Identification of the CBF Gene Family in Wheat and TaCBF14B Could Enhance the Drought Tolerance of Arabidopsis thaliana
Abstract
1. Introduction
2. Materials and Methods
2.1. Genome-Wide Identification of CBF Genes in Wheat
2.2. Gene Structure and Conserved Protein Motifs
2.3. Plant Materials and Growth Conditions
2.4. Gene Expression Analysis of TaCBF
2.5. Subcellular Localization Assays of TaCBF14B
2.6. Transcriptional Activity Assay
2.7. Arabidopsis Transformation
2.8. Drought Tolerance Assessment
2.9. Measurement of MDA and Proline Content and Analysis of POD Activity
2.10. ABA Treatment Analysis
2.11. Statistical Analysis
3. Results
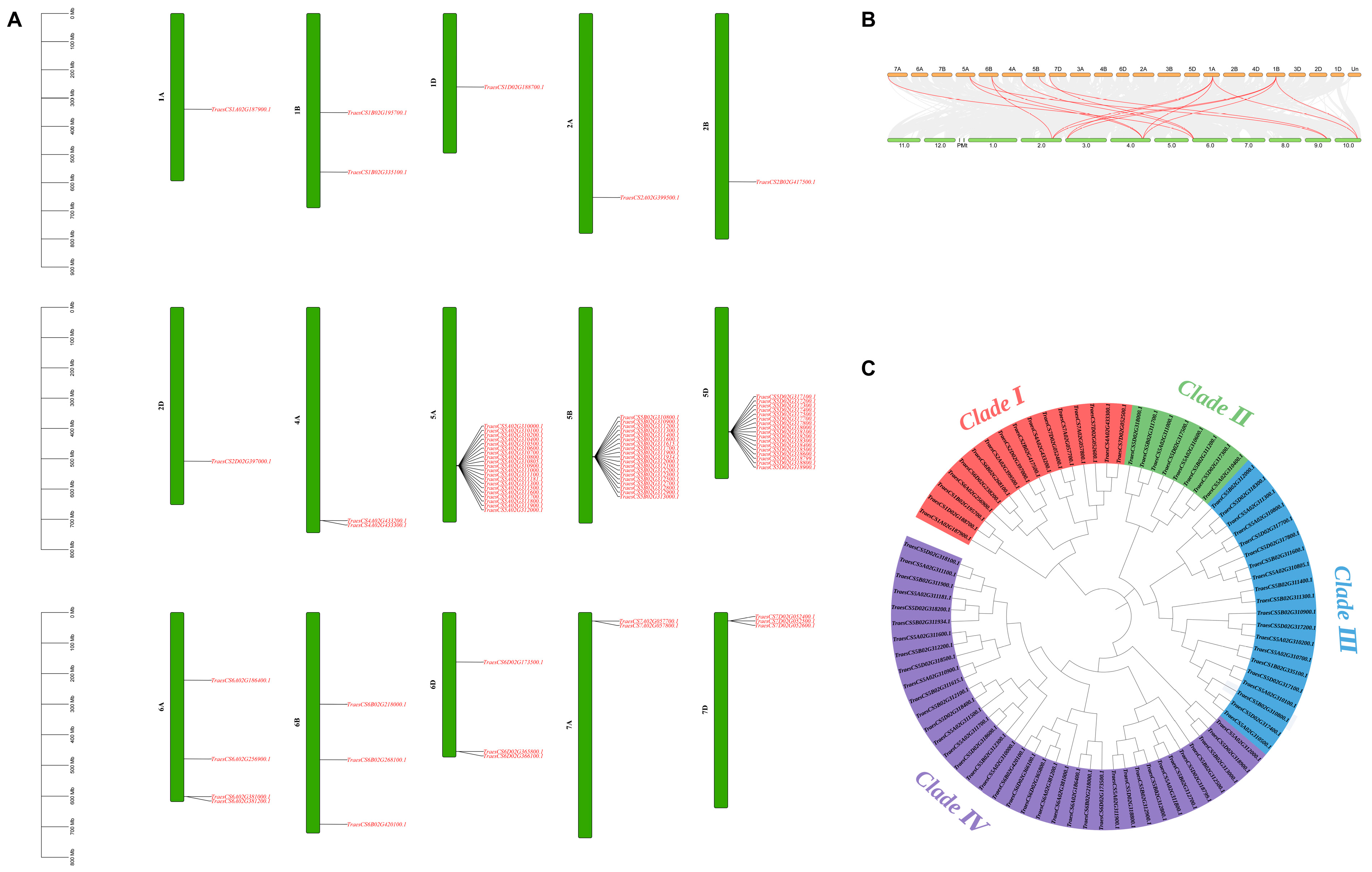
3.1. Genome-Wide Identification and Characterization of CBF Family Genes in Wheat
3.2. Analysis of Conserved Motifs and Gene Structure of TaCBFs
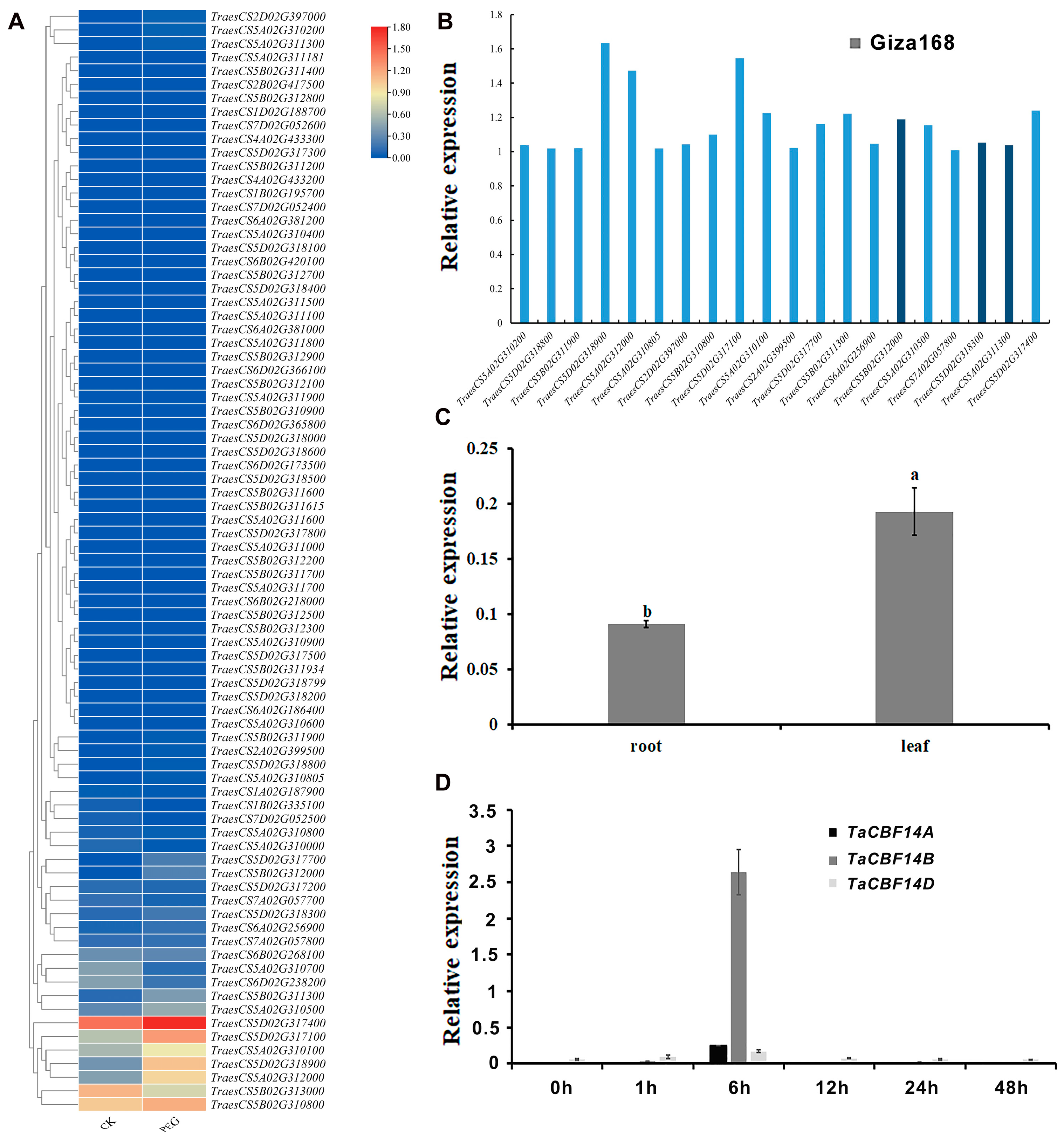
3.3. The TaCBF14B Gene Was Identified as Being Induced by Drought Stress
3.4. Subcellular Localization of the TaCBF14B Protein
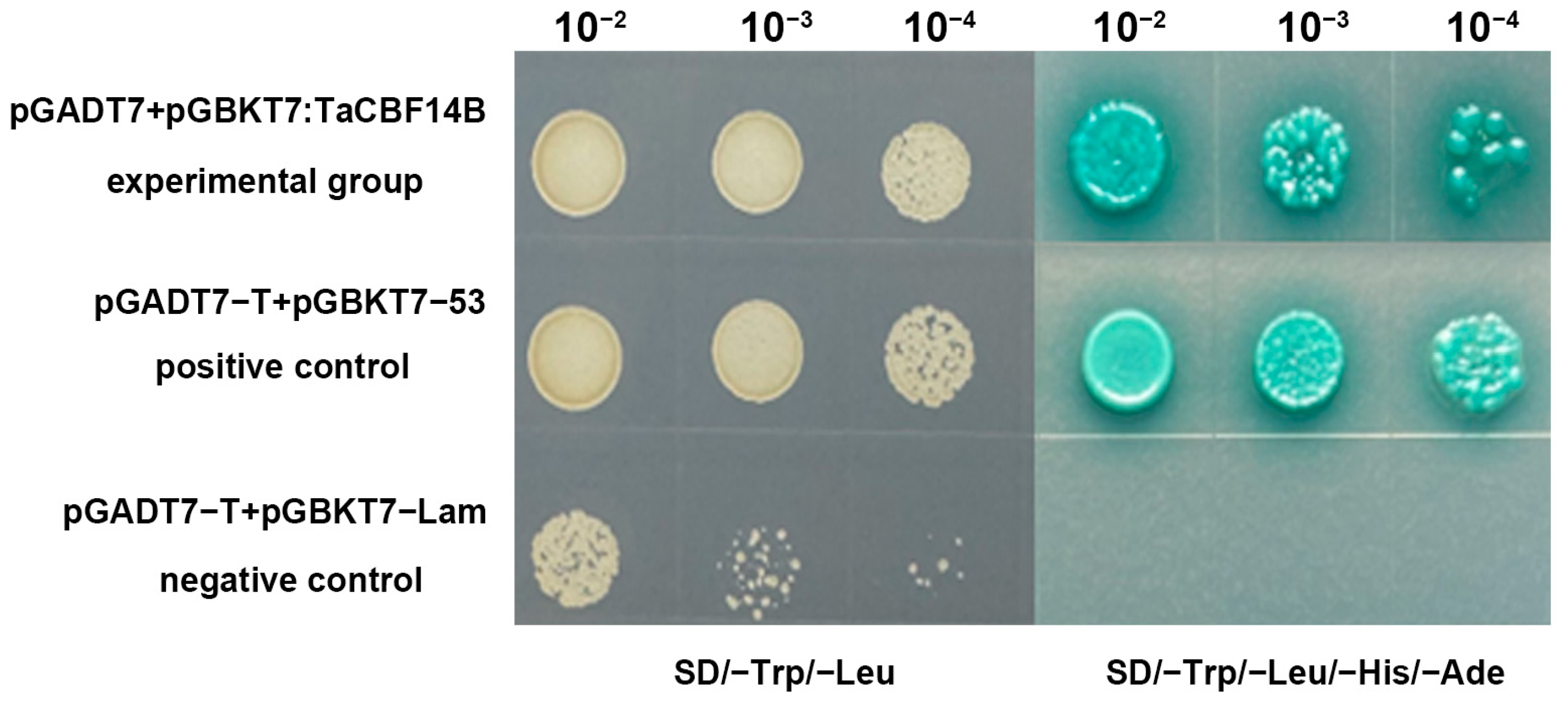
3.5. TaCBF14B Has Transactivation Activity in Yeast
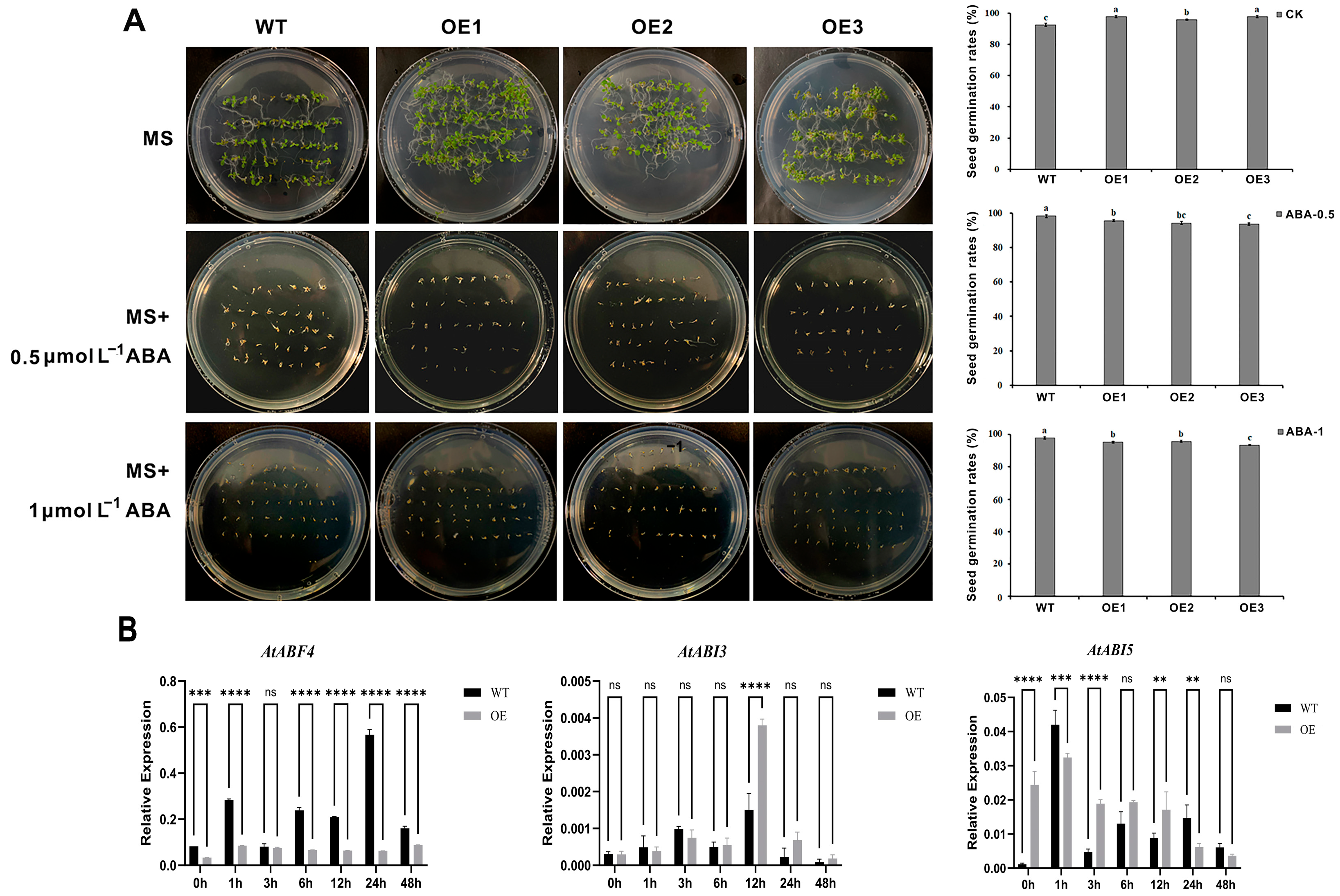
3.6. TaCBF14B Overexpression in Arabidopsis Showed Enhanced Tolerance to Drought Tolerance
3.7. Overexpression of TaCBF14B in Arabidopsis Contributes to ABA Hypersensitivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, W.; Zhang, J.; Zhang, N.; Xin, M.; Peng, H.; Hu, Z.; Ni, Z.; Du, J. The wheat NAC transcription factor TaNAC2L Is regulated at the transcriptional and post-translational levels and promotes heat Stress tolerance in transgenic Arabidopsis. PLoS ONE 2015, 10, e0135667. [Google Scholar] [CrossRef]
- Lu, P.L.; Chen, N.Z.; An, R.; Su, Z.; Qi, B.S.; Ren, F.; Chen, J.; Wang, X.C. A novel drought-inducible gene, ATAF1, encodes a NAC family protein that negatively regulates the expression of stress-responsive genes in Arabidopsis. Plant Mol. Biol. 2007, 63, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chi, M.; Liu, S.; Zhang, Y.; Song, J.; Xia, G.; Liu, S. TaGPAT6 enhances salt tolerance in wheat by synthesizing cutin and suberin monomers to form a diffusion barrier. J. Integr. Plant Biol. 2024, 67, 208–225. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, Z.; Jiang, L.; Chen, L.; Ma, J. Involvement of the ABA-and H2O2-Mediated Ascorbate-Glutathione Cycle in the Drought Stress Responses of Wheat Roots. Phyton-Int. J. Exp. Bot. 2024, 93, 329–342. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Food Agric Data. 2020. Available online: https://www.fao.org/faostat/ (accessed on 19 January 2025).
- Li, J.; Liu, X.; Chang, S.; Chu, W.; Lin, J.; Zhou, H.; Hu, Z.; Zhang, M.; Xin, M.; Yao, Y.; et al. The potassium transporter TaNHX2 interacts with TaGAD1 to promote drought tolerance via modulating stomatal aperture in wheat. Sci. Adv. 2024, 10, eadk4027. [Google Scholar] [CrossRef]
- Yin, H.; Sun, Q.; Lu, X.; Zhang, L.; Yuan, Y.; Gong, C.; He, X.; Ma, W.; Mu, P. Identification of the glutamine synthetase (GS) gene family in four wheat species and functional analysis of Ta4D.GSe in Arabidopsis thaliana. Plant Mol. Biol. 2022, 110, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, M.; Guo, J.; Wang, Y.; Min, D.; Jiang, Q.; Ji, H.; Huang, C.; Wei, W.; Xu, H. Overexpression of soybean DREB1 enhances drought stress tolerance of transgenic wheat in the field. J. Exp. Bot. 2020, 71, 1842–1857. [Google Scholar] [CrossRef]
- Alexandra, S.; Mark, S.; Ildikó, V.; Gábor, G.; Wendy, H.; Attila, V. Transgenic barley lines prove the involvement of TaCBF14 and TaCBF15 in the cold acclimation process and in frost tolerance. J. Exp. Bot. 2013, 64, 1849–1862. [Google Scholar] [CrossRef]
- Jaglo, K.R.; Kleff, S.; Amundsen, K.L.; Zhang, X.; Haake, V.; Zhang, J.Z.; Deits, T.; Thomashow, M.F. Components of the Arabidopsis C-repeat/dehydration-responsive element binding factor cold-response pathway are conserved in Brassica napus and other plant species. Plant Physiol. 2001, 127, 910–917. [Google Scholar] [CrossRef]
- Zhang, X.; Fowler, S.G.; Cheng, H.; Lou, Y.; Rhee, S.Y.; Stockinger, E.J.; Thomashow, M.F. Freezing-sensitive tomato has a functional CBF cold response pathway, but a CBF regulon that differs from that of freezing-tolerant Arabidopsis. Plant J. 2004, 39, 905–919. [Google Scholar] [CrossRef]
- Hu, Z.; Ban, Q.; Hao, J.; Zhu, X.; Cheng, Y.; Mao, J.; Lin, M.; Xia, E.; Li, Y. Genome-wide characterization of the C-repeat binding factor (CBF) gene family involved in the response to abiotic stresses in tea plant (Camellia sinensis). Front. Plant Sci. 2020, 11, 921. [Google Scholar] [CrossRef] [PubMed]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt-and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Siddiqua, M.; Braybrook, S.; Nassuth, A. Three grape CBF/DREB1 genes respond to low temperature, drought and abscisic acid. Plant Cell Environ. 2006, 29, 1410–1421. [Google Scholar] [CrossRef]
- Oh, S.J.; Kwon, C.W.; Choi, D.W.; Song, S.I.; Kim, J.K. Expression of barley HvCBF4 enhances tolerance to abiotic stress in transgenic rice. Plant Biotechnol. J. 2007, 5, 646–656. [Google Scholar] [CrossRef]
- Yan, J.; Liu, Y.; Yan, J.; Liu, Z.; Lou, H.; Wu, J. The salt-activated CBF1/CBF2/CBF3-GALS1 module fine-tunes galactan-induced salt hypersensitivity in Arabidopsis. J. Integr. Plant Biol. 2023, 65, 1904–1917. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Chong, L.; Hsu, C.-C.; Zhu, Y. Advances in mass spectrometry-based phosphoproteomics for elucidating abscisic acid signaling and plant responses to abiotic stress. J. Exp. Bot. 2022, 73, 6547–6557. [Google Scholar] [CrossRef]
- Xie, G.; Xu, R.; Chong, L.; Zhu, Y. Understanding drought stress response mechanisms in tomato. Veg. Res. 2024, 4, e001. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Y.; Yang, Y.; Song, C.; Wang, B.; Yang, S.; Guo, Y.; Gong, Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [CrossRef]
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 2009, 324, 1064–1068. [Google Scholar] [CrossRef]
- Park, S.Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.F.; et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Kizis, D.; Pagès, M. Maize DRE-binding proteins DBF1 and DBF2 are involved in rab17 regulation through the drought-responsive element in an ABA-dependent pathway. Plant J. 2002, 30, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Fujita, Y.; Sayama, H.; Kidokoro, S.; Maruyama, K.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 2010, 61, 672–685. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Q.; Li, Z.; Zhang, A.; Ling, H.; Tong, Y.; Liu, Z. Research achievement and prospect development on wheat genome. Bull. Chin. Acad. Sci. (Chin. Version) 2018, 33, 909–914. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, M.; Li, F.; Lv, H.; Li, C.; Xia, G. A proteomic study of the response to salinity and drought stress in an introgression strain of bread wheat. Mol. Cell. Proteom. 2009, 8, 2676–2686. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Qi, L.; Liu, Y.; Li, L. Effect of water stress on drought-resistance characteristics of wheat seedling with different fertilizer and water types. J. Agric. 2011, 1, 1–7. [Google Scholar]
- Zhang, T.; Wang, Z.; Yin, Y.; Cai, R.; Yan, S.; Li, W. Starch Content and Granule Size Distribution in Grains of Wheat in Relation to Post-Anthesis Water Deficits. J. Agron. Crop Sci. 2010, 196, 1–8. [Google Scholar] [CrossRef]
- Hao, P.; Zhu, J.; Gu, A.; Lv, D.; Ge, P.; Chen, G.; Li, X.; Yan, Y. An integrative proteome analysis of different seedling organs in tolerant and sensitive wheat cultivars under drought stress and recovery. Proteomics 2015, 15, 1544–1563. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Gomaa, M.A. Combining high tolerance to drought with high tolerance to salinity in Egyptian wheat (Triticum aestivum L.) cultivars. Cereal Res. Commun. 2022, 50, 717–732. [Google Scholar] [CrossRef]
- Guo, G.; Zhang, H.; Dong, W.; Xu, B.; Wang, Y.; Zhao, Q.; Liu, L.; Tang, X.; Liu, L.; Ye, Z.; et al. Overexpression of PbrGA2ox1 enhances pear drought tolerance through the regulation of GA3-inhibited reactive oxygen species detoxification and abscisic acid signaling. J. Integr. Agric. 2024, 23, 2989–3011. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, M.; Hu, J.; Wang, W.; Fu, X.; Liu, J.H. PtrABF of Poncirus trifoliata functions in dehydration tolerance by reducing stomatal density and maintaining reactive oxygen species homeostasis. J. Exp. Bot. 2015, 66, 5911–5927. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef]
- Li, Y.; Han, S.; Sun, X.; Khan, N.U.; Zhong, Q.; Zhang, Z.; Zhang, H.; Ming, F.; Li, Z.; Li, J. Variations in OsSPL10 confer drought tolerance by directly regulating OsNAC2 expression and ROS production in rice. J. Integr. Plant Biol. 2022, 65, 918–933. [Google Scholar] [CrossRef] [PubMed]
- Shaik, R.; Ramakrishna, W. Machine learning approaches distinguish multiple stress conditions using stress-responsive genes and identify candidate genes for broad resistance in rice. Plant Physiol. 2014, 164, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cheng, J.; Wu, J.; Chen, J.; Liu, D.; Wang, C.; Ma, S.; Guo, W.; Li, G.; Di, D.; et al. Variation in TaSPL6-D confers salinity tolerance in bread wheat by activating TaHKT1;5-D while preserving yield-related traits. Nat. Genet. 2024, 56, 1257–1269. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, M.; Zhang, H.; Huang, K.; Chen, M.; Chen, C.; Yang, X.; Li, Z.; Chen, H.; Ma, Z.; et al. Identification and Characterization of EDT1 conferring drought tolerance in Rice. J. Plant Biol. 2019, 62, 39–47. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought-and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, X.; Li, M.; Yang, H.; Fu, D.; Lv, J.; Ding, Y.; Gong, Z.; Shi, Y.; Yang, S. The direct targets of CBFs: In cold stress response and beyond. J. Integr. Plant Biol. 2021, 63, 1874–1887. [Google Scholar] [CrossRef]
- Badawi, M.; Danyluk, J.; Boucho, B.; Houde, M.; Sarhan, F. The CBF gene family in hexaploid wheat and its relationship to the phylogenetic complexity of cereal CBFs. Mol. Genet. Genom. 2007, 277, 533–554. [Google Scholar] [CrossRef]
- Vágújfalvi, A.; Aprile, A.; Miller, A.; Dubcovsky, J.; Delugu, G.; Galiba, G.; Cattivelli, L. The expression of several Cbf genes at the Fr-A2 locus is linked to frost resistance in wheat. Mol. Genet. Genom. 2005, 274, 506–514. [Google Scholar] [CrossRef]
- Knox, A.K.; Li, C.; Vágújfalvi, A.; Galiba, G.; Stockinger, E.J.; Dubcovsky, J. Identification of candidate CBF genes for the frost tolerance locus Fr-A m 2 in Triticum monococcum. Plant Mol. Biol. 2008, 67, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, E.J.; Gilmour, S.J.; Thomashow, M.F. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA 1997, 94, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, L.; Liu, M.; Peng, D.; Wei, A.; Hou, B.; Lei, Y.; Li, X. TaFDL2-1A confers drought stress tolerance by promoting ABA biosynthesis, ABA responses, and ROS scavenging in transgenic wheat. Plant J. 2022, 112, 722–737. [Google Scholar] [CrossRef]
- Liu, G.; Li, B.; Li, X.; Wei, Y.; He, C.; Shi, H. MaWRKY80 positively regulates plant drought stress resistance through modulation of abscisic acid and redox metabolism. Plant Physiol. Biochem. 2020, 156, 155–166. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Wu, Y.; Luo, Q.; Zhang, Y.; Qiu, D.; Han, J.; Su, P.; Xiong, Z.; Chang, J. TaSnRK2. 9, a sucrose non-fermenting 1-related protein kinase gene, positively regulates plant response to drought and salt stress in transgenic tobacco. Front. Plant Sci. 2019, 9, 2003. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, X.; Zhao, S.; Guo, Y. The PYR-PP2C-CKL2 module regulates ABA-mediated actin reorganization during stomatal closure. New Phytol. 2022, 233, 2168–2184. [Google Scholar] [CrossRef]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated role of ABA in seed maturation, dormancy, and germination. J. Adv. Res. 2022, 35, 199–214. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abudukerimu, Z.; Xu, Y.; Chen, S.; Tan, Y.; Li, C.; Niu, N.; Xie, Y.; He, Z.; Liu, X.; Xin, J.; et al. Identification of the CBF Gene Family in Wheat and TaCBF14B Could Enhance the Drought Tolerance of Arabidopsis thaliana. Agronomy 2025, 15, 1265. https://doi.org/10.3390/agronomy15061265
Abudukerimu Z, Xu Y, Chen S, Tan Y, Li C, Niu N, Xie Y, He Z, Liu X, Xin J, et al. Identification of the CBF Gene Family in Wheat and TaCBF14B Could Enhance the Drought Tolerance of Arabidopsis thaliana. Agronomy. 2025; 15(6):1265. https://doi.org/10.3390/agronomy15061265
Chicago/Turabian StyleAbudukerimu, Zubaidai, Yitu Xu, Shengjing Chen, Yuliu Tan, Caihong Li, Nan Niu, Yuxin Xie, Zihan He, Xiangyu Liu, Junwei Xin, and et al. 2025. "Identification of the CBF Gene Family in Wheat and TaCBF14B Could Enhance the Drought Tolerance of Arabidopsis thaliana" Agronomy 15, no. 6: 1265. https://doi.org/10.3390/agronomy15061265
APA StyleAbudukerimu, Z., Xu, Y., Chen, S., Tan, Y., Li, C., Niu, N., Xie, Y., He, Z., Liu, X., Xin, J., Yu, J., Li, J., Li, X., Wang, H., Wang, M., Golub, N., Zhang, Y., & Guo, W. (2025). Identification of the CBF Gene Family in Wheat and TaCBF14B Could Enhance the Drought Tolerance of Arabidopsis thaliana. Agronomy, 15(6), 1265. https://doi.org/10.3390/agronomy15061265





