using a pH meter (LE438, order NO. 51340242, Mettler Toledo, Shanghai, China). The ammonia nitrogen (AN) content was determined using the phenol-sodium hypochlorite colorimetric method, as described by Broderick et al. [15]. A lactic acid (LA) content determination kit (Nanjing Jiancheng Bioengineering, Nanjing, China) was used to determine filtrate LA levels. The organic acids (acetic acid, propionic acid, and butyric acid) were evaluated using a gas chromatography system (Agilent HP 6890 Series, Santa Clara, CA, USA) according to a previous study by Wu et al. [16].
Fresh silage samples were dried in a forced-air oven at 65 °C for 48 h and then weighed after being exposed to air for 24 h to ascertain the initial moisture content. Successively, the air-dried silage samples and all raw materials were ground using a pulverizer (FW 100, Taisite Instrument Co., Ltd., Tianjin, China) and passed through a 40-mesh sieve. The ground samples were stored at −20 °C until further analysis. The dry matter (DM) content of all samples was determined by drying the samples in an oven at 105 °C for 4 h (AOAC, method 934.01). CP content was analyzed using a Kjeldahl nitrogen analyzer (K9840; Hanon Instruments; Jinan, China) following the AOAC method 990.03. True-protein nitrogen (TPN) concentration was determined using the trichloroacetic acid method, as depicted by Licitra et al. [17], and nonprotein nitrogen (NPN) content was calculated as the difference between total nitrogen (TN) and TPN. EE content was determined using a Soxhlet apparatus (SOX406, Hanon Instruments, Shandong, PR China) according to AOAC method 954.02. Ash content was measured by burning samples in a muffle furnace (SX-G12123, Zhonghuan Furnace Corp., Tianjin, China) at 550 °C for 4 h. Crude fiber (CF) content was estimated using AOAC method 962.09. Neutral detergent fiber (NDF) and acid detergent fiber (ADF) were analyzed using an Alva F5800 fiber analyzer (Alva Instrument Co., Ltd., Jinan, China) following the method described by Van Soest [18]. Acid detergent lignin (ADL) was determined by treating the ADF residue with 72% sulfuric acid [19]. Hemicellulose (HC) content was calculated as the difference between NDF and ADF, and Cellulose (CEL) content was calculated as the difference between ADF and ADL. The biodegradation potential (BDP) of peanut vines and silages was estimated as the ratio of (HC + CEL) to ADL.
2.4. Characterization of the Microbial Community
2.4.1. Preparation of Microbial Samples
Ten grams of fresh silage samples were placed in a glass beaker containing 40 mL of sterile water. After homogenization for 2 min, two layers of sterile gauze were used to filter the mixture. To recover residual microorganisms, the gauze was rinsed three times using 40 mL of sterile water each time. The combined filtrate was centrifuged at 12,000× g for 15 min at 4 °C. The supernatant was discarded, and the precipitates were collected for further analysis.
2.4.2. DNA Extraction, Sequencing, and Bio-Information Analysis
Total DNA from the fermentative products was extracted using the MP-soil FastDNA™ Spin Kit for Soil (MP Biomedicals, Los Angeles, CA, USA) following the manufacturer’s instructions. The V3-V4 region of the 16S rDNA gene was amplified using primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). PCR amplification was performed in a 20 μL reaction system using TransStart Fastpfu DNA Polymerase (TransGen AP221-02, Beijing, China) under the following conditions: initial denaturation at 95 °C for 3 min, followed by 27 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 45 s, with a final extension at 72 °C for 10 min. The ITS2 region of the ITS gene was amplified using primers ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2R (5′-GCTGCGTTCTTCATCGATGC-3′). PCR amplification was conducted in a 20 μL reaction system using TaKaRa rTaq DNA Polymerase under the following conditions: initial denaturation at 95 °C for 3 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 45 s, with a final extension at 72 °C for 10 min. All PCR reactions were carried out on an ABI GeneAmp® 9700 PCR thermocycler (ABI, Los Angeles, CA, USA). The 16S rRNA and ITS2 gene amplicons were sequenced on the Illumina HiSeq sequencing platform, and the resulting data were analyzed using the Majorbio I-Sanger Cloud Platform (www.i-sanger.com, accessed on 20 July 2024, Majorbio, Shanghai, China). The 16S rRNA and ITS gene sequencing raw data for microbial community was submitted to the NCBI database with the accession number PRJNA1225790.
2.5. Statistical Analysis
The effect of the inoculant and substrate on the silage chemical composition and fermentation parameters were analyzed by means of the PROC GLM of SAS 9.4 (SAS Inst. Inc., Cary, NC, USA), the general linear model as follows: Yij = µ + Ti + Dj + (T + D)ij + εij, where Yij is the dependent variable; µ is the overall mean; Ti is the fixed effect of inoculant; Dj is the fixed effect of substrate; and (T + D)ij is the interaction effect between the inoculant and substrate. Data of the chemical composition, the BDP of peanut leaf, stalk, and plant harvested at different advanced times, and silage chemical composition and fermentation parameters, along with the addition of different inoculants in the same silage substrate were analyzed using PROC ANOVA of SAS 9.4 and then Duncan’s multiple comparison tests were used for the means. The effect on the substrate within each inoculant was analyzed by PROC TTEST of SAS 9.4. A significant difference is defined as a p-value below 0.05.
3. Results and Discussion
3.1. Chemical Composition and BDP of Peanut Leaves, Stalks, and Plants at Different Advanced Harvest Times
The chemical composition of peanut leaves, stalks, and plants from six samples harvested at different advanced intervals is presented in Figure 1. As illustrated in Figure 1A, the CP content in samples harvested at 0 days in advance (15.94%) was significantly higher than in those harvested 10 days in advance (14.37%) (p < 0.05). No significant differences were observed in the CP content of the stalks across the three advanced harvest intervals. Notably, the CP content in peanut plants harvested 10 and 5 days in advance was 10.75% and 10.69%, respectively, which was significantly higher than the CP content (9.31%) in plants harvested at 0 days in advance (p < 0.05). As depicted in Figure 1B–D, there were no significant differences in the EE, CF, and NDF contents of peanut leaves, stalks, and plants within the range of 0 to 10 days in advance of harvest. A significant difference was observed in the ADF content of peanut plants between the 0 days in advance (32.46%) and the 5 days (29.33%) and 10 days (28.41%) in advance harvest intervals (p < 0.05) (Figure 1E). The ADL content of peanut stalks harvested 10 days earlier was 7.90%, significantly lower than the 9.30% observed in those harvested at 0 days in advance. Furthermore, the ADL content of peanut plants harvested 10 and 5 days earlier was 5.34% and 6.37%, respectively, which were lower than the 8.08% content at 0 days in advance (p < 0.05). The analysis revealed no significant differences in the Ash content of peanut leaves and stalks across various advanced harvest times. However, the Ash content was 14.69% in the harvest conducted 5 days earlier, which was significantly higher than the values observed in the harvests conducted at 10 days (p < 0.05) (Figure 1G). The HC content of peanut stalks harvested at 0 days was 11.69% (Figure 1H), which is significantly greater than the 9.30% observed in stalks harvested 10 days earlier (p < 0.05). The CEL content of peanut vines was 21.48% and 20.88% for harvests conducted 10 days and 5 days in advance, respectively (Figure 1I), both of which were significantly lower than the 23.23% observed in the 0-day harvest (p < 0.05).

Figure 1.
Chemical composition and biodegradation potential peanut leaf, stalk, and plant at different advanced harvest times (as dry matter basis, %).(A) CP: crude protein; (B) EE: ether extract; (C) CF: crude fiber; (D) NDF: neutral detergent fiber; (E) ADF: acid detergent fiber; (F) ADL: acid detergent lignin; (G) Ash; (H) HC: Hemicellulose; (I) CEL: Cellulose; (J) BDP: biodegradation potential. Different lowercase letters indicate significant differences in advanced harvest time in leaf, stalk, or plant, respectively (p < 0.05).
The chemical composition of peanut vines reported in previous studies is summarized in Table 1, indicating CP and EE contents ranging from 7.16% to 12.20% and 2.06% to 3.69%, respectively, with most peanut vines containing less than 10% crude protein. In the current study, the mean CP and EE contents of peanut leaves, stalks, and whole plants were 15.23% and 3.56%, 5.35% and 1.00%, and 10.25% and 1.99%, respectively. The results presented above suggest that peanut leaves exhibit higher CP and EE content compared to peanut stalks. Consequently, it is essential to retain more leaves during the peanut harvesting process to optimize and enhance the nutritional value for feeding purposes. Furthermore, in this study, the NDF content of peanut vine plants was measured at 38.87%, 39.50%, and 41.77%, respectively. These values are lower than those reported in previous studies, except for the spring-grown peanut vine, as documented by Cai et al. [20]. Notably, the ADF (28.41%, 29.33%, and 32.46%) and CF (29.08%, 28.31%, and 28.61%) contents of peanut plants harvested at three different advanced times were also lower than previously reported results. The previous study demonstrated that the CP and EE contents in peanut vine harvested 10 days in advance both increased by 20%, and the contents of vitamin B2 and vitamin B6 are also significantly higher than those of normally harvested peanut vine (p < 0.05) [21]. Moreover, similar observations were reported by Zheng et al. [22], in which, harvesting 10 days in advance improved the content of CP in peanut vine. These findings are consistent with our current results.

Table 1.
Chemical composition of peanut vine published in previous studies (%, as DM basis).
As illustrated in Figure 1J, the BDP value of peanut plants harvested 10 days earlier (6.07) was significantly higher than those observed at 5 days (5.00) and 0 days (4.36) of earlier harvesting (p < 0.05). Although no significant differences were found in the BDP value of peanut leaves across the three sampling times, a decreasing trend in BDP value (7.21, 6.28, and 5.29) was observed with delayed harvest time. Typically, summer-grown peanuts are considered those that are sown following wheat harvest. In this study, the six peanut samples collected in September were derived from peanuts sown after the wheat harvest and thus can be classified as summer-grown peanuts. Utilizing the BDP calculation formula outlined by Ren et al. [8], the BDP values for spring-grown and summer-grown peanut vines were determined to be 3.75 and 3.74, respectively, as reported in the research by Cai et al. [20]. These values are lower than those observed in the current study, suggesting that peanuts harvested in advance, which retain more foliage, may possess enhanced nutritional and feed value.
3.2. Effect of Substrates and Exogenous Inoculant on Organoleptic Properties of Silages
The results of the organoleptic assessment of silage are presented in Figure 2A. The total organoleptic properties score provides a comprehensive evaluation of co-silage quality. The total score for PV-WB silage fell within the range of 16–20, indicating an “excellent” grade, except for the TTX group, which was rated as “moderate” (5–9). For PV-CM silage, all results from the exogenous inoculant treatment group achieved a total score within the “good” range (10–15). According to the previous study by Tang [30], the total score of peanut vine co-silage with molasses ranged from 16 to 20, indicating an “excellent” grade, which surpassed that of peanut vine single silage. These findings suggest that to enhance the quality of fresh peanut vine silage, it should be combined with other ingredients rich in water-soluble carbohydrate. However, to minimize costs, the incorporation of local agriculture by-products into peanut vine silage should be considered.
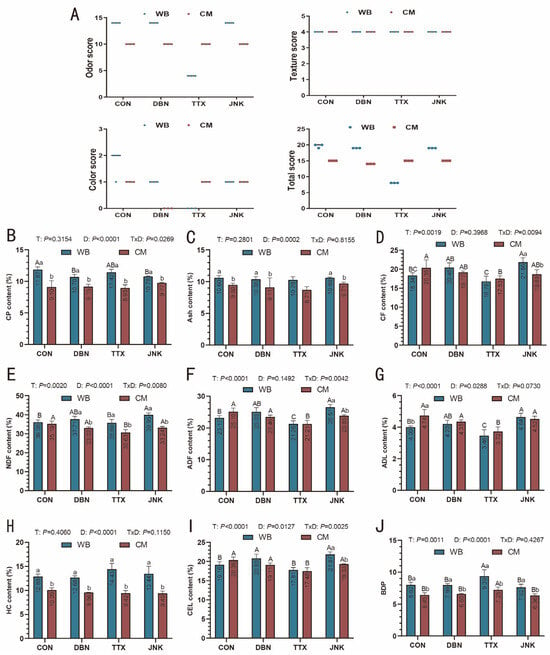
Figure 2.
(A) The organoleptic properties total score (each point represents three independent replicates of co-silage bags (n = 3) and the chemical composition biodegradation potential of the peanut vine co-silage with wheat bran or corn meal on DM basis, respectively (B–J). DM: dry matter; (B) CP: crude protein; (C) Ash; (D) CF: crude fiber; (E) NDF: neutral detergent fiber; (F) ADF: acid detergent fiber; (G) ADL: acid detergent lignin; (H) HC: Hemicellulose; (I) CEL: Cellulose; (J) BDP: biodegradation potential, WB: wheat bran; CM: corn meal. T: the influence of inoculant; D: the influence of silage substrate; T × D: the interaction between the inoculant and silage substrate. The significant difference (p < 0.05) between the different inoculant treatments on the same silage raw substrate is represented by the different capital letters (A–C). The significant difference (p < 0.05) between the different silage raw substrates on the same inoculant treatment is represented by the different lowercase letters (a–b).
3.3. Effect of Substrates and Exogenous Inoculant on Chemical Composition of Co-Silages
Figure 2B–I illustrates the impact of exogenous inoculants and silage substrates on the nutrient content of peanut vine mixed silage. On a dry matter basis, the CP content of PV-WB silage was 11.87% in the CON group, which was significantly higher than in the DBN (10.70%) and JNK (10.78%) groups (p < 0.05). In PV-CM silage, the CP contents were 9.10%, 9.15%, 8.93%, and 9.77% in the CON, DBN, TTX, and JNK groups, respectively. These values were significantly lower than those in the corresponding inoculant group for PV-WB silage. Regardless of the silage ingredients, the various inoculant treatments significantly influenced the contents of CF, NDF, ADF, and ADL (Figure 2D–G). For PV-WB silage, the CF content in the TTX group was 16.78%, which was significantly lower than the values observed in the DBN (20.46%) and JNK (21.86%) groups (p < 0.05). A similar trend was noted in PV-CM silage, where the CF content in the TTX group was 17.53%, significantly lower than that in the CON group (20.35%). Although no significant differences were found in CF content among the three inoculant treatments, the CF levels in the DBN and JNK groups were 1.64% and 1.15% lower, respectively, than in the TTX group. The contents of NDF, ADF, and ADL in peanut vine co-silage with wheat bran and corn meal were 35.69% and 30.63%, 21.26% and 21.21%, and 3.46% and 3.72% in the TTX group, respectively. These values were lower than those observed in the other inoculant treatments (DBN and JNK). PV-WB silage exhibited a higher HC content compared to PV-CM silage across all inoculant groups (p < 0.05). The TTX inoculant also resulted in a reduction in CEL content when compared to the DBN and JNK inoculant treatments (p < 0.05). Regardless of the ingredients mixed with peanut vine co-silage (Figure 2J), the TTX inoculant demonstrated the highest BDP value compared to the other treatments and the CON group (p < 0.05). Furthermore, we found that the BDP of PV-WB silage was greater than that obtained in PV-CM silage in the CON group and each inoculant group.
In the present study, the CP content of wheat bran used for co-silage was determined to be 18.41%, which is higher than that of corn meal at 10.41%. This higher CP content in wheat bran may contribute to the increased CP content observed in the peanut vine and wheat bran co-silage across various inoculant groups. Previous research has indicated that wheat bran, due to its high water-soluble carbohydrate content of 149.5 g kg−1 fresh weight [25], can serve as an effective water-holding material when mixed with high moisture content cabbage waste in co-silage. This approach offers a straightforward and cost-effective method for maintaining an optimal moisture level in the silage system [31]. In addition to wheat bran, other materials such as rice bran [12], maize straw [8], rice straw [32], and wheat straw [33] have also been utilized in previous studies for co-ensiling with high-moisture biomass. Qin and Shen [9] conducted a comparative analysis of the influence of adding corn stover and wheat bran on the chemical composition of peanut vine silage. Their findings revealed that the water-soluble carbohydrate and CP contents in peanut vine co-silage with wheat bran were significantly higher than in peanut vine silage alone. Conversely, the NDF and ADF amounts decreased markedly with the addition of wheat bran to the silage system. In a separate study, Li et al. [12] observed that the inclusion of wheat bran and rice bran in the Chinese cabbage silage system resulted in a reduction in CP content compared to the group with only Chinese cabbage silage. The CP content exhibited a gradual decline with increasing proportions of wheat bran and rice bran. Unfortunately, the researchers did not conduct a statistical analysis to assess the impact of wheat bran and rice bran addition on CP content in co-silage forage. Nevertheless, the results obtained by Li et al. [12] indicated that the CP amount in Chinese cabbage co-silage with wheat bran was more than double that of the Chinese cabbage and rice bran co-silage. Furthermore, the NDF and ADF contents in the wheat bran silage group were two-fold and three-fold lower, respectively, than those in the rice bran silage group. These results imply that wheat bran has potential as a water-holding coupling agent for high-moisture agricultural waste biomass and may enhance the feeding value of fresh agricultural waste. Table 2 displays data on silage forage from recently published papers. In comparison to the chemical composition of corn silage reported in previous studies, the CP content was higher in the peanut vine silage examined in this study. This increase is likely attributable to the elevated CP levels typically found in legume crops. Although soybean silage exhibited higher CP content, the NDF and ADF levels in peanut vine silage were significantly lower than those in soybean silage. This indicates that incorporating wheat bran into fresh peanut vine to produce high-quality silage offers dual benefits: enhancing the utilization of agricultural waste biomass and mitigating feed resource shortages.

Table 2.
Chemical composition of silage forage in previous studies (%, as DM basis).
3.4. Effect of Substrates and Exogenous Inoculant on Fermentation Characteristics of Co-Silage
The pH and variety of nitrogen-containing substances can serve as indicators of the microbial metabolite profile and overall silage quality. A pH range of 3.8 to 4.2 is indicative of well-preserved silage, as maintaining a low pH inhibits pathogenic microorganisms and enhances feed stability [37]. Figure 3A illustrates the pH levels of peanut vine co-silage with wheat bran and corn meal. In the study of PV-WB silage, the pH values were recorded as 4.52, 4.54, and 4.42 for the CON, BDN, and TTX groups, respectively, which were significantly lower than the pH value observed in the JNK group (4.85) (p < 0.05). In contrast, for PV-CM silage, the TTX group exhibited the lowest pH value of 4.45, whereas the JNK group demonstrated the highest pH value of 5.01. Although no significant differences were observed, the pH values of PV-WB silage were consistently lower than those of PV-CM silage in the CON, DBN, and TTX groups. Notably, the pH of PV-WB silage was significantly lower than that of PV-CM silage when the JNK inoculant was added. The reduction in pH in silage is attributed to the production of organic acids by Lactobacillus through carbohydrate fermentation. In the current study, the highest LA concentration in PV-WB silage was found in the JNK-inoculated group (8.88), followed by the TTX (8.46) and DBN (8.09) groups, with the lowest concentration in the CON group (6.20). Conversely, significant disparities in LA content were detected among the different inoculation treatments in PV-CM silage (p < 0.05). The highest LA content was recorded in the TTX-inoculated silage (10.01), followed by the CON (6.75) and DBN-inoculated (5.80) silages, the lowest LA content was observed in JNK-inoculated (4.23) silage. Furthermore, a significant difference in LA content was observed between wheat bran and corn meal substrates in JNK-inoculated silage (p < 0.05). Irrespective of the silage substrate, acetic acid (AA) concentrations in PV silage were significantly elevated with the addition of TTX and JNK inoculation in comparison with the CON group and DBN inoculation (p < 0.05). Silage containing corn meal exhibited a higher AA content than that containing wheat bran in both CON and TTX treatments (p < 0.05). Conversely, in JNK-inoculated silage, PV-WB silage demonstrated a greater AA content than PV-CM silage (p < 0.05). Additionally, propionic acid and butyric acid were not detected in this study, as is consistent with previous findings, potentially due to the inhibition of Clostridium and Propionibacterium activities during the anaerobic silage period [38,39].
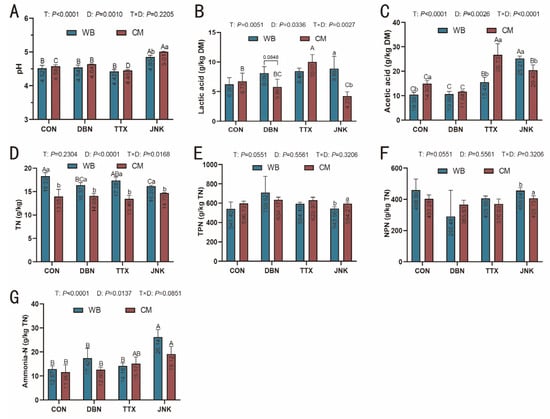
Figure 3.
(A) pH; (B) Lactic acid; (C) Acetic acid; (D) Total nitrogen (TN); (E) True-protein nitrogen (TPN); (F) Nonprotein nitrogen (NPN); (G) Ammonia-N in peanut vine co-silage with wheat bran or corn meal, respectively. T: the influence of inoculant; D: the influence of silage substrate; T × D: the interaction between the inoculant and silage substrate. The significant difference (p < 0.05) between the different inoculant treatments (T) on the same silage raw substrate is represented by the different capital letters (A–D). The significant difference (p < 0.05) between the different silage raw substrates on the same inoculant treatment is represented by the different lowercase letters (a–b).
Compared to the pH of whole-plant corn silage, the pH of peanut vine silage in this study was higher, which may be attributed to differences in the substrates used in silage [40]. The pH of silage is affected by numerous factors, with LA concentration and the buffering capacity of substrates being particularly significant. Generally, legumes exhibit buffering capacities more than twice that of corn [41]. Compared to corn silage, soybean silage does not typically reach the pH range of 3.8 to 4.2, primarily because of its lower content of water-soluble carbohydrate [35]. Previous studies have reported pH values for alfalfa silage ranging from 4.94 to 5.10 [42], and even reaching 5.32 in some instances [36]. During ensilage, LA is the predominant contributor to pH reduction, being approximately ten times more potent than other major acids such as acetic and propionic acids [43]. Consequently, given the differences in buffering capacity between legume and gramineous crop silages, a pH range from 4.3 to 5.0 is deemed suitable for legume silage [44]. In this perspective, the pH values observed for peanut vine co-silage with wheat bran and corn meal in this study were within the acceptable range.
The ensilage process is typically accompanied by protein hydrolysis, particularly in high-protein legume plants. The peptide bonds within proteins are cleaved by plant-derived proteases, resulting in the formation of small peptides and free amino acids. These amino acids are subsequently deaminated through microbial activity, yielding ammonia, amines, and other by-products [45]. The ratio of TPN to NPN within the total nitrogen content is indicative of the extent of protein degradation and utilization [44]. In the current study, the TN content of PV-WB silage was significantly higher than that of PV-CM silage (Figure 3D), likely due to the inherently greater TN content in wheat bran compared to corn meal (Supplementary Table S3). The TPN content in PV-CM silage was consistently higher than that in PV-WB silage (Figure 3E), with the exception of the DBN treatment. Notably, in the JNK treatment, the TPN content of PV-CM silage was significantly greater than that of PV-WB silage (p < 0.05). The degree of protein hydrolysis during silage fermentation is more accurately reflected by ammonia-N content compared to NPN [41]. In the present study, there was no notable difference in ammonia-N content between PV-WB and PV-CM silages (Figure 3G). However, it was observed that the JNK treatment resulted in the uppermost ammonia-N content, irrespective of the silage substrate used (p < 0.05). This finding provides insight into why the silage inoculated with JNK demonstrated the highest pH value compared to other inoculation treatments.
3.5. Effect of Substrates and Exogenous Inoculant on Microbial Community Diversity of Co-Silages
3.5.1. Alpha Diversity Indices of Microbial Community
The sequencing results of the bacteriome and mycobiome are detailed in Supplementary Table S4, with read counts ranging from 70,506 to 82,714 for the bacteriome and from 44,527 to 101,365 for the mycobiome, achieving a coverage of about 0.999 for all samples. As illustrated in Figure 4A,B, Venn analysis revealed that all treatments shared 52 OTUs for the bacteriome and 93 OTUs for the mycobiome. The PV-CM silage with TTX inoculant exhibited the highest number of unique bacteriome OTUs in comparison with other treatments. In contrast, the highest amount of unique mycobiome OTUs, totaling 87, was observed in PV-WB silage under the CON treatment. Figure 4C shows that the Chao1, Ace, and Shannon indices for the bacteriome in PV-WB silage were significantly higher than those in PV-CM silage with the addition of the JNK inoculant (p < 0.05). Conversely, the Simpson index in PV-WB silage was significantly lower than in PV-CM silage (p < 0.05). Additionally, the Shannon index in PV-WB silage with the DBN inoculant was significantly greater than that in PV-CM silage (p < 0.05), and the Simpson index in PV-WB silage was significantly lower than that in PV-CM silage (p < 0.05). The aforementioned results indicate that PV-WB silage exhibited greater bacteriome diversity compared to PV-CM silage. The application of the JNK inoculant, in contrast to the CON treatment, DBN, and TTX inoculation, significantly enhanced the Shannon indices for both PV-WB and PV-CM silages (p < 0.05) while reducing the Simpson index for PV-WB silage (p < 0.05). This suggests that the addition of the JNK inoculant may enhance microbial diversity in silage.
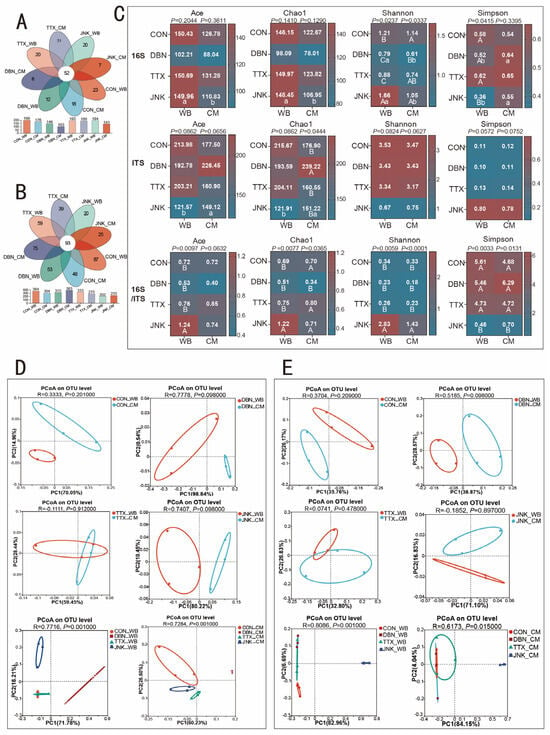
Figure 4.
(A) Venn analysis of the operational taxonomic units (OTUs) for bacteriome in peanut vine co-silage with WB and CM addition of inoculants. (B) Venn analysis of the operational taxonomic units (OTUs) for mycobiome in peanut vine co-silage with WB and CM addition of inoculants. (C) Alpha diversity indices of bacteriome and mycobiome, and the 16S/ITS Ace estimator, Chao1 estimator, Shannon diversity index, and Simpson diversity index ratios. The data are expressed as mean. (D) Bacteriome and (E) mycobiome community dissimilarities in peanut vine co-silage with wheat bran or corn meal, respectively, were calculated using principal coordinates analysis (PCoA), respectively.. WB: wheat bran; CM: corn meal. The significant difference (p < 0.05) between the different inoculant treatments on the same silage raw substrate is represented by the different capital letters (A–C). The significant difference (p < 0.05) between the different silage raw substrates on the same inoculant treatment is represented by the different lowercase letters (a–b).
Regarding the mycobiome, although no significant differences were detected, the Ace, Chao1, and Shannon indices were higher in the CON, DBN, and TTX groups compared to the JNK group, with the JNK-inoculated silage exhibiting the highest Simpson index. These findings imply that the JNK inoculant may reduce fungal diversity. The ratios of bacterial to fungal alpha diversity can be used to assess the balance between bacteriome and mycobiome diversity [31]. In this study, the influence of the silage substrate on this balance was not observed. However, the ratios of bacterial to fungal diversity indices, such as Ace, Chao1, and Shannon, were significantly higher in PV-WB silage treated with the JNK inoculant compared to other inoculation treatments. The Simpson ratio of 16S to ITS in the JNK inoculant was markedly lower than in other inoculant treatment groups, suggesting that bacterial diversity exceeded mycobiome diversity in silage with the inclusion of the JNK inoculant during ensilage.
To compare differences in microbial communities, we conducted a principal coordinates analysis (PCoA), as depicted in Figure 4D,E. The PCoA revealed no significant differences or separations between PV-WB silage and PV-CM silage within each inoculant group, suggesting that the bacteriome and mycobiome remained unchanged during the ensilage process between wheat bran and corn meal. However, for PV-WB and PV-CM silage, the bacteriome composition in the DBN inoculant group differed markedly from other treatments. Similarly, the JNK inoculant silage sample was significantly distinct from the DBN, TTX inoculant group, and CON group, indicating that the addition of inoculants influenced the mycobiome community during the silage process.
3.5.2. Abundance of Bacteriome and Mycobiome Community
The abundance of bacteriome communities at phylum and genus levels is presented in Figure 5A and Figure 5B, respectively. In all silage treatments, Firmicutes emerged as the predominant phylum, followed by Proteobacteria, Actinobacteria, and Cyanobacteria, respectively (Figure 5A). These findings align with those reported by Ren et al. [46], who observed that Firmicutes, specifically acid-producing hydrolytic bacteria, became the dominant phylum in cauliflower leaf silage following 30 days of ensiling. Ogunade et al. [47] similarly noted that Firmicutes and Proteobacteria constituted the major bacterial communities at the phylum level in silage. The observed decrease in Proteobacteria abundance post-ensiling may be attributed to the reduction in pH, as Proteobacteria generally favor neutral over acidic environments [10]. Notably, the relative abundance of Actinobacteriota was dramatically higher in PV-WB silage compared to PV-CM silage (p < 0.05), but this was only obtained in the CON and DBN treatments. This variation may be due to the nutrient characteristics of the raw substrates and the associated microbial communities influencing bacterial composition in different substrate silages [10].
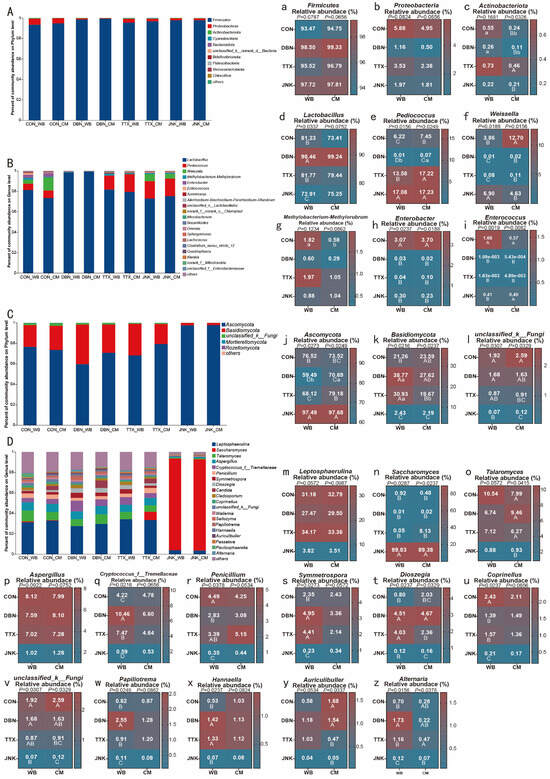
Figure 5.
(A,C) The relative abundance of bacteria and fungi at the phylum leveland (B,D) genus level for peanut vine co-silage with wheat bran or corn meal, respectively. WB: wheat bran; CM: corn meal. Statistical analysis results for the abundance of bacteria and fungi at the phylum level (a–c,j–l) and genus level (d–i,m–z). The significant difference (p < 0.05) between the different inoculant treatments on the same silage raw substrate is represented by the different capital letters (A–D). The significant difference (p < 0.05) between the different silage raw substrates on the same inoculant treatment is represented by the different lowercase letters (a–b).
At the genus level, Lactobacillus was the most dominant, followed by Pediococcus, Weissella, Methylobacterium-Methykorubrum, Enterobacter, and Enterococcus (Figure 5B). These findings were consistent with previous research, which identified the primary bacterial communities in fermented vegetables and silage as belonging to the genera Lactobacillus, Pediococcus [31], and Weissella [48,49]. It is well established that Lactobacillus predominates in reducing pH in silage by converting carbohydrates into organic acids under anaerobic conditions [50]. Noteworthy is the fact that the abundance of Lactobacillus in the JNK inoculant treatment was significantly less than that in the TTX and DBN inoculant treatments, and even lower than in the CON group (Figure 5d). This reduction may have contributed to the elevated pH levels observed in silage treated with the JNK inoculant, as discussed in Section 3.2. Furthermore, it has been reported that Pediococcus thrives in neutral environments and its metabolic activity is inhibited under excessively acidic conditions [51]. The results of this study corroborate this observation, as the addition of the JNK inoculant substantially increased the relative abundance of Pediococcus in the silage of two raw substrates, compared to the CON group and other inoculant groups (p < 0.05). Research indicates that Weissella plays a significant role in the beginning stages of lactic fermentation in ensiling. However, its tolerance for acidic conditions with low pH is inferior to that of Lactobacillus [52]. This suggests that as silage fermentation progresses, the LA production by Lactobacillus reduces the pH of the fermentation environment, leading to a decline in the abundance of Weissella and the predominance of Lactobacillus. This observation accorded with the results of the current study, where a higher abundance of Weissella was noted in the JNK inoculant treatment. Identifying the microbial communities associated with the surface of raw materials is crucial, as the silage process involves an interplay between the chemical composition and the microbial presence on the surface of raw materials in the absence of additional additives [53]. Several studies have documented the microbial communities on the surfaces of silage raw materials, including maize straw and cabbage waste [8], fresh cauliflower [46], and sweet potato vine and peanut straw [10]. Despite the high diversity of bacteria adhered to the surface of primary materials, the abundance of lactic acid-producing bacteria was initially low. However, these bacteria generally supplanted the acid-intolerant bacteria and became dominant in the subsequent fermentative process [10,49]. As a result, the composition of epiphytic microorganisms does not reliably predict the outcome of silage fermentation [51]. The potential competition between Enterobacter and LAB for nutrients can lead to the production of ammonia nitrogen and subsequent nutrient loss, rendering their presence in silage undesirable [54]. In this study, the relative abundance of Enterobacter decreased with the addition of inoculants, suggesting that the primary harmful bacteria were suppressed to some extent, aligning with findings from previous research [45]. Furthermore, the PV-WB silage in the control group exhibited a high abundance of Enterococcus. This observation is consistent with the findings of Guo et al. [55], which reported that Enterococcus was predominantly present in control silage and significantly higher than in silage inoculated with Lactobacillus. The results presented above indicate that while silage fermentation can be achieved by microorganisms inherent to the silage substrate under anaerobic conditions, the introduction of exogenous inoculants can further suppress the proliferation of harmful microorganisms.
Figure 5C,D illustrates the abundance of mycobiota communities at the phylum and genus levels, respectively. Ascomycota emerged as the dominant fungal phylum across all silage samples, succeeded by Basidiomycota. Dramatically, the relative abundance of Ascomycota exceeded 97% in the JNK inoculant treatment (Figure 5j), which was notably greater than the levels observed in the TTX and DBN inoculant treatments (p < 0.05). This finding is consistent with a previous study by Peng et al. [56], which reported that the relative abundance of Ascomycota and Basidiomycota exceeded 94% at the phylum level in purple prairie clover silage. Additionally, Egidi et al. [57] documented that Ascomycota is the dominant phylum in soil, attributed to their resilience to environmental stresses and enhanced resource utilization capabilities. This finding may partially elucidate why Ascomycota become the predominant fungi under acidic conditions in silage.
As shown in Figure 5D, Leptosphaerulina emerged as the dominant genus in silage samples, except for those treated with the JNK inoculant. Taxonomically, Leptosphaerulina belongs to the Ascomycota phylum, and certain species within this genus are known to parasitize plants, leading to diseases that adversely affect normal plant growth and development, such as leaf spot symptoms [58]. This phenomenon was also observed in the present study, where brownish maculae appeared on some fresh peanut leaves during silage preparation. Foliar diseases can lead to reduced leaf yield and diminished forage quality. Furthermore, Leptosphaerulina may produce toxins in feed, potentially harming livestock [59,60]. In comparison to the control group, the addition of DBN and TTX inoculants does not appear to reduce the abundance of Leptosphaerulina, which originates from fresh peanut vine. This observation suggests that while the introduction of Lactobacillus may enhance LA production and lower the pH in silage [61], it does not affect the fungal community associated with the raw material’s surface. Consequently, it is crucial to implement specific strategies to manage detrimental fungi present in silage raw materials to enhance silage quality and safety.
Notably, Saccharomyces, a member of the Ascomycota phylum, exhibited the highest abundance in the JNK inoculant treatment (p < 0.05), which may explain the decreased alpha diversity of mycobiota that was observed in this treatment, as discussed in Section 3.5.1. Previous studies have indicated that yeasts can convert LA into water and carbon dioxide, implicating them as primary agents of aerobic deterioration in silage [44]. This could explain the elevated pH levels observed in the JNK inoculant treatment, as noted in Section 3.4. Dry matter loss occurring in silage is frequently ascribed to the metabolic activity of yeast, which converts soluble carbohydrates into ethanol during anaerobic fermentation. Consequently, there is a negative correlation between the yeast population and the aerobic stability of silage [41]. Silages that have undergone aerobic deterioration are often rejected by livestock, potentially due to their adverse effects on physiological and immune functions [62]. It is widely accepted that yeast counts exceeding 5 log10 cfu/g DM lead to rapid deterioration upon exposure to air [63]. The current study has certain limitations, as it did not include assessments of aerobic stability or microbial counts. Future research on peanut vine silage could explore the addition of specific inoculants to mitigate aerobic deterioration, such as citric acid [31], high-biofilm-producing LAB [50], and sodium metabisulfite [37]. Furthermore, as illustrated in Figure 5D, there is greater fungal diversity in silage treated with DBN inoculant, TTX inoculant, and CON treatment compared to the JNK inoculant treatment. This is easily explained because of the higher abundance of dominant Saccharomyces, which results in a less varied mycobiome community [39]. Previous research reported that the genus Alternaria, frequently found in wheat feed, is capable of producing mycotoxins under pH 4.0 conditions [64]. This study similarly observed that the relative abundance of Alternaria in PV-WB silage with DBN and TTX inoculants was higher compared to PV-CM silage.
To further elucidate the specific bacterial and fungal genera present, LEfSe analysis was performed on silages with different inoculants (Figure 6A–D and Supplementary Figure S1A–D). Bacteria significantly enriched in the treatment group were identified as indicator bacteria [65]. In the case of PV-WB silage (Figure 6A), Enterobacter and Enterococcus were identified as indicator bacteria for the CON group; Lactobacillus for the DBN inoculant treatment; Staphylococcus for the TTX inoculant treatment; and Pediococcus and Weissella for the JNK inoculant treatment. In the analysis of PV-CM silage (Figure 6B), the indicator bacteria identified for the CON group included Weissella, Enterobacter, and Enterococcus. For the TTX inoculant treatment, the indicator bacteria were norank_f__Mitochondria and Marmoricola, while Pediococcus was identified for the JNK inoculant treatment. Similarly, the LEfSe analysis was conducted on the mycobiome of PV-WB silage (Figure 6C). The indicator mycobiome for the CON treatment comprised Penicillium, Coprinellus, and Agaricus. For the DBN inoculant treatment, Cryptococcus_f__Tremellaceae and Symmetrospora were identified, Sporidiobolus was the indicator for the TTX inoculant treatment, and Saccharomyces was identified for the JNK inoculant treatment. In the case of PV-CM silage (Figure 6D), the indicator mycobiome for the CON treatment included Auriculibuller and Mortierella. Talaromyces and Dioszegia were identified for the DBN inoculant treatment, Ustilaginoidea and Alternaria for the TTX inoculant treatment, and Saccharomyces for the JNK inoculant treatment.
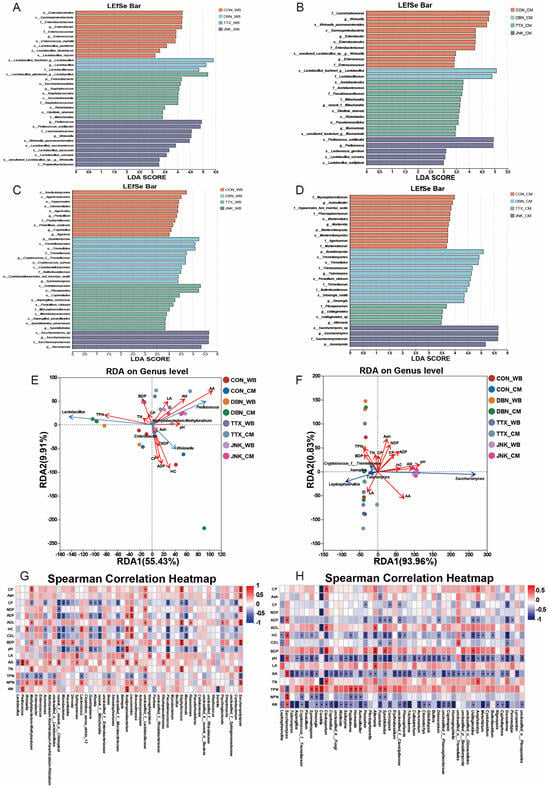
Figure 6.
(A,C) LEfSe analysis of bacteriome and mycobiome for PV-WB silage and (B,D) PV-CM silage. (E) Redundancy analysis of environmental factors with bacteriome and (F) mycobiome sequencing data at genus level. (G) Correlation analysis between bacteriome composition and silage chemical composition and silage fermentation indicators. (H) Correlation analysis between mycobiome composition and silage chemical composition and fermentation indicators. WB: wheat bran; CM: corn meal; CP: crude protein; CF: crude fiber; NDF: neutral detergent fiber; ADF: acid detergent fiber; ADL: acid detergent lignin; HC: Hemicellulose; CEL: Cellulose; BDP: biodegradation potential; LA: lactic acid; AA: acetic acid; TN: total nitrogen; TPN: true-protein nitrogen; NPN: nonprotein nitrogen; *, ** and *** means p < 0.05, p < 0.01 and p < 0.001, respectively.
3.6. Correlation Between Bacteriome, Mycobiome Community, and Silage Chemical Composition and Fermentation Indicators
The redundancy analysis (RDA) between the bacteriome and environmental factors, including silage chemical composition and fermentation indicators, is presented in Figure 6E. The first two axes of the RDA interpreted 55.43% and 9.91% of the variance between the bacteriome community and environmental factors. Acetic acid (r2 = 0.5910) emerged as the principal environmental factor influencing the succession of the dominant bacteriome (p < 0.05). In this study, the pH value, along with the concentrations of acetic acid, LA, and ammonia-N, exhibited a positive correlation with the abundances of Pediococcus and Weissella, while demonstrating a negative correlation with the abundance of Lactobacillus. This observation aligns with previous research by [12], which reported a negative correlation between Lactobacillus abundance and pH value. Similarly, Li et al. [12] reported that LA content was negatively correlated with Lactobacillus abundance, yet positively correlated with the abundances of Pediococcus and Weissella. These findings suggest that unlike Lactobacillus, Weissella and Pediococcus are less adept at tolerating acidic conditions during the silage process [52]. The current study found a positive relationship among LA, acetic acid, and pH value, which contradicts the outcomes of previous studies [12,66], but concurs with the findings reported by Bai et al. [48], who stated elevated levels of LA in conjunction with high pH values in silage. In addition, a positive correlation was identified among LA, acetic acid, pH value, and ammonia-N. It is hypothesized that the presence of ammonia-N may neutralize the acids, thereby inhibiting the decline in pH within the silage.
The RDA for mycobiome is presented in Figure 6F, where the first two axes explained 93.96% and 0.83% of the variance between the mycobiome community and environmental factors. Key environmental factors influencing the succession of the dominant mycobiome, identified with statistical significance (p < 0.05), include pH (r2 = 0.7971), ammonia-N (r2 = 0.5523), acetic acid (r2 = 0.4306), CEL (r2 = 0.2770), and ADF (r2 = 0.2674). The pH value, along with the amounts of acetic acid and ammonia-N, exhibited a positive relationship with the abundance of Saccharomyces while showing a negative association with the abundance of Leptosphaerulina, Cryptococcus_f__Tremellaceae, Talaromyces, and Aspergillus. Additionally, a positive association was observed between the LA amount and the abundance of Leptosphaerulina, Talaromyces, and Aspergillus. The results were in accordance with previous findings that Aspergillus abundance is negatively associated with pH value and ammonia-N, but positively associated with LA content, as reported by Li et al. [31]. This may partly be due to Aspergillus’s tendency not to consume LA, instead utilizing water-soluble carbohydrates and ammonia-N as a nitrogen substrate for growth.
To elucidate the relationship among the abundance of bacteriome, mycobiome, and environmental factors, we conducted a Spearman correlation analysis at the genus level. For the bacteriome (Figure 6G), the presence of negative correlation among orank_f__norank_o__Chloroplas, Microbacterium, Quadrisphaera, norank_f__Mitochondria, Pseudokineococcus, Marmoricola, and ADF, HC, and CEL contents (p < 0.05). A previous study by Chang et al. [67] demonstrated that Microbacterium isolated from the gut of Tuta absoluta can degrade Cellulose as a carbon source for growth, which may explain the negative correlation between Microbacterium abundance and ADF, HC, and CEL contents. Regarding the mycobiome (Figure 6H), a positive correlation was observed between Alternaria abundance and LA content, while a negative relationship was found between Alternaria abundance and pH in the present study (p < 0.05). This may be attributed to Alternaria’s ability to tolerate acidic environments [64]. In the present study, it was observed that acetic acid content exhibited a negative correlation with the majority of fungi, potentially contributing to the antifungal properties of acetic acid and subsequently inhibiting fungal growth in silage, thereby enhancing aerobic stability [41].
3.7. Co-Occurrence Network of Bacteriome and Mycobiome
Network correlation analysis was performed to assess the influences of various silage substrates and exogenous inoculants on the interactions between bacteria and fungi within the microbial ecosystem during the silage process (Figure 7A–H). Previous research has indicated that the size and complexity of the co-occurrence network can be represented by the number of nodes and edges [68]. In this study, the majority of nodes and edges was identified within the mycobiome across all silage samples. Specifically, on the one hand, for PV-WB silage samples, the node counts for bacteriome and mycobiome in the CON treatment were 8 and 42, respectively, while the addition of inoculants resulted in node counts of 4 and 44 (DBN), 8 and 42 (TTX), and 18 and 31 (JNK), respectively (Figure 7A–D). The edge counts within the bacteriome for PV-WB in the CON group and silage with the addition of DBN, TTX, and JNK inoculants were 8, 2, 8, and 64, respectively (Figure 7I–L). The edge counts for the corresponding treatments within the mycobiome were recorded as 286, 310, 334, and 159, respectively. The interkingdom interaction edge counts between the bacteriome and mycobiome for the CON, DBN, TTX, and JNK treatments were 120, 60, 94, and 163, respectively. On the other hand, counts for the bacteriome and mycobiome in PV-CM silage under the CON treatment were 8 and 41, while for the different inoculants, they were 3 and 46 (DBN), 6 and 43 (TTX), and 16 and 33 (JNK), respectively (Figure 7E–H). The edge counts within the bacteriome for PV-CM in the CON group and with the addition of DBN, TTX, and JNK inoculants were 21, 3, 4, and 34, respectively (Figure 7M–P), and the edge counts within the mycobiome were 266, 346, 321, and 191, respectively. The interkingdom interaction edge counts between the bacteriome and mycobiome for the CON, DBN, TTX, and JNK treatments were 91, 33, 97, and 185, respectively. In the present study, in the CON group, the silage including wheat bran increases the edge amount compared with adding the corn meal, which suggested that the inclusion of wheat bran improved commensalism or mutualism. This result was consistent with the previous study, in which the addtion of wheat bran improved the commensalism or mutualism of Chinese cabbage waste silage [12].
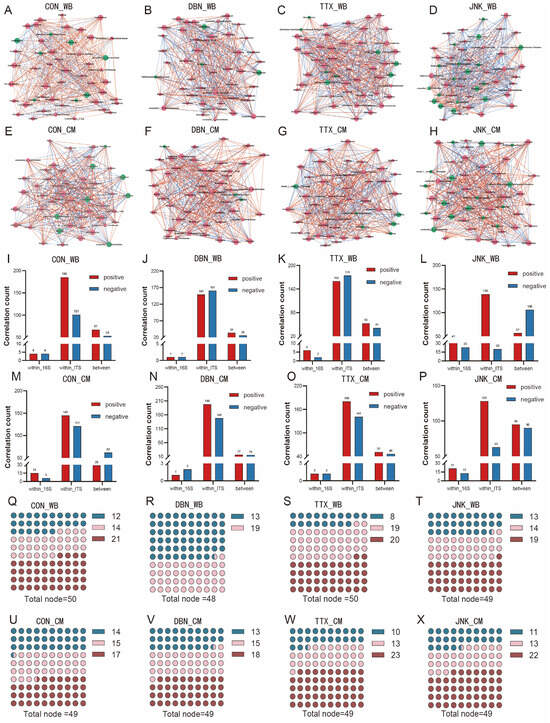
Figure 7.
Co-occurrence network based on Spearman analysis showing the correlation among bacteria–bacteria, fungi–fungi, and bacteria–fungi (A–H). Counts of interactions analyzed by Spearman analysis within the bacteriome and mycobiome, and between bacteriome and mycobiome based on the genus level (I–P). The node degree value that corresponds to the node size, and degree distribution proportions for each silage sample (Q–X). The node size reflects the connective degree of each node; the green node represents bacteria; and the red node represents fungi. The red edge represents a positive correlation, and the blue edge represents a negative correlation. WB: wheat bran; CM: corn meal.
To assess the impact of exogenous inoculants on the complexity of co-occurrence networks, we focused on a comparative analysis using the same silage substrate. For PV-WB silage, the application of the TTX inoculant increased total edge counts, whereas the DBN and JNK inoculants led to a decrease in total edge counts compared to the CON group. In addition, relative to the CON treatment, the inclusion of the JNK inoculant resulted in a greater number of bacterial nodes being identified, whereas the addition of the DBN inoculant appeared to reduce the bacterial node counts, albeit by only four nodes. The edge counts in the PV-CM silage sample increased with the addition of the DBN, TTX, and JNK inoculants compared to the CON treatment, with the TTX and JNK inoculants showing particularly notable increases of 44 and 32 edges, respectively, over the CON treatment. Similarly, the JNK inoculant was found to increase bacterial node counts in PV-CM silage, while the DBN and TTX inoculants resulted in reductions of five and two nodes, respectively, compared to the CON treatment. A significant number of intrakingdom interactions was observed within the mycobiome. The percentages of positive intrakingdom interactions within the mycobiome for the CON, DBN, TTX, and JNK treatments were 44.69%, 40.05%, 36.70%, and 36.01% for PV-WB silage, and 38.36%, 51.83%, 42.65%, and 31.22% for PV-CM silage, respectively. The percentages of negative interactions within the mycobiome under the CON, DBN, TTX, and JNK treatments were 24.40%, 43.28%, 39.91%, and 5.18% for PV-WB silage, and 32.01%, 38.74%, 33.41%, and 15.37% for PV-CM silage, respectively. Additionally, the proportions of positive and negative interkingdom interactions between bacterial and fungal communities were 16.18% and 12.80% (CON), 9.14% and 6.99% (DBN), 12.16% and 9.40% (TTX), and 14.77% and 27.46% (JNK) in PV-WB silage. The corresponding values for PV-CM silage were 7.67% and 16.40%, 4.45% and 4.19%, 12.09% and 10.90%, and 23.17% and 21.95%, respectively. The data indicate that numerous negative associations within the fungal communities in the CON, DBN, and TTX treatments suggest significant competition for resources among microbiomes originating from the silage substrate surface during the ensilage process [31]. The previous study implied that a large and complex co-occurrence network had greater stability when exposed to an exogenous stimulus [69]. In this study, the peanut vine co-silage with wheat bran including the TTX inoculant had more edges among all treatments, which might be suggested that supplementing the wheat bran and TTX inoculant was better suited to improve fresh peanut vine ensilage.
4. Conclusions
The present study demonstrated that harvesting 5 days earlier could enhance the retention of CP and decrease the ADF and ADL contents of PV plants. Furthermore, the addition of the TTX inoculant increased lactic and acetic acid concentrations, thus causing a lower pH. The bacterial community across all silage samples was predominantly composed of the genera Lactobacillus, Pediococcus, and Weissella. The addition of an exogenous inoculant, in contrast to silage without inoculant, was found to inhibit the abundance of Enterobacter and Enterococcus during the silage process. In summary, harvesting peanut vines five days earlier can enhance the retention of CP content while reducing ADF and ADL content, thereby increasing the feed value. Furthermore, co-ensiling fresh peanut vines with wheat bran, in conjunction with the application of the TTX inoculant, can enhance the preservation of nutrients and improve the fermentation parameters of peanut vine silage.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15061271/s1. Figure S1: The LEfSe analysis of bacteriome and mycobiome for PV-WB silage (A and C) and PV-CM silage (B and D); Table S1: Chemical composition of peanut vine, wheat bran, and corn meal used for co-silage in this study (%, as DM basis); Table S2: Counts of lactic acid bacteria and yeasts in TTX, DBN, and JNK inoculants, respectively. (CFU/g); Table S3: Contents of total nitrogen (TN), true protein nitrogen (TPN), and nonprotein nitrogen (NPN) of peanut vine, wheat bran, and corn meal, respectively; Table S4: General information of sequence bacteriome and mycobiome.
Author Contributions
K.L.: Conceptualization, Methodology, Data Curation, Visualization, Writing—Original Draft. C.T.: Methodology, Data Curation, Investigation. J.W.: Methodology, Data Curation, Visualization. Y.L.: Methodology, Software. X.J.: Methodology, Visualization. R.Z.: Validation, Writing—Review and Editing, Funding Acquisition. L.C.: Investigation, Formal Analysis, Writing—Review and Editing, Resources, Funding Acquisition. H.Z.: Conceptualization, Resources, Supervision, Funding Acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (2022YFD1300605), the Fundamental Research Funds for the Chinese Academy of Agricultural Sciences (2024-YWF-ZX-05) in China, and the Shandong Provincial Science and Technology SME Innovation Capacity Enhancement Project (2022TSGC2029).
Data Availability Statement
Data will be made available from the corresponding author upon reasonable request.
Acknowledgments
We thank Xiaogang Yan (Jilin Academy of Agri-cultural Sciences) who provided the inoculant, and we would like to thank Xintiandi Grass Industry Company of Zhengyang County of Henan provinces for providing experimental sites.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fang, Q.; Zhang, X.; Dai, G.; Tong, B.; Wang, H.; Oenema, O.; Zanten, H.; Gerber, P.J.; Hou, Y. Low-opportunity-cost feed can reduce land-use-related environmental impacts by about one-third in China. Nat. Food 2023, 4, 677–685. [Google Scholar] [CrossRef] [PubMed]
- (FAO), Food and Agriculture Organization of the United Nations. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 19 September 2024).
- Bi, Y.Y. Study on Straw Resource Evaluation and Utilization in China; Chinese Academy of Agricultural Sciences: Beijing, China, 2010. [Google Scholar]
- Zhang, S.J.; Zhu, C.H.; Guo, J.; Tang, Q.P.; Li, H.F.; Zou, J.M. Metabolizable energy and fiber digestibility of uncommon feedstuffs for geese. Poult. Sci. 2013, 92, 1812–1817. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Hu, Z.C.; Chen, Y.Q.; Wu, H.C.; Wang, Y.W.; Wu, F.; Gu, F.W. Integration of agricultural machinery and agronomy for mechanised peanut production using the vine for animal feed. Biosyst. Eng. 2022, 219, 135–152. [Google Scholar] [CrossRef]
- Zhang, L.; Shang, Y.; Li, J.; Fu, T.; Lian, H.; Gao, T.; Shi, Y.; Li, M. Comparison of feeding diets including dried or ensiled peanut vines as forage sources on the growth performance, ruminal fermentation, and bacterial community in young Holstein bulls. Anim. Sci. J. 2021, 93, e13675. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, H.; Hu, Z.; Chen, Y.; Cao, M.; Yu, Z.; Mao, E. Characteristics of Agricultural Dust Emissions from Harvesting Operations: Case Study of a Whole-Feed Peanut Combine. Agriculture 2021, 11, 1068. [Google Scholar] [CrossRef]
- Ren, H.; Feng, Y.; Liu, T.; Li, J.; Wang, Z.; Fu, S.; Zheng, Y.; Peng, Z. Effects of different simulated seasonal temperatures on the fermentation characteristics and microbial community diversities of the maize straw and cabbage waste co-ensiling system. Sci. Total Environ. 2020, 708, 135113. [Google Scholar] [CrossRef]
- Qin, M.Z.; Shen, Y.X. Effect of Application of a Bacteria Inoculant and Wheat Bran on Fermentation Quality of Peanut Vine Ensiled Alone or with Corn Stover. J. Integr. Agric. 2013, 12, 556–560. [Google Scholar] [CrossRef]
- He, L.; Wang, Y.; Guo, X.; Chen, X.; Zhang, Q. Evaluating the Effectiveness of Screened Lactic Acid Bacteria in Improving Crop Residues Silage: Fermentation Parameter, Nitrogen Fraction, and Bacterial Community. Front. Microbiol. 2022, 13, 680988. [Google Scholar] [CrossRef]
- Wang, W.; Nie, Y.; Tian, H.; Quan, X.; Li, J.; Shan, Q.; Li, H.; Cai, Y.; Ning, S.; Santos Bermudez, R.; et al. Microbial Community, Co-Occurrence Network Relationship and Fermentation Lignocellulose Characteristics of Broussonetia papyrifera Ensiled with Wheat Bran. Microorganisms 2022, 10, 2015. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Zhang, S.; Xing, J.; Song, C.; Meng, Q.; Li, J.; Jia, S.; Shan, A. Anaerobic fermentation featuring wheat bran and rice bran realizes the clean transformation of Chinese cabbage waste into livestock feed. Front. Microbiol. 2023, 14, 1108047. [Google Scholar] [CrossRef]
- Li, J.; Meng, Q.; Wang, C.; Song, C.; Lyu, Y.; Li, J.; Shan, A. The interaction between temperature and citric acid treatment in the anaerobic fermentation of Chinese cabbage waste. J. Clean Prod. 2023, 383, 135502. [Google Scholar] [CrossRef]
- Liu, W. Effects of Foliar Selenium Application in Different Periods on Hay and Silage Quality and Spatial Transformation of Selenium in Cyperus Esculentus; Northeast Agricultural University: Harbin, China, 2022. [Google Scholar]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and In Vitro Media1. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Wu, W.; Xie, J.; Zhang, H. Dietary fibers influence the intestinal SCFAs and plasma metabolites profiling in growing pigs. Food Funct. 2016, 7, 4644–4654. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Robinson, P.H.; Swanepoel, N.; Heguy, J.M.; Price, T.; Meyer, D.M. ‘Shrink’ losses in commercially sized corn silage piles: Quantifying total losses and where they occur. Sci. Total Environ. 2016, 542, 530–539. [Google Scholar] [CrossRef]
- Cai, A.M.; Xue, X.; Zhao, J.H.; Lian, H.X.; Fu, T.; Gao, T.Y. Evaluation of Nutrient Value and Comparison of Rumen Degradation Rate between Spring-Grown Peanut Vine and Summer-Grown Peanut Vine. Chin. J. Anim. Nutr. 2019, 31, 1823–1832. [Google Scholar]
- Liu, T.Y.; Guo, X.; Guo, L.X. The effects of cutting on yield and quality of peanut vine. Ecol. Domest. Anim. 2002, 23, 38–40. [Google Scholar]
- Zheng, X.L.; Ye, H.L.; Wang, J.H.; Xu, G.Z.; Weng, B.Q. Effect of vine cutting time on yield and quality of peanuts and vine. Fujian J. Agric. Sci. 2011, 26, 234–237. [Google Scholar]
- Wang, Q.; Wang, R.; Wang, C.; Dong, W.; Zhang, Z.; Zhao, L.; Zhang, X. Effects of Cellulase and Lactobacillus plantarum on Fermentation Quality, Chemical Composition, and Microbial Community of Mixed Silage of Whole-Plant Corn and Peanut Vines. Appl. Biochem. Biotechnol. 2022, 194, 2465–2480. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Zhu, M.; Wang, C. The In Vitro Digestion and Fermentation Characteristics of Feedstuffs Inoculated With Cecal or Colic Fluid of Dezhou Donkey. J. Equine Vet. Sci. 2022, 110, 103864. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, B.; Xiao, J.; Guo, M.; Zhao, S.; Hu, M.; Cui, Y.; Li, D.; Wang, C.; Ma, S.; et al. Effects of Different Roughage Diets on Fattening Performance, Meat Quality, Fatty Acid Composition, and Rumen Microbe in Steers. Front. Nutr. 2022, 9, 885069. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.C.; Li, W.J.; Wang, W.K.; Wang, Y.L.; Zhang, F.; Lv, L.K.; Yang, H.J. Foxtail millet (Setaria italica L.) silage compared peanut vine hay (Arachis hypogaea L.) exhibits greater feed efficiency via enhancing nutrient digestion and promoting rumen fermentation more efficiently in feedlotting lambs. Small Rumin. Res. 2022, 215, 106704. [Google Scholar] [CrossRef]
- Li, J.; Lian, H.; Zheng, A.; Zhang, J.; Dai, P.; Niu, Y.; Gao, T.; Li, M.; Zhang, L.; Fu, T. Effects of Different Roughages on Growth Performance, Nutrient Digestibility, Ruminal Fermentation, and Microbial Community in Weaned Holstein Calves. Front. Vet. Sci. 2022, 9, 864320. [Google Scholar] [CrossRef]
- Tawila, M.; Omer, H.; Gad, S. Partial Replacing of Concentrate Feed Mixture by Potato Processing Waste in Sheep Rations. Am-Euras. J. Agric. & Environ. Sci. 2008, 4, 156–164. [Google Scholar]
- Fu, C.; Batima, N.E.B.H.T.; Ma, H.H.; Sun, Q.Y.; Zhou, Y.; Wang, G.L.; Li, S.Y.; Xu, B.; Wang, L.Y. Construction of Nutrient Component Prediction Model of Peanut Vines Based on Near Infrared Spectroscopy. Chin. J. Anim. Nutr. 2023, 35, 4697–4705. [Google Scholar]
- Tang, M.Q. Effects of Different Additives on the Quality of Peanut Seed Silage and Its Effect on Growth Performance in Fattening Sheep; Henan Agricultural University: Zhengzhou, China, 2020. [Google Scholar]
- Li, J.; Meng, Q.; Xing, J.; Wang, C.; Song, C.; Ma, D.; Shan, A. Citric acid enhances clean recycling of Chinese cabbage waste by anaerobic fermentation. J. Clean Prod. 2022, 348, 131366. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Yuan, X.-J.; Guo, G.; Li, J.-F.; Bai, Y.-F.; Shao, T. Effects of molasses on the fermentation characteristics of mixed silage prepared with rice straw, local vegetable by-products and alfalfa in Southeast China. J. Integr. Agric. 2017, 16, 664–670. [Google Scholar] [CrossRef]
- Hillion, M.-L.; Moscoviz, R.; Trably, E.; Leblanc, Y.; Bernet, N.; Torrijos, M.; Escudié, R. Co-ensiling as a new technique for long-term storage of agro-industrial waste with low sugar content prior to anaerobic digestion. Waste Manag. 2018, 71, 147–155. [Google Scholar] [CrossRef]
- Toledo, A.F.; Dondé, S.C.; Silva, A.P.; Cezar, A.M.; Coelho, M.G.; Tomaluski, C.R.; Virgínio, G.F.; Costa, J.H.C.; Bittar, C.M.M. Whole-plant flint corn silage inclusion in total mixed rations for pre- and postweaning dairy calves. J. Dairy Sci. 2023, 106, 6185–6197. [Google Scholar] [CrossRef]
- Silva, T.B.P.; Del Valle, T.A.; Ghizzi, L.G.; Silva, G.G.; Gheller, L.S.; Marques, J.A.; Dias, M.S.S.; Nunes, A.T.; Grigoletto, N.T.S.; Takiya, C.S.; et al. Partial replacement of corn silage with whole-plant soybean and black oat silages for dairy cows. J. Dairy Sci. 2021, 104, 9842–9852. [Google Scholar] [CrossRef]
- Chen, L.; Bao, X.; Guo, G.; Huo, W.; Xu, Q.; Wang, C.; Li, Q.; Liu, Q. Effects of Hydrolysable Tannin with or without Condensed Tannin on Alfalfa Silage Fermentation Characteristics and In Vitro Ruminal Methane Production, Fermentation Patterns, and Microbiota. Animals 2021, 11, 1967. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Lee, Y.H.; Lee, W.H.; Oh, Y.-K.; Park, K.; Kwak, W.S. Long-term anaerobic conservation of fruit and vegetable discards without or with moisture adjustment after aerobic preservation with sodium metabisulfite. Waste Manag. 2019, 87, 258–267. [Google Scholar] [CrossRef]
- Huang, P.; Chen, M.; Chen, D.; Zang, M.; Zhang, W.; Lin, X.; Han, H.; Zhang, Q. Effects of Neolamarckia cadamba Leaf Extract on Dynamic Fermentation Characteristics and Bacterial Community of Stylosanthes guianensis Silage. Fermentation 2024, 10, 347. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Xing, Y.; Zhou, W.; Pian, R.; Yang, F.; Chen, X.; Zhang, Q. Bacterial diversity and fermentation quality of Moringa oleifera leaves silage prepared with lactic acid bacteria inoculants and stored at different temperatures. Bioresour. Technol. 2019, 284, 349–358. [Google Scholar] [CrossRef]
- Restelatto, R.; Novinski, C.O.; Pereira, L.M.; Silva, E.P.A.; Volpi, D.; Zopollatto, M.; Schmidt, P.; Faciola, A.P. Chemical composition, fermentative losses, and microbial counts of total mixed ration silages inoculated with different Lactobacillus species. J. Anim. Sci. 2019, 97, 1634–1644. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- You, L.J.; Bao, W.C.; Yao, C.Q.; Zhao, F.Y.; Jin, H.; Huang, W.Q.; Li, B.H.; Kwok, L.Y.; Liu, W.J. Changes in chemical composition, structural and functional microbiome during alfalfa (Medicago sativa) ensilage with Lactobacillus plantarum PS-8. Anim. Nutr. 2022, 9, 100–109. [Google Scholar] [CrossRef]
- Benjamim da Silva, É.; Polukis, S.A.; Smith, M.L.; Voshell, R.S.; Leggett, M.J.; Jones, P.B.; Kung, L. The use of Lentilactobacillus buchneri PJB1 and Lactiplantibacillus plantarum MTD1 on the ensiling of whole-plant corn silage, snaplage, and high-moisture corn. J. Dairy Sci. 2024, 107, 883–901. [Google Scholar] [CrossRef]
- He, L.; Lv, H.; Chen, N.; Wang, C.; Zhou, W.; Chen, X.; Zhang, Q. Improving fermentation, protein preservation and antioxidant activity of Moringa oleifera leaves silage with gallic acid and tannin acid. Bioresour. Technol. 2020, 297, 122390. [Google Scholar] [CrossRef]
- He, L.; Wang, C.; Xing, Y.; Zhou, W.; Pian, R.; Chen, X.; Zhang, Q. Ensiling characteristics, proteolysis and bacterial community of high-moisture corn stalk and stylo silage prepared with Bauhinia variegate flower. Bioresour. Technol. 2020, 296, 122336. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Feng, Y.; Pei, J.; Li, J.; Wang, Z.; Fu, S.; Zheng, Y.; Li, Z.; Peng, Z. Effects of Lactobacillus plantarum additive and temperature on the ensiling quality and microbial community dynamics of cauliflower leaf silages. Bioresour. Technol. 2020, 307, 123238. [Google Scholar] [CrossRef] [PubMed]
- Ogunade, I.M.; Jiang, Y.; Pech Cervantes, A.A.; Kim, D.H.; Oliveira, A.S.; Vyas, D.; Weinberg, Z.G.; Jeong, K.C.; Adesogan, A.T. Bacterial diversity and composition of alfalfa silage as analyzed by Illumina MiSeq sequencing: Effects of Escherichia coli O157:H7 and silage additives. J. Dairy Sci. 2018, 101, 2048–2059. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Franco, M.; Ding, Z.; Hao, L.; Ke, W.; Wang, M.; Xie, D.; Li, Z.; Zhang, Y.; Ai, L.; et al. Effect of Bacillus amyloliquefaciens and Bacillus subtilis on fermentation, dynamics of bacterial community and their functional shifts of whole-plant corn silage. J. Anim. Sci. Biotechnol. 2022, 13, 7. [Google Scholar] [CrossRef]
- Xu, J.; Peng, S.; Xiong, Y.; Zheng, Z.; Liu, M.; Xu, J.; Chen, W.; Liu, M.; Kong, J.; Wang, C.; et al. A review on fermented vegetables: Microbial community and potential upgrading strategy via inoculated fermentation. Compr. Rev. Food. Sci. Food Saf. 2024, 23, e13362. [Google Scholar] [CrossRef]
- Yang, C.; Huang, B.; Lin, J.; Yang, Q.; Guo, Y.; Liu, D.; Sun, B. Isolation and screening of high biofilm producing lactic acid bacteria, and exploration of its effects on the microbial hazard in corn straw silage. J. Hazard. Mater. 2024, 480, 136009. [Google Scholar] [CrossRef]
- Dong, Z.; Shao, T.; Li, J.; Yang, L.; Yuan, X. Effect of alfalfa microbiota on fermentation quality and bacterial community succession in fresh or sterile Napier grass silages. J. Dairy Sci. 2020, 103, 4288–4301. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, X.; Li, J.; Dong, Z.; Shao, T. Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresour. Technol. 2019, 275, 280–287. [Google Scholar] [CrossRef]
- Wang, S.; Shao, T.; Li, J.; Zhao, J.; Dong, Z. Fermentation Profiles, Bacterial Community Compositions, and Their Predicted Functional Characteristics of Grass Silage in Response to Epiphytic Microbiota on Legume Forages. Front. Microbiol. 2022, 13, 830888. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Xing, Y.; Zheng, Y.; Zhou, W.; Pian, R.; Yang, F.; Chen, X.; Zhang, Q.; Marco, M.L. Dynamics of Bacterial Community and Fermentation Quality during Ensiling of Wilted and Unwilted Moringa oleifera Leaf Silage with or without Lactic Acid Bacterial Inoculants. mSphere 2019, 4, e00341-19. [Google Scholar] [CrossRef]
- Guo, L.; Yao, D.; Li, D.; Lin, Y.; Bureenok, S.; Ni, K.; Yang, F. Effects of Lactic Acid Bacteria Isolated From Rumen Fluid and Feces of Dairy Cows on Fermentation Quality, Microbial Community, and in vitro Digestibility of Alfalfa Silage. Front. Microbiol. 2019, 10, 2998. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Jin, L.; Niu, Y.D.; Huang, Q.; McAllister, T.A.; Yang, H.E.; Denise, H.; Xu, Z.; Acharya, S.; Wang, S.; et al. Condensed Tannins Affect Bacterial and Fungal Microbiomes and Mycotoxin Production during Ensiling and upon Aerobic Exposure. Appl. Environ. Microbiol. 2018, 84, e02274-17. [Google Scholar] [CrossRef]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef]
- Liang, J.; Li, G.; Hou, L.; Zhao, M.; Cai, L. Leptosphaerulina species isolated from golf turfgrass in China, with description of L. macrospora, sp. nov. Mycologia 2021, 113, 956–967. [Google Scholar] [CrossRef]
- Huo, H.; Huangfu, J.; Song, P.; Zhang, D.; Shi, Z.; Zhao, L.; Li, Z.; Zhou, H. Isolation, Identification and Characterization of Leptosphaerulina trifolii, the Causative Agent of Alfalfa Leptosphaerulina Leaf Spot in Inner Mongolia, China. Agronomy 2024, 14, 1156. [Google Scholar] [CrossRef]
- Liu, X.P.; Jing, X.M.; Yan, H.X.; Li, G.L.; Luo, Y.H. First Report of Leptosphaerulina Leaf Spot Caused by Leptosphaerulina trifolii on Alfalfa in Heilongjiang Province, China. Plant Dis. 2019, 103, 2673. [Google Scholar] [CrossRef]
- Xu, S.; Yang, J.; Qi, M.; Smiley, B.; Rutherford, W.; Wang, Y.; McAllister, T.A. Impact of Saccharomyces cerevisiae and Lactobacillus buchneri on microbial communities during ensiling and aerobic spoilage of corn silage1. J. Anim. Sci. 2019, 97, 1273–1285. [Google Scholar] [CrossRef]
- Huhtanen, P.; Rinne, M.; Nousiainen, J. Evaluation of the factors affecting silage intake of dairy cows: A revision of the relative silage dry-matter intake index. Animal 2007, 1, 758–770. [Google Scholar] [CrossRef]
- Jonsson, A.; Pahlow, G. Systematic classification and biochemical characterization of yeast growing in grass silage inoculated with lactobacillus cultures. Anim. Res. Dev. A Biannu. Collect. Recent Ger. Contrib. Concern. Dev. Through Anim. Res. 1984, 20, 7–22. [Google Scholar]
- Brzonkalik, K.; Hümmer, D.; Syldatk, C.; Neumann, A. Influence of pH and carbon to nitrogen ratio on mycotoxin production by Alternaria alternata in submerged cultivation. AMB Express 2012, 2, 28. [Google Scholar] [CrossRef]
- Li, M.; Zi, X.; Sun, R.; Ou, W.; Chen, S.; Hou, G.; Zhou, H. Co-Ensiling Whole-Plant Cassava with Corn Stalk for Excellent Silage Production: Fermentation Characteristics, Bacterial Community, Function Profile, and Microbial Ecological Network Features. Agronomy 2024, 14, 501. [Google Scholar] [CrossRef]
- Du, G.; Zhang, G.; Shi, J.; Zhang, J.; Ma, Z.; Liu, X.; Yuan, C.; Li, X.; Zhang, B. Keystone Taxa Lactiplantibacillus and Lacticaseibacillus Directly Improve the Ensiling Performance and Microflora Profile in Co-Ensiling Cabbage Byproduct and Rice Straw. Microorganisms 2021, 9, 1099. [Google Scholar] [CrossRef]
- Chang, L.S.; Wang, W.Q.; Yang, Y.; Yang, H.; Yang, J.B.; Du, G.Z.; Zhang, T.F.; Yi, X.G.; Xiao, G.L.; Chen, B. Study on the composition of culturable gut bacteria in the larvae of Yunnan population of Tuta absoluta and the degradation for macromolecular compounds. J. Environ. Entomol. 2022, 44, 1240–1251. [Google Scholar]
- Banerjee, S.; Kirkby, C.A.; Schmutter, D.; Bissett, A.; Kirkegaard, J.A.; Richardson, A.E. Network analysis reveals functional redundancy and keystone taxa amongst bacterial and fungal communities during organic matter decomposition in an arable soil. Soil Biol. Biochem. 2016, 97, 188–198. [Google Scholar] [CrossRef]
- Lei, L.; Gu, J.; Wang, X.; Song, Z.; Wang, J.; Yu, J.; Hu, T.; Dai, X.; Xie, J.; Zhao, W. Microbial succession and molecular ecological networks response to the addition of superphosphate and phosphogypsum during swine manure composting. J. Environ. Manag. 2021, 279, 111560. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
