Biochar Herbicide Protection Pods for Mitigating Herbicide Sensitivity in Tomato Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Background
2.2. Visual Injury Analysis
2.3. Growth Parameters
2.4. Enzymatic Activity Assays
2.5. Data Collection and Analysis
3. Results
3.1. Fresh Weight and Plant Length
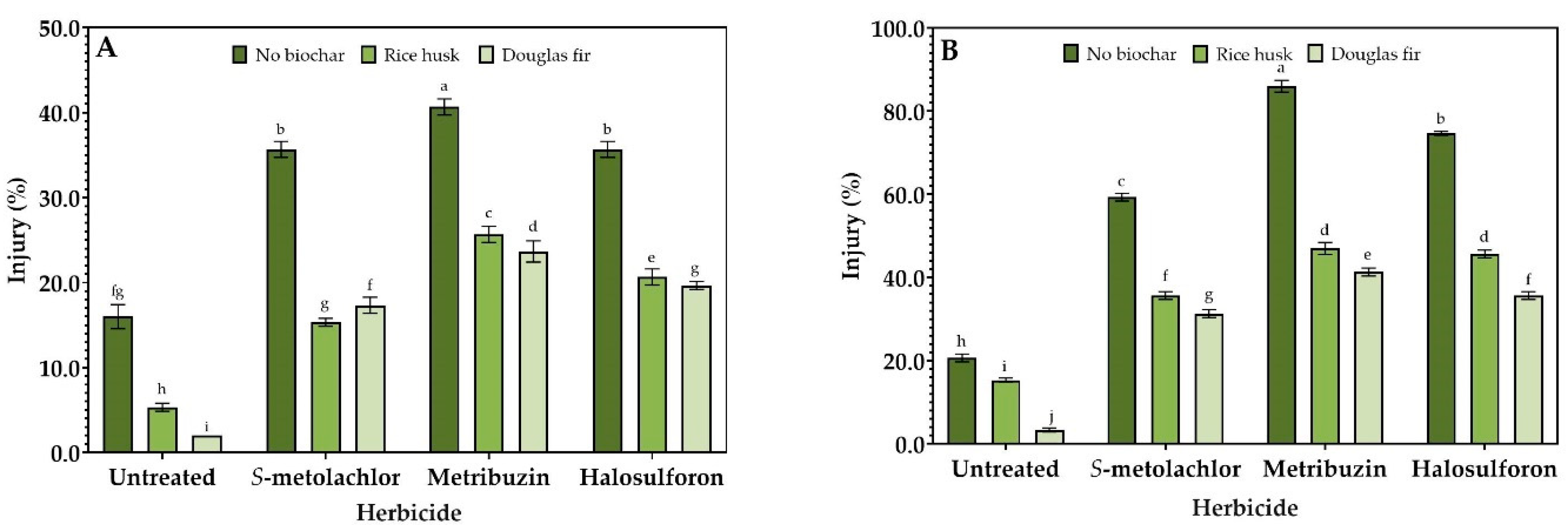
3.2. Injury%
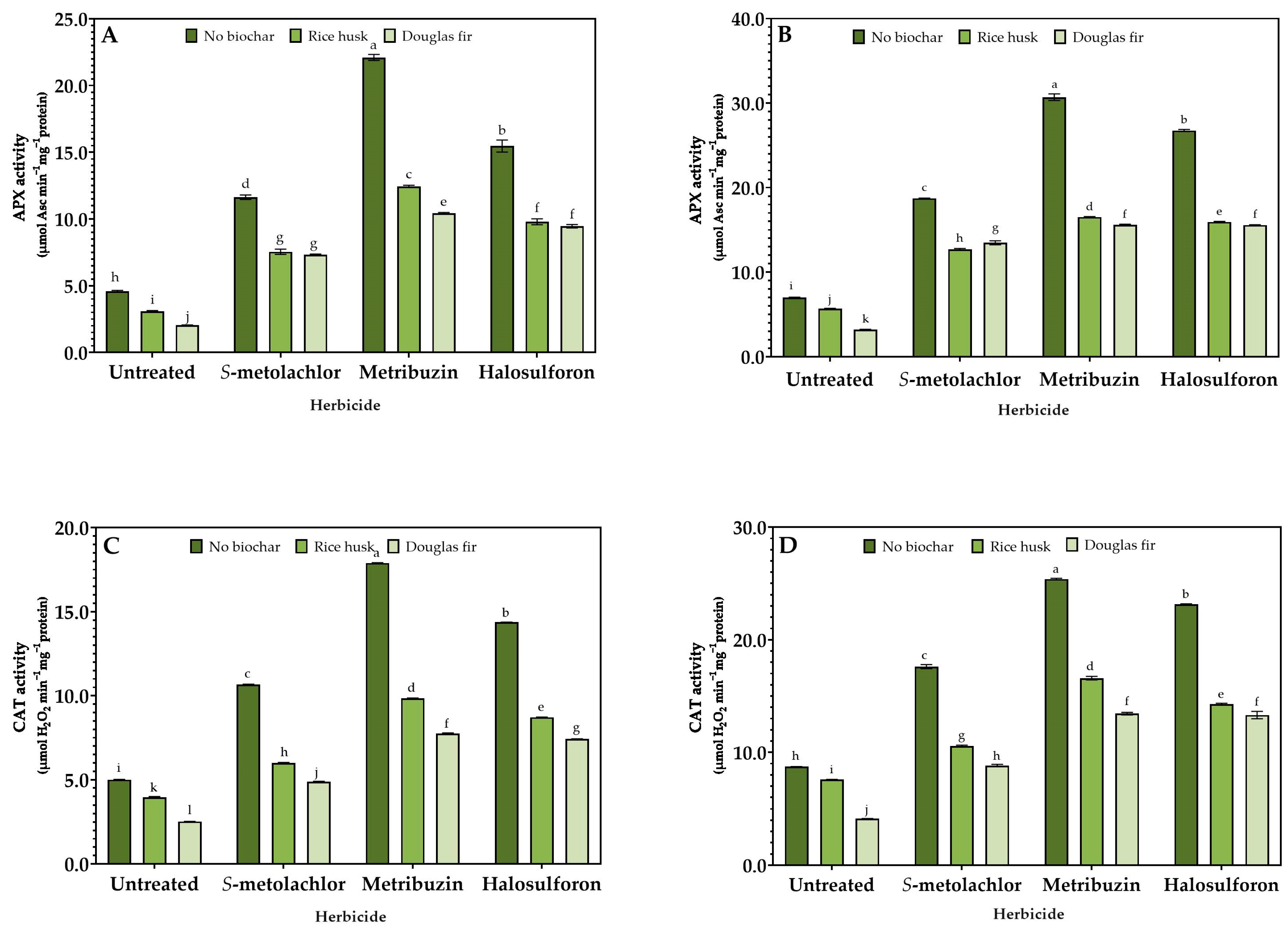
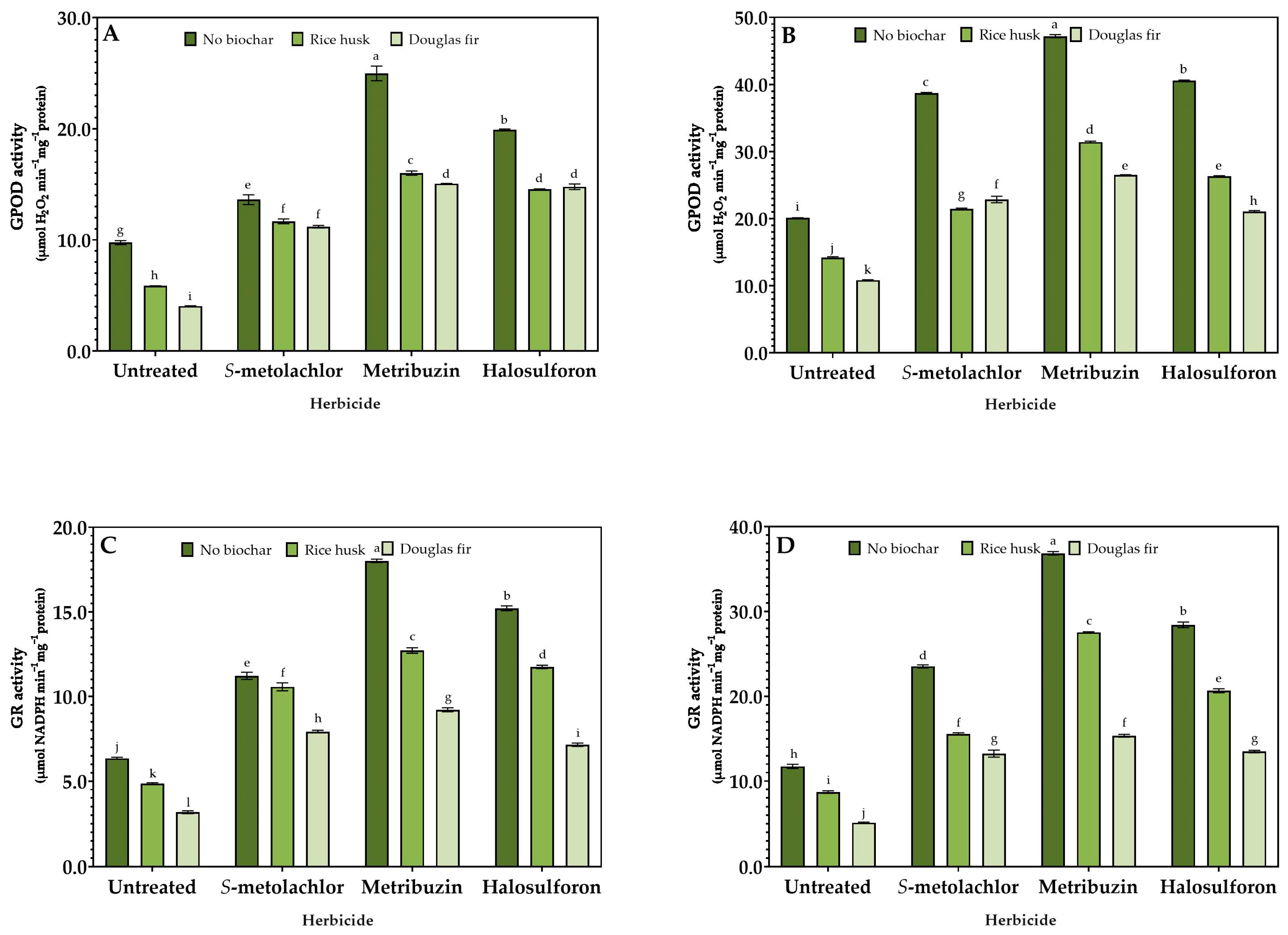
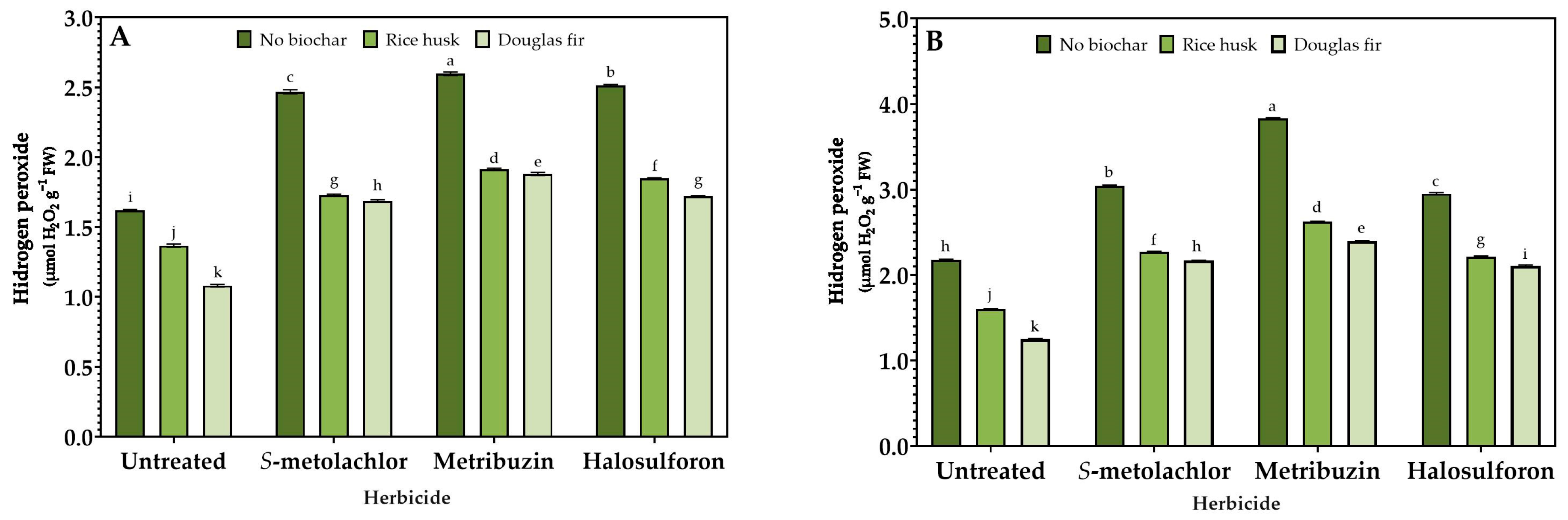
3.3. Stress Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maham, S.G.; Rahimi, A.; Subramanian, S.; Smith, D.L. The environmental impacts of organic greenhouse tomato production based on the nitrogen-fixing plant (Azolla). J. Clean. Prod. 2020, 245, 118679. [Google Scholar] [CrossRef]
- USDA. Vegetable and Pulses Outlook, April 2024; USDA: Washington, DC, USA, 2024. [Google Scholar]
- Qu, Z.M.; Chen, Q.; Feng, H.J.; Hao, M.; Niu, G.L.; Liu, Y.L.; Li, C.L. Interactive effect of irrigation and blend ratio of controlled release potassium chloride and potassium chloride on greenhouse tomato production in the Yellow River Basin of China. Agric. Water Manag. 2022, 261, 107346. [Google Scholar] [CrossRef]
- Yan, F.L.; Zhang, F.C.; Fan, X.K.; Fan, J.L.; Wang, Y.; Zou, H.Y.; Wang, H.D.; Li, G.D. Determining irrigation amount and fertilization rate to simultaneously optimize grain yield, grain nitrogen accumulation and economic benefit of drip-fertigated spring maize in northwest China. Agric. Water Manag. 2021, 243, 106440. [Google Scholar] [CrossRef]
- Tiwari, R.; Bashyal, M.; Kanissery, R. Weed management strategies for tomato plasticulture production in Florida. Plants 2022, 11, 3292. [Google Scholar] [CrossRef]
- Ustuner, T.; Sakran, A.; Almhemed, K. Effect of herbicides on living organisms in the ecosystem and available alternative control methods. Int. J. Sci. Res. Publ. 2020, 10, 633–641. [Google Scholar]
- Clark, J. Tupelo Organic Farmer Loses Crop to Herbicide Drift. Dly. J. 2013. Available online: https://www.djournal.com/news/tupelo-organic-farmer-loses-crop-to-herbicide-rift/article_b0bf99b1-989a-51af-9148-747cb69332cf.html (accessed on 27 March 2025).
- Vasseghian, Y.; Nadagouda, M.M.; Aminabhavi, T.M. Biochar-enhanced bioremediation of eutrophic waters impacted by algal blooms. J. Environ. Manag. 2024, 367, 122044. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science and Technology, 1st ed.; Routledge: New York, NY, USA, 2009. [Google Scholar]
- Li, C.; Tao, J.; Wu, Z. Gaseous ozone regulates reactive oxygen species metabolism and ascorbate–glutathione cycle to delay the senescence of fresh-cut red pitaya (Selenicereus undatus) fruit. Sci. Hortic. 2023, 312, 111839. [Google Scholar] [CrossRef]
- Yasin, M.U.; Haider, Z.; Munir, R.; Zulfiqar, U.; Rehman, M.; Javaid, M.H.; Ahmad, I.; Nana, C.; Saeed, M.S.; Ali, B.; et al. The synergistic potential of biochar and nanoparticles in phytoremediation and enhancing cadmium tolerance in plants. Chemosphere 2024, 354, 141672. [Google Scholar] [CrossRef]
- Faloye, O.T.; Alatise, M.O.; Ajayi, A.E.; Ewulo, B.S. Effects of biochar and inorganic fertilizer applications on growth, yield and water use efficiency of maize under deficit irrigation. Agric. Water Manag. 2019, 217, 165–178. [Google Scholar] [CrossRef]
- Wu, K.Q.; Zhao, W.J.; Ma, F.; Ma, H.; Cao, W. Effect of biochar and attapulgite amendment on saline soil hydraulic parameters and shrinkage. Environ. Prog. Sustain. Energy 2023, 42, e14060. [Google Scholar] [CrossRef]
- Hou, J.; Pugazhendhi, A.; Sindhu, R.; Vinayak, V.; Thanh, N.C.; Brindhadevi, K.; Yuan, D. An assessment of biochar as a potential amendment to enhance plant nutrient uptake. Environ. Res. 2022, 214, 113909. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Wang, F.; Yang, X. The impact of the planting of forest biomass energy plants under the embedded Internet of Things technology on the biodiversity of the local environmental ecology. Environ. Technol. Innov. 2021, 24, 101894. [Google Scholar] [CrossRef]
- Guo, L.L.; Yu, H.W.; Kharbach, M.; Zhang, W.; Wang, J.W.; Niu, W.Q. Biochar improves soil-tomato plant, tomato production, and economic benefits under reduced nitrogen application in northwestern China. Plants 2021, 10, 759. [Google Scholar] [CrossRef]
- Wang, Y.F.; Pan, F.B.; Wang, G.S.; Zhang, G.D.; Wang, Y.L.; Chen, X.S.; Mao, Z.Q. Effects of biochar on photosynthesis and antioxidative system of Malus hupehensis Rehd seedlings under replant conditions. Sci. Hortic. 2014, 175, 9–15. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, J.; Guo, L.; Fang, H.; Liu, X.; Liang, K.; Siddique, K.H. Effects of two biochar types on mitigating drought and salt stress in tomato seedlings. Agronomy 2023, 13, 1039. [Google Scholar] [CrossRef]
- Khorram, M.S.; Zhang, Q.; Lin, D.; Zheng, Y.; Fang, H.; Yu, Y. Biochar: A review of its impact on pesticide behavior in soil environments and its potential applications. J. Environ. Sci. 2016, 44, 269–279. [Google Scholar] [CrossRef]
- Madsen, M.D.; Davies, K.W.; Mummey, D.L.; Svejcar, T.J. Improving restoration of exotic annual grass-invaded rangelands through activated carbon seed enhancement technologies. Rangel. Ecol. Manag. 2014, 67, 61–67. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Thamaraiselvi, K.; Namasivayam, C. Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresour. Technol. 2001, 76, 63–65. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Azevedo, R.A.; Alas, R.M.; Smith, R.J.; Lea, P.J. Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild-type and a catalase-deficient mutant of barley. Physiol. Plant. 1998, 104, 280–292. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Urbanek, H.; Kuźniak-Gębarowska, E.; Herka, K. Elicitation of defence responses in bean leaves by Botrytis cinerea polygalacturonase. Acta Physiol. Plant. 1991, 13, 43–50. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A.J.P.S. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, K.; Wu, Y.; Yu, H.; Cao, W.; Ma, H. Effects of biochar amendment on greenhouse tomato quality, nutrient uptake and use efficiency under various irrigation and fertilization regimes. Sci. Hortic. 2024, 337, 113441. [Google Scholar] [CrossRef]
- Zhao, R.; Ma, X.; Xu, J.; Zhang, Q. Removal of the pesticide imidacloprid from aqueous solution by biochar derived from peanut shell. BioResources 2018, 13, 5656–5669. [Google Scholar] [CrossRef]
- Hasnain, M.; Munir, N.; Abideen, Z.; Zulfiqar, F.; Koyro, H.W.; El-Naggar, A.; Caçador, I.; Duarte, B.; Rinklebe, J.; Yong, J.W.H. Biochar-plant interaction and detoxification strategies under abiotic stresses for achieving agricultural resilience: A critical review. Ecotoxicol. Environ. 2023, 249, 114408. [Google Scholar] [CrossRef]
- Ibrahim, R.I.; Alkhudairi, U.A.; Alhusayni, S.A. Alleviation of herbicide toxicity in Solanum lycopersicum L.—An antioxidant stimulation approach. Plants 2022, 11, 2261. [Google Scholar] [CrossRef]
- Tognetti, V.B.; Bielach, A.; Hrtyan, M. Redox regulation at the site of primary growth: Auxin, cytokinin and ROS crosstalk. Plant Cell Environ. 2017, 40, 2586–2605. [Google Scholar] [CrossRef]
- Khanna-Chopra, R. Leaf senescence and abiotic stresses share reactive oxygen species-mediated chloroplast degradation. Protoplasma 2012, 249, 469–481. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol. 2016, 171, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.S.; Li, G.T.; Andersen, M.N.; Liu, F.L. Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Manag. 2014, 138, 37–44. [Google Scholar] [CrossRef]
- Kamyab, H.; Chelliapan, S.; Khalili, E.; Rezania, S.; Balasubramanian, B.; Taheri, M.M.; Simancas-Racines, D.; Rajendran, S.; Yusuf, M. Biochar as a carrier for plant growth-promoting bacteria in phytoremediation of pesticides. J. Hazard. Mater. Adv. 2025, 18, 100673. [Google Scholar] [CrossRef]
- Qiu, Y.; Pang, H.; Zhou, Z.; Zhang, P.; Feng, Y.; Sheng, D.G. Competitive biodegradation of dichlobenil and atrazine coexisting in soil amended with a char and citrate. Environ. Pollut. 2009, 157, 2964–2969. [Google Scholar] [CrossRef]
- Yu, X.Y.; Ying, G.G.; Kookana, R.S. Sorption and desorption behaviors of diuron in soils amended with charcoal. J. Agric. Food Chem. 2006, 54, 8545–8550. [Google Scholar] [CrossRef]
- Zhang, P.; Sheng, G.; Feng, Y.; Miller, D.M. Role of wheat-residue-derived char in the biodegradation of benzonitrile in soil: Nutritional stimulation versus adsorptive inhibition. Environ. Sci. Technol. 2005, 39, 5442–5448. [Google Scholar] [CrossRef]
- Yang, Y.; Sheng, G.; Huang, M. Bioavailability of diuron in soil containing wheat-straw-derived char. Sci. Total Environ. 2006, 354, 170–178. [Google Scholar] [CrossRef]
- Yu, X.Y.; Ying, G.G.; Kookana, R.S. Reduced plant uptake of pesticides with biochar additions to soil. Chemosphere 2009, 76, 665–671. [Google Scholar] [CrossRef]
- Yang, X.B.; Ying, G.G.; Peng, P.A.; Wang, L.; Zhao, J.L.; Zhang, L.J.; Yuan, P.; He, H.P. Influence of biochars on plant uptake and dissipation of two pesticides in an agricultural soil. J. Agric. Food Chem. 2010, 58, 7915–7921. [Google Scholar] [CrossRef]
- Traxler, C.; Gaines, T.A.; Küpper, A.; Luemmen, P.; Dayan, F.E. The nexus between reactive oxygen species and the mechanism of action of herbicides. J. Biol. Chem. 2023, 299, 105267. [Google Scholar] [CrossRef]
- Sonalin Rath, S.; Das, S. Oxidative stress-induced DNA damage and DNA repair mechanisms in mangrove bacteria exposed to climatic and heavy metal stressors. Environ. Pollut. 2023, 339, 122722. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.K.; Noman, A.; Alhaithloul, H.A.S.; Adeel, M.; Rui, Y.; Shah, T.; Zhu, S.; Shang, J. Goethite-modified biochar ameliorates the growth of rice (Oryza sativa L.) plants by suppressing Cd and As-induced oxidative stress in Cd and As co-contaminated paddy soil. Sci. Total Environ. 2020, 717, 137086. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.; Aqeel, M. miRNA-based heavy metal homeostasis and plant growth. Environ. Sci. Pollut. 2017, 24, 10068–10082. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, S.S.; Corpas, F.J.; Ortega-Villasante, C.; Hernandez, L.E.; Tuteja, N.; Sofo, A.; Hasanuzzaman, M.; Fujita, M. Recent Insights into the double role of hydrogen peroxide in Plants. Front. Plant Sci. 2022, 13, 843274. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.; Asaf, S.; Waqas, M.; Park, J.; Asif, S.; Kim, N.; Lee, I.; Kim, K. Drought and UV Radiation Stress Tolerance in Rice Is Improved by Overaccumulation of Non-Enzymatic Antioxidant Flavonoids. Antioxidants 2022, 11, 917. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Yan, Y.; Ma, R.; Zhang, X.; Wang, J.; Shen, Y.; Ullah, H.; Lu, L. Removal of organophosphorus flame retardant by biochar-coated nZVI activating persulfate: Synergistic mechanism of adsorption and catalytic degradation. Environ. Pollut. 2023, 331, 121880. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.; Rahman, A.; Mahmud, J.A.; Suzuki, T.; Fujita, M. Insights into spermine-induced combined high temperature and drought tolerance in mung bean: Osmoregulation and roles of antioxidant and glyoxalase system. Protoplasma 2017, 254, 445–460. [Google Scholar] [CrossRef]
- Fijalkowski, K.L.; Kwarciak-Kozlowska, A. Phytotoxicity assay to assess sewage sludge phytoremediation rate using guaiacol peroxidase activity (GPX): A comparison of four growth substrates. J. Environ. Manag. 2020, 263, 110413. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Z.; Chen, X.; Li, E.; Li, Y.; Zhang, C.; Hou, X. BcWRKY22 ativa BcCAT2 para aumentar a atividade da catalase (CAT) e reduzir o acúmulo de peróxido de hidrogênio (H2O2), promovendo a termotolerância na couve chinesa não cabeçuda (Brassica campestris ssp. chinensis). Antioxidants 2023, 12, 1710. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Yousaf, B.; Liu, G.; Wang, R.; Zia-ur-Rehman, M.; Rizwan, M.S.; Imtiaz, M.; Shakoor, A. Investigating the potential influence of biochar and traditional organic amendments on the bioavailability and transfer of Cd in the soil–plant system. Environ. Earth Sci. 2016, 75, 374. [Google Scholar] [CrossRef]
- Cheng, J.; Lee, X.; Gao, W.; Chen, Y.; Pan, W.; Tang, Y. Effect of biochar on the bioavailability of difenoconazole and microbial community composition in a pesticide-contaminated soil. Appl. Soil Ecol. 2017, 121, 185–192. [Google Scholar] [CrossRef]

| Types of Biochar Treatment with Herbicide Application | ||
|---|---|---|
| No Biochar | Rice Husk (RH) * HPPs | Douglas Fir (DF) HPPs |
| Control (no herbicide) | RH (no herbicide) | DF (no herbicide) |
| Control + S-metolachlor | RH + S-metolachlor | DF + metolachlor |
| Control + metribuzin | RH + metribuzin | DF + metribuzin |
| Control + halosulfuron | RH + halosulfuron | DF + halosulfuron |
| Herbicide | Trade Name | Rates Used (2× Standard Rates) |
|---|---|---|
| S-metolachlor | Dual II Magnum | 6.72 kg ha−1 |
| Metribuzin | Glory | 1.12 kg ha−1 |
| Halosulfuron | Profine | 0.044 kg ha−1 |
| Injury% | Symptomology |
|---|---|
| 0–10 | No injuries were seen, and no reduction in growth or yellowing of leaves seen |
| 11–30 | Little to moderate injury and yellowing of leaves |
| 31–50 | Yellowing at the base; basal leaves are more yellowish, and leaf curling is seen; growth reduction is moderate |
| 51–70 | Yellowing of leaves is seen, more with growth reduction; moderate to severe injuries are seen |
| 71–95 | Severely injured, with almost all yellow leaves and no growth seen |
| 96–100 | Almost dead or dead plant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sil, S.; Souza, F.R.d.; Bullard, B.; Mlsna, T.; Tseng, T.-M. Biochar Herbicide Protection Pods for Mitigating Herbicide Sensitivity in Tomato Plants. Agronomy 2025, 15, 1188. https://doi.org/10.3390/agronomy15051188
Sil S, Souza FRd, Bullard B, Mlsna T, Tseng T-M. Biochar Herbicide Protection Pods for Mitigating Herbicide Sensitivity in Tomato Plants. Agronomy. 2025; 15(5):1188. https://doi.org/10.3390/agronomy15051188
Chicago/Turabian StyleSil, Sandipan, Fernanda Reolon de Souza, Bailey Bullard, Todd Mlsna, and Te-Ming Tseng. 2025. "Biochar Herbicide Protection Pods for Mitigating Herbicide Sensitivity in Tomato Plants" Agronomy 15, no. 5: 1188. https://doi.org/10.3390/agronomy15051188
APA StyleSil, S., Souza, F. R. d., Bullard, B., Mlsna, T., & Tseng, T.-M. (2025). Biochar Herbicide Protection Pods for Mitigating Herbicide Sensitivity in Tomato Plants. Agronomy, 15(5), 1188. https://doi.org/10.3390/agronomy15051188






