Effects and Mechanism of Nitrogen Regulation on Seed Yield and Quality of Rapeseed (Brassica napus L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Site and Soil
2.2. Materials, Experimental Design, and Field Management
2.3. Growth and Physiological Indexes
2.4. Activity and Gene Expression Level of the Enzymes Related to Nitrogen Metabolism and Fatty Acids Biosynthesis
2.5. Statistical Analysis
3. Results
3.1. Effects of Nitrogen Application on Seed Yield of Rapeseed
3.1.1. Seed Yield and Nitrogen Accumulation in Plants of Different Rapeseed Varieties at Different Levels of Nitrogen Application
3.1.2. Correlation Analysis of Nitrogen Levels, Nitrogen Accumulation in Plants, and Seed Yield Components of Rapeseed
3.1.3. Path Coefficient Analysis of Nitrogen Application Effect on Seed Yield of Rapeseed
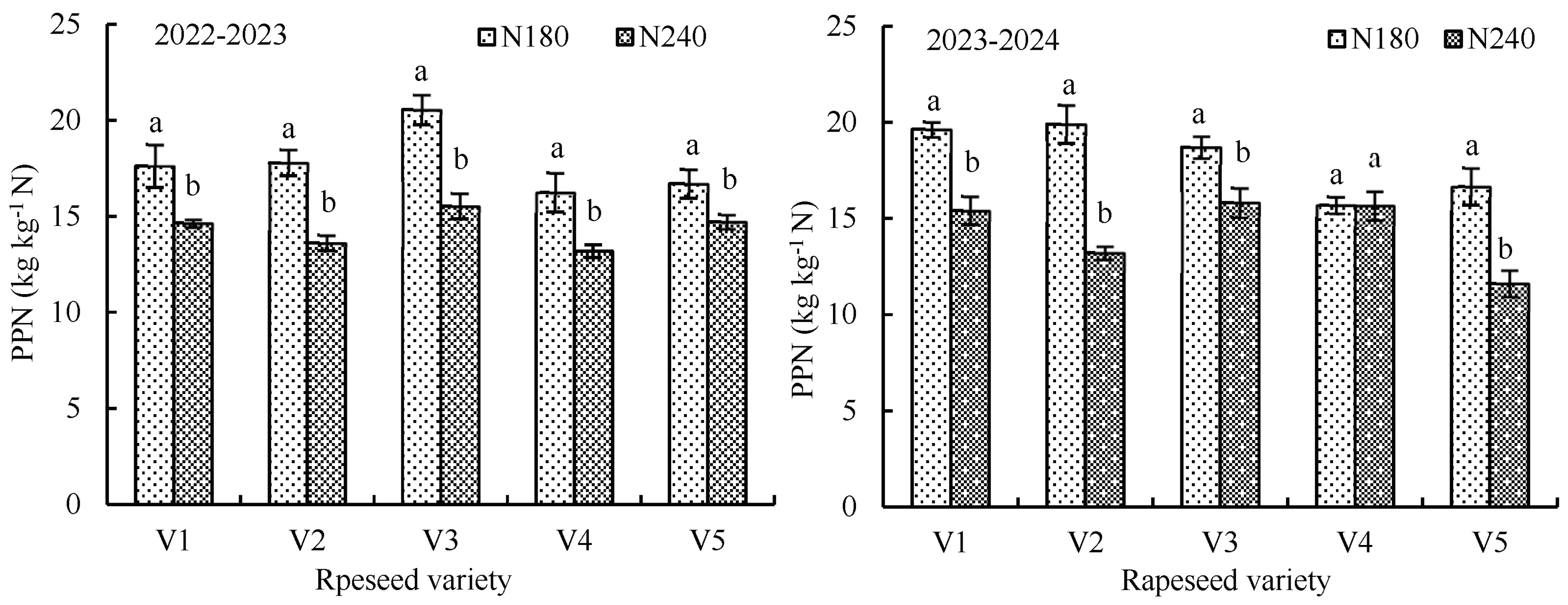
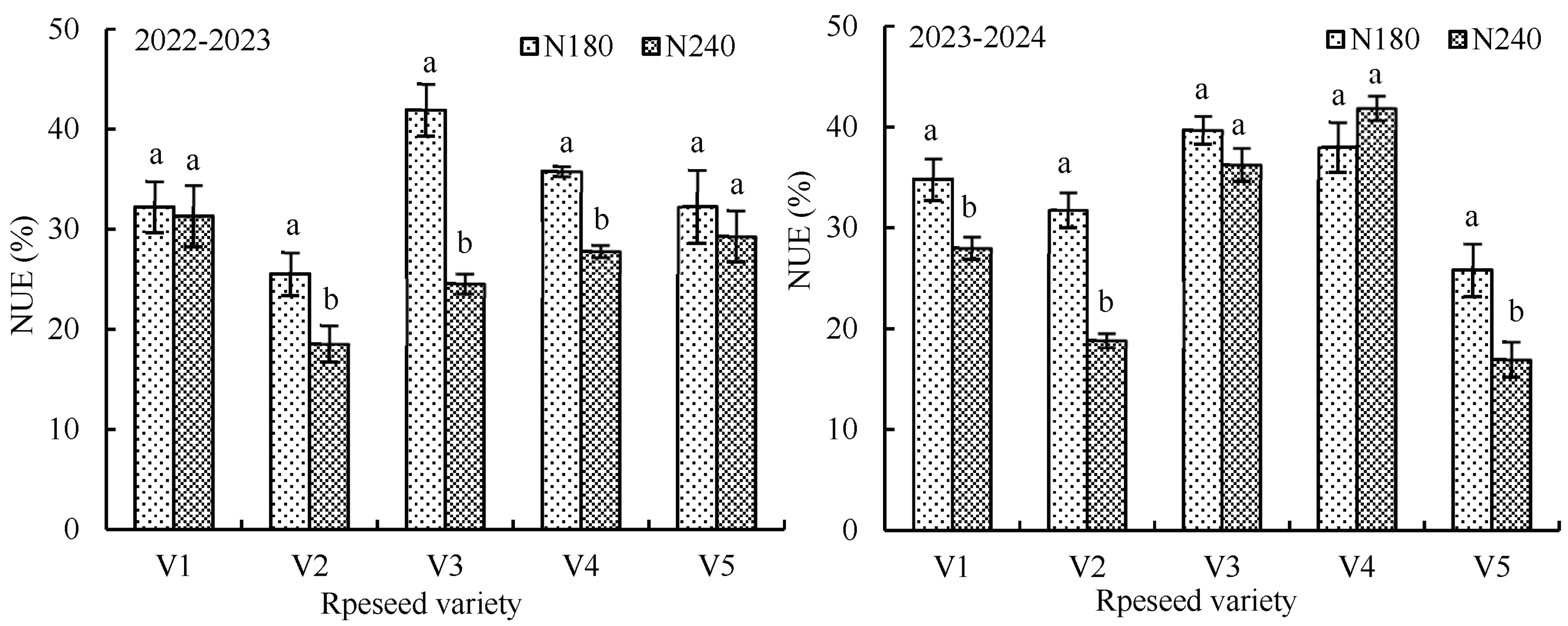
3.1.4. Influence of Nitrogen Application on Nitrogen Utilization Efficiency of Rapeseed
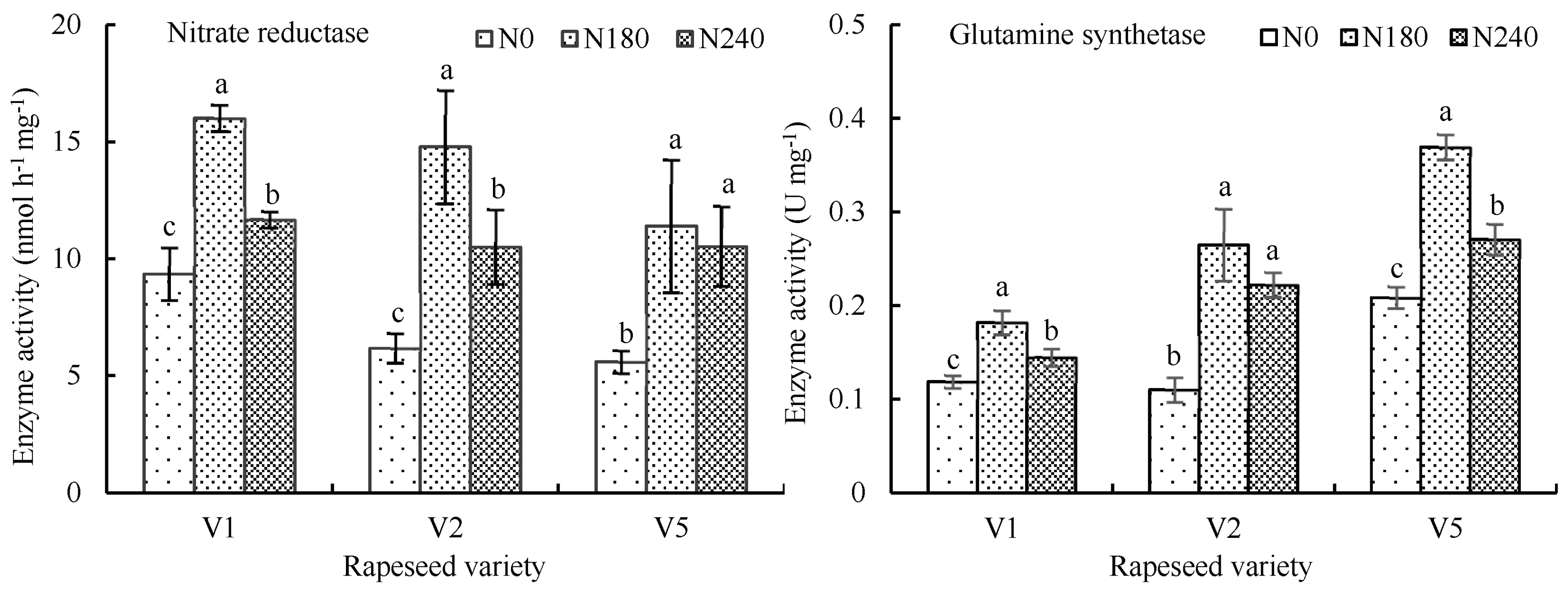
3.1.5. Influence of Nitrogen Application on Activity and Gene Expression of the Key Enzymes Related to Nitrogen Metabolism
3.2. Effects of Nitrogen Application on Seed Quality of Rapeseed
3.2.1. Effects of Nitrogen Application on Seed Quality of Rapeseed
3.2.2. Correlation Relationships Between Nitrogen Application, Plant Nitrogen Accumulation, and Seed Quality of Rapeseed
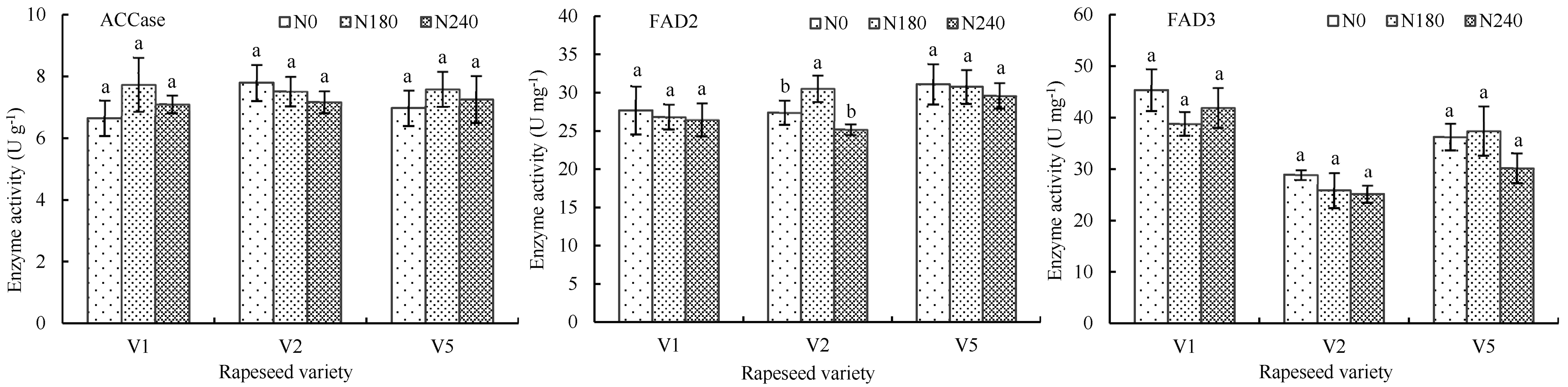
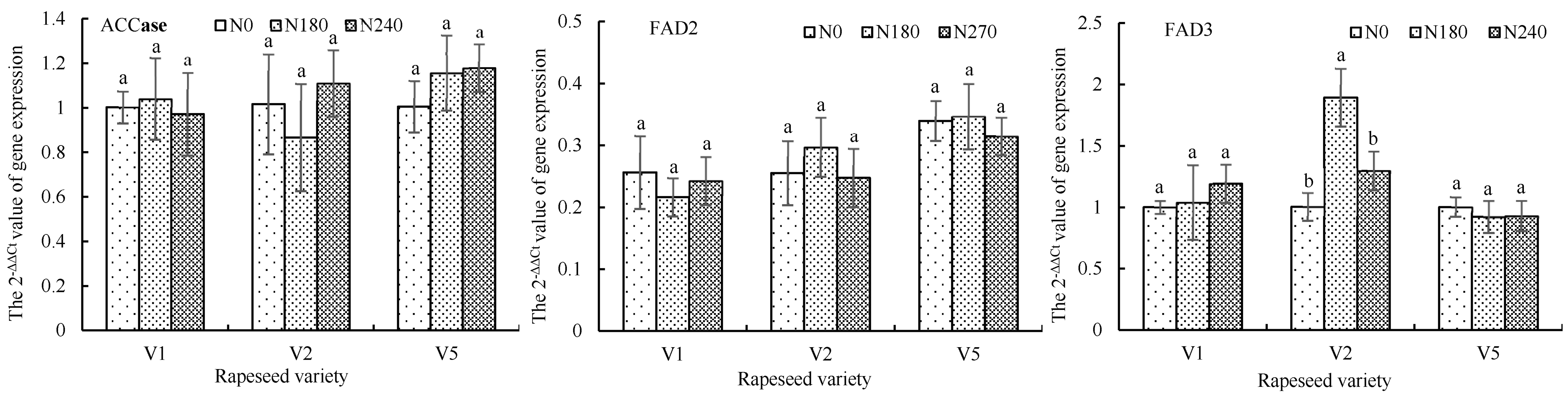
3.2.3. Influence of Nitrogen Application on Activity and Gene Expression of Key Enzymes Related to Fatty Acids Synthesis
4. Discussion
4.1. Effects of Nitrogen Application on Seed Yield of Rapeseed
4.2. The Key Enzymes Related to Nitrogen Metabolism
4.3. Effects of Nitrogen Application on Seed Quality of Rapeseed
4.4. The Key Enzymes Related to Fatty Acids Synthesis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACCase | acetyl-CoA carboxylase |
| Branch-num | number of branches per plant |
| FAD2 | oleic acid desaturase |
| FAD3 | omega-3 fatty acid desaturase |
| GS | glutamine synthetase |
| N0 | 0 kg ha−1 nitrogen application |
| N180 | 180 kg ha−1 nitrogen application |
| N240 | 240 kg ha−1 nitrogen application |
| N-acc | nitrogen accumulation in flowering plants |
| N-acr | nitrogen accumulation in ripened plants |
| NMR | the nuclear magnetic resonance spectroscopy |
| NR | nitrate reductase |
| NUE | nitrogen utilization efficiency |
| PPN | partial productivity of nitrogen fertilizer |
| RT-qPCR | real-time fluorescence quantitative PCR |
| Seed-num | number of seeds per silique |
| Silique-num | number of siliques per plant |
References
- Stephenson, A.L.; Dennis, J.S.; Scott, S.A. Improving the sustainability of the production of biodiesel from oilseed rape in the UK. Process Saf. Environ. 2008, 86, 427–440. [Google Scholar] [CrossRef]
- Sulek, M.W.; Kulczychi, A.; Malysa, A. Assessment of lubricity of compositions of fuel oil with biocomponents derived from rape-seed. Wear 2010, 268, 104–108. [Google Scholar] [CrossRef]
- Yahbi, M.; Nabloussi, A.; Maataoui, A.; Alami, N.E.; Boutagayout, A.; Daoui, K. Effects of nitrogen rates on yield, yield components, and other related attributes of different rapeseed (Brassica napus L.) varieties. OCL-Oilseeds Fats Crops Lipids 2022, 29, 8. [Google Scholar] [CrossRef]
- Öztürk, Ö. Effects of source and rate of nitrogen fertilizer on yield, yield components and quality of winter rapeseed (Brassica napus L.). Chil. J. Agric. Res. 2010, 70, 132–141. [Google Scholar] [CrossRef]
- Kuai, J.; Sun, Y.; Zhou, M.; Zhang, P.; Zuo, Q.; Wu, J.; Zhou, G. The effect of nitrogen application and planting density on the radiation use efficiency and the stem lignin metabolism in rapeseed (Brassica napus L.). Field Crops Res. 2016, 199, 89–98. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, S.; Kuai, J.; Noman, A.; Shahid, M.; Din, M.; Ali, A.; Zhou, G. Alteration in yield and oil quality traits of winter rapeseed by lodging at different planting density and nitrogen rates. Sci. Rep. 2018, 8, 634. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, S.; Kuai, J.; Ullah, S.; Fahad, S.; Zhou, G. Optimization of nitrogen rate and planting density for improving yield, nitrogen use efficiency, and lodging resistance in oilseed rape. Front. Plant Sci. 2017, 8, 532. [Google Scholar] [CrossRef]
- Chen, G.; Li, C.; Hu, M.; He, X.; Yang, H.; Zhang, Q.; Wu, C.; Duan, Q.; Peng, L.; Zhang, Y.; et al. Evaluating rice lipid content, yield, and quality in response to nitrogen application rate and planting density. Front. Plant Sci. 2024, 15, 1469264. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, D.; Wang, Z.; Chen, Y.; Yang, Z.; Wu, C.; Yang, H.; Chen, L. Nitrogen application modifies the seed and oil yields and fatty acid composition of nicotiana tabacum. Hortscience 2020, 55, 1898–1902. [Google Scholar] [CrossRef]
- Gu, H.; Li, J.; Lu, Z.; Li, X.; Cong, R.; Ren, T.; Lu, J. Effects of combined application of nitrogen and potassium on oil concentration and fatty acid component of oilseed rape (Brassica napus L.). Field Crops Res. 2024, 306, 109229. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Vick, B.; Ebelhar, M.W.; Buehring, N.; Astatkie, T. Nitrogen applications modify seed and oil yields and fatty acid composition of winter mustard. Ind. Crops Prod. 2012, 36, 28–32. [Google Scholar] [CrossRef]
- Zapletalová, A.; Ducsay, L.; Varga, L.; Sitkey, J.; Javoreková, S.; Hozlár, P. Influence of Nitrogen Nutrition on Fatty Acids in Oilseed Rape (Brassica napus L.). Plants 2022, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Salmenkallio, M.; Sopanen, T. Amino acid and peptide uptake in the scutella of germinating grains of barley, wheat, rice, and maize. Plant Physiol. 1989, 89, 1285–1291. [Google Scholar] [CrossRef]
- Sohlenkamp, C.; Wood, C.C.; Roeb, G.W.; Udvardi, M.K. Characterization of Arabidopsis AtAMT2, a high-affinity ammonium transporter of the plasma membrane. Plant Physiol. 2002, 130, 1788–1796. [Google Scholar] [CrossRef]
- Zhan, N.; Xu, K.; Ji, G.; Yan, G.; Chen, B.; Wu, X.; Cai, G. Research progress in high-efficiency utilization of nitrogen in rapeseed. Int. J. Mol. Sci. 2023, 24, 7752. [Google Scholar] [CrossRef]
- Miflin, B.J. The location of nitrite reductase and other enzymes related to amino acid biosynthesis in the plastids of root and leaves. Plant Physiol. 1974, 54, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, H.S. Regulation of nitrate reductase activity in higher plants. Phytochemistry 1980, 19, 725–733. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.-I.; Konishi, M.; Yanagisawa, S.; Omata, T. Nitrite transport activity of a novel HPP family protein conserved in cyanobacteria and chloroplasts. Plant Cell Physiol. 2014, 55, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Nikolau, B.J.; Ohlrogge, J.B.; Wurtele, E.S. Plant biotin-containing carboxylases. Arch. Biochem. Biophys. 2003, 414, 211–222. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, X.; Su, Y.; Chen, Y.; Liu, Y.; Sun, M.; Qi, G. Transcriptome analysis reveals dynamic fat accumulation in the Walnut Kernel. Int. J. Genom. 2018, 2018, 8931651. [Google Scholar] [CrossRef] [PubMed]
- Modiri, S.; Zahiri, H.S.; Vali, H.; Noghabi, K.A. Evaluation of transcription profile of acetyl-CoA carboxylase (ACCase) and acyl-ACP synthetase (AAS) to reveal their roles in induced lipid accumulation of Synechococcus sp. HS01. Renew. Energy 2018, 129, 347–356. [Google Scholar] [CrossRef]
- Dar, A.A.; Choudhury, A.R.; Kancharla, P.K.; Arumugam, N. The FAD2 gene in plants: Occurrence, regulation, and role. Front. Plant Sci. 2017, 8, 1789. [Google Scholar] [CrossRef]
- O’Neill, C.M.; Gill, S.; Hobbs, D.; Morgan, C.; Bancroft, I. Natural variation for seed oil composition in Arabidopsis thaliana. Phytochemistry 2003, 64, 1077–1090. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Adnan, M.; Morton, G.; Hadi, S. Analysis of rpoS and bolA gene expression under various stress-induced environments in planktonic and biofilm phase using 2−ΔΔCT method. Mol. Cell. Biochem. 2011, 357, 275–282. [Google Scholar] [CrossRef]
- Bogard, M.; Jourdan, M.; Allard, V.; Martre, P.; Perretant, M.R.; Ravel, C.; Heumez, E.; Orford, S.; Snape, J.; Griffiths, S.; et al. Anthesis date mainly explained correlations between post-anthesis leaf senescence, grain yield, and grain protein concentration in a winter wheat population segregating for flowering time QTLs. J. Exp. Bot. 2011, 62, 3621–3636. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Serret, M.D.; Elazab, A.; Bort, P.J.; Araus, J.L.; Aranjuelo, I.; Sanz-Sáez, Á. Wheat ear carbon assimilation and nitrogen remobilization contribute significantly to grain yield. J. Integr. Plant Biol. 2016, 58, 914–926. [Google Scholar] [CrossRef]
- Yang, L.; Guo, S.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Use of the stable nitrogen isotope to reveal the source-sink regulation of nitrogen uptake and remobilization during grain filling phase in maize. PLoS ONE 2016, 11, e162201. [Google Scholar] [CrossRef]
- Bouchet, A.; Laperche, A.; Bissuel-Belaygue, C.; Snowdon, R.; Nesi, N.; Stahl, A. Nitrogen use efficiency in rapeseed. A review. Agron. Sustain. Dev. 2016, 36, 38. [Google Scholar] [CrossRef]
- Ma, B.L.; Herath, A.W. Timing and rates of nitrogen fertiliser application on seed yield, quality and nitrogen-use efficiency of canola. Crop Pasture Sci. 2016, 67, 167–180. [Google Scholar] [CrossRef]
- Sieling, K.; Böttcher, U.; Kage, H. Effect of sowing method and N application on seed yield and N use efficiency of winter oilseed rape. Agronomy 2017, 7, 21. [Google Scholar] [CrossRef]
- Sathee, L.; Krishna, G.K.; Adavi, S.B.; Jha, S.K.; Jain, V. Role of protein phosphatases in the regulation of nitrogen nutrition in plants. Physiol. Mol. Biol. Plants 2021, 27, 2911–2922. [Google Scholar] [CrossRef]
- Ahmad, A.; Sivakami, S.; Nandula, R.; Ali, A.; Raghuram, N. Effect of nitrate, nitrite, ammonium, glutamate, glutamine and 2-oxoglutarate on the RNA levels and enzyme activities of nitrate reductase and nitrite reductase in rice. Physiol. Mol. Biol. Plants 2007, 13, 17–25. [Google Scholar]
- Rohilla, P.; Yadav, J.P. Nitrate reductase structure, role and factors affecting its regulation: A review. Plant Arch. 2020, 20, 5787–5793. [Google Scholar]
- Fu, Y.-F.; Zhang, Z.-W.; Yang, X.-Y.; Wang, C.-Q.; Lan, T.; Tang, X.-Y.; Chen, G.-D.; Zeng, J.; Yuan, S. Nitrate reductase is a key enzyme responsible for nitrogen-regulated auxin accumulation in Arabidopsis roots. Biochem. Biophys. Res. Commun. 2020, 532, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.; Bio, A.F.M.; Domínguez-Valdivia, M.D.; Aparicio-Tejo, P.M.; Lamsfus, C.; Martins-Loução, M.A. How does glutamine synthetase activity determine plant tolerance to ammonium? Planta 2006, 223, 1068–1080. [Google Scholar] [CrossRef]
- Yin, H.; Yang, F.; He, X.; Du, X.; Mu, P.; Ma, W. Advances in the functional study of glutamine synthetase in plant abiotic stress tolerance response. Crop J. 2022, 10, 917–923. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, D.; Ma, G.; Wang, C.; Xie, X.; Kang, G. Responses of glutamine synthetase activity and gene expression to nitrogen levels in winter wheat cultivars with different grain protein content. J. Cereal Sci. 2017, 74, 187–193. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, H.; Liu, Q.; Rong, X.; Peng, J.; Xie, G.; Zhang, Y.; Chen, L.; Guan, C.; Gu, J. Responses of Seed Yield and Quality to Nitrogen Application Levels in Two Oilseed Rape (Brassica napus L.) Varieties Differing in Nitrogen Efficiency. Plant Prod. Sci. 2012, 15, 265–269. [Google Scholar] [CrossRef]
- Tian, C.; Zhou, X.; Liu, Q.; Peng, J.; Zhang, Z.; Song, H.; Ding, Z.; Zhran, M.A.; Eissa, M.A.; Kheir, A.M.S.; et al. Increasing yield, quality and profitability of winter oilseed rape (Brassica napus) under combinations of nutrient levels in fertiliser and planting density. Crop Pasture Sci. 2020, 71, 1010–1019. [Google Scholar] [CrossRef]
- Li, D.W.; Xie, W.H.; Hao, T.B.; Cai, J.X.; Zhou, T.B.; Balamurugan, S.; Yang, W.D.; Liu, J.S.; Li, H.Y. Constitutive and chloroplast targeted expression of acetyl-CoA carboxylase in oleaginous microalgae elevates fatty acid biosynthesis. Mar. Biotechnol. 2018, 20, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.L.; Wu, G.T.; Lang, C.X.; Liu, R.H. Influence on Brassica seed oil content by transformation with heteromeric acetyl-CoA carboxylase (ACCase) Gene. Mol. Plant Breed. 2017, 15, 920–927. (In Chinese) [Google Scholar]
- Tezaki, S.; Iwama, R.; Kobayashi, S.; Shiwa, Y.; Yoshikawa, H.; Ohta, A.; Horiuchi, H.; Fukuda, R. Δ12-fatty acid desaturase is involved in growth at low temperature in yeast Yarrowia lipolytica. Biochem. Biophys. Res. Commun. 2017, 488, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xing, G.; Niu, L.; He, H.; Guo, D.; Du, Q.; Qian, X.; Yao, Y.; Li, H.; Zhong, X.; et al. Improved oil quality in transgenic soybean seeds by RNAi-mediated knockdown of GmFAD2-1B. Transgenic Res. 2018, 27, 155–166. [Google Scholar] [CrossRef]
- Yin, Z.J.; Liu, H.L.; Dong, X.B.; Tian, L.H.; Xiao, L.; Xu, Y.N.; Qu, L.Q. Increasing α-linolenic acid content in rice bran by embryo-specific expression of ω3/Δ15-desaturase gene. Mol. Breed. 2014, 33, 987–996. [Google Scholar] [CrossRef]
- Yurchenko, O.P.; Park, S.; Ilut, D.C.; Inmon, J.J.; Millhollon, J.C.; Liechty, Z.; Page, J.T.; Jenks, M.A.; Chapman, K.D.; Udall, J.A.; et al. Genome-wide analysis of the omega-3 fatty acid desaturase gene family in Gossypium. BMC Plant Biol. 2014, 14, 312. [Google Scholar] [CrossRef]

| Gene | Primer Sequence (5′-3′) | Size (bp) | |
|---|---|---|---|
| NR | Forward | GCAAGTTCTGGTGCTGGTGTTTC | 123 |
| Reverse | AGATGAGTTTTTCAGGCTGGGTG | ||
| GS | Forward | AAACAGAGCAGCAGCAAAGTCAG | 116 |
| Reverse | CGGTCAGTGAAAGGTTTGGTGTC | ||
| EF1-α | Forward | GCCTGGTATGGTTGTGACCT | 202 |
| Reverse | GAAGTTAGCAGCACCCTTGG |
| Gene | Primer Sequence (5′-3′) | Size (bp) | |
|---|---|---|---|
| ACCase | Forward | AGGACTTGCCAATCTTCTAAAC | 158 |
| Reverse | AGCTTCTTTCACCGTAGGACAC | ||
| FAD2 | Forward | CACCACGCCTTCAGCGACTAC | 162 |
| Reverse | CTTCTTCTTGGGGACAAACACTTC | ||
| FAD3 | Forward | TTCCCACAAATCCCTCACTATCA | 132 |
| Reverse | ACTTGCCACCAAACTTTCCACC | ||
| EF1-α | Forward | GCCTGGTATGGTTGTGACCT | 202 |
| Reverse | GAAGTTAGCAGCACCCTTGG |
| Growing Season | Rapeseed Variety | N-app (kg ha−1) | N-acc (kg ha−1) | Branch-num | Seed-num | 1000-Seed Weight (g) | Silique-num | Seed Yield (kg ha−1) |
|---|---|---|---|---|---|---|---|---|
| 2022–2023 | V1 | 0 | 45.7 ± 3.44 c | 1.77 ± 0.322 b | 20.2 ± 1.34 b | 4.03 ± 0.116 a | 76.5 ± 9.46 c | 1907.8 ± 96.61 c |
| 180 | 79.1 ± 9.29 b | 3.60 ± 0.520 a | 23.7 ± 0.53 a | 3.90 ± 0.058 a | 122.2 ± 3.10 b | 3170.2 ± 201.98 b | ||
| 240 | 124.7 ± 16.10 a | 3.90 ± 0.265 a | 24.9 ± 0.67 a | 3.97 ± 0.058 a | 149.1 ± 7.81 a | 3514.7 ± 48.21 a | ||
| V2 | 0 | 39.5 ± 6.15 b | 1.50 ± 0.200 c | 19.7 ± 0.63 b | 3.50 ± 0.173 a | 82.0 ± 6.93 c | 1848.5 ± 77.22 b | |
| 180 | 88.8 ± 8.71 a | 3.50 ± 0.173 b | 22.8 ± 0.95 a | 3.40 ± 0.100 a | 133.8 ± 10.45 b | 3201.7 ± 123.24 a | ||
| 240 | 97.5 ± 6.38 a | 4.17 ± 0.153 a | 23.3 ± 0.48 a | 3.43 ± 0.058 a | 159.1 ± 8.71 a | 3265.7 ± 97.96 a | ||
| V3 | 0 | 31.9 ± 3.83 c | 2.40 ± 0.400 b | 18.5 ± 1.13 a | 3.57 ± 0.153 a | 95.0 ± 6.84 b | 1943.2 ± 148.11 b | |
| 180 | 96.2 ± 11.73 b | 4.73 ± 0.231 a | 19.3 ± 1.45 a | 3.53 ± 0.153 a | 166.1 ± 10.16 a | 3698.0 ± 142.20 a | ||
| 240 | 124.0 ± 8.80 a | 4.97 ± 0.208 a | 19.9 ± 0.49 a | 3.63 ± 0.116 a | 174.0 ± 8.95 a | 3727.0 ± 161.21 a | ||
| V4 | 0 | 35.8 ± 7.10 b | 1.43 ± 0.322 c | 18.1 ± 0.76 c | 3.33 ± 0.058 a | 76.0 ± 5.37 c | 1734.9 ± 111.93 b | |
| 180 | 82.2 ± 7.99 a | 4.37 ± 0.116 b | 23.8 ± 0.85 a | 3.30 ± 0.100 a | 134.1 ± 6.26 b | 2924.1 ± 183.53 a | ||
| 240 | 94.3 ± 8.67 a | 5.00 ± 0.100 a | 21.7 ± 1.35 b | 3.17 ± 0.116 a | 162.6 ± 11.08 a | 3163.8 ± 79.82 a | ||
| V5 | 0 | 38.4 ± 5.68 b | 1.93 ± 0.231 c | 21.2 ± 0.72 b | 4.27 ± 0.058 a | 82.5 ± 7.95 c | 1890.2 ± 77.09 c | |
| 180 | 83.8 ± 10.12 a | 3.90 ± 0.361 b | 21.8 ± 0.90 ab | 4.13 ± 0.116 a | 112.0 ± 7.58 b | 3004.7 ± 135.94 b | ||
| 240 | 78.2 ± 4.88 a | 4.63 ± 0.404 a | 23.2 ± 0.41 a | 4.13 ± 0.208 a | 146.6 ± 6.39 a | 3532.9 ± 88.01 a | ||
| 2023–2024 | V1 | 0 | 44.6 ± 6.10 b | 3.37 ± 0.116 c | 20.6 ± 0.83 b | 4.40 ± 0.132 a | 72.0 ± 8.26 b | 2099.7 ± 154.83 b |
| 180 | 109.0 ± 11.45 a | 5.67 ± 0.208 a | 22.6 ± 0.80 a | 4.35 ± 0.05 a | 121.4 ± 1.41 a | 3533.9 ± 69.81 a | ||
| 240 | 104.0 ± 9.26 a | 5.13 ± 0.252 b | 23.3 ± 0.65 a | 4.27 ± 0.029 a | 119.2 ± 10.65 a | 3700.2 ± 177.89 a | ||
| V2 | 0 | 44.1 ± 5.31 c | 3.53 ± 0.231 c | 21.3 ± 0.79 a | 3.87 ± 0.076 a | 79.3 ± 9.28 b | 2286.3 ± 118.12 c | |
| 180 | 98.3 ± 6.40 b | 4.40 ± 0.300 b | 22.8 ± 0.42 a | 3.88 ± 0.153 a | 133.8 ± 6.25 a | 3584.1 ± 179.08 a | ||
| 240 | 123.6 ± 4.94 a | 5.13 ± 0.322 a | 22.8 ± 0.96 a | 3.93 ± 0.076 a | 119.4 ± 10.44 a | 3305.9 ± 151.95 a | ||
| V3 | 0 | 35.0 ± 9.83 c | 3.90 ± 0.265 b | 18.8 ± 1.01 a | 4.15 ± 0.002 a | 77.6 ± 4.81 b | 1705.7 ± 80.99 c | |
| 180 | 100.3 ± 7.70 b | 5.93 ± 0.252 a | 19.6 ± 0.50 a | 4.35 ± 0.087 a | 158.7 ± 1.56 a | 3368.2 ± 102.76 b | ||
| 240 | 135.9 ± 10.81 a | 6.20 ± 0.300 a | 19.4 ± 0.89 a | 4.37 ± 0.076 a | 170.9 ± 10.28 a | 3798.8 ± 183.11 a | ||
| V4 | 0 | 29.1 ± 7.33 b | 2.87 ± 0.153 b | 17.7 ± 0.62 b | 3.80 ± 0.001 a | 69.1 ± 9.12 b | 1252.1 ± 90.31 c | |
| 180 | 89.3 ± 5.69 a | 5.03 ± 0.208 a | 22.2 ± 0.43 a | 3.80 ± 0.050 a | 116.9 ± 10.40 a | 2826.7 ± 76.84 b | ||
| 240 | 83.8 ± 5.86 a | 4.87 ± 0.153 a | 22.7 ± 0.56 a | 3.75 ± 0.001 a | 108.7 ± 3.35 a | 3761.8 ± 178.75 a | ||
| V5 | 0 | 37.0 ± 4.84 b | 3.00 ± 0.100 b | 21.0 ± 0.64 b | 4.78 ± 0.161 a | 77.3 ± 6.75 b | 1943.3 ± 149.93 b | |
| 180 | 102.4 ± 7.08 a | 4.33 ± 0.208 a | 23.8 ± 1.05 a | 4.60 ± 0.150 a | 95.2 ± 4.40 a | 2999.4 ± 173.50 a | ||
| 240 | 94.6 ± 9.57 a | 4.53 ± 0.306 a | 23.1 ± 0.69 a | 4.47 ± 0.202 a | 104.5 ± 6.48 a | 2788.4 ± 165.85 a |
| Factor | N-app | N-acc | Branch-num | Seed-num | 1000-Seed Weight | Silique-num |
|---|---|---|---|---|---|---|
| N-acc | 0.920 ** | — | — | — | — | — |
| Branch-num | 0.800 ** | 0.817 ** | — | — | — | — |
| Seed-num | 0.631 ** | 0.528 ** | 0.352 | — | — | — |
| 1000-seed weight | −0.060 | 0.050 | 0.196 | 0.121 | — | — |
| Silique-num | 0.820 ** | 0.819 ** | 0.712 ** | 0.293 | −0.259 | — |
| Seed yield | 0.922 ** | 0.911 ** | 0.810 ** | 0.562 ** | −0.014 | 0.848 ** |
| Effect | N-app (x1) | N-acc (x2) | Branch-num (x3) | Seed-num (x4) | 1000-Seeds Weight (x5) | Silique-num (x6) |
|---|---|---|---|---|---|---|
| Direct effect | 0.254 | 0.225 | 0.137 | 0.136 | 0.031 | 0.325 |
| Through x1 | — | — | — | — | — | — |
| Through x2 | 0.207 | — | — | — | — | — |
| Through x3 | 0.110 | 0.112 | — | — | — | — |
| Through x4 | 0.086 | 0.072 | 0.048 | — | — | — |
| Through x5 | −0.002 | 0.002 | 0.006 | 0.004 | — | — |
| Through x6 | 0.267 | 0.266 | 0.231 | 0.095 | −0.084 | — |
| Growing Season | Rapeseed Variety | N-app (kg ha−1) | N-acc (kg ha−1) | Oil Content (%) | Protein Content (%) | Oleic Acid Content (%) | Linoleic Acid Content (%) | Linolenic Acid Content (%) |
|---|---|---|---|---|---|---|---|---|
| 2022–2023 | V1 | 0 | 45.7 ± 3.44 c | 44.3 ± 0.99 a | 21.2 ± 0.84 a | 60.0 ± 2.97 a | 15.8 ± 0.22 a | 9.27 ± 0.265 a |
| 180 | 79.1 ± 9.29 b | 44.6 ± 0.53 a | 20.4 ± 0.25 a | 60.2 ± 1.83 a | 15.9 ± 0.48 a | 9.23 ± 0.544 a | ||
| 240 | 124.7 ± 16.10 a | 42.7 ± 1.60 a | 22.1 ± 1.28 a | 58.4 ± 2.69 a | 16.5 ± 0.31 a | 8.98 ± 0.217 a | ||
| V2 | 0 | 39.5 ± 6.15 b | 45.3 ± 0.15 b | 20.4 ± 0.54 a | 63.7 ± 1.87 a | 14.5 ± 0.54 a | 8.83 ± 0.479 a | |
| 180 | 88.8 ± 8.71 a | 45.8 ± 0.30 ab | 19.5 ± 0.50 ab | 58.3 ± 3.15 a | 14.8 ± 0.87 a | 9.00 ± 0.325 a | ||
| 240 | 97.5 ± 6.38 a | 46.3 ± 0.45 a | 19.3 ± 0.39 b | 57.4 ± 5.12 a | 15.4 ± 0.64 a | 9.40 ± 0.340 a | ||
| V3 | 0 | 31.9 ± 3.83 c | 45.7 ± 1.79 a | 19.9 ± 1.26 a | 64.1 ± 2.62 ab | 14.7 ± 0.40 a | 8.60 ± 0.272 a | |
| 180 | 96.2 ± 11.73 b | 44.8 ± 1.50 a | 20.0 ± 1.29 a | 60.3 ± 3.25 b | 14.7 ± 0.84 a | 8.80 ± 0.162 a | ||
| 240 | 124.0 ± 8.80 a | 46.6 ± 1.59 a | 18.5 ± 1.58 a | 67.3 ± 2.59 a | 14.0 ± 0.62 a | 8.50 ± 0.256 a | ||
| V4 | 0 | 35.8 ± 7.10 b | 46.3 ± 1.14 a | 19.3 ± 0.65 a | 63.9 ± 1.59 a | 14.6 ± 0.27 a | 8.94 ± 0.301 ab | |
| 180 | 82.2 ± 7.99 a | 46.7 ± 0.52 a | 18.4 ± 0.23 a | 62.9 ± 2.05 a | 14.3 ± 0.39 a | 9.14 ± 0.093 a | ||
| 240 | 94.3 ± 8.67 a | 47.1 ± 1.07 a | 18.2 ± 0.74 a | 65.0 ± 0.91 a | 14.7 ± 0.43 a | 8.61 ± 0.280 b | ||
| V5 | 0 | 38.4 ± 5.68 b | 47.9 ± 0.90 a | 19.2 ± 0.37 a | 64.2 ± 2.76 a | 12.7 ± 0.60 a | 8.49 ± 0.213 a | |
| 180 | 83.8 ± 10.12 a | 47.7 ± 0.39 a | 19.5 ± 0.28 a | 64.9 ± 0.10 a | 12.5 ± 0.54 a | 8.62 ± 0.169 a | ||
| 240 | 78.2 ± 4.88 a | 46.7 ± 2.03 a | 19.5 ± 0.93 a | 61.4 ± 2.53 a | 13.1 ± 1.47 a | 8.64 ± 0.347 a | ||
| 2023–2024 | V1 | 0 | 44.6 ± 6.10 b | 48.6 ± 0.44 a | 19.7 ± 0.33 a | 65.8 ± 5.20 a | 17.1 ± 0.83 a | 8.58 ± 0.245 a |
| 180 | 109.0 ± 11.45 a | 48.4 ± 1.18 a | 20.2 ± 1.08 a | 64.0 ± 2.88 a | 17.5 ± 0.42 a | 8.46 ± 0.250 a | ||
| 240 | 104.0 ± 9.26 a | 48.1 ± 0.22 a | 20.5 ± 0.17 a | 65.7 ± 2.06 a | 17.9 ± 0.73 a | 8.53 ± 0.437 a | ||
| V2 | 0 | 44.1 ± 5.31 c | 48.6 ± 0.64 a | 19.3 ± 0.82 a | 67.5 ± 2.22 a | 16.9 ± 0.58 a | 8.41 ± 0.296 a | |
| 180 | 98.3 ± 6.40 b | 48.5 ± 0.56 a | 19.2 ± 0.49 a | 67.1 ± 0.77 a | 16.5 ± 0.41 a | 7.62 ± 0.360 b | ||
| 240 | 123.6 ± 4.94 a | 47.5 ± 0.81 a | 20.5 ± 0.81 a | 68.7 ± 0.74 a | 16.6 ± 0.17 a | 7.82 ± 0.185 b | ||
| V3 | 0 | 35.0 ± 9.83 c | 49.1 ± 1.34 a | 18.2 ± 1.22 a | 68.9 ± 0.92 a | 15.9 ± 0.28 a | 7.48 ± 0.312 a | |
| 180 | 100.3 ± 7.70 b | 48.6 ± 0.27 a | 18.6 ± 0.30 a | 69.3 ± 0.53 a | 16.2 ± 0.72 a | 7.10 ± 0.308 a | ||
| 240 | 135.9 ± 10.81 a | 48.7 ± 0.08 a | 18.4 ± 0.31 a | 68.5 ± 0.40 a | 15.5 ± 0.61 a | 7.23 ± 0.136 a | ||
| V4 | 0 | 29.1 ± 7.33 b | 49.6 ± 0.43 a | 18.9 ± 0.41 a | 68.3 ± 0.68 a | 16.6 ±0.56 a | 7.52 ± 0.271 a | |
| 180 | 89.3 ± 5.69 a | 49.2 ± 0.87 a | 18.9 ± 0.74 a | 67.8 ± 0.57 a | 16.7 ± 0.44 a | 7.47 ± 0.257 a | ||
| 240 | 83.8 ± 5.86 a | 48.6 ± 0.91 a | 19.4 ± 0.66 a | 68.2 ± 1.05 a | 16.8 ± 0.54 a | 7.48 ± 0.204 a | ||
| V5 | 0 | 37.0 ± 4.84 b | 51.4 ± 1.03 a | 18.8 ± 0.74 a | 68.3 ± 1.26 a | 14.8 ± 0.23 a | 8.22 ± 0.252 a | |
| 180 | 102.4 ± 7.08 a | 50.6 ± 0.76 a | 19.7 ± 0.39 a | 66.9 ± 2.82 a | 14.2 ± 0.79 a | 7.43 ± 0.340 b | ||
| 240 | 94.6 ± 9.57 a | 50.2 ± 0.57 a | 20.1 ± 0.77 a | 69.5 ± 0.34 a | 15.1 ± 0.63 a | 7.30 ± 0.135 b |
| Index | N-app | N-acc | Protein Content | Oil Content | Oleic Acid Content | Linoleic Acid Content |
|---|---|---|---|---|---|---|
| N-acc | 0.920 ** | — | — | — | — | — |
| Protein content | 0.053 | 0.111 | — | — | — | — |
| Oil content | −0.091 | −0.092 | −0.540 ** | — | — | — |
| Oleic acid content | −0.089 | −0.028 | −0.476 ** | 0.808 ** | — | — |
| Linoleic acid content | 0.048 | 0.144 | 0.248 | 0.131 | 0.213 | — |
| Linolenic acid content | −0.116 | −0.170 | 0.381 * | −0.748 ** | −0.856 ** | −0.249 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wang, X.; Yang, J.; Zhang, Z.; Chen, M. Effects and Mechanism of Nitrogen Regulation on Seed Yield and Quality of Rapeseed (Brassica napus L.). Agronomy 2025, 15, 1232. https://doi.org/10.3390/agronomy15051232
Wang C, Wang X, Yang J, Zhang Z, Chen M. Effects and Mechanism of Nitrogen Regulation on Seed Yield and Quality of Rapeseed (Brassica napus L.). Agronomy. 2025; 15(5):1232. https://doi.org/10.3390/agronomy15051232
Chicago/Turabian StyleWang, Chunli, Xiaojun Wang, Jianli Yang, Zhi Zhang, and Miaomiao Chen. 2025. "Effects and Mechanism of Nitrogen Regulation on Seed Yield and Quality of Rapeseed (Brassica napus L.)" Agronomy 15, no. 5: 1232. https://doi.org/10.3390/agronomy15051232
APA StyleWang, C., Wang, X., Yang, J., Zhang, Z., & Chen, M. (2025). Effects and Mechanism of Nitrogen Regulation on Seed Yield and Quality of Rapeseed (Brassica napus L.). Agronomy, 15(5), 1232. https://doi.org/10.3390/agronomy15051232





