Effects of Litter Input on Soil Enzyme Activities and Their Stoichiometric Ratios in Sandy Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Location
2.2. Experimental Design
2.3. Research Methods
2.3.1. Determination of Litter Chemical Properties
2.3.2. Soil Chemical Properties Determination
2.3.3. Soil Enzyme Activity Determination and Stoichiometric Ratio Calculation
2.4. Statistical Analysis
3. Results
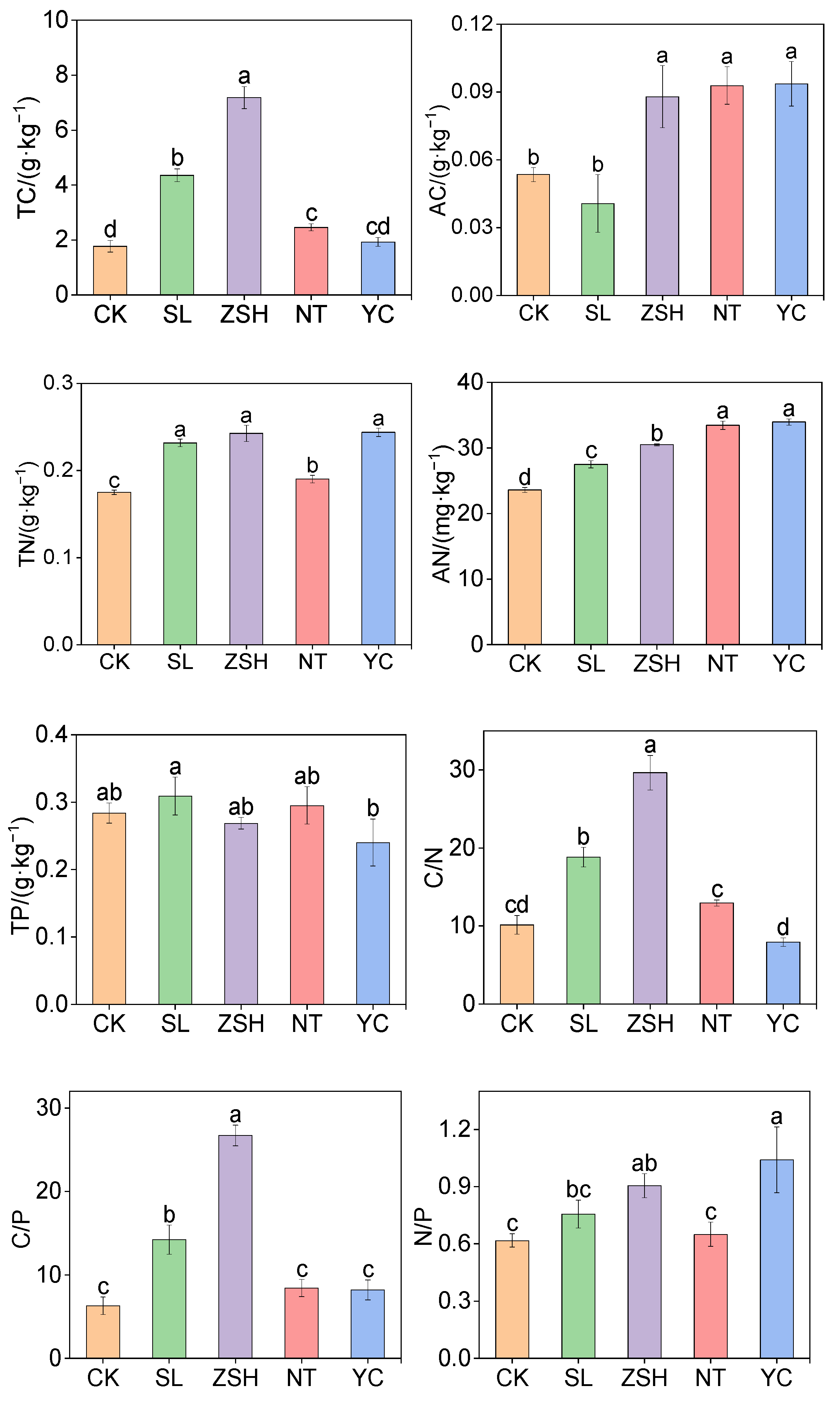
3.1. Effects of Litter Input on Soil Physical and Chemical Properties
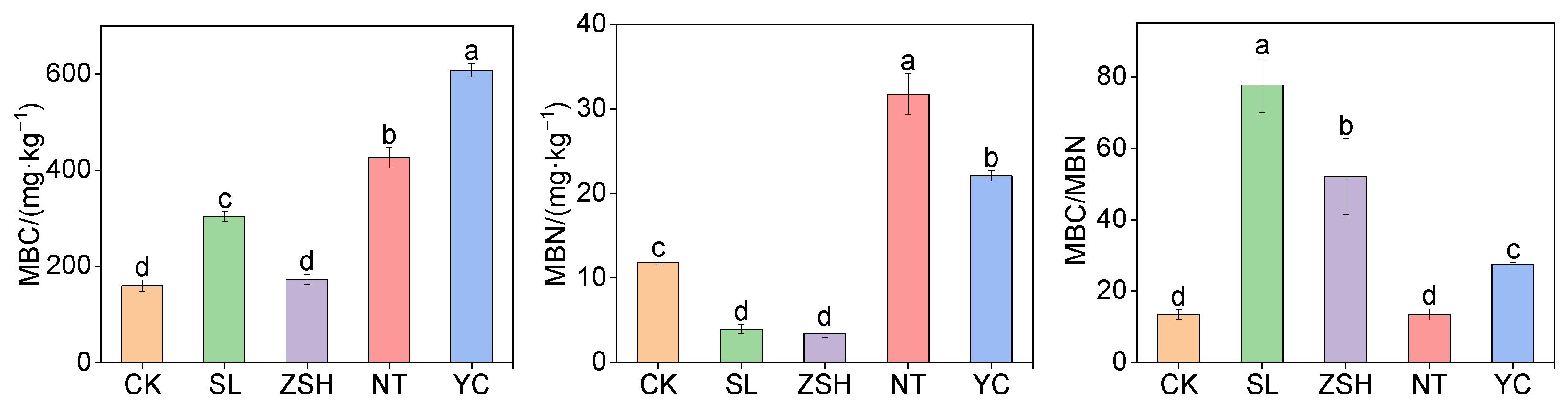
3.2. Effects of Litter Input on Soil Microbial Biomass and Its Stoichiometric Ratio
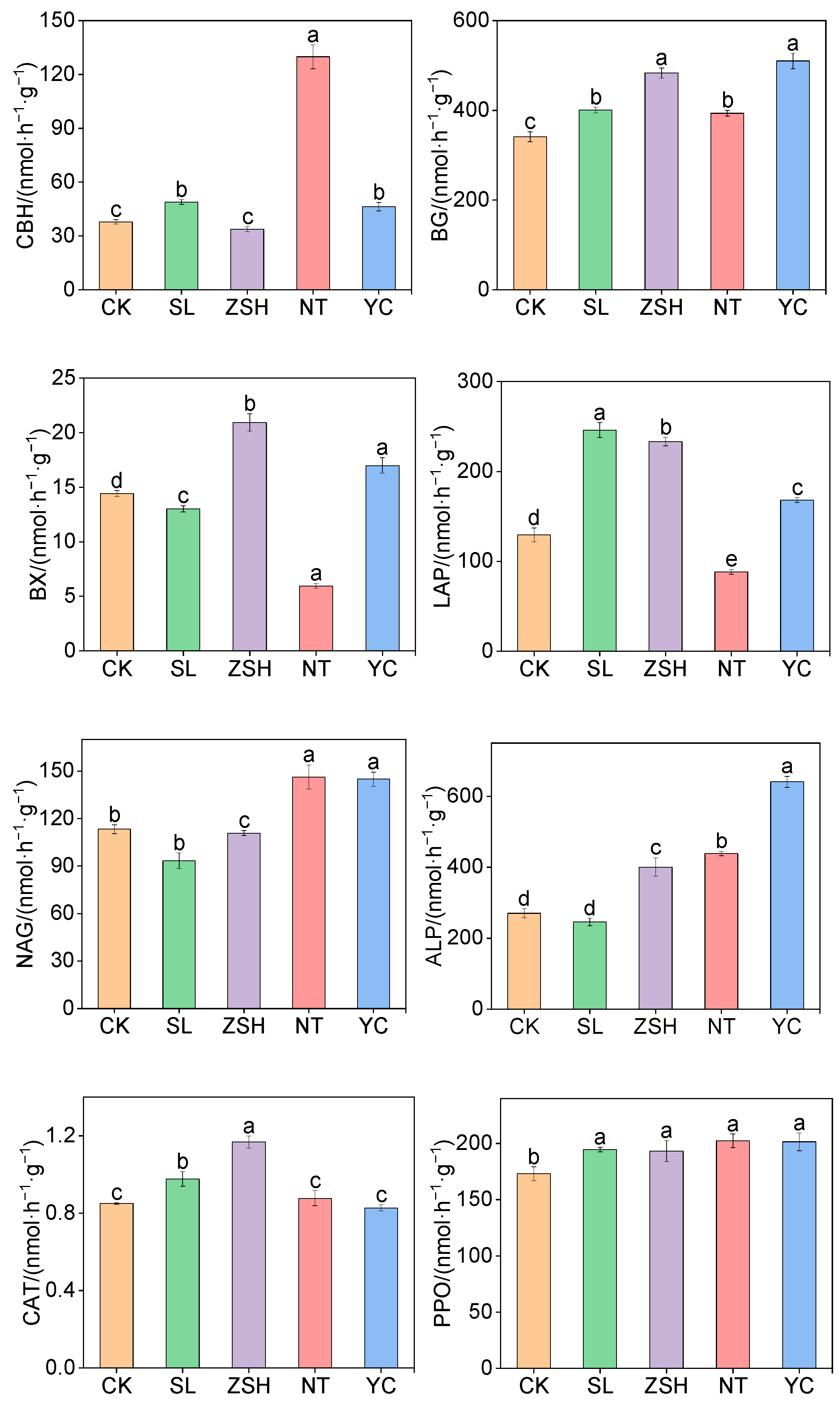
3.3. Effects of Litter Input on Soil Enzyme Activities and Their Stoichiometric Ratios
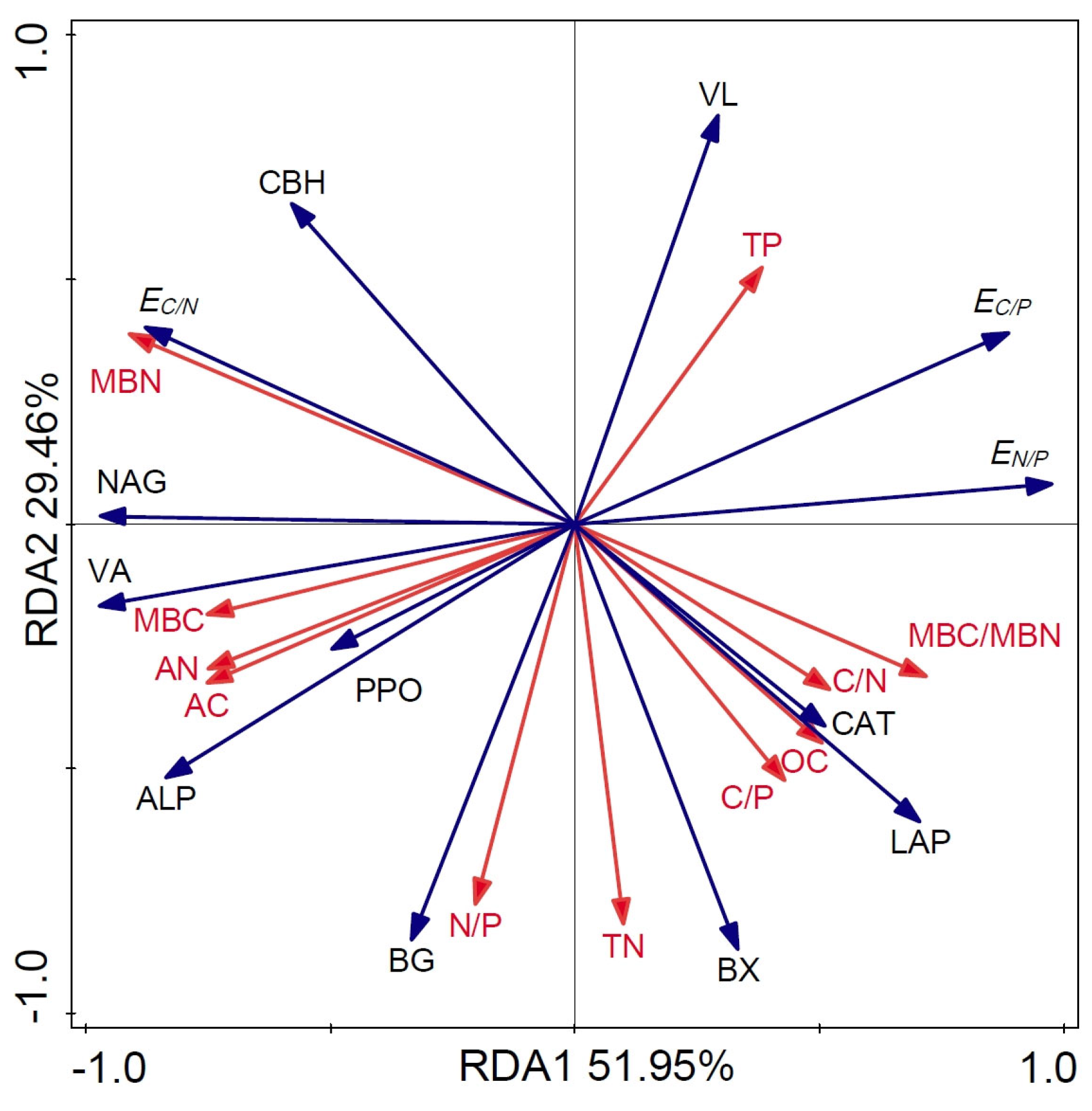
3.4. Effects of Soil Factors on Enzyme Activity and Enzyme Stoichiometric Ratio
4. Discussion
4.1. Effects of Litter Input Changes on Soil Chemical Properties
4.2. Effects of Litter Input Changes on Enzyme Activities and Stoichiometric Ratios
4.3. Main Factors Affecting Microbial Enzyme Activity and Its Stoichiometric Ratio
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fei, B.Q.; Ma, H.R.; Yin, J.; Zhang, L.G.; Li, J.; Xiu, X.M.; Zhou, D.Z.; Pang, Y.J.; Zhang, Y.D.; Jia, X.H.; et al. Landscape Dynamics of the Mu Us Sandy Land Based on Multi-Source Remote Sensing Images. Land 2024, 13, 977. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Huang, M.; Xiao, C.J.; Qi, S.S.; Du, W.Y.; Zhu, D.Y.; Altan, O. Integrating Remote Sensing and Spatiotemporal Analysis to Characterize Artificial Vegetation Restoration Suitability in Desert Areas: A Case Study of Mu Us Sandy Land. Remote Sens. 2022, 14, 4736. [Google Scholar] [CrossRef]
- Yuan, J.; Wu, F.Z.; Peng, C.H.; Peñuelas, J.; Vallicrosa, H.; Sardans, J.; Peng, Y.; Wu, Q.Q.; Li, Z.M.; Heděnec, P.; et al. Global spectra of plant litter carbon, nitrogen and phosphorus concentrations and returning amounts. J. Ecol. 2024, 112, 717–729. [Google Scholar] [CrossRef]
- Luo, X.X.; Qiu, K.Y.; Jin, T.; Bao, P.A.; Huang, Y.Y. The effects of carbon, nitrogen, and potassium addition on the decomposition characteristics of litter in desert grasslands. Acta Prataculturae Sin. 2025, 34, 41–53. [Google Scholar]
- Wang, L.Y.; Zhou, G.N.; Zhu, X.Y.; Gao, B.J.; Xu, H.D. Effects of litter on soil organic carbon and microbial functional diversity. Acta Ecol. Sin. 2021, 41, 2709–2718. [Google Scholar]
- Li, Q.; Wang, F.C.; Liu, R.; Hu, X.F.; Wang, H.M.; Chen, F.S. Aboveground litter input alters the effects of understory vegetation removal on soil microbial communities and enzyme activities along a 60-cm profile in a subtropical plantation forest. Appl. Soil Ecol. 2022, 176, 104489. [Google Scholar] [CrossRef]
- Sun, J.X.; Xu, G.R.; Cheng, X.D.; Wang, Y.Y.; Wu, J.Q. Effects of warming and grazing on soil physicochemical properties and enzyme activities in Salinized Grassland in Hexi Corridor. J. Soil Water Conserv. 2025, 39, 309–317+347. [Google Scholar]
- Huang, B.B.; Xing, Y.J.; Luo, W.; Yan, G.Y.; Liu, G.C.; Wang, X.C.; Wang, Q.G. Effects of long-term nitrogen addition and throughfall reduction on extracellular enzyme activity and ecoenzymatic stoichiometry in a temperate forest. J. Soil Sci. Plant Nutr. 2024, 24, 1534–1546. [Google Scholar] [CrossRef]
- Chen, Z. Effects of Orchard Litter Decomposition on the Stoichiometry Characteristics of Soileco-Enzymes. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2019. [Google Scholar]
- Barnes, A.D.; Potapov, A.; Yang, J.N.; Zhu, M.Y.; Chen, X.Y.; Hu, F.; Liu, M.Q. Altered litter stoichiometry drives energy dynamics of food webs through changing multiple facets of soil biodiversity. Soil Biol. Biochem. 2024, 191, 109331. [Google Scholar]
- Lu, Y.M.; Xu, E.L.; Wu, D.M.; Lu, S.X.; Liu, X.F.; Guo, J.F. Effects of Double Addition or Removal of Litter on Soil Hydrolases Activities and Their Stoichiometry in Castanopsis carlesii Fore. J. Soil Water Conserv. 2021, 35, 313–320. [Google Scholar]
- Li, J.W.; Jing, W.; Yu, J.Y.; Wang, K.B.; Li, J.P.; Cui, Y.X.; Shangguan, Z.P.; Deng, L. Soil enzyme activity and stoichiometry in response to precipitation changes in terrestrial ecosystems. Soil Biol. Biochem. 2024, 191, 109321. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Shah, J.J.F. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.L.; Contosta, A.R.; Cusack, D.; Frey, S.D.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.H.; Huang, C.B.; Wang, K.B.; Liu, Q.Y.; Liu, Y.L.; Hai, X.Y.; Shangguan, Z.P. Drivers of soil microbial metabolic limitation changes along a vegetation restoration gradient on the loess plateau, China. Geoderma 2019, 353, 188–200. [Google Scholar] [CrossRef]
- Cui, Y.X.; Bing, H.J.; Fang, L.C.; Jiang, M.; Shen, G.T.; Yu, J.L.; Wang, X.; Zhu, H.; Wu, Y.H.; Zhang, X.C. Extracellular enzyme stoichiometry reveals the carbon and phosphorus limitations of microbial metabolisms in the rhizosphere and bulk soils in alpine ecosystems. Plant Soil 2021, 458, 7–20. [Google Scholar] [CrossRef]
- Fang, H.; Mo, J.M.; Peng, S.L.; Li, Z.; Wang, H. Cumulative effects of nitrogen additions on litter decomposition in three tropical forests in southern China. Plant Soil 2007, 297, 233–242. [Google Scholar] [CrossRef]
- Gong, J.R.; Zhu, C.C.; Yang, L.L.; Yang, B.; Wang, B.; Baoyin, T.T.; Liu, M.; Zhang, Z.H.; Shi, J.Y. Effects of nitrogen addition on above-and belowground litter decomposition and nutrient dynamics in the litter-soil continuum in the temperate steppe of inner Mongolia, China. J. Arid Environ. 2020, 172, 104036. [Google Scholar] [CrossRef]
- Gong, J.R.; Zhang, Z.H.; Zhu, C.C.; Shi, J.Y.; Zhang, W.Y.; Song, L.Y.; Li, Y.; Zhang, S.Q.; Dong, J.J.; Li, X.B. The response of litter decomposition to phosphorus addition in typical temperate grassland in inner Mongolia. J. Arid Environ. 2022, 197, 104677. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Zechmeister-Boltenstern, S.; Richter, A. Stoichiometric imbalances between terrestrial decomposer communities and their resources: Mechanisms and implications of microbial adaptations to their resources. Front. Microbiol. 2014, 5, 22. [Google Scholar] [CrossRef]
- Liu, Y.; Xian, S.; Chen, Y.M.; Wang, L.F.; Chen, Q.M.; Zhang, J.; Xu, Z.F.; Zhang, L.; Xiao, J.J.; Zhu, P.; et al. Litter chemical quality strongly affects forest floor microbial groups and ecoenzymatic stoichiometry in the subalpine forest. Ann. For. Sci. 2019, 76, 106. [Google Scholar] [CrossRef]
- Liu, R.; Chen, F.S.; Fang, X.M.; Wan, S.Z.; Bu, W.S.; Wang, H.M.; Li, J.J. Effects of litter addition and removal on soil hydrolytic enzyme activities and ecoenzymatic stoichiometry in Chinese fir plantation. Acta Ecol. Sin. 2020, 40, 739–5750. [Google Scholar]
- Li, J.N.; Niu, X.M.; Wang, P.; Yang, J.J.; Liu, J.W.; Wu, D.H.; Guan, P.T. Soil degradation regulates the effects of litter decomposition on soil microbial nutrient limitation: Evidence from soil enzymatic activity and stoichiometry. Front. Plant Sci. 2023, 13, 1090954. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shi, R.K.; Li, D.H.; Xu, R.Q.; Wu, D.F.; Miao, R.H. Effects of Litter input on forest soil chemometric characteristics. J. Shandong Agric. Univ. (Nat. Sci. Ed.) 2024, 55, 70–75. [Google Scholar]
- Zhao, J. Effects of Nitrogen Addition and Litter on Soil Microorganism and Enzyme Activities. Master’s Thesis, Beijing Forestry University, Beijing, China, 2016. [Google Scholar]
- Błońska, E.; Piaszczyk, W.; Staszel, K.; Lasota, J. Enzymatic activity of soils and soil organic matter stabilization as an effect of components released from the decomposition of litter. Appl. Soil Ecol. 2021, 157, 103723. [Google Scholar] [CrossRef]
- Ai, L.; Wu, F.Z.; Fan, X.B.; Yang, J.; Wu, Q.X.; Zhu, J.J.; Ni, X.Y. Short-term responses of soil enzyme activities and stoichiometry to litter input in Castanopsis carlesii and Cunninghamia lanceolata plantations. Chin. J. Appl. Ecol. 2024, 35, 631–638. [Google Scholar]
- Yang, Y.L. Effects of Litterfall on the Dynamics of Soil Nitrogen and Phosphorusin Subalpine Forests. Ph.D. Thesis, Journal of Sichuan Agricultural University, Chengdu, China, 2020. [Google Scholar]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; Beijing Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Jones, D.L.; Willett, V.B. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Li, H.C.; Pan, H.L.; Feng, Q.H.; Ma, W.B.; Deng, D.Z. Effects of litter quantity on soil enzymes and stoichiometric ratios of spruce plantations with different stand densities in the subalpine region of western Sichuan. Chin. J. Ecol. 2024, 43, 2967–2978. [Google Scholar]
- Li, X.R.; Lin, H.; Cao, P.L.; Lin, W.S.; Liu, X.F.; Zhong, X.F.; Guo, J.F. Long-term nitrogen application exacerbates soil phosphorus limitation at different depths in subtropical natural castanopsis carlesii forest: Based on soil extracellular enzymes and their stoichiometric ratios. Soils 2024, 56, 963–974. [Google Scholar]
- Cui, Y.X.; Wang, X.; Zhang, X.C.; Ju, W.L.; Duan, C.J.; Guo, X.B.; Wang, Y.Q.; Fang, L.C. Soil moisture mediates microbial carbon and phosphorus metabolism during vegetation succession in a semiarid region. Soil Biol. Biochem. 2020, 147, 107814. [Google Scholar] [CrossRef]
- Sheng, Y.Y.; Bai, Y.F.; Jin, Y.D.; Liu, Y.Y.; Zhao, C.Y.; Liu, C.; Li, Y.Q.; Liu, R.; Jiang, C.Q.; Zhang, Z.W.; et al. Effects of litter additions on the soil nutrients and microbial properties in Cunninghamia lanceolata plantations of different stand ages. J. Cent. South Univ. For. Technol. 2022, 42, 114–125. [Google Scholar]
- Lu, S.X.; Xu, E.L.; Wu, D.M.; Lu, Y.M.; Guo, J.F.; Yang, Y.S. Response of soil microbial community composition on litterfall input in a castanopsis carlesii plantation. J. For. Environ. 2020, 40, 16–23. [Google Scholar]
- Wei, C.C.; Liu, X.F.; Lin, C.F.; Li, X.F.; Li, Y.; Zhang, Y.X. Response of soil enzyme activities to litter input changes in two secondary castanopsis carlessii forests in subtropical China. Chin. J. Plant Ecol. 2018, 42, 692–702. [Google Scholar]
- Su, Z.X.; Su, B.Q.; Shangguan, Z.P. Advances in effects of plant litter decomposition on the stability of soil organic carbon. Res. Soil Water Conserv. 2022, 29, 406–413. [Google Scholar]
- Zhou, L.; Sun, Z.J.; Nie, T.T.; Yu, B.J.; Zheng, L.; Zhou, C.H. Effects of litter additions on soil carbon, nitrogen and phosphorus contents and their stoichiometric characteristics in sagebrush desert grassland. Acta Agrestia Sin. 2024, 32, 462–469. [Google Scholar]
- Chen, Z.J.; Xiao, Y.T.; Dong, X.D.; Wang, W.J.; Wang, J.; Zhai, W.F.; Tian, M.; Han, S.J. Effects of sediment deposition on soil stoichiometric ratios in the middle and lower reaches of the Yellow River. Chin. J. Ecol. 2022, 41, 1334–1341. [Google Scholar]
- Hui, D.F.; Yang, X.X.; Deng, Q.; Liu, Q.; Wang, X.; Yang, H.; Ren, H. Soil C:N:P stoichiometry in tropical forests on Hainan Island of China:Spatial and vertical variations. Catena 2021, 201, 105228. [Google Scholar] [CrossRef]
- Springob, G.; Kirchmann, H. Bulk soil C to N ratio as a simplemeasure of net N mineralization from stabilized soil organic matterin sandy arable soils. Soil Biol. Biochem. 2003, 35, 629–632. [Google Scholar] [CrossRef]
- Ma, X.Y.; Gong, L.; Zhu, H.Q.; Zhang, T.; Yin, K.J.; Lu, X.Y. Effects of different carbon inputs on soil stoichiometry in tianshan mountains. Environ. Sci. 2023, 44, 2715–2723. [Google Scholar]
- Lü, J.L.; Yan, M.J.; Song, B.L.; Guan, J.H.; Shi, W.Y.; Du, S. Ecological stoichiometry characteristics of soil carbon, nitrogen, and phosphorus in an oak forest and a black locust plantation in the Loess hilly region. Acta Ecol. Sin. 2017, 37, 3385–3393. [Google Scholar]
- Chen, S.L.; Li, T.Y.; He, B.H.; Liu, Y.M.; Liu, Y.M.; Tang, R.D.; Tang, L. Soil Carbon, Nitrogen and Phosphorus Stoichiometric Characteristics of Typical Torreya grandis Intercropping Systems on Sloping Farmland in the Three Gorges Reservoir Area. J. Soil Water Conserv. 2023, 37, 220–226. [Google Scholar]
- Tian, H.Q.; Chen, G.S.; Zhang, C.; Melillo, J.M.; Hall, C.A.S. Pattern and variation of C:N:P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio:a new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Robert, L.; Sinsabaugh; Mary, E.; Stromberger; Matthew, D.; Wallenstein; Michael, N.; et al. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Yan, L.J.; Wang, H.Y.; Li, G.; Wu, J.Q. Effects of four typical vegetations on soil nutrient and enzymes activities in loess hilly region. J. Soil Water Conserv. 2019, 33, 190–196+204. [Google Scholar]
- Zhou, X.Q.; Chen, C.R.; Wang, Y.F.; Xu, Z.H.; Han, H.Y.; Li, L.H.; Wan, S.Q. Warming and increased precipitation have differential effects on soil extracellular enzyme activities in a temperate grassland. Sci. Total Environ. 2013, 444, 552–558. [Google Scholar] [CrossRef]
- Sun, C.L.; Qiu, M.S.; Huang, C.X.; Wang, Y.W. Characteristics of soil extracellular enzyme activities and their stoichiometry during rocky desertification in southwestern Guizhou, China. Chin. J. Plant Ecol. 2022, 46, 834–845. [Google Scholar] [CrossRef]
- Yan, Y.C.; Gong, J.R.; Zhang, S.Q.; Zhang, W.Y.; Dong, X.D.; Yang, G.S. Effects of nitrogen addition on soil active organic carbon in a temperate grassland of Nei Mongol, China. Chin. J. Plant Ecol. 2024, 48, 229–241. [Google Scholar]
- Wu, F.Y.; Wu, Y.S.; Chen, X.H.; Feng, J.; Lu, L.Y.; Casina; Wang, C.Y.; Meng, Y.F. Spatial-temporal variation of water use efficiency in three species of sand-fixing shrubs on the Ordos Plateau. Chin. J. Plant Ecol. 2024, 48, 1180–1191. [Google Scholar]
- Men, X.; Bao, Y.; Wu, M.; Liao, C.; Cheng, X. Soil enzyme activities responded differently to short-term litter input manipulation under coniferous and broad-leaved forests in the subalpine area of Southwest China. For. Ecol. Manag. 2023, 546, 121360. [Google Scholar] [CrossRef]
- Zhu, Z.H. Effect of Mixed Tree Leaf Litter Decomposition on Nutrient Release in the Loess Plateau. Master’s Thesis, Northwest A&F University, Yanglin, China, 2012. [Google Scholar]
- Yang, S.L. Comparison of Changes in Protective Enzymes and Metabolites and Drought Tolerance of Several Species of Desert Plants. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2018. [Google Scholar]
- Bell, C.; Carrillo, Y.; Boot, C.M.; Rocca, J.D.; Pendall, E.; Wallenstein, M.D. Rhizosphere stoichiometry: Are C:N:P ratios of plants, soils, and enzymes conserved at the plant species-level? New Phytol. 2014, 201, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Cai, T.P.; Du, J.; Yuan, L.Q.; Chen, J.; Xu, X.N. Effects of nitrogen addition and resin tapping on soil enzyme activities and their stoichiometry in a slash pine plantation. Chin. J. Ecol. 2022, 41, 227–235. [Google Scholar]
- Song, S.Y.; Chen, Y.M.; Wang, T.; Liao, Y.M.; Zhang, S.; Xu, X. Characteristics of soil enzyme activity and stoichiometry in a Picea likiangensis var. linzhiensis plantation with different ages. Chin. J. Appl. Environ. Biol. 2023, 29, 178–185. [Google Scholar]
- Liu, J.B.; Chen, J.; Chen, G.S.; Jin, G.; Li, Y.Q. Enzyme stoichiometry indicates the variation of microbial nutrient requirements at different soil depths in subtropical forests. PLoS ONE 2020, 15, e0220599. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.C.; Guo, Q.; Xia, Y.; Yang, L.M.; Fan, Y.X.; Yang, Y.S. Characterizations of soil enzyme activities and stoichiometry in three subtropical forest stands. Chin. J. Appl. Ecol. 2024, 35, 1501–1508. [Google Scholar]
- Luo, P.; Chen, H.; Xiao, K.C.; Yang, L.Q.; Wen, L.; Li, D.J. Effects of Topography, Tree Species and Soil Properties on Soil Enzyme Activity in Karst Regions. Environ. Sci. 2017, 38, 2577–2585. [Google Scholar]
- Allison, S.D.; Weintraub, M.N.; Gartner, T.B.; Waldrop, M.P. Evolutionary-economic principles as regulators of soil enzyme production and ecosystem function. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 229–243. [Google Scholar]
- Kiikkila, O.; Kanerva, S.; Kitunen, V.; Smolander, A. Soil microbial activity in relation to dissolved organic matter properties under different tree species. Plant Soil 2014, 377, 169–177. [Google Scholar] [CrossRef]


| Longitude and Latitude | Altitude/m | Basic Situation | |
|---|---|---|---|
| YC | 38°51′58.55″ N, 109°15′31.04″ E | 1266.78 | Average height 0.7 m, canopy width 0.25 m × 0.30 m, total coverage 90% |
| NT | 38°57′21.04″ N, 109°07′44.00″ E | 1308.67 | Average height 1.5 m, canopy width 2.65 m × 2.30 m, total coverage 80% |
| ZSH | 38°52′19.30″ N, 109°14′06.68″ E | 1281.18 | Average height 1.2 m, crown width 1.52 m × 1.02 m, total coverage 85% |
| SL | 38°51′58.57″ N, 109°15′31.04″ E | 1247.77 | Average height 1.8 m, canopy width 1.30 m × 1.40 m, total coverage 88% |
| PTC/g·kg−1 | PTN/g·kg−1 | PTP/g·kg−1 | |
| YC | 409.90 ± 16.19 a | 39.74 ± 5.23 ab | 1.70 ± 0.02 b |
| NT | 436.57 ± 17.30 a | 44.69 ± 2.49 a | 1.73 ± 0.04 b |
| ZSH | 426.06 ± 21.04 a | 44.96 ± 5.03 a | 1.91 ± 0.02 a |
| SL | 425.31 ± 29.92 a | 29.34 ± 6.52 b | 1.45 ± 0.04 c |
| Soil Chemical Properties | Explanation/% | Contribution Rate% | F | p |
|---|---|---|---|---|
| MBN | 47.7 | 48.6 | 11.9 | 0.002 ** |
| TN | 21.1 | 21.5 | 8.1 | 0.002 ** |
| MBC/MBN | 15.8 | 16.1 | 11.3 | 0.002 ** |
| OC | 6.4 | 6.5 | 7.0 | 0.002 ** |
| MBC | 1.9 | 1.9 | 2.4 | 0.066 |
| AN | 1.6 | 1.7 | 2.4 | 0.040 * |
| C/N | 0.7 | 0.7 | 1.0 | 0.390 |
| AC | 0.4 | 0.4 | 0.5 | 0.756 |
| N/P | 0.2 | 0.2 | 0.2 | 0.912 |
| TP | 1.5 | 1.5 | 2.1 | 0.130 |
| C/P | 0.9 | 0.9 | 1.4 | 0.298 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Zhang, S.; Yang, Z.; Xu, H.; Huang, H.; Wang, C.; Zhang, L. Effects of Litter Input on Soil Enzyme Activities and Their Stoichiometric Ratios in Sandy Soil. Agronomy 2025, 15, 1152. https://doi.org/10.3390/agronomy15051152
Gao H, Zhang S, Yang Z, Xu H, Huang H, Wang C, Zhang L. Effects of Litter Input on Soil Enzyme Activities and Their Stoichiometric Ratios in Sandy Soil. Agronomy. 2025; 15(5):1152. https://doi.org/10.3390/agronomy15051152
Chicago/Turabian StyleGao, Haiyan, Shengnan Zhang, Zhiguo Yang, Hongbin Xu, Haiguang Huang, Chunying Wang, and Lei Zhang. 2025. "Effects of Litter Input on Soil Enzyme Activities and Their Stoichiometric Ratios in Sandy Soil" Agronomy 15, no. 5: 1152. https://doi.org/10.3390/agronomy15051152
APA StyleGao, H., Zhang, S., Yang, Z., Xu, H., Huang, H., Wang, C., & Zhang, L. (2025). Effects of Litter Input on Soil Enzyme Activities and Their Stoichiometric Ratios in Sandy Soil. Agronomy, 15(5), 1152. https://doi.org/10.3390/agronomy15051152





