Impacts of Shrub Encroachment on Vegetation Community and Soil Characteristics in Coastal Wetlands of the Abandoned Yellow River Course
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Design and Vegetation Survey
2.3. Sample Collection and Processing
2.4. Data Processing
3. Results
3.1. Vegetation Community Characteristics Across Shrub Encroachment Stages
3.2. Analysis of Soil Physicochemical Properties Across Shrub Encroachment Stages
3.3. Soil Nutrient Contents and Stoichiometric Characteristics
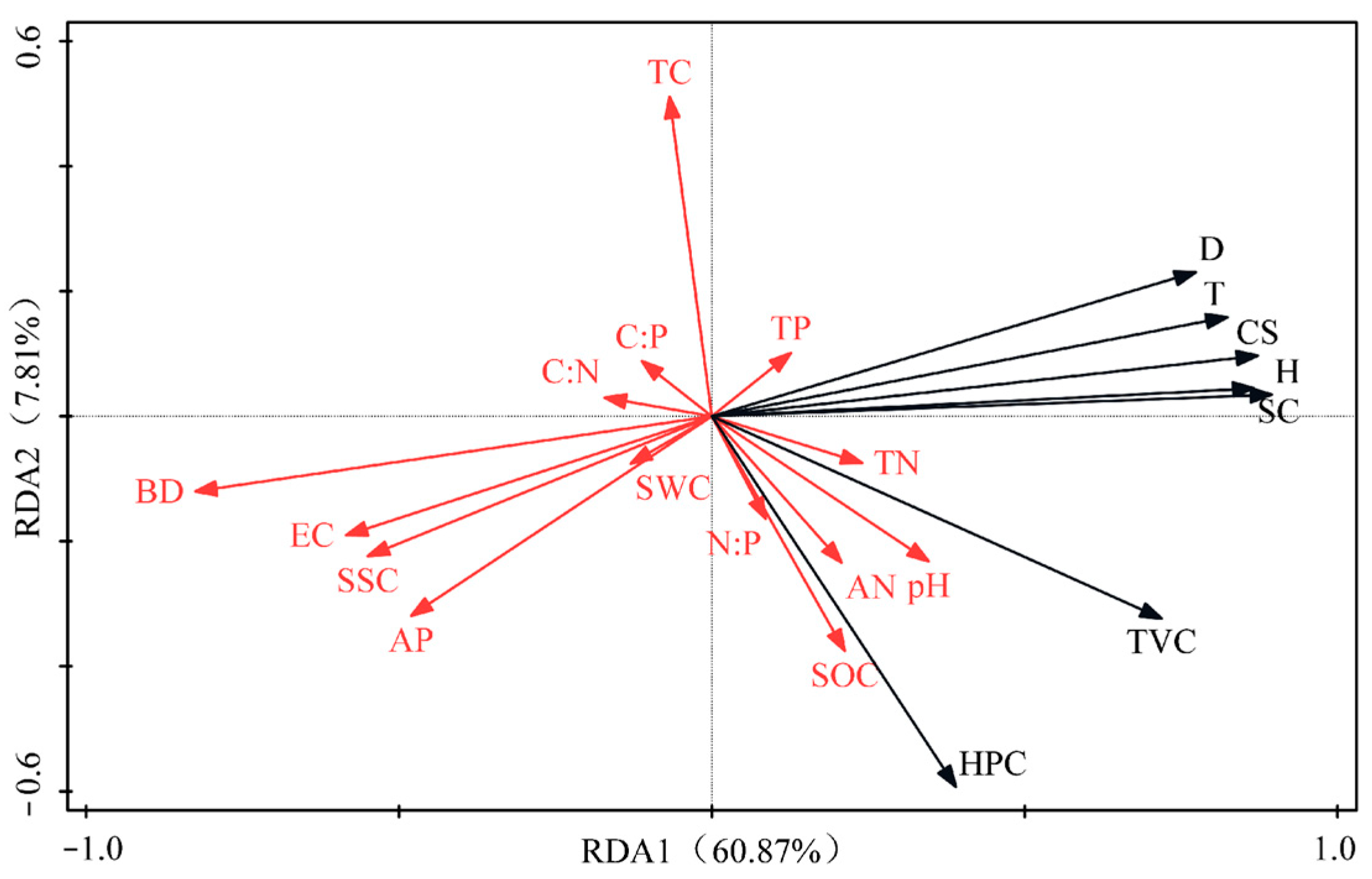
3.4. Correlation Analysis Between Vegetation Characteristics and Soil Properties
4. Discussion
4.1. Impacts of Shrub Encroachment on Vegetation Community Characteristics
4.2. Alterations in Soil Physicochemical Properties
4.3. Stoichiometric Shifts in Soil C-N-P Cycling
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Parameters | Explains % | Contribution % | Pseudo-F | p |
|---|---|---|---|---|
| BD | 41.70 | 58.40 | 75.70 | 0.002 |
| SWC | 8.40 | 11.70 | 17.60 | 0.002 |
| TC | 5.60 | 7.90 | 13.20 | 0.002 |
| AP | 4.20 | 5.90 | 10.70 | 0.002 |
| SOC | 3.30 | 4.60 | 9.10 | 0.002 |
| TN | 2.80 | 4.00 | 8.40 | 0.004 |
| SSC | 1.80 | 2.50 | 5.60 | – |
| pH | 1.60 | 2.20 | 5.10 | 0.010 |
| RCN | 0.60 | 0.90 | 2.00 | 0.130 |
| AN | 0.60 | 0.80 | 1.80 | 0.180 |
| RNP | 0.50 | 0.60 | 1.50 | 0.218 |
| TP | 0.10 | 0.20 | 0.40 | 0.718 |
| RCP | 0.20 | 0.30 | 0.70 | 0.518 |
| EC | <0.1 | <0.1 | <0.1 | 0.984 |
References
- Ratajczak, Z.; D’Odorico, P.; Nippert, J.B.; Collins, S.L.; Brunsell, N.A.; Ravi, S. Changes in spatial variance during a grassland to shrubland state transition. J. Ecol. 2017, 105, 750–760. [Google Scholar] [CrossRef]
- Brantley, S.T.; Young, D.R. Shrub expansion stimulates soil C and N storage along a coastal soil chronosequence. Glob. Change Biol. 2010, 16, 2052–2061. [Google Scholar] [CrossRef]
- Zhang, J.M.; Zhu, N.; Cai, Y.R.; Wang, C.; Liu, J.H.; Li, L.; Luo, Y.H.; Xu, D.W.; Yan, Y.C. Effects of Caragana microphylla on herbaceous community characteristic. Acta Ecol. Sin. 2023, 43, 8830–8839. [Google Scholar]
- D’Odorico, P.; Okin, G.S.; Bestelmeyer, B.T. A synthetic review of feedbacks and drivers of shrub encroachment in arid grasslands. Ecohydrology 2012, 5, 520–530. [Google Scholar] [CrossRef]
- Archer, S.R.; Andersen, E.M.; Predick, K.I.; Schwinning, S.; Woods, S.R. Woody Plant Encroachment: Causes and Consequences. In Rangeland Systems; Springer: Cham, Switzerland, 2017; pp. 25–84. [Google Scholar]
- Ding, J.Y.; Eldridge, D. The success of woody plant removal depends on encroachment stage and plant traits. Nat. Plants 2023, 9, 58–67. [Google Scholar] [CrossRef]
- Zhai, L.; Zhang, B.; Sen Roy, S.; Fuller, D.O.; Sternberg, L.D.L. Remote sensing of unhelpful resilience to sea level rise caused by mangrove expansion: A case study of islands in Florida Bay, USA. Ecol. Indic. 2019, 97, 51–58. [Google Scholar] [CrossRef]
- Saintilan, N.; Rogers, K. Woody plant encroachment of grasslands: A comparison of terrestrial and wetland settings. New Phytol. 2015, 205, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.L. Research process on the mechanism and ecological effects of shrub encroachment in coastal wetland ecosystems. Chin. Coast. Sci. 2019, 6, 58–64. [Google Scholar]
- Arkema, K.K.; Guannel, G.; Verutes, G.; Wood, S.A.; Guerry, A.; Ruckelshaus, M.; Kareiva, P.; Lacayo, M.; Silver, J.M. Coastal habitats shield people and property from sea-level rise and storms. Nat. Clim. Change 2013, 3, 913–918. [Google Scholar] [CrossRef]
- Zinnert, J.C.; Stallins, J.A.; Brantley, S.T.; Young, D.R. Crossing Scales: The Complexity of Barrier-Island Processes for Predicting Future Change. Bioscience 2017, 67, 39–52. [Google Scholar] [CrossRef]
- McLeod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Kwon, M.J.; Ballantyne, A.; Ciais, P.; Qiu, C.J.; Salmon, E.; Raoult, N.; Guenet, B.; Göckede, M.; Euskirchen, E.S.; Nykänen, H.; et al. Lowering water table reduces carbon sink strength and carbon stocks in northern peatlands. Glob. Change Biol. 2022, 28, 6752–6770. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.W.; Wu, J.J.; Bi, X.L. Spatial characteristics of soil δ13C and δ15N reveal shrub-induced successional process in a coastal wetland. Estuar. Coast. Shelf Sci. 2020, 236, 106621. [Google Scholar] [CrossRef]
- Balwan, W.K.; Kour, S. Wetland- An Ecological Boon for the Environment. East Afr. Sch. J. Agric. Life Sci. 2021, 4, 38–43. [Google Scholar] [CrossRef]
- Yang, L.S.; Feng, Q.; Yin, Z.L.; Wen, X.H.; Si, J.H.; Li, C.B.; Deo, R.C. Identifying separate impacts of climate and land use/cover change on hydrological processes in upper stream of Heihe River, Northwest China. Hydrol. Process. 2017, 31, 1100–1112. [Google Scholar] [CrossRef]
- Han, J.Q.; Wu, N.; Wu, Y.R.; Zhou, S.W.; Bi, X.L. Spatial effects of shrub encroachment on wetland soil pH and salinity in the Yellow River Delta, China. J. Coast. Conserv. 2024, 28, 60. [Google Scholar] [CrossRef]
- Gao, X.L.; Li, X.G.; Zhao, L.; Kuzyakov, Y. Shrubs magnify soil phosphorus depletion in Tibetan meadows: Conclusions from C:N:P stoichiometry and deep soil profiles. Sci. Total Environ. 2021, 785, 147320. [Google Scholar] [CrossRef]
- Naorem, A.; Jayaraman, S.; Dalal, R.C.; Patra, A.; Rao, C.S.; Lal, R. Soil Inorganic Carbon as a Potential Sink in Carbon Storage in Dryland Soils-A Review. Agriculture 2022, 12, 1256. [Google Scholar] [CrossRef]
- Wang, X.T.; Jiang, Z.X.; Li, Y.; Kong, F.L.; Xi, M. Inorganic carbon sequestration and its mechanism of coastal saline-alkali wetlands in Jiaozhou Bay, China. Geoderma 2019, 351, 221–234. [Google Scholar] [CrossRef]
- Shao, P.S.; Li, T.; Dong, K.K.; Yang, H.J.; Sun, J.K. Microbial residues as the nexus transforming inorganic carbon to organic carbon in coastal saline soils. Soil Ecol. Lett. 2022, 4, 328–336. [Google Scholar] [CrossRef]
- Soliveres, S.; Maestre, F.T.; Eldridge, D.J.; Delgado-Baquerizo, M.; Quero, J.L.; Bowker, M.A.; Gallardo, A. Plant diversity and ecosystem multifunctionality peak at intermediate levels of woody cover in global drylands. Glob. Ecol. Biogeogr. 2014, 23, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Wang, Y.B.; Xiang, G.H.; Chi, Y.G.; Lu, S.B.; Zheng, S.X. Effects of Caragana microphylla encroachment on community structure and ecosystem function of a typical steppe. Chin. J. Plant Ecol. 2020, 44, 33–43. [Google Scholar] [CrossRef]
- Ding, J.Y.; Yin, C.C.; Han, Y.; Zhao, W.W. Research progress and perspectives on the impact of shrub encroachment on ecosystem multifunctionality. Acta Ecol. Sin. 2023, 43, 8257–8267. [Google Scholar]
- Zinnert, J.C.; Via, S.M.; Nettleton, B.P.; Tuley, P.A.; Moore, L.J.; Stallins, J.A. Connectivity in coastal systems: Barrier island vegetation influences upland migration in a changing climate. Glob. Change Biol. 2019, 25, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Zhou, L.H.; Li, H.; Zhao, X.; Yang, Y.H.; Zhu, Y.K.; Hu, H.F.; Chen, L.Y.; Zhang, P.J.; Shen, H.H.; et al. Shrub encroachment decreases soil inorganic carbon stocks in Mongolian grasslands. J. Ecol. 2020, 108, 678–686. [Google Scholar] [CrossRef]
- Zhao, Q.Q.; Bai, J.H.; Liu, Q.; Lu, Q.Q.; Gao, Z.Q.; Wang, J.J. Spatial and Seasonal Variations of Soil Carbon and Nitrogen Content and Stock in a Tidal Salt Marsh with Tamarix chinensis China. Wetlands 2016, 36, S145–S152. [Google Scholar] [CrossRef]
- Qin, D.H.; Gao, M.; Wu, X.Q.; Du, X.Y.; Bi, X.L. Seasonal changes in soil TN and SOC in a seawall-reclaimed marsh in the Yellow River Delta, China. J. Coast. Conserv. 2015, 19, 79–84. [Google Scholar] [CrossRef]
- Shi, L.; Liu, Q.S.; Huang, C.; Gao, X.; Li, H.; Liu, G.H. Mapping quasi-circular vegetation patch dynamics in the Yellow River Delta, China, between 1994 and 2016. Ecol. Indic. 2021, 126, 107656. [Google Scholar] [CrossRef]
- Wei, N.; Zhao, L.P.; Tan, S.T.; Zhao, F.R. Research progress on shrub encroachment in grasslands. Ecol. Sci. 2019, 38, 208–216. [Google Scholar]
- Liu, M.F.; Wang, D.; Tian, X.P.; Wu, Y.R.; Bi, X.L. Multifractal parameters reveal the impacts of shrub encroachment on soil particle size distribution (PSD) in a coastal wetland of the Yellow River Delta. J. Coast. Conserv. 2024, 28, 63. [Google Scholar] [CrossRef]
- Li, D.X.; Li, Y.N.; Xie, Y.L.; Cui, B.S.; Ning, Z.H.; Zhang, S.Y.; Bi, Z.G.; Fu, S.Q.; Che, C.G. Effects of ecological restoration on soil biogenic elements and their ecological stoichiometry in the Yellow River Delta, China. Front. Mar. Sci. 2022, 9, 993202. [Google Scholar] [CrossRef]
- Sun, J.; Xia, J.B.; Dong, B.T.; Gao, F.L.; Chen, P.; Zhao, W.L.; Li, C.R. Root morphology and growth characteristics of Tamarix chinensis with different densities on the beach of the Yellow River Delta. Acta Ecol. Sin. 2021, 41, 3775–3783. [Google Scholar]
- Naito, A.T.; Cairns, D.M. Patterns and processes of global shrub expansion. Prog. Phys. Geogr. 2011, 35, 423–442. [Google Scholar] [CrossRef]
- Yang, W.; Qu, G.P.; Kelly, A.R.; Wu, G.L.; Zhao, J.X. Positive effects of leguminous shrub encroachment on multiple ecosystem functions of alpine meadows and steppes greatly depended on increasing soil nutrient. Catena 2024, 236, 107745. [Google Scholar] [CrossRef]
- Yip, K.; Liu, R.; Wu, J.; Hau, B.C.H.; Lin, Y.Y.; Zhang, H.S. Community-based plant diversity monitoring of a dense-canopy and species-rich tropical forest using airborne LiDAR data. Ecol. Indic. 2024, 158, 111346. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; Agricultural Press: Beijing, China, 2005; pp. 20–144.
- Wood, L.K.; Hays, S.; Zinnert, J.C. Decreased temperature variance associated with biotic composition enhances coastal shrub encroachment. Sci. Rep. 2020, 10, 8210. [Google Scholar] [CrossRef]
- Woods, N.N.; Zinnert, J.C. Shrub encroachment of coastal ecosystems depends on dune elevation. Plant Ecol. 2024, 225, 1047–1057. [Google Scholar] [CrossRef]
- Devaney, J.L.; Lehmann, M.; Feller, I.C.; Parker, J.D. Mangrove microclimates alter seedling dynamics at the range edge. Ecology 2017, 98, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Hu, J.; Zhou, Q.P.; Cao, Q.H.; Sun, M.L.; Chen, X.L.; Yang, L.X. Effects of typical shrub-encroached grassland on vegetation characteristics and soil nutrients in the Zoige Plateau. Acta Agrestia Sin. 2022, 30, 901–908. [Google Scholar]
- An, Q.Q.; Qiao, W.Y.; Li, W.J.; Chang, X.F. Effect of shrub encroachment on grassland community structure and above-ground biomass on the Loess Plateau. Acta Bot. Boreali-Occident. Sin. 2021, 41, 664–671. [Google Scholar]
- Mahaut, L.; Choler, P.; Denelle, P.; Garnier, E.; Thuiller, W.; Kattge, J.; Lemauviel-Lavenant, S.; Lavorel, S.; Munoz, F.; Renard, D. Trade-offs and synergies between ecosystem productivity and stability in temperate grasslands. Glob. Ecol. Biogeogr. 2023, 32, 561–572. [Google Scholar] [CrossRef]
- Zhang, J.H.; Huang, Y.M. Biodiversity and stability mechanisms: Understanding and future research. Acta Ecol. Sin. 2016, 36, 3859–3870. [Google Scholar]
- Zhu, J.T.; Yu, J.J.; Wang, P.; Zhang, Y.C.; Yu, Q. Interpreting the groundwater attributes influencing the distribution patterns of groundwater-dependent vegetation in northwestern China. Ecohydrology 2012, 5, 628–636. [Google Scholar] [CrossRef]
- Bao, D.H.; Sun, X.M.; Zhang, Z.M.; Wang, W.B.; Yang, T.; Wang, Y.; Wang, J.; Su, J.H. Effects of shrub encroachment of alpine meadows on vegetation community structure and soil physicochemical properties. Acta Ecol. Sin. 2024, 44, 6208–6218. [Google Scholar]
- Wen, Y.Y.; Zhu, J.; Wang, H.; Zhang, M.D.; Lu, S.B.; Zheng, S.X. Population characteristics of Caragana microphylla and the influencing soil factors in shrub-encroached grassland of Inner Mongolia, China. Chin. J. Appl. Ecol. 2024, 35, 1525–1533. [Google Scholar]
- Eldridge, D.J.; Bowker, M.A.; Maestre, F.T.; Roger, E.; Reynolds, J.F.; Whitford, W.G. Impacts of shrub encroachment on ecosystem structure and functioning: Towards a global synthesis. Ecol. Lett. 2011, 14, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Yin, L.K.; Pan, B.R. A review on the study of salt glands of Tamarix. Acta Bot. Boreali-Occident. Sin. 2003, 23, 190–194. [Google Scholar]
- Pausch, J.; Kuzyakov, Y. Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob. Change Biol. 2018, 24, 1–12. [Google Scholar] [CrossRef]
- Du, Z.; Zheng, H.; Penuelas, J.; Sardans, J.; Deng, D.Z.; Cai, X.H.; Gao, D.C.; Nie, S.R.; He, Y.M.; Lü, X.T.; et al. Shrub encroachment leads to accumulation of C, N, and P in grassland soils and alters C:N:P stoichiometry: A meta-analysis. Sci. Total Environ. 2024, 951, 175534. [Google Scholar] [CrossRef]
- Song, X.J.; Li, S.N.; Wei, W.; Guo, J.; Yu, Y.L.; Liu, Z.W. Distribution Characteristics of Root System of Tamarix chinensis in Yellow River Delta and Its Influence Factors. Wetland Sci. 2017, 15, 716–723. [Google Scholar]
- Kautz, T.; Amelung, W.; Ewert, F.; Gaiser, T.; Horn, R.; Jahn, R.; Javaux, M.; Kemna, A.; Kuzyakov, Y.; Munch, J.C.; et al. Nutrient acquisition from arable subsoils in temperate climates: A review. Soil Biol. Biochem. 2013, 57, 1003–1022. [Google Scholar] [CrossRef]
- Blaser, W.J.; Shanungu, G.K.; Edwards, P.J.; Venterink, H.O. Woody encroachment reduces nutrient limitation and promotes soil carbon sequestration. Ecol. Evol. 2014, 4, 1423–1438. [Google Scholar] [CrossRef] [PubMed]
- Rowley, M.C.; Grand, S.; Verrecchia, É. Calcium-mediated stabilisation of soil organic carbon. Biogeochemistry 2018, 137, 27–49. [Google Scholar] [CrossRef]
- Qu, F.Z.; Meng, L.; Fu, Z.Y.; Sun, J.K.; Liu, J.T.; Song, A.Y. Influences of anthropogenic cultivation on C, N and P stoichiometry of reed-dominated coastal wetlands in the Yellow River Delta. Acta Ecol. Sin. 2018, 38, 1731–1738. [Google Scholar] [CrossRef]
- Cai, W.T.; Lai, L.M.; Li, H.Y.; Zhou, J.H.; Guan, T.Y.; Zhang, X.L.; Gao, N.N.; Zheng, Y.R. Progress of research on shrub encroachment in grassland. Chin. J. Appl. Environ. Biol. 2016, 22, 531–537. [Google Scholar]
- Meng, L.; Qu, F.Z.; Bi, X.L.; Xia, J.B.; Li, Y.Z.; Wang, X.H.; Yu, J.B. Elemental stoichiometry (C, N, P) of soil in the Yellow River Delta nature reserve: Understanding N and P status of soil in the coastal estuary. Sci. Total Environ. 2021, 751, 141737. [Google Scholar] [CrossRef]
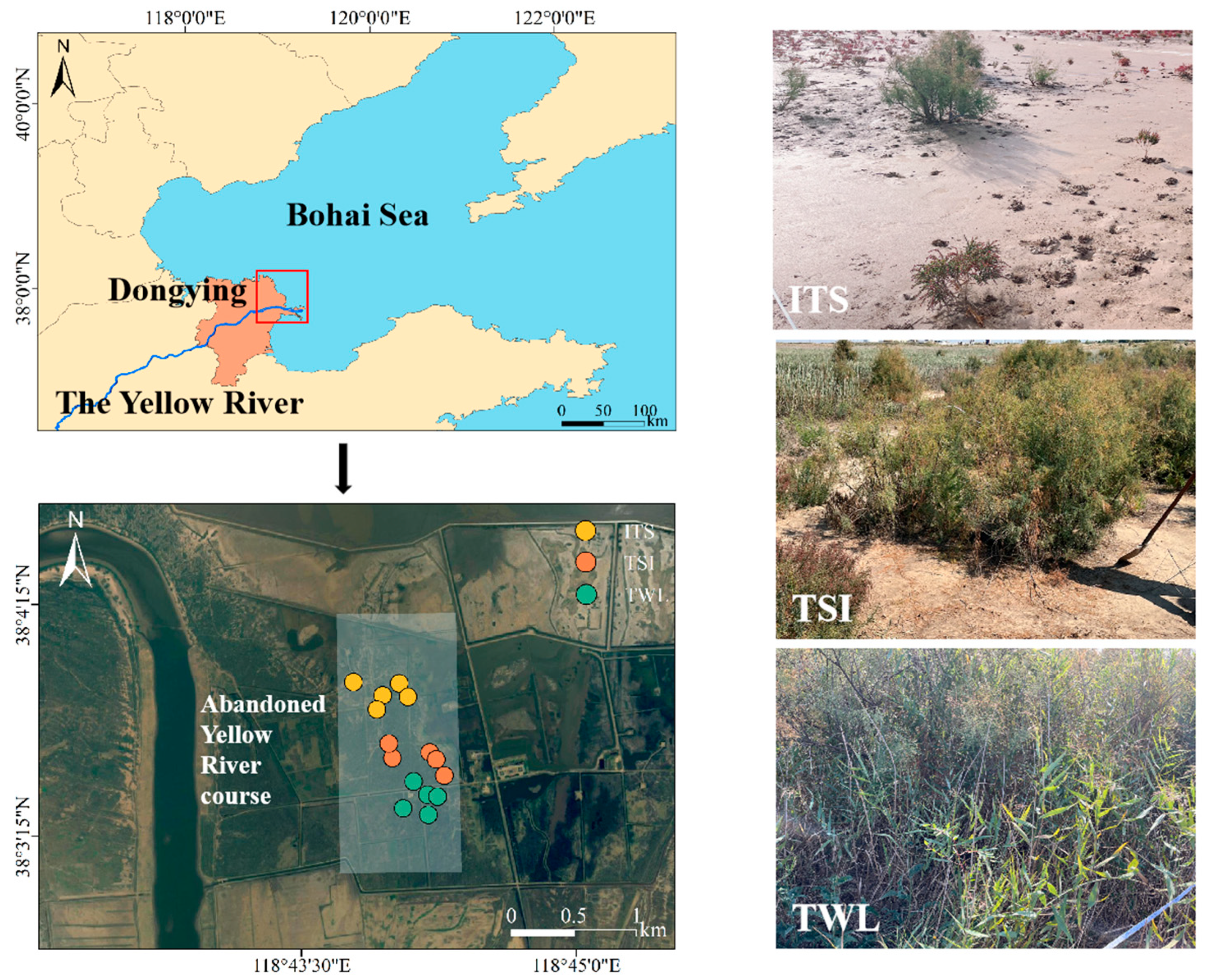
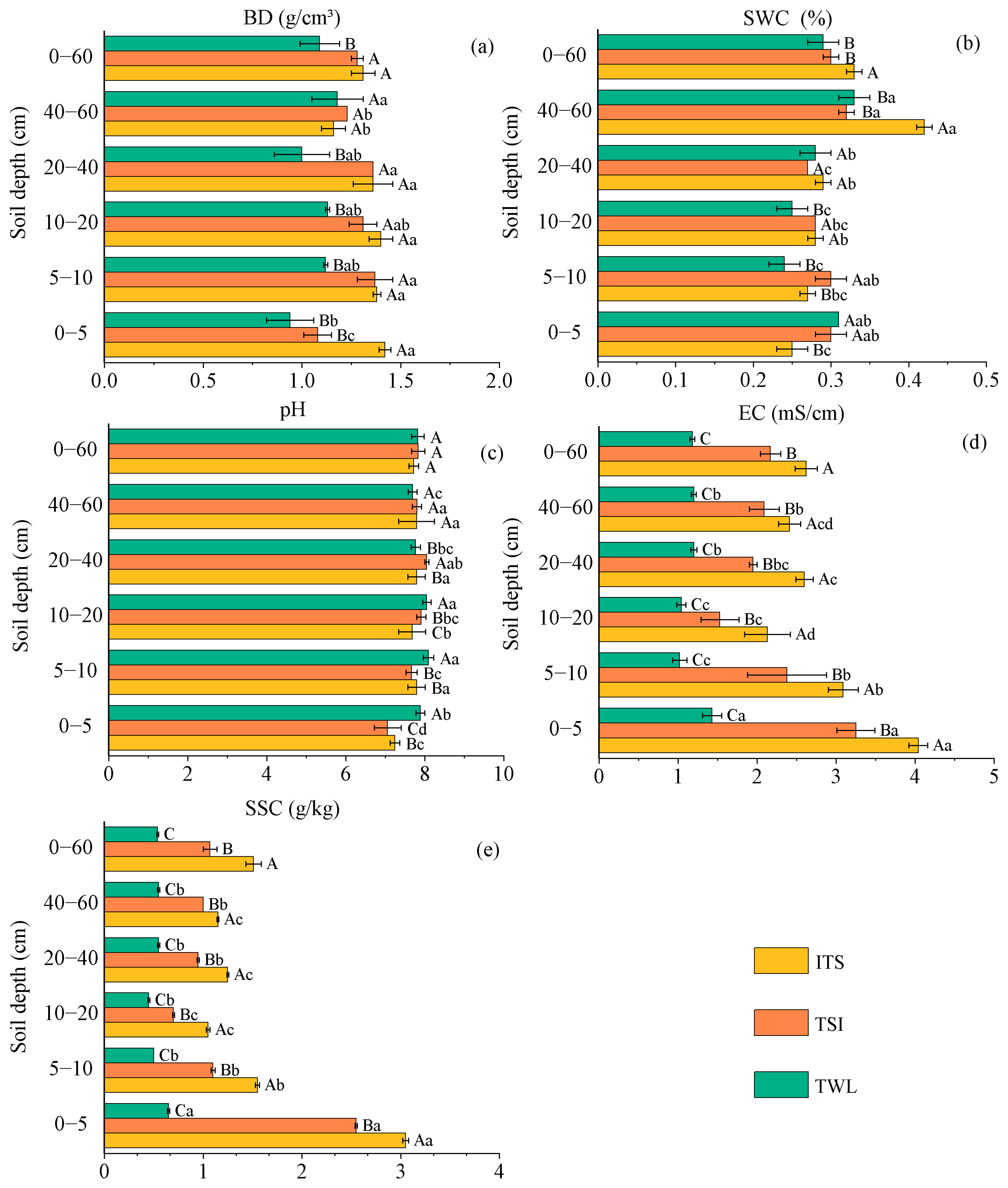

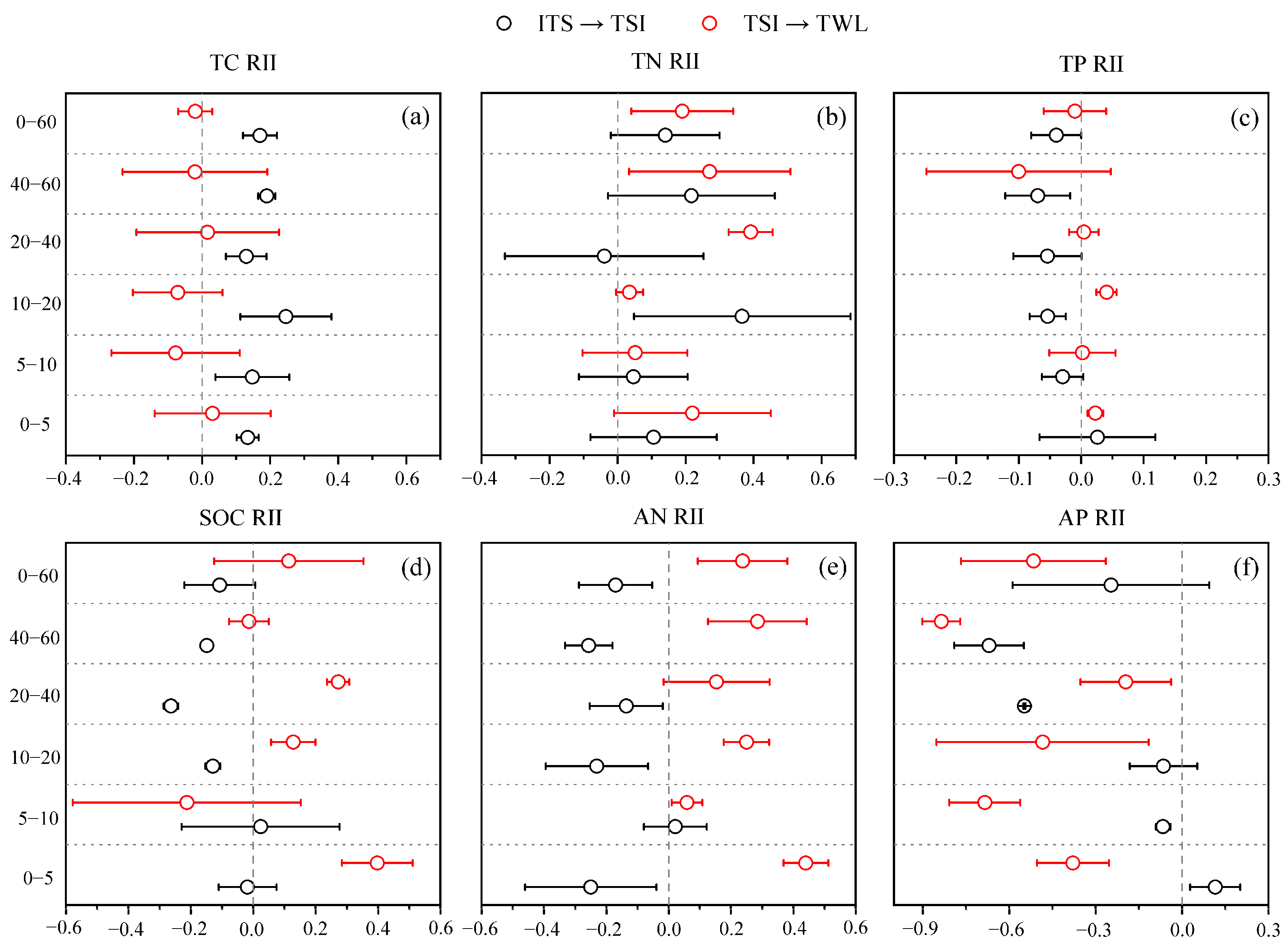
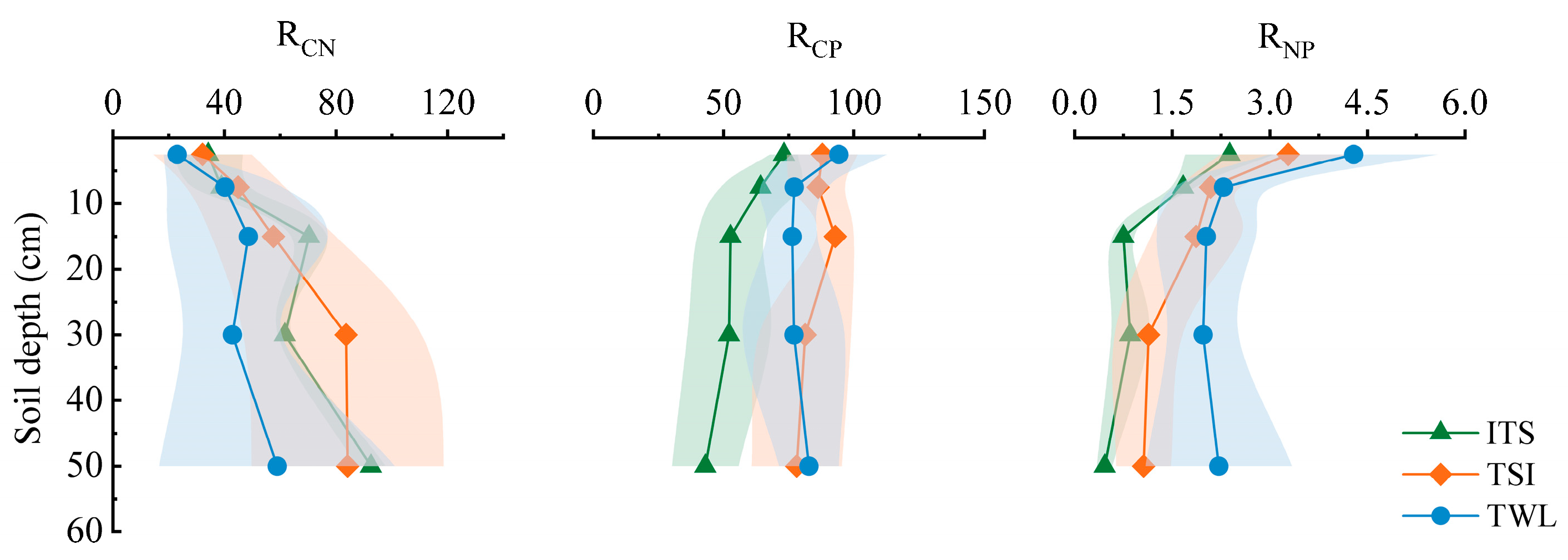

| Parameters | Shrub Encroachment Stage | ||
|---|---|---|---|
| ITS | TSI | TWL | |
| Total vegetation cover (%) | 30.75 ± 24.25 b | 80.00 ± 10.00 a | 100.00 ± 0.00 a |
| Herbaceous cover (%) | 26.25 ± 23.75 a | 61.00 ± 21.00 a | 59.00 ± 13.00 a |
| Shrub cover (%) | 7.00 ± 3.00 c | 30.00 ± 0.00 b | 57.50 ± 7.50 a |
| Shrub density (clumps/100 m2) | 4.50 ± 0.50 b | 7.00 ± 0.00 ab | 9.50 ± 2.50 a |
| Shrub height (cm) | 121.50 ± 7.50 b | 165.29 ± 47.65 a | 271.83 ± 33.02 a |
| Shrub crown width (cm) | 125.00 ± 11.81 c | 202.14 ± 72.86 b | 291.29 ± 82.60 a |
| Branches per shrub (branches/clump) | 1.00 ± 0.00 c | 2.00 ± 1.03 b | 2.29 ± 0.82 a |
| Species richness | 2.50 ± 0.71 a | 4.50 ± 0.71 a | 7.50 ± 2.12 a |
| Shannon-Wiener (H′) | 0.55 ± 0.47 a | 0.86 ± 0.32 a | 1.28 ± 0.16 a |
| Simpson (λ) | 0.56 ± 0.34 a | 0.57 ± 0.16 a | 0.64 ± 0.01 a |
| Pielou evenness (E) | 0.08 ± 0.11 a | 0.44 ± 0.42 a | 0.52 ± 0.23 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Luo, M.; Qu, F.; Sun, B.; Yu, Y.; Meng, L. Impacts of Shrub Encroachment on Vegetation Community and Soil Characteristics in Coastal Wetlands of the Abandoned Yellow River Course. Agronomy 2025, 15, 1258. https://doi.org/10.3390/agronomy15051258
Liu J, Luo M, Qu F, Sun B, Yu Y, Meng L. Impacts of Shrub Encroachment on Vegetation Community and Soil Characteristics in Coastal Wetlands of the Abandoned Yellow River Course. Agronomy. 2025; 15(5):1258. https://doi.org/10.3390/agronomy15051258
Chicago/Turabian StyleLiu, Jiaxuan, Mengjiao Luo, Fanzhu Qu, Bowen Sun, Yang Yu, and Ling Meng. 2025. "Impacts of Shrub Encroachment on Vegetation Community and Soil Characteristics in Coastal Wetlands of the Abandoned Yellow River Course" Agronomy 15, no. 5: 1258. https://doi.org/10.3390/agronomy15051258
APA StyleLiu, J., Luo, M., Qu, F., Sun, B., Yu, Y., & Meng, L. (2025). Impacts of Shrub Encroachment on Vegetation Community and Soil Characteristics in Coastal Wetlands of the Abandoned Yellow River Course. Agronomy, 15(5), 1258. https://doi.org/10.3390/agronomy15051258






