1. Introduction
According to the 2023 Intergovernmental Panel on Climate Change (IPCC) report on climate change, global temperatures are expected to reach or exceed 1.5 °C of warming by 2030, projecting increasing heatwaves, longer warm seasons, and shorter cold seasons [
1]. Furthermore, South Korea continuously experienced increasing temperatures, demonstrated by a long-term warming trend with more frequent hot events [
2]. The rising temperatures pose a significant threat to crop production in South Korea’s agricultural market. According to the 2023 crop production survey in South Korea [
3], high temperatures increased damage levels to Chinese cabbage (
Brassica rapa) from insects between September and October. Higher temperatures can accelerate the physiology and metabolism of insects, which may lead to increased population growth rates and greater crop damage [
4]. The beet armyworm (
Spodoptera exigua) is one of the most serious pests impacting Chinese cabbage in South Korea. In response to a changing climate, maintaining the yield stability of Chinese cabbage has become a critical consideration for farmers and stakeholders to limit exposure to production risks and ensure supply stability.
Agricultural modeling systems play a crucial role in ensuring yield stability amid highly variable and unpredictable environmental conditions. The modeling system can effectively assist both farmers’ decisions in crop management (e.g., pesticide application and harvest timing) and stakeholders’ decisions in agricultural planning processes (e.g., crop price determination). Chinese cabbage, a primary vegetable produced in South Korea, serves mainly as the key ingredient in Kimchi, the traditional fermented vegetable dish. Despite its significance, the area harvested for Chinese cabbage in South Korea has been progressively declining over the past decade, which increased the likelihood of price fluctuations due to changes in production [
5]. Developing a Chinese cabbage modeling system is essential to mitigate production risks primarily caused by yield instability in unexpected climatic conditions. Optimizing crop management will not only stabilize yields, but also minimize price risks [
6].
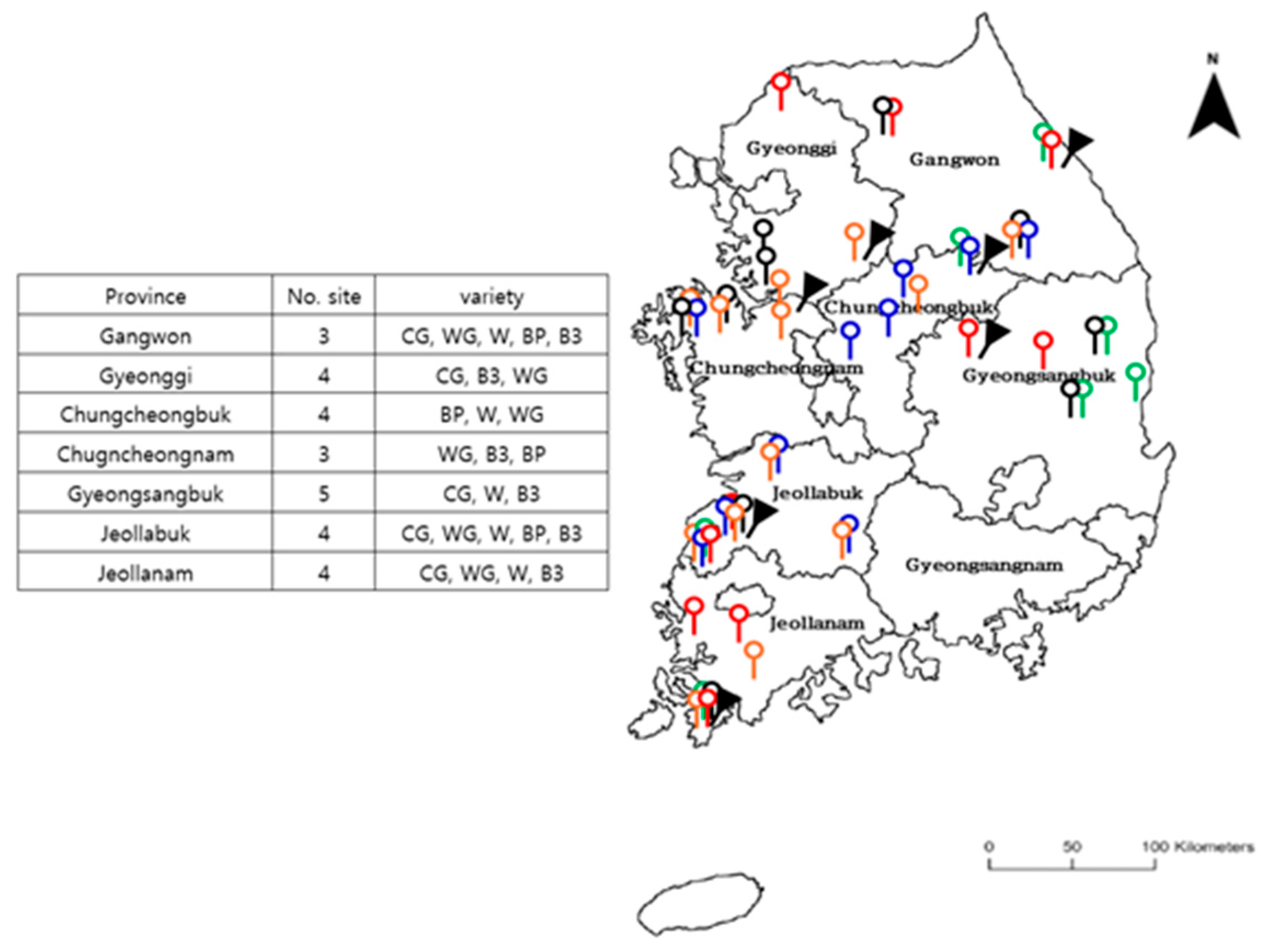
A heading type of Chinese cabbage, commonly used for making kimchi, is cultivated year-round with varieties bred for summer, fall, winter, and spring weather conditions [
7]. Since Chinese cabbage requires only 50–85 days to mature, it can be grown throughout the year in open field conditions [
8]. The fall production of Chinese cabbage is critical for price stability in the Korean agricultural market, especially given the high demand in November [
9]. There are over 100 varieties of Chinese cabbage grown in the fall season, each exhibiting distinct physiological characteristics and varying tolerance levels to biotic (insects, diseases, etc.) and abiotic (drought, cold, among others) stresses. Since there is insufficient field data for modeling each variety of Chinese cabbage, it is crucial to group varieties based on similar morphological characteristics. By grouping varieties with similar characteristics, a more universally applicable model that is not only simpler to use and manage, but also facilitates the accumulation of data, is created.
In this study, a hybrid modeling system will be developed to simulate the interaction between Chinese cabbage crop production and insect pest management under various climate change scenarios. Unlike major grain crops such as corn and soybean, the modeling of vegetable growth, specifically Chinese cabbage, is less developed. Most modeling studies evaluated the impacts of climate change on main crops such as corn [
10], sorghum [
11], and soybean [
12]. Few studies attempted to simulate Chinese cabbage production in Korea [
9,
13]. Previous studies focused either on cabbage production at a specific location [
13] or on the economic yields (revenue) of various varieties at different locations [
9] in Korea. There is still a lack of understanding of the interactions among environmental conditions (e.g., soil, temperature), biotic stress factors, and crop yields. To evaluate climate risks impacting crops at a national level, a comprehensive approach is needed, incorporating climate modeling and agricultural data that cover entire regions [
12].
This study aims to develop a multi-modeling system to understand the impacts of climate change on Chinese cabbage production and insect-related damages in diverse environmental conditions. In this study, the model is developed using Chinese cabbage yield data collected from multiple locations across South Korea, and the developed model will be used to evaluate the overall impact on crop yields. The simulation results of this study will provide farmers with valuable guidelines for selecting the appropriate varieties, managing cropping practices, and preventing insect damages under varying environmental conditions.
4. Discussion
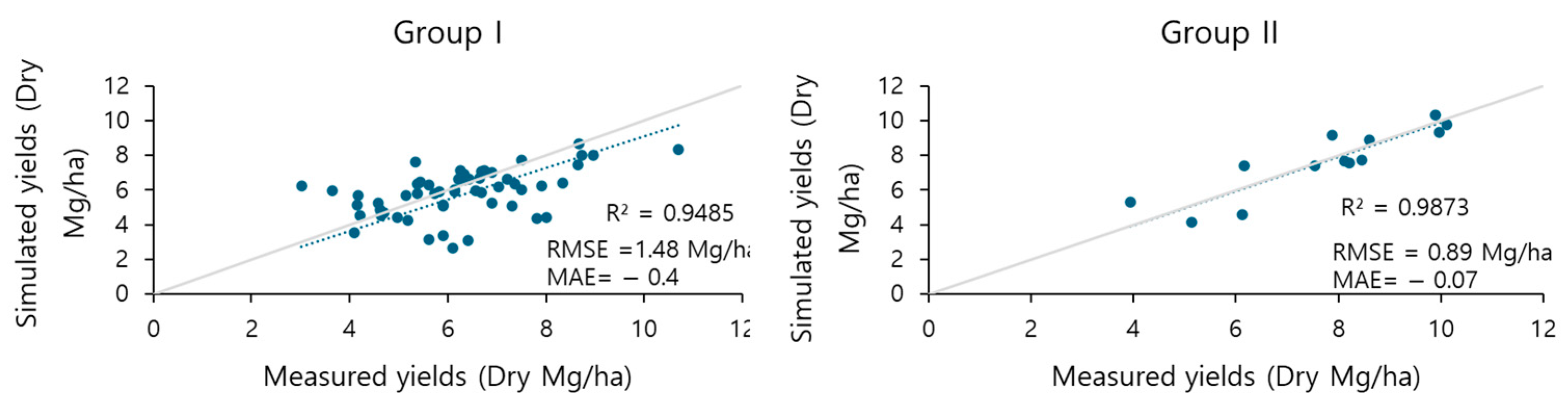
In this study, multiple modeling systems contributing to a crop growth model and an insect development model were developed. Chinese cabbage, an important economic horticulture crop in Korea, has several commercial varieties. In this study, the five most common varieties were used. Two distinct cabbage groups, Group I and Group II, were identified based on morphological characteristics collected from 4-year field studies. The “Whistle” variety was exclusive to Group II. This variety exhibited a higher number of leaves and greater height, resulting in a larger yield than the Group I varieties. Based on the clustering analysis, two cabbage parameter sets were established. A total of 67 yield data points were used for the calibration and validation of the crop growth model, which was successfully calibrated with R
2 = 0.94 for Group I and R
2 = 0.98 for Group II [
29]. Following the development of the crop growth model, an insect model was formulated to predict insect population dynamics and evaluate potential impacts on crop yield.
Larval and egg duration prediction models were developed based on five prior studies that examined the effects of temperature changes on larval development in the beet armyworm (
Spodoptera exigua). The beet armyworm is a significant pest that consumes leaves during its larval (caterpillar) stage. According to Kim et al. [
24], approximately 80 larvae can damage about 63.2% of the foliage in a group of 20 Chinese cabbage leaves. Furthermore, Chowdary et al. [
30] reported a doubling in the population of beet armyworm on host plants within 3–7 days. The larval duration model developed in this study aims to prevent pest outbreaks during the cabbage growing season and to mitigate economic losses in cabbage production. According to the larval and egg duration models, the periods of egg and larval duration decrease as temperatures increase, corroborating similar findings in other insect species reported by Lindroth et al. [
31] and Williams et al. [
32]. Elevated temperatures can directly influence the physiology and metabolism of host plants, which in turn indirectly impacts insect herbivores [
33]. In environments with higher temperatures, the chemical composition of plant leaves changes unfavorably for foraging larvae [
34,
35]. Throughout this process, the concentration of phenolic compounds increases while that of nitrogen decreases. Protein deficiency influenced larval feeding behavior; larvae consumed food faster and in greater quantities, enabling them to mature more rapidly [
36]. This observation corroborated the simulation findings that higher temperatures reduce larval lifespan.
Higher temperatures could also affect the survival rates of larvae. Increased temperatures resulted in significantly reduced survival rates by interrupting larval development [
33,
37]. However, heat tolerance levels vary by species. The beet armyworm exhibits high tolerance to elevated temperatures [
38]. More than 95% of larvae survived at temperatures of 30 °C on maize and 27 °C on soybean. In scenarios of climate change, temperatures exceeded those of the reference period. The average temperature in August was 3 °C higher than during the reference period, and the duration of larval development was shorter, leading to increased insect occurrences during this month. Given the higher survival rates of the beet armyworm at elevated temperatures, the insect population is likely to increase under future climate conditions, resulting in greater plant damage. Similar results were observed by Tanyi et al. [
39], who studied the effects of climate variability on insect pests of cabbage. They tested the rates of cabbage infestation on different planting dates. The study reported that cabbage infestation was highly corrected with climate dynamics, including rainfall and temperature. In yield simulations, cabbage yields under both SSP245 and SSP585 scenarios gradually decreased with the increase in temperature stress days. Additionally, the SSP585 climate scenarios led to a more rapid temperature increase than SSP245, which resulted in greater yield losses between 2031 and 2080. If carbon dioxide (CO
2) levels and temperatures continue to rise, simulation results indicate that farmers will face significant yield losses in Chinese cabbage, potentially leading to insufficient supply and increased prices.
This study utilized a hybrid modeling approach, incorporating a crop growth model with an insect model, to simulate the climate change impacts of climate change on Chinese cabbage yields and damages from insects. However, the model could not predict direct plant damage from insects due to insufficient experimental data. Future research should include a field study to explore the effects of varying temperatures on the development rates of the beet armyworm on Chinese cabbage, which would enhance model accuracy. In addition, the insect model can be improved by using the biological data of additional other insect species. Additionally, this model can predict the distribution of other species based on the environmental condition and evaluate yield damage more effectively.