Research on the Synergistic Mechanism of Maize–Soybean Rotation and Bio-Organic Fertiliser in Cold Regions
Abstract
1. Introduction
2. Materials and Methods
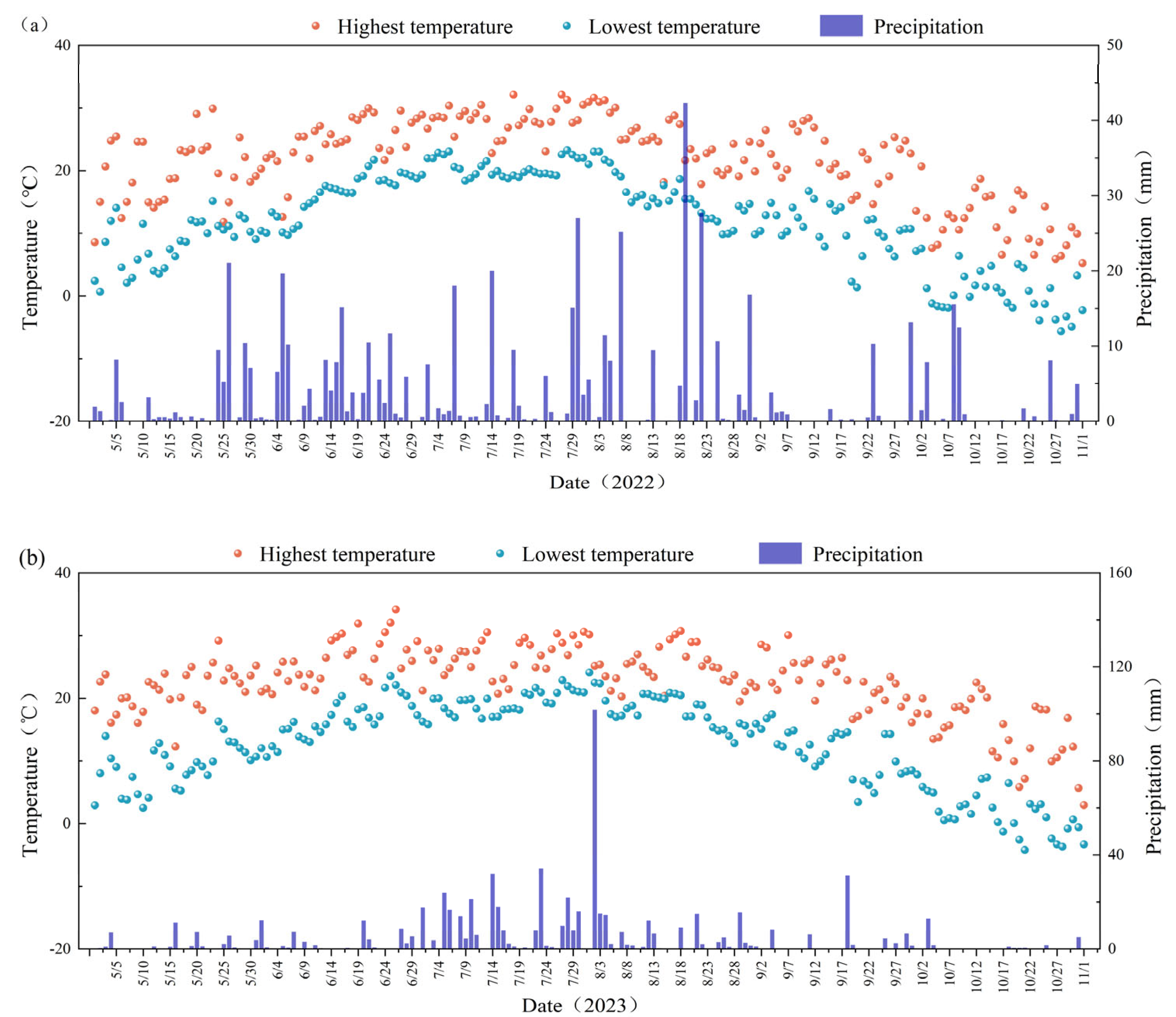
2.1. Overview of the Experimental Site
2.2. Experimental Materials
2.3. Experimental Design
2.4. Sample Collection and Analytical Methods
2.5. Data Processing
3. Results and Discussion
3.1. Effect of Different Fertiliser Treatments on Yield, Quality, and Economic Efficiency of Soybean and Maize
3.2. Effects of Different Fertilisation Treatments on Soil Physicochemical Properties
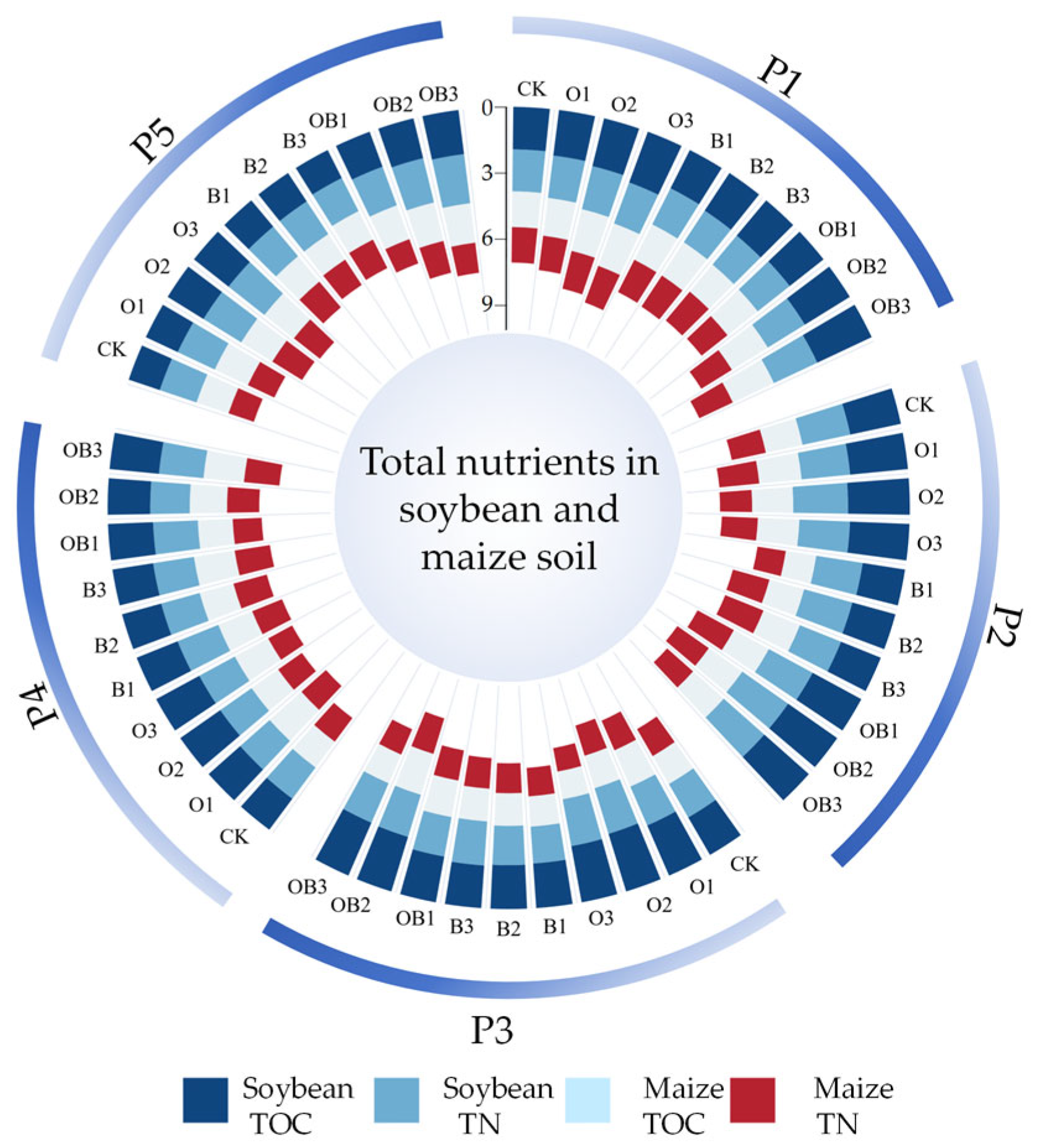
3.2.1. Effects on Soil Total Nutrients
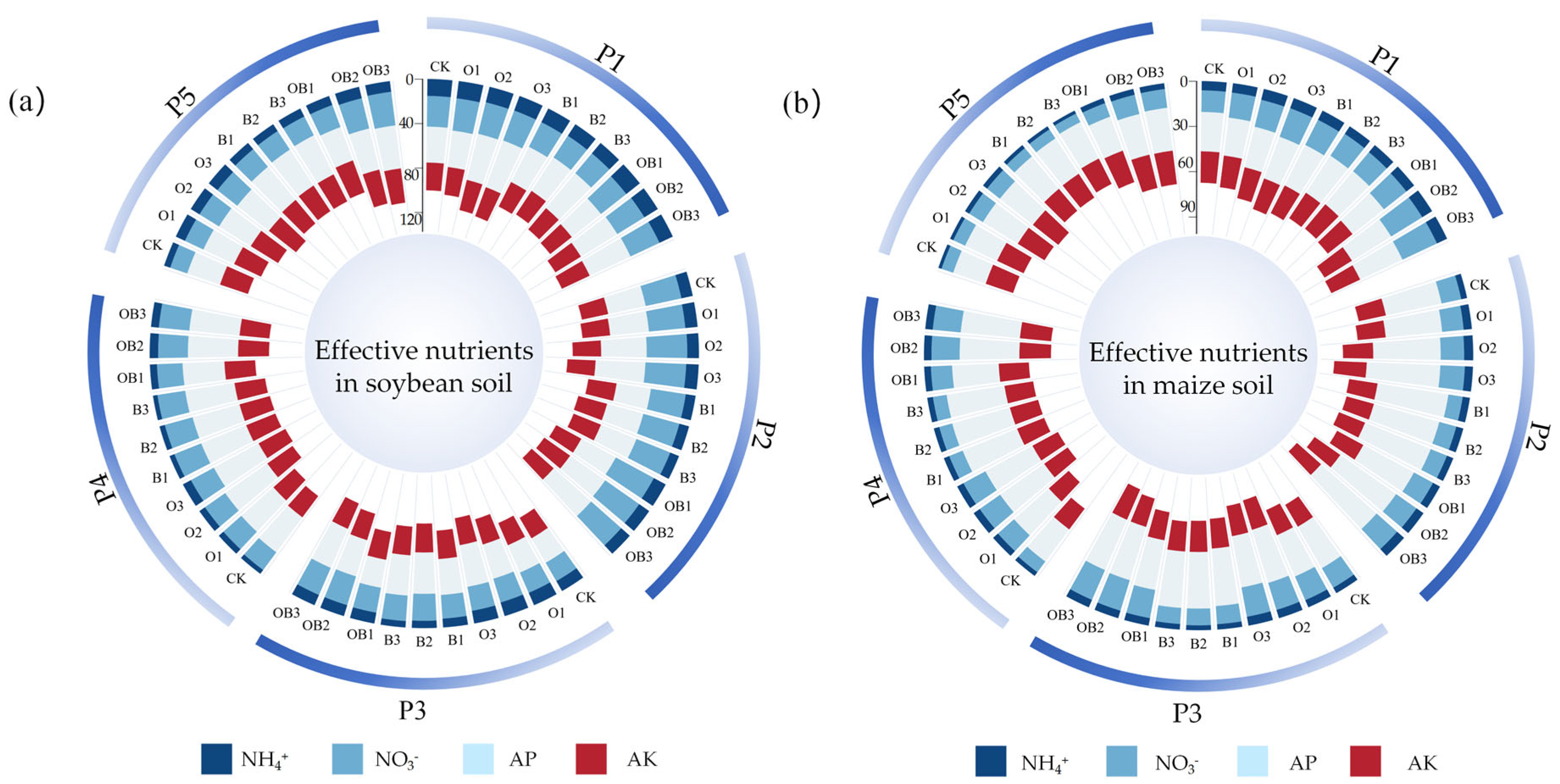
3.2.2. Effects on Available Soil Nutrients
- (1)
- Reduced microbial activity due to low temperatures—in Heilongjiang, autumn temperatures drop significantly, inhibiting microbial metabolism, including that of Bacillus subtilis, thus reducing the mineralisation of organic phosphorus and potassium [45];
- (2)
- Crop nutrient uptake—maize has a high demand for phosphorus and potassium during its late growth stages, which may have masked treatment-related differences in soil nutrient supply;
- (3)
- Nutrient fixation and balance—phosphorus tends to become fixed in the soil, while potassium may be lost through leaching or stabilised by crop uptake, resulting in a dynamic equilibrium that reduces the apparent impact of fertiliser additions.
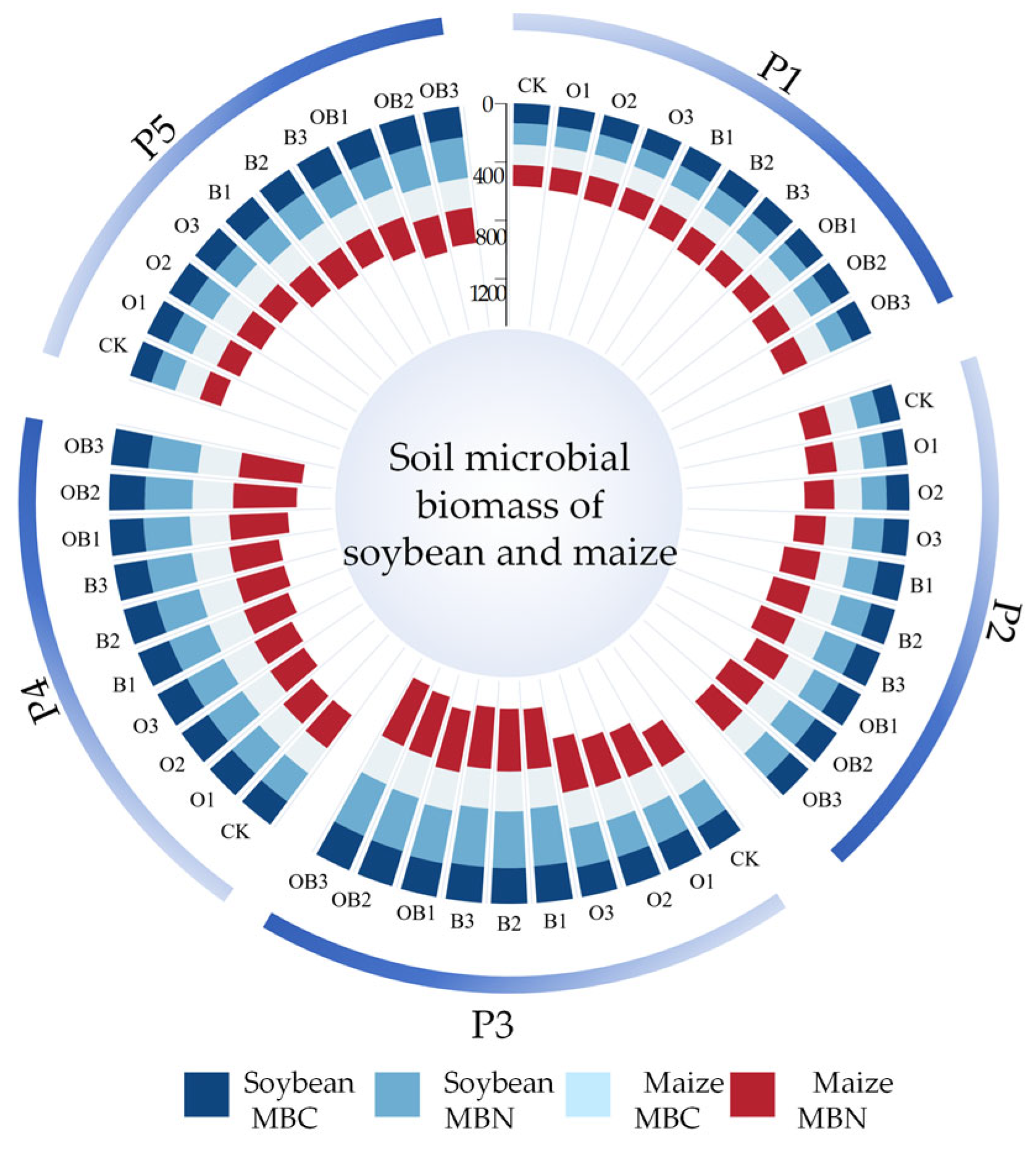
3.3. Effect of Different Fertilisation Treatments on Soil Biological Properties
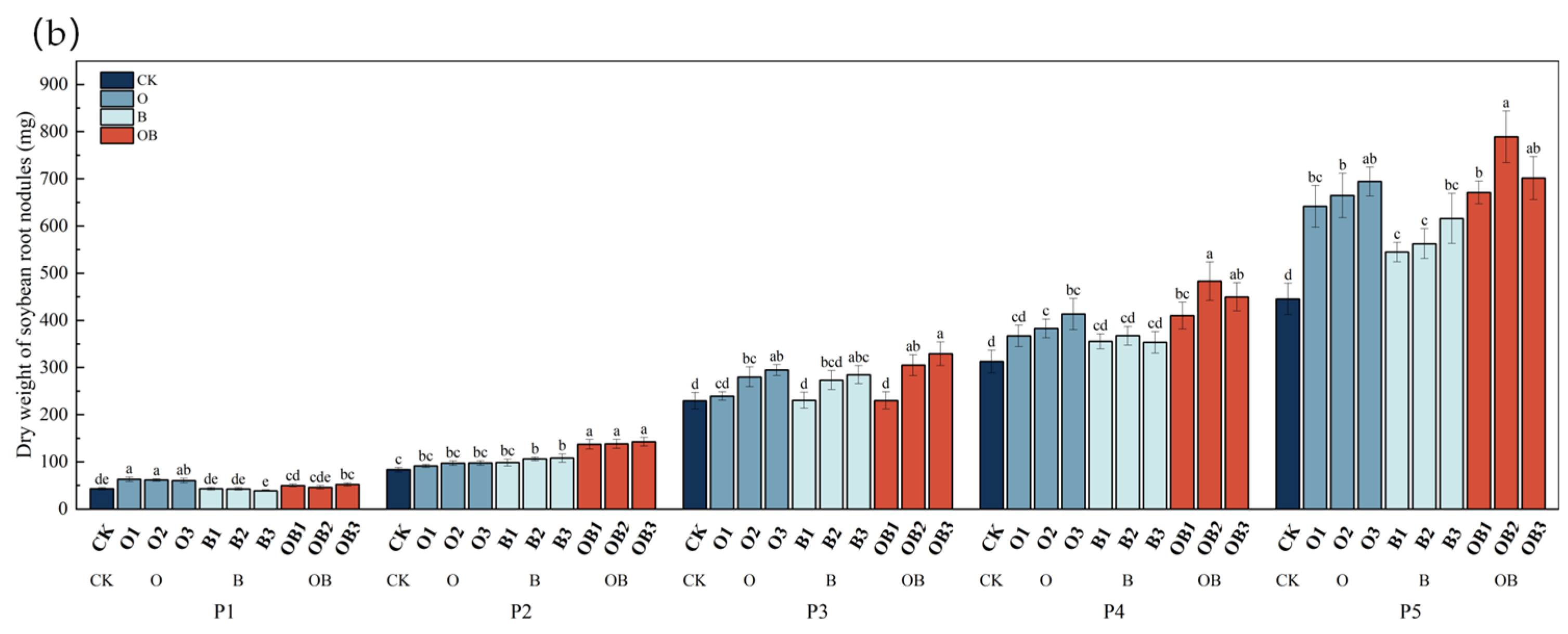
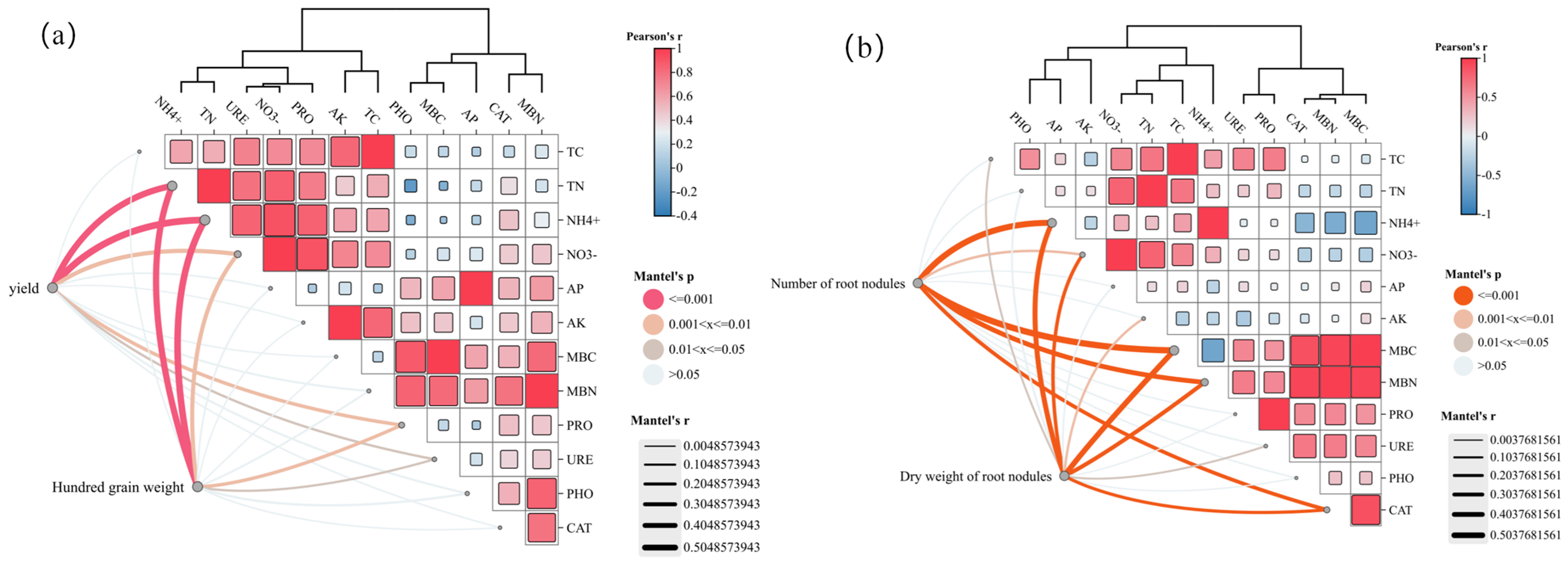
3.3.1. Effects on Soybean Root Nodules
3.3.2. Effects on Microbial Biomass
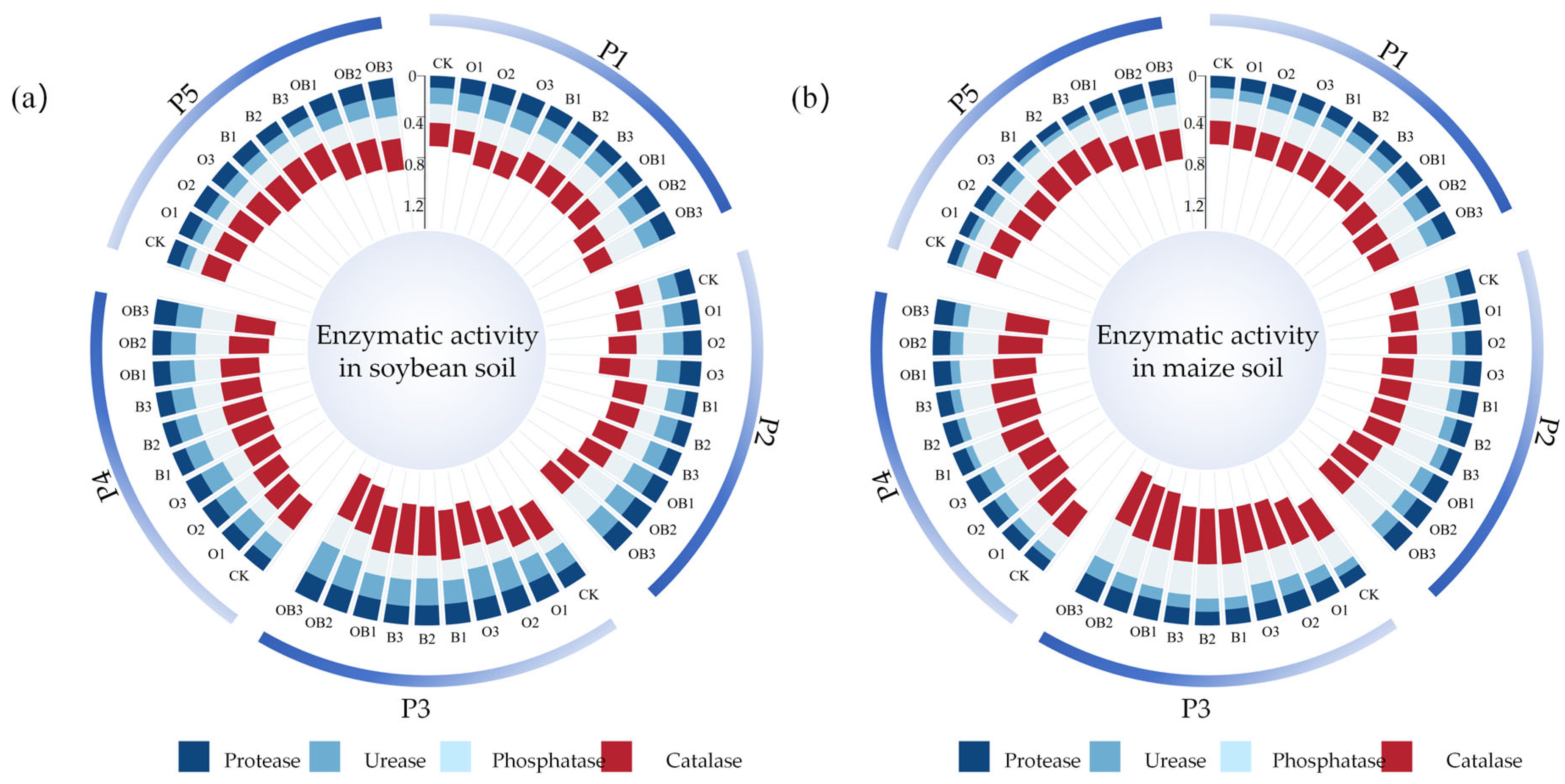
3.3.3. Effects on Soil Functional Enzyme Activities
- (1)
- The application of an organic fertiliser significantly boosting the soil organic matter cycling efficiency;
- (2)
- The introduction of Bacillus subtilis, which contributed to a more stable microbial environment and enhanced urease activity through increased synthesis and activation [54].
- (1)
- Bacillus subtilis plays a crucial role in regulating soil enzyme activities within the bio-organic fertiliser system;
- (2)
- The application of bio-organic fertiliser in the maize–soybean rotation system exerts a sustained effect: enzyme activities were significantly enhanced during the initial soybean growing season and remained high during the subsequent maize season;
- (3)
- From a long-term perspective, bio-organic fertiliser containing Bacillus subtilis can continuously support soil fertility in rotation-based farming systems [55].
3.4. Synergistic Mechanism of Maize–Soybean Rotation and Bio-Organic Fertiliser in Cold Regions
- (1)
- Organic nitrogen in bio-organic fertilisers exerts a prolonged effect in the soil, offering a more sustained nutrient supply compared with chemical fertilisers;
- (2)
- The rich organic carbon content of the fertiliser facilitates the accumulation of potassium and provides essential nutrients for microbial growth and development;
- (3)
- Bacillus subtilis improves the activity of functional soil microorganisms and key enzymes, thereby promoting the cycling of carbon and nitrogen and the mobilisation of available phosphorus;
- (4)
- Bio-organic fertilisers improve the soil microbial environment, enhancing the nitrogen-fixing function of soybean and mitigating the inhibitory effects of low temperatures on rhizobia in cold-region soils.
3.5. Optimisation of Fertilisation Strategies for Quality and Efficiency Improvement
4. Conclusions
- (1)
- Bio-organic fertiliser significantly enhanced production efficiency. The OB2 treatment resulted in the highest soybean and maize yields, 100-grain weights, and economic benefits. Compared with CK, soybean yield and 100-grain weight increased by 26.56% and 11.51%, respectively, while maize yield and 100-grain weight increased by 26.69% and 14.67%, respectively. The overall economic benefit improved by 23.04%. However, the substitution ratio of bio-organic fertiliser does not show a linear positive effect: further increasing its application amount led to diminished yield gains and reduced economic returns due to higher input costs.
- (2)
- The OB2 treatment significantly improved both total and available nutrient contents in the soil throughout the soybean and maize growth periods. It also promoted the accumulation of microbial biomass carbon and nitrogen and enhanced the activity of key soil enzymes. These positive effects were sustained in the subsequent maize rotation season. The treatment also markedly stimulated the development of soybean root nodules. Soil nutrients were significantly higher under OB2 treatment compared with CK at the flowering stage of soybean and the male pulling stage of maize. This indicates that the bio-organic fertiliser can meet the nutrients during the period of high crop growth demand. At the soybean maturity stage, nodule dry weight and number were increased by 77.15% and 32.11%, respectively, compared with CK. However, excessive application of bio-organic fertiliser inhibited nodule formation. Although nutrient availability in the later stages of crop growth was reduced due to low temperatures and crop uptake, Bacillus subtilis continued to improve the microbial environment and provided a sustained nutrient supply under cold-region conditions.
- (3)
- Crop yield and 100-grain weight were most responsive to the soil nitrogen supply capacity. The synergistic effect of bio-organic fertiliser in cold-region maize–soybean rotations is primarily driven by: the fertiliser’s own nitrogen supply capacity, the improvement of the soil environment through abundant organic carbon and functional microorganisms, enhanced nitrogen fixation in soybean, and the alleviation of temperature-induced inhibition on rhizobia. According to the GRA-TOPSIS model, the OB2 treatment showed the most significant effect in improving yield and quality in the maize–soybean rotation system in cold regions. Substituting 20% of chemical nitrogen fertiliser with bio-organic fertiliser during the soybean growth period was identified as the optimal fertilisation strategy. These findings provide a theoretical basis for improving production efficiency and supporting the sustainable development of farmland in cold-region maize–soybean rotation systems.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dai, Z.-Z.; Chang, S.; Zhao, G.-R.; Duan, J.-J.; Liang, H.-Y.; Zhu, Z.-Y.; Liu, S.-L.; Feng, Y.-Z.; Wang, X. Unlocking China’s Grain Yield Potential: Harnessing Technological and Spatial Synergies in Diverse Cropping Systems. Agric. Syst. 2025, 226, 104308. [Google Scholar] [CrossRef]
- Feng, L.; Luo, G.; Ioppolo, G.; Zhang, X.; Liao, W. Prevention and Control of Soil Pollution toward Sustainable Agricultural Land Use in China: Analysis from Legislative and Judicial Perspectives. Land Use Policy 2025, 151, 107497. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Zhang, K.; Jeong, J.; Zeng, Z.; Zang, H. Corrigendum to “Does Crop Rotation Yield More in China? A Meta-Analysis” [Field Crops Research, Volume 245, 1 January 2020, 107659]. Field Crops Res. 2024, 313, 109404. [Google Scholar] [CrossRef]
- Dang, K.; Ma, Y.; Liang, H.; Fan, Z.; Guo, S.; Li, Z.; Li, H.; Zhang, S. Distinct Planting Patterns Exert Legacy Effects on the Networks and Assembly of Root-Associated Microbiomes in Subsequent Crops. Sci. Total Environ. 2024, 946, 174276. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, X.; Liu, Z.; Shi, H.; Zhai, B.; Zhu, Y. Divergent Responses of Soil Physicochemical Properties in 6-m Profiles to Long-Term Overfertilization in Rainfed Apple Orchards on China’s Loess Plateau. Agric. Ecosyst. Environ. 2024, 361, 108817. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, P.; Qiao, Y.; Li, Y.; Liu, S.; Gao, C.; Liu, C.; Shao, J.; Yu, H.; Zhao, Z.; et al. The Potential for Soil C Sequestration and N Fixation under Different Planting Patterns Depends on the Carbon and Nitrogen Content and Stability of Soil Aggregates. Sci. Total Environ. 2023, 897, 165430. [Google Scholar] [CrossRef]
- Raza, S.; Miao, N.; Wang, P.; Ju, X.; Chen, Z.; Zhou, J.; Kuzyakov, Y. Dramatic Loss of Inorganic Carbon by Nitrogen-induced Soil Acidification in Chinese Croplands. Glob. Change Biol. 2020, 26, 3738–3751. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Ciampitti, I.A.; Cui, J.; Fang, K.; Zhao, S.; He, P.; Zhou, W. Benefits and Trade-Offs of Replacing Inorganic Fertilizer by Organic Substrate in Crop Production: A Global Meta-Analysis. Sci. Total Environ. 2024, 925, 171781. [Google Scholar] [CrossRef] [PubMed]
- De Notaris, C.; Enggrob, E.E.; Olesen, J.E.; Sørensen, P.; Rasmussen, J. Faba Bean Productivity, Yield Stability and N2-Fixation in Long-Term Organic and Conventional Crop Rotations. Field Crops Res. 2023, 295, 108894. [Google Scholar] [CrossRef]
- Yang, R.; Qi, Y.; Yang, L.; Chen, T.; Deng, A.; Zhang, J.; Song, Z.; Ge, B. Rotation Regimes Lead to Significant Differences in Soil Macrofaunal Biodiversity and Trophic Structure with the Changed Soil Properties in a Rice-Based Double Cropping System. Geoderma 2022, 405, 115424. [Google Scholar] [CrossRef]
- Chahal, I.; Hooker, D.C.; Deen, B.; Janovicek, K.; Van Eerd, L.L. Long-Term Effects of Crop Rotation, Tillage, and Fertilizer Nitrogen on Soil Health Indicators and Crop Productivity in a Temperate Climate. Soil Tillage Res. 2021, 213, 105121. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Delgado-Baquerizo, M.; Quero, J.L.; Ochoa, V.; Gozalo, B.; García-Palacios, P.; Escolar, C.; García-Gómez, M.; Prina, A.; Bowker, M.A.; et al. Surface Indicators Are Correlated with Soil Multifunctionality in Global Drylands. J. Appl. Ecol. 2020, 57, 424–435. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Beillouin, D.; Lambers, H.; Yang, Y.; Smith, P.; Zeng, Z.; Olesen, J.E.; Zang, H. Global Systematic Review with Meta-Analysis Reveals Yield Advantage of Legume-Based Rotations and Its Drivers. Nat. Commun. 2022, 13, 4926. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Sun, L.; Wang, T.; Li, C.; Chen, B. Sustainability Evaluation of Soybean-maize Rotation Systems in the Loess Plateau Region of Shaanxi, China. J. Clean. Prod. 2019, 210, 1229–1237. [Google Scholar] [CrossRef]
- Giller, K.E. The Food Security Conundrum of Sub-Saharan Africa. Glob. Food Secur. 2020, 26, 100431. [Google Scholar] [CrossRef]
- Luo, B.; Zhou, J.; Yao, W.; Wang, Y.; Guillaume, T.; Yuan, M.; Han, D.; Bilyera, N.; Wang, L.; Zhao, L.; et al. Maize and Soybean Rotation Benefits Soil Quality and Organic Carbon Stock. J. Environ. Manag. 2024, 372, 123352. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.; Chu, J.; Zhao, H.; Zhao, J.; Zang, H.; Yang, Y.; Zeng, Z. Improving Soil Quality and Wheat Yield through Diversified Crop Rotations in the North China Plain. Soil Tillage Res. 2024, 244, 106231. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Yao, W.; Shao, J.; Peixoto, L.; Yang, Y.; Zeng, Z.; Olesen, J.E.; Zang, H. Legume-Based Rotation Benefits Crop Productivity and Agricultural Sustainability in the North China Plain. Soil Tillage Res. 2025, 250, 106502. [Google Scholar] [CrossRef]
- Ratajczak, K.; Becher, M.; Kalembasa, S.; Faligowska, A.; Kalembasa, D.; Symanowicz, B.; Panasiewicz, K.; Szymańska, G.; Sulewska, H. Quantitative Determination of Nitrogen Fixed by Soybean and Its Uptake by Winter Wheat as Aftercrops Within Sustainable Agricultural Systems. Sustainability 2024, 16, 10153. [Google Scholar] [CrossRef]
- Ji, L.; Ni, K.; Wu, Z.; Zhang, J.; Yi, X.; Yang, X.; Ling, N.; You, Z.; Guo, S.; Ruan, J. Effect of Organic Substitution Rates on Soil Quality and Fungal Community Composition in a Tea Plantation with Long-Term Fertilization. Biol. Fertil. Soils 2020, 56, 633–646. [Google Scholar] [CrossRef]
- Luo, L.; Li, L.; Raza, A.; Zhao, C.; Pang, X.; Zhang, J.; Müller, C.; Yin, C. Organic Fertilizer and Bacillus Amyloliquefaciens Promote Soil N Availability via Changing Different Mineralization–Immobilization Turnover Rates in Acidic Soils. Agric. Ecosyst. Environ. 2024, 366, 108950. [Google Scholar] [CrossRef]
- Bacenetti, J.; Lovarelli, D.; Fiala, M. Mechanisation of Organic Fertiliser Spreading, Choice of Fertiliser and Crop Residue Management as Solutions for Maize Environmental Impact Mitigation. Eur. J. Agron. 2016, 79, 107–118. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, S.; Zhang, J.; Zhao, S.; Lu, H.; Li, L.; Liu, L.; Wang, G. Incorporating Crop Rotation into the Winter Wheat-Summer Maize System to Enhance Soil Multifunctionality and Sustainable Grain Production in the North China Plain. Field Crops Res. 2025, 325, 109834. [Google Scholar] [CrossRef]
- Lestari, P.G.; Sinaga, A.O.Y.; Marpaung, D.S.S.; Nurhayu, W.; Oktaviani, I. Application of Organic Fertilizer for Improving Soybean Production under Acidic Stress. Oil Crop Sci. 2024, 9, 46–52. [Google Scholar] [CrossRef]
- Bahuguna, A.; Sharma, S.; Yadav, J. Effect of Different Organic Sources on Physical, Chemical and Biological Properties of Soil in Inceptisols of Varanasi. Int. J. Plant Soil Sci. 2021, 33, 41–52. [Google Scholar] [CrossRef]
- Steinmuller, H.E.; Breithaupt, J.L.; Rovai, A.S.; Engelbert, K.M.; Smoak, J.M.; Chambers, L.G.; Radabaugh, K.R.; Moyer, R.P.; Chappel, A.; Vaughn, D.R.; et al. Using Loss-on-Ignition to Estimate Total Nitrogen Content of Mangrove Soils. Geoderma 2024, 448, 116956. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, J.; Huang, Z.; Zhang, X.; Duan, H.; Yu, A.; Yang, H.; Fan, C.; Chen, G.; Li, X. Aboveground Plants Influence Heterogeneously Soil Organic Carbon (SOC) and Its Labile Fractions after Mixed Afforestation: Three Afforestation Types of Masson’s Pine in the Upper Yangtze River, China. Sci. Total Environ. 2024, 957, 177293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Su, S.; Duan, C.; Feng, H.; Chau, H.W.; He, J.; Li, Y.; Hill, R.L.; Wu, S.; Zou, Y. Effects of Partial Organic Fertilizer Replacement Combined with Rainwater Collection System on Soil Water, Nitrate-Nitrogen and Apple Yield of Rainfed Apple Orchard in the Loess Plateau of China: A 3-Year Field Experiment. Agric. Water Manag. 2022, 260, 107295. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Ma, Y.; Guan, P.; Sun, Q.; Wang, Z.; Wang, Z.; Wang, Z.; Shi, M. Key Factors Influencing Wheat Grain Zinc and Manganese Concentration in Areas with Different Soil Available Phosphorus. Field Crops Res. 2024, 317, 109558. [Google Scholar] [CrossRef]
- Xia, H.; Wang, J.; Riaz, M.; Babar, S.; Li, Y.; Wang, X.; Xia, X.; Liu, B.; Jiang, C. Co-Application of Biochar and Potassium Fertilizer Improves Soil Potassium Availability and Microbial Utilization of Organic Carbon: A Four-Year Study. J. Clean. Prod. 2024, 469, 143211. [Google Scholar] [CrossRef]
- Kumwimba, M.N.; Akter, S.; Li, X.; Dzakpasu, M.; Ifon, B.E.; Manirakiza, B.; Muyembe, D.K.; Zhang, Y.; Huang, J.; Guadie, A. Nutrient and Sediment Retention by Riparian Vegetated Buffer Strips: Impacts of Buffer Length, Vegetation Type, and Season. Agric. Ecosyst. Environ. 2024, 369, 109050. [Google Scholar] [CrossRef]
- Tian, Y.; Schindlbacher, A.; Malo, C.U.; Shi, C.; Heinzle, J.; Kwatcho Kengdo, S.; Inselsbacher, E.; Borken, W.; Wanek, W. Long-Term Warming of a Forest Soil Reduces Microbial Biomass and Its Carbon and Nitrogen Use Efficiencies. Soil Biol. Biochem. 2023, 184, 109109. [Google Scholar] [CrossRef]
- Elrys, A.S.; Wen, Y.; Feng, D.; El-Mekkawy, R.M.; Kong, M.; Qin, X.; Lu, Q.; Dan, X.; Zhu, Q.; Tang, S.; et al. Cadmium Inhibits Carbon and Nitrogen Cycling through Soil Microbial Biomass and Reduces Soil Nitrogen Availability. J. Hazard. Mater. 2025, 489, 137524. [Google Scholar] [CrossRef]
- Afrin, S.; Tamanna, T.; Shahajadi, U.F.; Bhowmik, B.; Jui, A.H.; Miah, M.d.A.S.; Bhuiyan, M.N.I. Characterization of Protease-Producing Bacteria from Garden Soil and Antagonistic Activity against Pathogenic Bacteria. Microbe 2024, 4, 100123. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, W.; Zhang, P.; Zhang, M.; Yu, F.; Yu, L.; Liu, W.; Zhang, W.; Zhang, M. Combined Bamboo-Derived Biochar and DCD Improves the Catalytic Potentials of Ureases in Forest and Agricultural Soils. Ind. Crops Prod. 2025, 226, 120761. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Bao, J.; Yang, L.; Liu, B.; Wang, X.; Zhang, C.; Zhang, J.; Liu, Y.; Wang, L. Natural Restoration Has Better Soil Acid Phosphatase Kinetics than Artificial Plantations. Acta Oecologica 2025, 126, 104062. [Google Scholar] [CrossRef]
- Yeboah, J.O.; Shi, G.; Shi, W. Effect of Heavy Metal Contamination on Soil Enzymes Activities. J. Geosci. Environ. Prot. 2021, 9, 135–154. [Google Scholar] [CrossRef]
- He, S.; Du, J.; Wang, Y.; Cui, L.; Liu, W.; Xiao, Y.; Ran, Q.; Li, L.; Zhang, Z.; Tang, L.; et al. Differences in Background Environment and Fertilization Method Mediate Plant Response to Nitrogen Fertilization in Alpine Grasslands on the Qinghai-Tibetan Plateau. Sci. Total Environ. 2024, 906, 167272. [Google Scholar] [CrossRef]
- Bryk, M. Resolving Compactness Index of Pores and Solid Phase Elements in Sandy and Silt Loamy Soils. Geoderma 2018, 318, 109–122. [Google Scholar] [CrossRef]
- Luo, L.; Zhao, C.; Wang, E.; Raza, A.; Yin, C. Bacillus amyloliquefaciens as an Excellent Agent for Biofertilizer and Biocontrol in Agriculture: An Overview for Its Mechanisms. Microbiol. Res. 2022, 259, 127016. [Google Scholar] [CrossRef]
- Peng, L.; Deng, S.; Wu, Y.; Yi, W.; Zhang, Y.; Yao, X.; Xing, P.; Gu, Q.; Qi, J.; Tang, X. A Rapid Increase of Soil Organic Carbon in Paddy Fields after Applying Organic Fertilizer with Reduced Inorganic Fertilizer and Water-Saving Irrigation Is Linked with Alterations in the Structure and Function of Soil Bacteria. Agric. Ecosyst. Environ. 2025, 379, 109353. [Google Scholar] [CrossRef]
- Mohammadbagheri, L.; Nasr-Esfahani, M.; Abdossi, V.; Naderi, D. Genetic Diversity and Biochemical Analysis of Capsicum Annuum (Bell Pepper) in Response to Root and Basal Rot Disease, Phytophthora capsici. Phytochemistry 2021, 190, 112884. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Cai, X.; Cai, L.; Dong, B.; Haider, F.U.; Bo, Y.; Hu, Z.; Li, A.; Xue, Q. Soil and Microbial Biomass Response to Land-Use Changes in the Loess Plateau. Sustainability 2024, 16, 10496. [Google Scholar] [CrossRef]
- Shao, D.; He, Y.; Zhai, Y.; Yang, X.; Guo, Z.; Tan, J.; Wei, M. Mechanisms of Tomato Growth Promotion in Three Soils after Applying Bacillus Combinations. Soil Tillage Res. 2025, 249, 106477. [Google Scholar] [CrossRef]
- Sheng, M.; Hu, W.; Liu, C.-Q.; Niu, M.; Jin, R.; Deng, J.; Wu, L.; Li, P.; Yan, Z.; Zhu, Y.-G.; et al. Characteristics and Assembly Mechanisms of Bacterial and Fungal Communities in Soils from Chinese Forests across Different Climatic Zones. Catena 2024, 245, 108306. [Google Scholar] [CrossRef]
- Almeida, L.F.; Correndo, A.A.; Hefley, T.; Hintz, G.; Prasad, P.V.V.; Licht, M.; Casteel, S.; Singh, M.; Naeve, S.; Bais, J.; et al. Assessing the Influence of Environmental Drivers on Soybean Seed Yield and Nitrogen Fixation Estimates and Uncertainties in the United States. Eur. J. Agron. 2025, 162, 127428. [Google Scholar] [CrossRef]
- Jiang, D.; Jiang, Z.; Liu, S.; Hu, Y.; Deng, S.; Wang, J.; Shi, L.; Liu, Y.; Qu, J.; Zhang, Y. Inhibition Mechanism of Atrazine on Soybean Growth Insight from the Plant Nitrogen Fixation and Rhizobia Diversity Inhabiting in Nodules and Rhizosphere Soil. Appl. Soil Ecol. 2024, 195, 105236. [Google Scholar] [CrossRef]
- Laik, R.; Eltahira, E.B.A.; Pramanick, B.; Nidhi; Singh, S.K.; van Es, H. Enhancing Soil Health in Rice Cultivation: Optimized Zn Application and Crop Residue Management in Calcareous Soils. Sustainability 2025, 17, 489. [Google Scholar] [CrossRef]
- Feng, W.; Sánchez-Rodríguez, A.R.; Bilyera, N.; Wang, J.; Wang, X.; Han, Y.; Ma, B.; Zhang, H.; Li, F.Y.; Zhou, J.; et al. Mechanisms of Biochar-Based Organic Fertilizers Enhancing Maize Yield on a Chinese Chernozem: Root Traits, Soil Quality and Soil Microorganisms. Environ. Technol. Innov. 2024, 36, 103756. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, D.; Cheng, H.; Ren, L.; Jin, X.; Fang, W.; Yan, D.; Li, Y.; Wang, Q.; Cao, A. Organic Fertilizers Activate Soil Enzyme Activities and Promote the Recovery of Soil Beneficial Microorganisms after Dazomet Fumigation. J. Environ. Manag. 2022, 309, 114666. [Google Scholar] [CrossRef]
- Yang, J.; Ren, Y.; Jia, M.; Huang, S.; Guo, T.; Liu, B.; Liu, H.; Zhao, P.; Wang, L.; Jie, X. Improving Soil Quality and Crop Yield of Fluvo-Aquic Soils through Long-Term Organic-Inorganic Fertilizer Combination: Promoting Microbial Community Optimization and Nutrient Utilization. Environ. Technol. Innov. 2025, 37, 104050. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, X.; Cai, L. Effects of Different Straw Incorporation Amounts on Soil Organic Carbon, Microbial Biomass, and Enzyme Activities in Dry-Crop Farmland. Sustainability 2024, 16, 10588. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, J.; Zhang, L.; Sun, G.; Zheng, J. Organic Fertilizer Substitution Increased Soil Organic Carbon through the Association of Microbial Necromass C with Iron Oxides. Soil Tillage Res. 2025, 248, 106402. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, Q.; Gao, Y.-N.; Long, Q.-G.; Yan, W.-J.; Zhang, P.-D. Restoration of Degraded Seagrass Meadows: Effects of Plant Growth-Promoting Rhizobacteria (PGPR) Inoculation on Zostera marina Growth, Rhizosphere Microbiome and Ecosystem Functionality. J. Environ. Manag. 2024, 371, 123286. [Google Scholar] [CrossRef]
- Wu, S.; Li, K.; Diao, T.; Sun, Y.; Sun, T.; Wang, C. Influence of Continuous Fertilization on Heavy Metals Accumulation and Microorganism Communities in Greenhouse Soils under 22 Years of Long-Term Manure Organic Fertilizer Experiment. Sci. Total Environ. 2025, 959, 178294. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Ortale, R. Unifying Clustering and Representation Learning for Unsupervised Text Analysis: A Bayesian Knowledge-Enhanced Approach. Inf. Fusion 2025, 117, 102886. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X.; Shi, X.; Li, C.; Zhang, C. Adsorption–Desorption Behaviors of Enrofloxacin and Trimethoprim and Their Interactions with Typical Microplastics in Aqueous Systems. Sustainability 2025, 17, 516. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, Y.; Li, Y.; Liu, L.; Zhou, J.; Dai, C.; Cai, Z.; Huang, X. Application of Cellulose-Rich Organic Resource Improves Soil Quality and Plant Growth by Recruiting Beneficial Microorganisms. Appl. Soil Ecol. 2025, 207, 105909. [Google Scholar] [CrossRef]
- Jiang, Z.; Shao, Q.; Li, Y.; Cao, B.; Li, J.; Ren, Z.; Qu, J.; Zhang, Y. Noval Bio-Organic Fertilizer Containing Arthrobacter sp. DNS10 Alleviates Atrazine-Induced Growth Inhibition on Soybean by Improving Atrazine Removal and Nitrogen Accumulation. Chemosphere 2023, 313, 137575. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Z.; Wu, D.; Wang, H.; Li, J.; Bi, M.; Zhang, Y. Development of a Novel Bio-Organic Fertilizer for the Removal of Atrazine in Soil. J. Environ. Manag. 2019, 233, 553–560. [Google Scholar] [CrossRef]
- Pan, T.; Chen, Y.; Wang, L.; Hafeez, A.; Muramoto, J.; Shennan, C.; Cai, Y.; Tian, J.; Cai, K. Integrated Anaerobic Soil Disinfestation and Bio-Organic Fertilizers to Alleviate Continuous Cropping Obstacles: Improving Soil Health and Changing Bacterial Communities. Agric. Ecosyst. Environ. 2025, 385, 109562. [Google Scholar] [CrossRef]
- Zhaoxiang, W.; Huihu, L.; Qiaoli, L.; Changyan, Y.; Faxin, Y. Application of Bio-Organic Fertilizer, Not Biochar, in Degraded Red Soil Improves Soil Nutrients and Plant Growth. Rhizosphere 2020, 16, 100264. [Google Scholar] [CrossRef]
- Hafez, M.; Popov, A.I.; Rashad, M. Integrated Use of Bio-Organic Fertilizers for Enhancing Soil Fertility–Plant Nutrition, Germination Status and Initial Growth of maize (Zea mays L.). Environ. Technol. Innov. 2021, 21, 101329. [Google Scholar] [CrossRef]
- Mendonça, S.; Piovesana, G.F.; Pissolito, V. Geoethics and Sustainability: Addressing Challenges in Environmental Education for Achieving the SDGs. Sustainability 2025, 17, 574. [Google Scholar] [CrossRef]
- Han, J.; Li, G.; Zhong, W.; Yang, Y.; Ge, R.; Li, M.; Chen, Z.; Huang, L. Assessment of Carboniferous Volcanic Horizontal Wells after Fracturing Based on Gray Correlation, Hierarchical Analysis and Fuzzy Evaluation. Fluid Dyn. Mater. Process. 2024, 20, 2757–2773. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, F.; Li, B.; Liu, W.; Zhang, K.; Liu, Z. Comprehensive Analysis of Compatibility and Low-Temperature Rheological Properties of SBS Modified Asphalt Binder Using Entropy Weight-TOPSIS Method. Constr. Build. Mater. 2024, 457, 139364. [Google Scholar] [CrossRef]
- Bellini, F.; Barzegar, Y.; Barzegar, A.; Marrone, S.; Verde, L.; Pisani, P. Sustainable Water Quality Evaluation Based on Cohesive Mamdani and Sugeno Fuzzy Inference System in Tivoli (Italy). Sustainability 2025, 17, 579. [Google Scholar] [CrossRef]
- Stoilova, S.; Pulevski, I. An Integrated Two-Step Optimization Model and Aggregative Multi-Criteria Approach for Establishing Sustainable Tram Transportation Plan. Sustainability 2025, 17, 543. [Google Scholar] [CrossRef]

| Soil Properties | pH | SOC 1 (g/kg) | TN 2 (g/kg) | NH4+ 3 (mg/kg) | NO3− 4 (mg/kg) | AP 5 (mg/kg) | AK 6 (mg/kg) |
|---|---|---|---|---|---|---|---|
| Value | 6.8 ± 0.12 | 18.16 ± 2.44 | 1.61 ± 0.16 | 4.22 ± 0.41 | 1.30 ± 0.20 | 11.34 ± 0.31 | 147.70 ± 10.76 |
| Basic Physical and Chemical Properties | Effective Number of Live Bacteria (cfu/g) | Nitrogen Content (g/kg) | Organic Matter Content (g/kg) |
|---|---|---|---|
| Value | ≥0.2 × 1010 | 39.40 | 402.24 |
| No. | Treatment Name | Treatment Number | Nitrogen Application (kg/hm) | Additions of Bio-Organic Fertiliser Components Corresponding to Each Treatment (kg/hm) |
|---|---|---|---|---|
| 1 | Normal application of nitrogen fertiliser | CK | 60 | 0 |
| 2 | Bio-organic fertiliser replaces 10% of nitrogen fertiliser | OB1 | 54 | 152.28 |
| 3 | Bacillus subtilis powder replaces 10% of nitrogen fertiliser | B1 | 54 | 152.28 × 10−3 |
| 4 | Inactivated organic fertiliser substrate replacing 10% of nitrogen fertiliser | O1 | 54 | 152.28 |
| 5 | Bio-organic fertiliser replaces 10% of nitrogen fertiliser | OB2 | 48 | 304.57 |
| 6 | Bacillus subtilis powder replaces 10% of nitrogen fertiliser | B2 | 48 | 304.57 × 10−3 |
| 7 | Inactivated organic fertiliser substrate replacing 10% of nitrogen fertiliser | O2 | 48 | 304.57 |
| 8 | Bio-organic fertiliser replaces 10% of nitrogen fertiliser | OB3 | 42 | 456.85 |
| 9 | Bacillus subtilis powder replaces 10% of nitrogen fertiliser | B3 | 42 | 456.85 × 10−3 |
| 10 | Inactivated organic fertiliser substrate replacing 10% of nitrogen fertiliser | O3 | 42 | 456.85 |
| Treat | Soybean Yield (t/ha) | Soybean Hundred-Grain Weight (g/100 Grains) | Maize Yield (kg/ha) | Maize Hundred-Grain Weight (g/100 Grains) |
|---|---|---|---|---|
| CK | 46.66 ± 1.95 cde | 16.33 ± 0.30 b | 108,929.7 ± 6034.65 bc | 25.22 ± 1.22 bcd |
| O1 | 51.40 ± 3.13 bcd | 17.37 ± 0.39 ab | 110,568.15 ± 6508.05 bc | 26.35 ± 1.10 abc |
| O2 | 53.04 ± 3.49 abc | 16.97 ± 0.32 b | 134,484.9 ± 9044.1 a | 26.94 ± 1.33 ab |
| O3 | 53.22 ± 2.80 abc | 17.98 ± 0.34 ab | 132,162.3 ± 5629.95 a | 28.05 ± 1.24 ab |
| B1 | 44.79 ± 2.38 de | 15.68 ± 0.75 b | 102,061.95 ± 6655.35 c | 26.59 ± 1.54 abc |
| B2 | 48.72 ± 2.89 cde | 15.76 ± 0.83 b | 101,168.7 ± 5082.6 c | 22.88 ± 1.08 d |
| B3 | 42.90 ± 2.15 e | 15.42 ± 0.36 b | 99,343.05 ± 4733.55 c | 23.72 ± 0.89 cd |
| OB1 | 51.23 ± 2.10 bcd | 16.88 ± 0.37 b | 123,995.25 ± 5283.3 ab | 27.96 ± 1.21 ab |
| OB2 | 59.05 ± 3.59 a | 18.21 ± 0.34 a | 138,001.35 ± 5870.55 a | 28.92 ± 1.14 a |
| OB3 | 55.53 ± 2.25 ab | 17.48 ± 0.41 ab | 137,213.1 ± 8187.45 a | 28.67 ± 1.65 a |
| Treat | Total Output Value (CNY/ha) | Save Nitrogen Fertiliser (CNY/ha) | Increase Costs (CNY/ha) | Economic Benefits (CNY/ha) |
|---|---|---|---|---|
| CK | 31,424.08 | / | / | 31,424.08 |
| O1 | 33,299.29 | 46.95 | 609.15 | 32,737.09 |
| O2 | 37,214.89 | 93.90 | 1218.30 | 36,090.49 |
| O3 | 36,953.72 | 140.85 | 1827.45 | 35,267.12 |
| B1 | 29,815.95 | 46.95 | / | 29,862.90 |
| B2 | 31,052.99 | 93.90 | / | 31,146.89 |
| B3 | 28,781.08 | 140.85 | / | 28,921.93 |
| OB1 | 35,117.73 | 46.95 | 609.15 | 34,555.53 |
| OB2 | 39,789.69 | 93.90 | 1218.30 | 38,665.29 |
| OB3 | 38,461.61 | 140.85 | 1827.45 | 36,775.01 |
| Treat | TOPSIS Mark | GRA Mark | Final Mark | Rank |
|---|---|---|---|---|
| OB2 | 0.173324 | 0.104724 | 0.278048 | 1 |
| OB3 | 0.169757 | 0.100814 | 0.270570 | 2 |
| O3 | 0.117343 | 0.100074 | 0.217417 | 3 |
| OB1 | 0.115919 | 0.101025 | 0.216944 | 4 |
| O2 | 0.104099 | 0.100285 | 0.204384 | 5 |
| B1 | 0.081487 | 0.099123 | 0.180610 | 6 |
| B2 | 0.076891 | 0.097432 | 0.174023 | 7 |
| B3 | 0.075228 | 0.097749 | 0.172977 | 8 |
| O1 | 0.058868 | 0.099651 | 0.158519 | 9 |
| CK | 0.027385 | 0.099123 | 0.126508 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Tian, H.; Sun, N.; Wang, H.; Tang, S.; Chen, S.; Wang, X.; Ren, S.; Zuo, X.; Zhao, X. Research on the Synergistic Mechanism of Maize–Soybean Rotation and Bio-Organic Fertiliser in Cold Regions. Agronomy 2025, 15, 1256. https://doi.org/10.3390/agronomy15051256
Wang Z, Tian H, Sun N, Wang H, Tang S, Chen S, Wang X, Ren S, Zuo X, Zhao X. Research on the Synergistic Mechanism of Maize–Soybean Rotation and Bio-Organic Fertiliser in Cold Regions. Agronomy. 2025; 15(5):1256. https://doi.org/10.3390/agronomy15051256
Chicago/Turabian StyleWang, Zijian, Hao Tian, Nan Sun, Haocheng Wang, Songyan Tang, Shengjie Chen, Xuebing Wang, Shiwei Ren, Xiangyuan Zuo, and Xingbo Zhao. 2025. "Research on the Synergistic Mechanism of Maize–Soybean Rotation and Bio-Organic Fertiliser in Cold Regions" Agronomy 15, no. 5: 1256. https://doi.org/10.3390/agronomy15051256
APA StyleWang, Z., Tian, H., Sun, N., Wang, H., Tang, S., Chen, S., Wang, X., Ren, S., Zuo, X., & Zhao, X. (2025). Research on the Synergistic Mechanism of Maize–Soybean Rotation and Bio-Organic Fertiliser in Cold Regions. Agronomy, 15(5), 1256. https://doi.org/10.3390/agronomy15051256






