Antimicrobial Peptides and Their Potential Applications in Plant Protection
Abstract
:1. Introduction
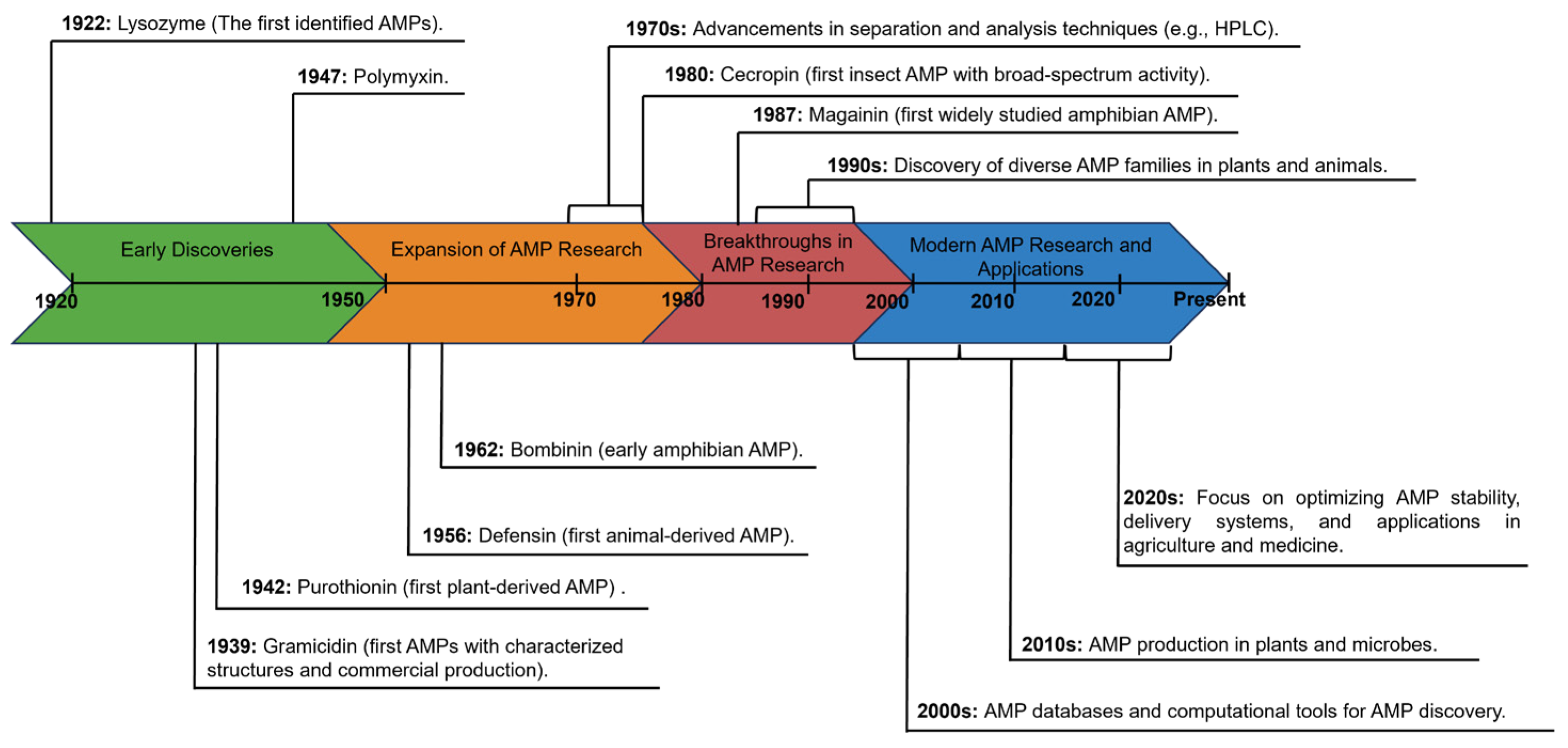
2. The Discovery History of AMPs
3. Classification of AMPs
3.1. Source-Based Classification of AMPs
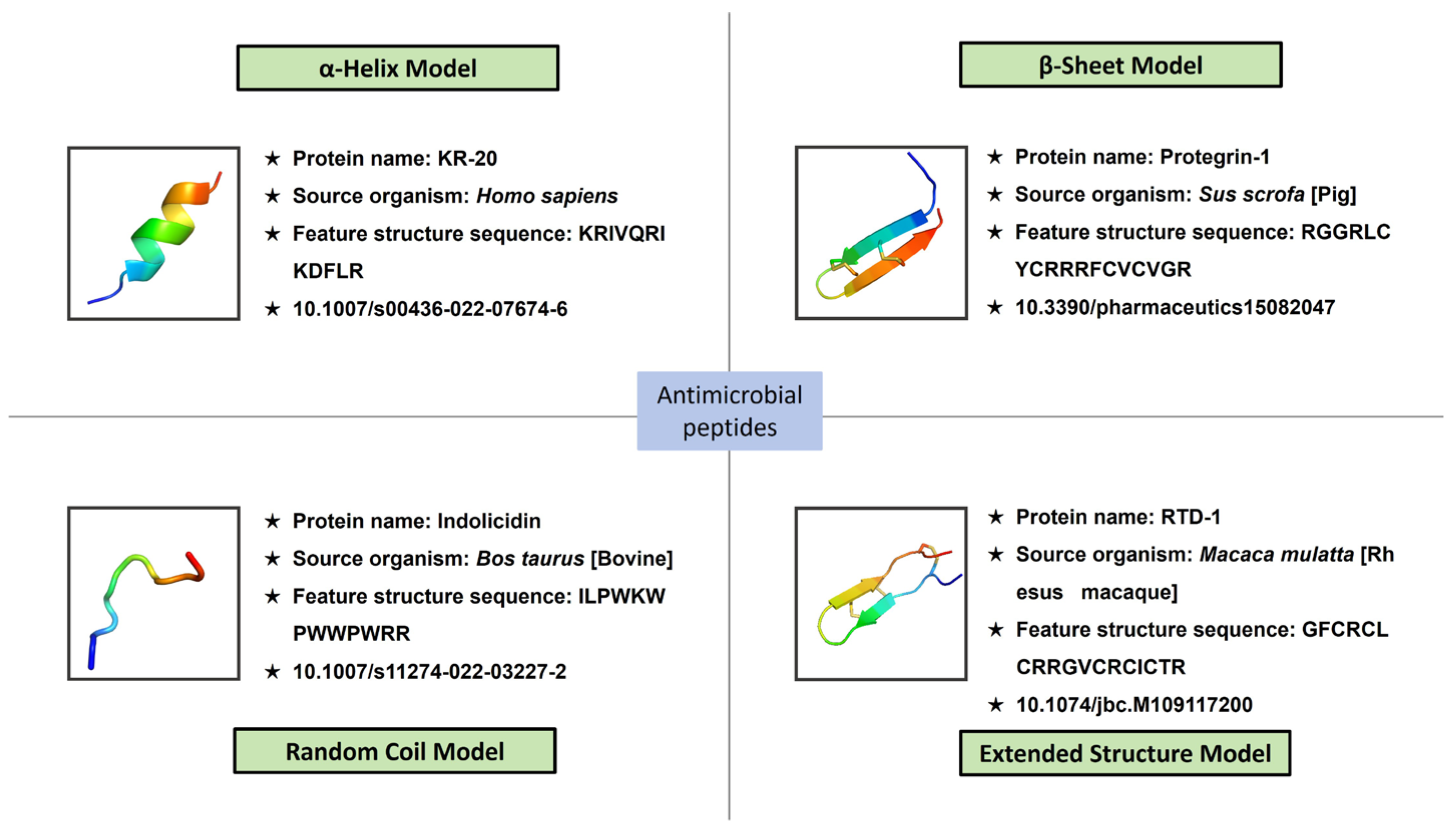
3.2. Structure-Based Classification of AMPs
3.3. Activity-Based Classification of AMPs
3.4. Classification of AMPs Based on Amino Acid-Rich Species
4. Antimicrobial Mechanisms of AMPs
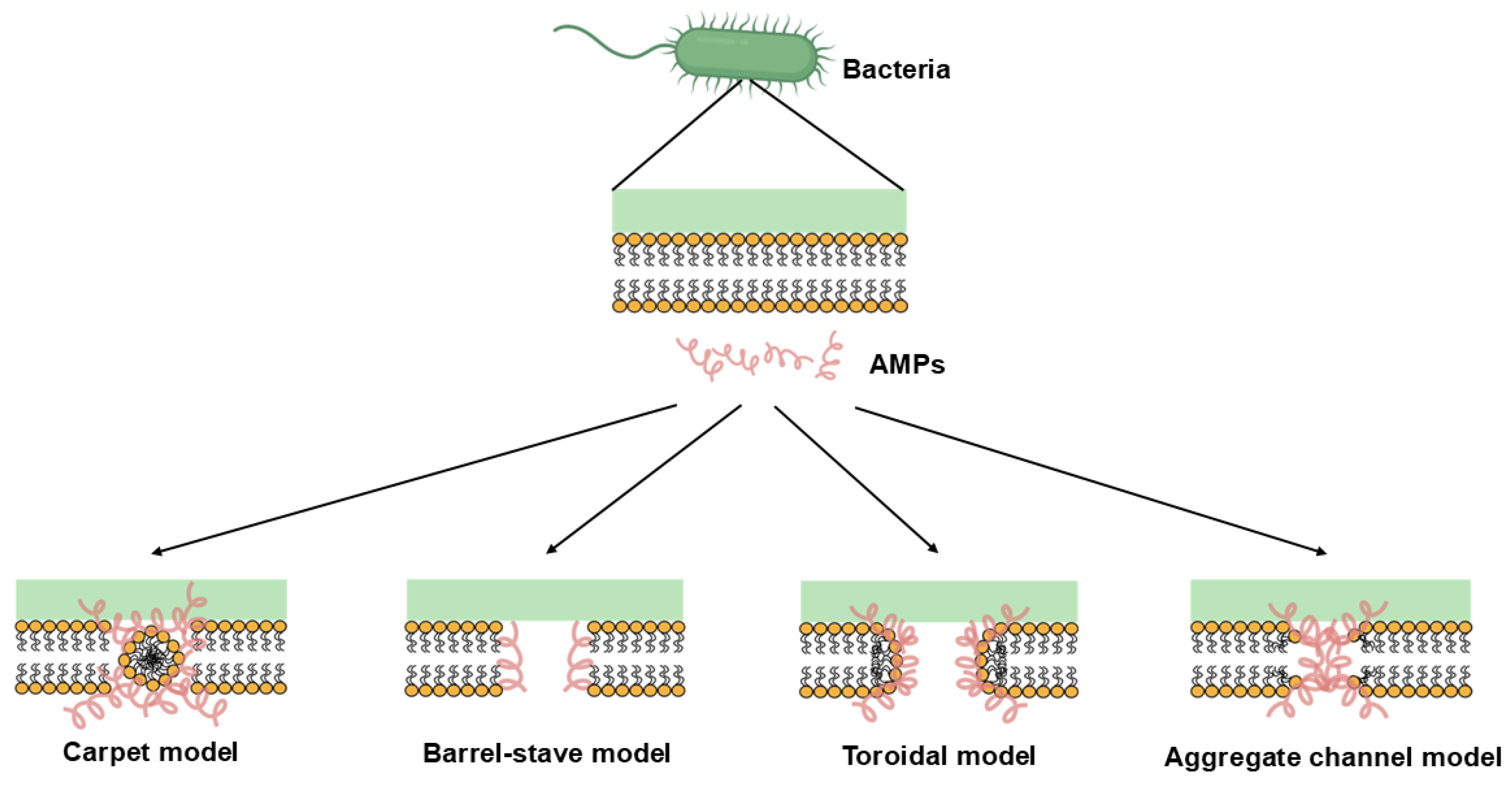
4.1. Membrane-Targeting Mechanism
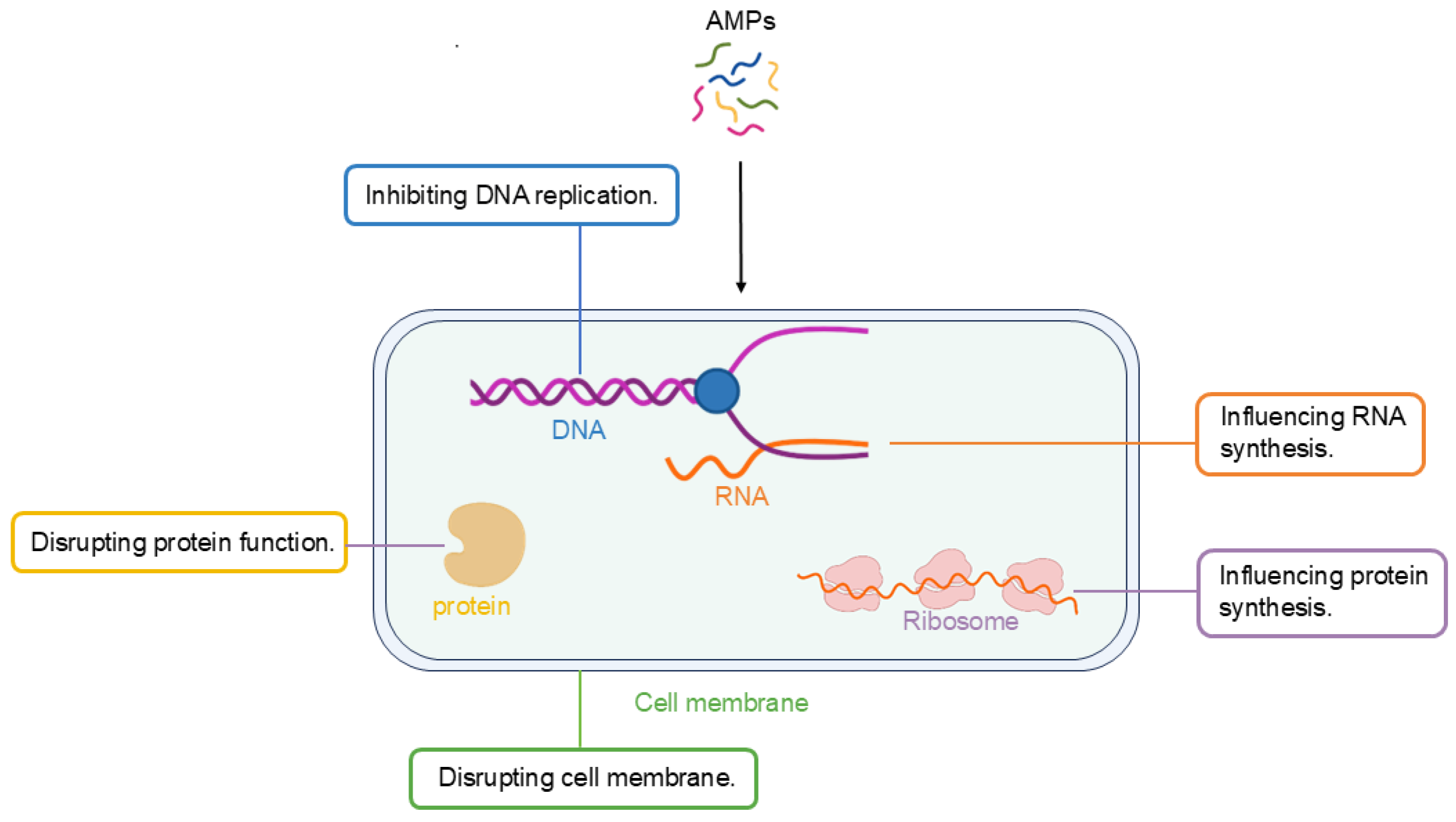
4.2. Non-Membrane-Targeting Mechanism
5. Strategies for AMP Identification and Improvement
5.1. Identification of AMPs
5.1.1. Traditional and Experimental Approaches
5.1.2. Bioinformatics and Omics-Based Strategies
5.2. Improvement of AMPs
5.2.1. Structural Modifications to Enhance Activity and Stability
5.2.2. Optimization for Targeted Delivery
6. Production of AMPs
6.1. Plant-Based Expression
6.2. Bacteria-Based Expression
6.3. Yeast-Based Expression
7. Application Strategies of AMPs in Plant Protection
7.1. Direct Application of AMP Products
7.2. Plant Expression of AMP Genes
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Zasloff, M.; Martin, B.; Chen, H.-C. Antimicrobial activity of synthetic magainin peptides and several analogues. Proc. Natl. Acad. Sci. USA 1988, 85, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Luong, H.X.; Thanh, T.T.; Tran, T.H. Antimicrobial peptides–Advances in development of therapeutic applications. Life Sci. 2020, 260, 118407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-M.; Ye, D.-X.; Liu, Y.; Zhang, X.-Y.; Zhou, Y.-L.; Zhang, L.; Yang, X.-L. Peptides, new tools for plant protection in eco-agriculture. Adv. Agrochem 2023, 2, 58–78. [Google Scholar] [CrossRef]
- Liu, Y.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W. Antimicrobial peptides and their application in food packaging. Trends Food Sci. Technol. 2021, 112, 471–483. [Google Scholar] [CrossRef]
- Iqbal, A.; Khan, R.S.; Shehryar, K.; Imran, A.; Ali, F.; Attia, S.; Shah, S.; Mii, M. Antimicrobial peptides as effective tools for enhanced disease resistance in plants. Plant Cell Tissue Organ Cult. 2019, 139, 1–15. [Google Scholar] [CrossRef]
- Nazarian-Firouzabadi, F.; Torres, M.D.T.; de la Fuente-Nunez, C. Recombinant production of antimicrobial peptides in plants. Biotechnol. Adv. 2024, 71, 108296. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Araujo, K.; Sánchez, J.N.; Kund, G.; Trumble, J.; Roper, C.; Godfrey, K.E.; Jin, H. A stable antimicrobial peptide with dual functions of treating and preventing citrus Huanglongbing. Proc. Natl. Acad. Sci. USA 2021, 118, e2019628118. [Google Scholar] [CrossRef]
- Nandi, A.K. Application of antimicrobial proteins and peptides in developing disease-resistant plants. Plant Pathog. Resist. Biotechnol. 2016, 51–70. [Google Scholar] [CrossRef]
- Fadiji, T.; Rashvand, M.; Daramola, M.O.; Iwarere, S.A. A review on antimicrobial packaging for extending the shelf life of food. Processes 2023, 11, 590. [Google Scholar] [CrossRef]
- Chipman, D.M.; Sharon, N. Mechanism of Lysozyme Action: Lysozyme is the first enzyme for which the relation between structure and function has become clear. Science 1969, 165, 454–465. [Google Scholar] [CrossRef]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Dubos, R.J. Studies on a bactericidal agent extracted from a soil bacillus: I. Preparation of the agent. Its activity in vitro. J. Exp. Med. 1939, 70, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Gallo, R.L. Antimicrobial peptides: Old molecules with new ideas. J. Investig. Dermatol. 2012, 132, 887–895. [Google Scholar] [CrossRef]
- Balls, A.; Hale, W.; Harris, T. A crystalline protein obtained from a lipoprotein of wheat flour. Cereal Chem. 1942, 19, 279–288. [Google Scholar]
- Hirsch, J.G. Phagocytin: A bactericidal substance from polymorphonuclear leucocytes. J. Exp. Med. 1956, 103, 589. [Google Scholar] [CrossRef]
- Groves, M.; Peterson, R.; Kiddy, C. Polymorphism in the red protein isolated from milk of individual cows. Nature 1965, 207, 1007–1008. [Google Scholar] [CrossRef]
- Zeya, H.; Spitznagel, J.K. Antibacterial and enzymic basic proteins from leukocyte lysosomes: Separation and identification. Science 1963, 142, 1085–1087. [Google Scholar] [CrossRef]
- Hultmark, D.; STEINER, H.; Rasmuson, T.; Boman, H.G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 1980, 106, 7–16. [Google Scholar] [CrossRef]
- Boman, H.G. Antibacterial peptides: Key components needed in immunity. Cell 1991, 65, 205–207. [Google Scholar] [CrossRef]
- Tang, S.S.; Prodhan, Z.H.; Biswas, S.K.; Le, C.F.; Sekaran, S.D. Antimicrobial peptides from different plant sources: Isolation, characterisation, and purification. Phytochemistry 2018, 154, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Olga, K.; Marina, K.; Alexey, A.; Anton, S.; Vladimir, Z.; Igor, T. The role of plant antimicrobial peptides (AMPs) in response to biotic and abiotic environmental factors. Biol. Commun. 2020, 65, 187–199. [Google Scholar]
- De Caleya, R.F.; Gonzalez-Pascual, B.; García-Olmedo, F.; Carbonero, P. Susceptibility of phytopathogenic bacteria to wheat purothionins in vitro. Appl. Microbiol. 1972, 23, 998–1000. [Google Scholar] [CrossRef]
- Salas, C.E.; Badillo-Corona, J.A.; Ramírez-Sotelo, G.; Oliver-Salvador, C. Biologically active and antimicrobial peptides from plants. BioMed Res. Int. 2015, 2015, 102129. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Xu, C.; Ren, F.; Peng, C.; Wu, G.; Zhao, J. Purification, characterization, and molecular cloning of the gene of a seed-specific antimicrobial protein from pokeweed. Plant Physiol. 2000, 122, 1015–1024. [Google Scholar] [CrossRef]
- Marcus, J.P.; Green, J.L.; Goulter, K.C.; Manners, J.M. A family of antimicrobial peptides is produced by processing of a 7S globulin protein in Macadamia integrifolia kernels. Plant J. 1999, 19, 699–710. [Google Scholar] [CrossRef]
- Terras, F.R.; Eggermont, K.; Kovaleva, V.; Raikhel, N.V.; Osborn, R.W.; Kester, A.; Rees, S.B.; Torrekens, S.; Van Leuven, F.; Vanderleyden, J. Small cysteine-rich antifungal proteins from radish: Their role in host defense. Plant Cell 1995, 7, 573–588. [Google Scholar]
- Zottich, U.; Da Cunha, M.; Carvalho, A.O.; Dias, G.B.; Silva, N.C.; Santos, I.S.; do Nacimento, V.V.; Miguel, E.C.; Machado, O.L.; Gomes, V.M. Purification, biochemical characterization and antifungal activity of a new lipid transfer protein (LTP) from Coffea canephora seeds with α-amylase inhibitor properties. Biochim. Biophys. Acta Gen. Subj. 2011, 1810, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Berrocal-Lobo, M.; Segura, A.; Moreno, M.; López, G.; Garcıa-Olmedo, F.; Molina, A. Snakin-2, an antimicrobial peptide from potato whose gene is locally induced by wounding and responds to pathogen infection. Plant Physiol. 2002, 128, 951–961. [Google Scholar] [CrossRef]
- Zhao, P.; Yang, H.; Sun, Y.; Zhang, J.; Gao, K.; Wu, J.; Zhu, C.; Yin, C.; Chen, X.; Liu, Q.; et al. Targeted MYC2 stabilization confers citrus Huanglongbing re-sistance. Science 2025, 388, 191–198. [Google Scholar] [CrossRef]
- Niu, L.; Zhong, X.; Zhang, Y.; Yang, J.; Xing, G.; Li, H.; Liu, D.; Ma, R.; Dong, Y.; Yang, X. Enhanced tolerance to Phytophthora root and stem rot by over-expression of the plant antimicrobial peptide CaAMP1 gene in soybean. BMC Genet. 2020, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Jiang, X.; Xu, L.; Lei, T.; Peng, A.; He, Y.; Yao, L.; Chen, S. Transgenic citrus expressing synthesized cecropin B genes in the phloem exhibits decreased susceptibility to Huanglongbing. Plant Mol. Biol. 2017, 93, 341–353. [Google Scholar] [CrossRef]
- Hao, G.; Stover, E.; Gupta, G. Overexpression of a modified plant thionin enhances disease resistance to citrus canker and huanglongbing (HLB). Front. Plant Sci. 2016, 7, 1078. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.; Turton, K.L.; Kainth, T.; Kumar, A.; Wieden, H.-J. Strategies for improving antimicrobial peptide production. Biotechnol. Adv. 2022, 59, 107968. [Google Scholar] [CrossRef] [PubMed]
- Mirzaee, H.; Peralta, N.L.N.; Carvalhais, L.C.; Dennis, P.G.; Schenk, P.M. Plant-produced bacteriocins inhibit plant pathogens and confer disease resistance in tomato. New Biotechnol. 2021, 63, 54–61. [Google Scholar] [CrossRef]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, T.K. Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial peptides: An update on classifications and databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef]
- Ageitos, J.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef]
- Otvos, J.L. Antibacterial peptides isolated from insects. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2000, 6, 497–511. [Google Scholar]
- Tincu, J.A.; Taylor, S.W. Antimicrobial peptides from marine invertebrates. Antimicrob. Agents Chemother. 2004, 48, 3645–3654. [Google Scholar] [CrossRef]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, antimicrobial peptides from bacterial origin: Overview of their biology and their impact against multidrug-resistant bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Karimi, A.; Fallah, F.; Akhavan, M. Overview of ribosomal and non-ribosomal antimicrobial peptides produced by Gram positive bacteria. Cell. Mol. Biol. 2017, 63, 20–32. [Google Scholar] [CrossRef]
- Hill, C.; Draper, L.A.; Ross, R.; Cotter, P.D. Lantibiotic immunity. Curr. Protein Pept. Sci. 2008, 9, 39–49. [Google Scholar] [CrossRef]
- Koehbach, J.; Craik, D.J. The vast structural diversity of antimicrobial peptides. Trends Pharmacol. Sci. 2019, 40, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Maróti, G.; Kereszt, A.; Kondorosi, E.; Mergaert, P. Natural roles of antimicrobial peptides in microbes, plants and animals. Res. Microbiol. 2011, 162, 363–374. [Google Scholar] [CrossRef]
- Liu, Z.; Brady, A.; Young, A.; Rasimick, B.; Chen, K.; Zhou, C.; Kallenbach, N.R. Length effects in antimicrobial peptides of the (RW)n series. Antimicrob. Agents Chemother. 2007, 51, 597–603. [Google Scholar] [CrossRef]
- Ringstad, L.; Schmidtchen, A.; Malmsten, M. Effect of peptide length on the interaction between consensus peptides and DOPC/DOPA bilayers. Langmuir 2006, 22, 5042–5050. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-H.; Hall, K.N.; Aguilar, M.-I. Antimicrobial peptide structure and mechanism of action: A focus on the role of membrane structure. Curr. Top. Med. Chem. 2016, 16, 25–39. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Algboory, H.L.; Kadum, H.; Mohammed, N.K.; Saari, N.; Hassan, Z.; Hussin, A.S.M. Antifungal activity determination for the peptides generated by Lactobacillus plantarum TE10 against Aspergillus flavus in maize seeds. Food Control 2020, 109, 106898. [Google Scholar] [CrossRef]
- Jung, Y.; Kong, B.; Moon, S.; Yu, S.-H.; Chung, J.; Ban, C.; Chung, W.-J.; Kim, S.-G.; Kweon, D.-H. Envelope-deforming antiviral peptide derived from influenza virus M2 protein. Biochem. Biophys. Res. Commun. 2019, 517, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Abbassi, F.; Raja, Z.; Oury, B.; Gazanion, E.; Piesse, C.; Sereno, D.; Nicolas, P.; Foulon, T.; Ladram, A. Antibacterial and leishmanicidal activities of temporin-SHd, a 17-residue long membrane-damaging peptide. Biochimie 2013, 95, 388–399. [Google Scholar] [CrossRef]
- Rivas, L.; Rojas, V. Cyanobacterial peptides as a tour de force in the chemical space of antiparasitic agents. Arch. Biochem. Biophys. 2019, 664, 24–39. [Google Scholar] [CrossRef]
- Mattiuzzo, M.; Bandiera, A.; Gennaro, R.; Benincasa, M.; Pacor, S.; Antcheva, N.; Scocchi, M. Role of the Escherichia coli SbmA in the antimicrobial activity of proline-rich peptides. Mol. Microbiol. 2007, 66, 151–163. [Google Scholar] [CrossRef]
- Seefeldt, A.C.; Nguyen, F.; Antunes, S.; Pérébaskine, N.; Graf, M.; Arenz, S.; Inampudi, K.K.; Douat, C.; Guichard, G.; Wilson, D.N. The proline-rich antimicrobial peptide Onc112 inhibits translation by blocking and destabilizing the initiation complex. Nat. Struct. Mol. Biol. 2015, 22, 470–475. [Google Scholar] [CrossRef]
- Imjongjirak, C.; Amphaiphan, P.; Charoensapsri, W.; Amparyup, P. Characterization and antimicrobial evaluation of SpPR-AMP1, a proline-rich antimicrobial peptide from the mud crab Scylla paramamosain. Dev. Comp. Immunol. 2017, 74, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Lointier, M.; Aisenbrey, C.; Marquette, A.; Tan, J.H.; Kichler, A.; Bechinger, B. Membrane pore-formation correlates with the hydrophilic angle of histidine-rich amphipathic peptides with multiple biological activities. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183212. [Google Scholar] [CrossRef]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial peptides from plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef] [PubMed]
- Erdem Büyükkiraz, M.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2022, 132, 1573–1596. [Google Scholar] [CrossRef]
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial mechanisms and clinical application prospects of antimicrobial peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef]
- Pirtskhalava, M.; Vishnepolsky, B.; Grigolava, M. Physicochemical Features and Peculiarities of Interaction of Antimicrobial Peptides with the Membrane. arXiv 2020, arXiv:2005.04104. [Google Scholar]
- Pirtskhalava, M.; Vishnepolsky, B.; Grigolava, M. Transmembrane and antimicrobial peptides. Hydrophobicity, amphiphilicity and propensity to aggregation. arXiv 2013, arXiv:1307.6160. [Google Scholar]
- Sato, H.; Feix, J.B. Peptide-membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1245–1256. [Google Scholar] [CrossRef]
- Hazam, P.K.; Goyal, R.; Ramakrishnan, V. Peptide based antimicrobials: Design strategies and therapeutic potential. Prog. Biophys. Mol. Biol. 2019, 142, 10–22. [Google Scholar] [CrossRef]
- López-Meza, J.E.; Ochoa-Zarzosa, A.; Aguilar, J.A.; Loeza-Lara, P.D. Antimicrobial peptides: Diversity and perspectives for their biomedical application. Biomed. Eng. Trends Res. Technol. 2011, 1094, 275–304. [Google Scholar]
- Lohner, K.; Prossnigg, F. Biological activity and structural aspects of PGLa interaction with membrane mimetic systems. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Murase, O.; Fujii, N.; Miyajima, K. Translocation of a channel-forming antimicrobial peptide, magainin 2, across lipid bilayers by forming a pore. Biochemistry 1995, 34, 6521–6526. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Mardirossian, M.; Grzela, R.; Giglione, C.; Meinnel, T.; Gennaro, R.; Mergaert, P.; Scocchi, M. The host antimicrobial peptide Bac71-35 binds to bacterial ribosomal proteins and inhibits protein synthesis. Chem. Biol. 2014, 21, 1639–1647. [Google Scholar] [CrossRef]
- Le, C.-F.; Gudimella, R.; Razali, R.; Manikam, R.; Sekaran, S.D. Transcriptome analysis of Streptococcus pneumoniae treated with the designed antimicrobial peptides, DM3. Sci. Rep. 2016, 6, 26828. [Google Scholar] [CrossRef] [PubMed]
- He, S.-w.; Zhang, J.; Li, N.-q.; Zhou, S.; Yue, B.; Zhang, M. A TFPI-1 peptide that induces degradation of bacterial nucleic acids, and inhibits bacterial and viral infection in half-smooth tongue sole, Cynoglossus semilaevis. Fish Shellfish Immunol. 2017, 60, 466–473. [Google Scholar] [CrossRef]
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob. Agents Chemother. 2017, 61, 10. [Google Scholar] [CrossRef]
- Shu, G.; Chen, Y.; Liu, T.; Ren, S.; Kong, Y. Antimicrobial peptide cathelicidin-BF inhibits platelet aggregation by blocking protease-activated receptor 4. Int. J. Pept. Res. Ther. 2019, 25, 349–358. [Google Scholar] [CrossRef]
- Li, L.; Sun, J.; Xia, S.; Tian, X.; Cheserek, M.J.; Le, G. Mechanism of antifungal activity of antimicrobial peptide APP, a cell-penetrating peptide derivative, against Candida albicans: Intracellular DNA binding and cell cycle arrest. Appl. Microbiol. Biotechnol. 2016, 100, 3245–3253. [Google Scholar] [CrossRef]
- Cruz, G.F.; de Araujo, I.; Torres, M.D.; de la Fuente-Nunez, C.; Oliveira, V.X.; Ambrosio, F.N.; Lombello, C.B.; Almeida, D.V.; Silva, F.D.; Garcia, W. Photochemically-generated silver chloride nanoparticles stabilized by a peptide inhibitor of cell division and its antimicrobial properties. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2464–2474. [Google Scholar] [CrossRef]
- Waghu, F.H.; Barai, R.S.; Gurung, P.; Idicula-Thomas, S. CAMPR3: A database on sequences, structures and signatures of antimicrobial peptides. Nucleic Acids Res. 2016, 44, D1094–D1097. [Google Scholar] [CrossRef] [PubMed]
- Pirtskhalava, M.; Amstrong, A.A.; Grigolava, M.; Chubinidze, M.; Alimbarashvili, E.; Vishnepolsky, B.; Gabrielian, A.; Rosenthal, A.; Hurt, D.E.; Tartakovsky, M. DBAASP v3: Database of antimicrobial/cytotoxic activity and structure of peptides as a resource for development of new therapeutics. Nucleic Acids Res. 2021, 49, D288–D297. [Google Scholar] [CrossRef]
- Shi, G.; Kang, X.; Dong, F.; Liu, Y.; Zhu, N.; Hu, Y.; Xu, H.; Lao, X.; Zheng, H. DRAMP 3.0: An enhanced comprehensive data repository of antimicrobial peptides. Nucleic Acids Res. 2022, 50, D488–D496. [Google Scholar] [CrossRef]
- Piotto, S.P.; Sessa, L.; Concilio, S.; Iannelli, P. YADAMP: Yet another database of antimicrobial peptides. Int. J. Antimicrob. Agents 2012, 39, 346–351. [Google Scholar] [CrossRef]
- Singh, S.; Chaudhary, K.; Dhanda, S.K.; Bhalla, S.; Usmani, S.S.; Gautam, A.; Tuknait, A.; Agrawal, P.; Mathur, D.; Raghava, G.P. SATPdb: A database of structurally annotated therapeutic peptides. Nucleic Acids Res. 2016, 44, D1119–D1126. [Google Scholar] [CrossRef] [PubMed]
- Jhong, J.-H.; Chi, Y.-H.; Li, W.-C.; Lin, T.-H.; Huang, K.-Y.; Lee, T.-Y. dbAMP: An integrated resource for exploring antimicrobial peptides with functional activities and physicochemical properties on transcriptome and proteome data. Nucleic Acids Res. 2019, 47, D285–D297. [Google Scholar] [CrossRef]
- Fang, P.; Yu, S.; Ma, X.; Hou, L.; Li, T.; Gao, K.; Wang, Y.; Sun, Q.; Shang, L.; Liu, Q. Applications of tandem mass spectrometry (MS/MS) in antimicrobial peptides field: Current state and new applications. Heliyon 2024, 10, e28484. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.X.; Bishop-Hurley, S.L.; Cooper, M.A. Development of anti-infectives using phage display: Biological agents against bacteria, viruses, and parasites. Antimicrob. Agents Chemother. 2012, 56, 4569–4582. [Google Scholar] [CrossRef]
- Ashby, M.; Petkova, A.; Gani, J.; Mikut, R.; Hilpert, K. Use of peptide libraries for identification and optimization of novel antimicrobial peptides. Curr. Top. Med. Chem. 2017, 17, 537–553. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, H.; Deng, L. Ensemble machine learning and predicted properties promote antimicrobial peptide identification. Interdiscip. Sci. Comput. Life Sci. 2024, 16, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Lata, S.; Mishra, N.K.; Raghava, G.P. AntiBP2: Improved version of antibacterial peptide prediction. BMC Bioinform. 2010, 11, S19. [Google Scholar] [CrossRef]
- Yan, J.; Bhadra, P.; Li, A.; Sethiya, P.; Qin, L.; Tai, H.K.; Wong, K.H.; Siu, S.W. Deep-AmPEP30: Improve short antimicrobial peptides prediction with deep learning. Mol. Ther. Nucleic Acids 2020, 20, 882–894. [Google Scholar] [CrossRef]
- Rezaei Javan, R.; Van Tonder, A.J.; King, J.P.; Harrold, C.L.; Brueggemann, A.B. Genome sequencing reveals a large and diverse repertoire of antimicrobial peptides. Front. Microbiol. 2018, 9, 2012. [Google Scholar] [CrossRef]
- Huang, K.-Y.; Chang, T.-H.; Jhong, J.-H.; Chi, Y.-H.; Li, W.-C.; Chan, C.-L.; Robert Lai, K.; Lee, T.-Y. Identification of natural antimicrobial peptides from bacteria through metagenomic and metatranscriptomic analysis of high-throughput transcriptome data of Taiwanese oolong teas. BMC Syst. Biol. 2017, 11, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Azkargorta, M.; Bregón-Villahoz, M.; Escobes, I.; Ibáñez-Pérez, J.; Iloro, I.; Iglesias, M.; Diez-Zapirain, M.; Rabanal, A.; Prieto, B.; Moragues, M.-D. In-depth proteomics and natural peptidomics analyses reveal antibacterial peptides in human endometrial fluid. J. Proteom. 2020, 216, 103652. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Y.; Xue, Y.; Huang, Y.; Li, X.; Chen, X.; Xu, Y.; Zhang, D.; Zhang, P.; Zhao, J. Identification of potent antimicrobial peptides via a machine-learning pipeline that mines the entire space of peptide sequences. Nat. Biomed. Eng. 2023, 7, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Wong, F.; Collins, J.J.; de la Fuente-Nunez, C. Machine learning for antimicrobial peptide identification and design. Nat. Rev. Bioeng. 2024, 2, 392–407. [Google Scholar] [CrossRef]
- Chen, E.H.-L.; Weng, C.-W.; Li, Y.-M.; Wu, M.-C.; Yang, C.-C.; Lee, K.-T.; Chen, R.P.-Y.; Cheng, C.-P. De novo design of antimicrobial peptides with a special charge pattern and their application in combating plant pathogens. Front. Plant Sci. 2021, 12, 753217. [Google Scholar] [CrossRef]
- Vasco, A.V.; Brode, M.; Méndez, Y.; Valdés, O.; Rivera, D.G.; Wessjohann, L.A. Synthesis of lactam-bridged and lipidated cyclo-peptides as promising anti-phytopathogenic agents. Molecules 2020, 25, 811. [Google Scholar] [CrossRef]
- Ng-Choi, I.; Soler, M.; Güell, I.; Badosa, E.; Cabrefiga, J.; Bardaji, E.; Montesinos, E.; Planas, M.; Feliu, L. Antimicrobial peptides incorporating non-natural amino acids as agents for plant protection. Protein Pept. Lett. 2014, 21, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xu, H.; Xia, J.; Ma, J.; Xu, J.; Li, Y.; Feng, J. D-and unnatural amino acid substituted antimicrobial peptides with improved proteolytic resistance and their proteolytic degradation characteristics. Front. Microbiol. 2020, 11, 563030. [Google Scholar] [CrossRef]
- Badrhadad, A.; Nazarian-Firouzabadi, F.; Ismaili, A. Fusion of a chitin-binding domain to an antibacterial peptide to enhance resistance to Fusarium solani in tobacco (Nicotiana tabacum). 3 Biotech 2018, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Badea, G.; Lăcătuşu, I.; Badea, N.; Ott, C.; Meghea, A. Use of various vegetable oils in designing photoprotective nanostructured formulations for UV protection and antioxidant activity. Ind. Crops Prod. 2015, 67, 18–24. [Google Scholar] [CrossRef]
- López-Vargas, E.R.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; de Alba Romenus, K.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Foliar application of copper nanoparticles increases the fruit quality and the content of bioactive compounds in tomatoes. Appl. Sci. 2018, 8, 1020. [Google Scholar] [CrossRef]
- Felippim, E.C.; Marcato, P.D.; Maia Campos, P.M.B.G. Development of photoprotective formulations containing nanostructured lipid carriers: Sun protection factor, physical-mechanical and sensorial properties. AAPS PharmSciTech 2020, 21, 311. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and controlled delivery for bioactive compounds: Outlining challenges for new “smart-foods” for health. Foods 2018, 7, 72. [Google Scholar] [CrossRef]
- Irigoyen, S.; Ramasamy, M.; Pant, S.; Niraula, P.; Bedre, R.; Gurung, M.; Rossi, D.; Laughlin, C.; Gorman, Z.; Achor, D. Plant hairy roots enable high throughput identification of antimicrobials against Candidatus Liberibacter spp. Nat. Commun. 2020, 11, 5802. [Google Scholar] [CrossRef]
- Gao, J.; Na, H.; Zhong, R.; Yuan, M.; Guo, J.; Zhao, L.; Wang, Y.; Wang, L.; Zhang, F. One step synthesis of antimicrobial peptide protected silver nanoparticles: The core-shell mutual enhancement of antibacterial activity. Colloids Surf. B Biointerfaces 2020, 186, 110704. [Google Scholar] [CrossRef]
- Meikle, T.G.; Zabara, A.; Waddington, L.J.; Separovic, F.; Drummond, C.J.; Conn, C.E. Incorporation of antimicrobial peptides in nanostructured lipid membrane mimetic bilayer cubosomes. Colloids Surf. B Biointerfaces 2017, 152, 143–151. [Google Scholar] [CrossRef]
- Chatzidaki, M.D.; Papadimitriou, K.; Alexandraki, V.; Balkiza, F.; Georgalaki, M.; Papadimitriou, V.; Tsakalidou, E.; Xenakis, A. Reverse micelles as nanocarriers of nisin against foodborne pathogens. Food Chem. 2018, 255, 97–103. [Google Scholar] [CrossRef]
- Shuai, J.; Guan, F.; He, B.; Hu, J.; Li, Y.; He, D.; Hu, J. Self-assembled nanoparticles of symmetrical cationic peptide against citrus pathogenic bacteria. J. Agric. Food Chem. 2019, 67, 5720–5727. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Luo, X.; Zhang, Y.; Tian, P.; Xiao, Y.; Li, S.; Yang, X.; Li, F.; Zhang, D.; Zhang, S. Broad-spectrum nano-bactericide utilizing antimicrobial peptides and bimetallic Cu-Ag nanoparticles anchored onto multiwalled carbon nanotubes for sustained protection against persistent bacterial pathogens in crops. Int. J. Biol. Macromol. 2024, 265, 131042. [Google Scholar] [CrossRef]
- Ghidey, M.; Islam, S.A.; Pruett, G.; Kearney, C.M. Making plants into cost-effective bioreactors for highly active antimicrobial peptides. New Biotechnol. 2020, 56, 63–70. [Google Scholar] [CrossRef]
- Łojewska, E.; Sakowicz, T.; Kowalczyk, A.; Konieczka, M.; Grzegorczyk, J.; Sitarek, P.; Skała, E.; Czarny, P.; Śliwiński, T.; Kowalczyk, T. Production of recombinant colicin M in Nicotiana tabacum plants and its antimicrobial activity. Plant Biotechnol. Rep. 2020, 14, 33–43. [Google Scholar] [CrossRef]
- Shams, M.V.; Nazarian-Firouzabadi, F.; Ismaili, A.; Shirzadian-Khorramabad, R. Production of a recombinant dermaseptin peptide in nicotiana tabacum hairy roots with enhanced antimicrobial activity. Mol. Biotechnol. 2019, 61, 241–252. [Google Scholar] [CrossRef]
- Khan, R.S.; Nishihara, M.; Yamamura, S.; Nakamura, I.; Mii, M. Transgenic potatoes expressing wasabi defensin peptide confer partial resistance to gray mold (Botrytis cinerea). Plant Biotechnol. 2006, 23, 179–183. [Google Scholar] [CrossRef]
- Cabanos, C.; Ekyo, A.; Amari, Y.; Kato, N.; Kuroda, M.; Nagaoka, S.; Takaiwa, F.; Utsumi, S.; Maruyama, N. High-level production of lactostatin, a hypocholesterolemic peptide, in transgenic rice using soybean A1aB1b as carrier. Transgenic Res. 2013, 22, 621–629. [Google Scholar] [CrossRef]
- Liu, Y.; Kamesh, A.C.; Xiao, Y.; Sun, V.; Hayes, M.; Daniell, H.; Koo, H. Topical delivery of low-cost protein drug candidates made in chloroplasts for biofilm disruption and uptake by oral epithelial cells. Biomaterials 2016, 105, 156–166. [Google Scholar] [CrossRef]
- Paškevičius, Š.; Starkevič, U.; Misiūnas, A.; Vitkauskienė, A.; Gleba, Y.; Ražanskienė, A. Plant-expressed pyocins for control of Pseudomonas aeruginosa. PLoS ONE 2017, 12, e0185782. [Google Scholar] [CrossRef]
- Tusé, D.; Tu, T.; McDonald, K.A. Manufacturing economics of plant-made biologics: Case studies in therapeutic and industrial enzymes. BioMed Res. Int. 2014, 2014, 256135. [Google Scholar] [CrossRef]
- Hoelscher, M.P.; Forner, J.; Calderone, S.; Krämer, C.; Taylor, Z.; Loiacono, F.V.; Agrawal, S.; Karcher, D.; Moratti, F.; Kroop, X. Expression strategies for the efficient synthesis of antimicrobial peptides in plastids. Nat. Commun. 2022, 13, 5856. [Google Scholar] [CrossRef] [PubMed]
- Bock, R. Engineering plastid genomes: Methods, tools, and applications in basic research and biotechnology. Annu. Rev. Plant Biol. 2015, 66, 211–241. [Google Scholar] [CrossRef] [PubMed]
- Oey, M.; Lohse, M.; Kreikemeyer, B.; Bock, R. Exhaustion of the chloroplast protein synthesis capacity by massive expression of a highly stable protein antibiotic. Plant J. 2009, 57, 436–445. [Google Scholar] [CrossRef]
- Bock, R.; Warzecha, H. Solar-powered factories for new vaccines and antibiotics. Trends Biotechnol. 2010, 28, 246–252. [Google Scholar] [CrossRef]
- Hoelscher, M.; Tiller, N.; Teh, A.Y.-H.; Wu, G.-Z.; Ma, J.K.; Bock, R. High-level expression of the HIV entry inhibitor griffithsin from the plastid genome and retention of biological activity in dried tobacco leaves. Plant Mol. Biol. 2018, 97, 357–370. [Google Scholar] [CrossRef]
- Patiño-Rodríguez, O.; Ortega-Berlanga, B.; Llamas-González, Y.Y.; Flores-Valdez, M.A.; Herrera-Díaz, A.; Montes-de-Oca-Luna, R.; Korban, S.S.; Alpuche-Solís, Á.G. Transient expression and characterization of the antimicrobial peptide protegrin-1 in Nicotiana tabacum for control of bacterial and fungal mammalian pathogens. Plant Cell Tissue Organ Cult. 2013, 115, 99–106. [Google Scholar] [CrossRef]
- Chahardoli, M.; Fazeli, A.; Niazi, A.; Ghabooli, M. Recombinant expression of LFchimera antimicrobial peptide in a plant-based expression system and its antimicrobial activity against clinical and phytopathogenic bacteria. Biotechnol. Biotechnol. Equip. 2018, 32, 714–723. [Google Scholar] [CrossRef]
- Kmiec, B.; Teixeira, P.F.; Berntsson, R.P.-A.; Murcha, M.W.; Branca, R.M.; Radomiljac, J.D.; Regberg, J.; Svensson, L.M.; Bakali, A.; Langel, Ü. Organellar oligopeptidase (OOP) provides a complementary pathway for targeting peptide degradation in mitochondria and chloroplasts. Proc. Natl. Acad. Sci. USA 2013, 110, E3761–E3769. [Google Scholar] [CrossRef]
- Moberg, P.; Ståhl, A.; Bhushan, S.; Wright, S.J.; Eriksson, A.; Bruce, B.D.; Glaser, E. Characterization of a novel zinc metalloprotease involved in degrading targeting peptides in mitochondria and chloroplasts. Plant J. 2003, 36, 616–628. [Google Scholar] [CrossRef]
- Flavia Cancado Viana, J.; Campos Dias, S.; Luiz Franco, O.; Lacorte, C. Heterologous production of peptides in plants: Fusion proteins and beyond. Curr. Protein Pept. Sci. 2013, 14, 568–579. [Google Scholar] [CrossRef]
- Company, N.; Nadal, A.; Ruiz, C.; Pla, M. Production of phytotoxic cationic α-helical antimicrobial peptides in plant cells using inducible promoters. PLoS ONE 2014, 9, e109990. [Google Scholar] [CrossRef] [PubMed]
- Emadpour, M.; Karcher, D.; Bock, R. Boosting riboswitch efficiency by RNA amplification. Nucleic Acids Res. 2015, 43, e66. [Google Scholar] [CrossRef]
- Verhounig, A.; Karcher, D.; Bock, R. Inducible gene expression from the plastid genome by a synthetic riboswitch. Proc. Natl. Acad. Sci. USA 2010, 107, 6204–6209. [Google Scholar] [CrossRef] [PubMed]
- Holásková, E.; Galuszka, P.; Mičúchová, A.; Šebela, M.; Öz, M.T.; Frébort, I. Molecular farming in barley: Development of a novel production platform to produce human antimicrobial peptide LL-37. Biotechnol. J. 2018, 13, 1700628. [Google Scholar] [CrossRef]
- Vriens, K.; Cammue, B.P.; Thevissen, K. Antifungal plant defensins: Mechanisms of action and production. Molecules 2014, 19, 12280–12303. [Google Scholar] [CrossRef] [PubMed]
- Herrera Diaz, A.; Kovacs, I.; Lindermayr, C. Inducible expression of the de-novo designed antimicrobial peptide SP1-1 in tomato confers resistance to Xanthomonas campestris pv. vesicatoria. PLoS ONE 2016, 11, e0164097. [Google Scholar] [CrossRef]
- Peng, A.; Zhang, J.; Zou, X.; He, Y.; Xu, L.; Lei, T.; Yao, L.; Li, Q.; Chen, S. Pyramiding the antimicrobial PR1aCB and AATCB genes in ‘Tarocco’blood orange (Citrus sinensis Osbeck) to enhance citrus canker resistance. Transgenic Res. 2021, 30, 635–647. [Google Scholar] [CrossRef]
- Pavlova, O.; Severinov, K. Posttranslationally modified microcins. Russ. J. Genet. 2006, 42, 1380–1389. [Google Scholar] [CrossRef]
- Sang, Y.; Blecha, F. Antimicrobial peptides and bacteriocins: Alternatives to traditional antibiotics. Anim. Health Res. Rev. 2008, 9, 227–235. [Google Scholar] [CrossRef]
- Kaur, J.; Kumar, A.; Kaur, J. Strategies for optimization of heterologous protein expression in E. coli: Roadblocks and reinforcements. Int. J. Biol. Macromol. 2018, 106, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef]
- Sampaio de Oliveira, K.B.; Leite, M.L.; Rodrigues, G.R.; Duque, H.M.; da Costa, R.A.; Cunha, V.A.; de Loiola Costa, L.S.; da Cunha, N.B.; Franco, O.L.; Dias, S.C. Strategies for recombinant production of antimicrobial peptides with pharmacological potential. Expert Rev. Clin. Pharmacol. 2020, 13, 367–390. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Ge, H.; He, H.; Liu, Y.; Zhai, C.; Feng, L.; Yi, L. The heterologous expression strategies of antimicrobial peptides in microbial systems. Protein Expr. Purif. 2017, 140, 52–59. [Google Scholar] [CrossRef]
- Parachin, N.S.; Mulder, K.C.; Viana, A.A.B.; Dias, S.C.; Franco, O.L. Expression systems for heterologous production of antimicrobial peptides. Peptides 2012, 38, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, J.; Emrich, C.A.; Jeans, C.; Faulkner, M.; Riggs, P.; Berkmen, M. SHuffle, a novel Escherichia coli protein expression strain capable of correctly folding disulfide bonded proteins in its cytoplasm. Microb. Cell Factories 2012, 11, 56. [Google Scholar] [CrossRef]
- Samuelson, J.C.; Causey, T.B.; Berkmen, M. Disulfide-bonded protein production in E. coli: Involvement of disulfide bond isomerase improves protein folding. Genet. Eng. Biotechnol. News 2012, 32, 35. [Google Scholar] [CrossRef]
- Ren, G.; Ke, N.; Berkmen, M. Use of the SHuffle strains in production of proteins. Curr. Protoc. Protein Sci. 2016, 85, 5.26.1–5.26.21. [Google Scholar] [CrossRef]
- Robinson, M.-P.; Ke, N.; Lobstein, J.; Peterson, C.; Szkodny, A.; Mansell, T.J.; Tuckey, C.; Riggs, P.D.; Colussi, P.A.; Noren, C.J. Efficient expression of full-length antibodies in the cytoplasm of engineered bacteria. Nat. Commun. 2015, 6, 8072. [Google Scholar] [CrossRef]
- Ke, N.; Berkmen, M. Production of disulfide-bonded proteins in Escherichia coli. Curr. Protoc. Mol. Biol. 2014, 108, 16.11 B. 11–16.11 B. 21. [Google Scholar] [CrossRef]
- Terpe, K. Overview of bacterial expression systems for heterologous protein production: From molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 2006, 72, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.R.; Byregowda, S.M.; Veeregowda, B.M.; Balamurugan, V. An overview of heterologous expression host systems for the production of recombinant proteins. Adv. Anim. Vet. Sci. 2016, 4, 346–356. [Google Scholar] [CrossRef]
- Maamar, H.; Dubnau, D. Bistability in the Bacillus subtilis K-state (competence) system requires a positive feedback loop. Mol. Microbiol. 2005, 56, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.C.; Lee, W.; Tran, L.; Wong, S.L. Engineering a Bacillus subtilis expression-secretion system with a strain deficient in six extracellular proteases. J. Bacteriol. 1991, 173, 4952–4958. [Google Scholar] [CrossRef]
- Ÿztürk, S.; Ÿalık, P.; Ÿzdamar, T.H. Fed-Batch Biomolecule Production by Bacillus subtilis: A State of the Art Review. Trends Biotechnol. 2016, 34, 329–345. [Google Scholar] [CrossRef]
- He, Q.; Fu, A.Y.; Li, T.J. Expression and one-step purification of the antimicrobial peptide cathelicidin-BF using the intein system in Bacillus subtilis. J. Ind. Microbiol. Biotechnol. 2015, 42, 647–653. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Wei, D.; Wang, J.; Shan, A.; Li, Z. Expression of plectasin in Bacillus subtilis using SUMO technology by a maltose-inducible vector. J. Ind. Microbiol. Biotechnol. 2015, 42, 1369–1376. [Google Scholar] [CrossRef]
- Pohl, S.; Harwood, C.R. Heterologous protein secretion by bacillus species from the cradle to the grave. Adv. Appl. Microbiol. 2010, 73, 1–25. [Google Scholar] [CrossRef]
- Guoyan, Z.; Yingfeng, A.; Zabed, H.; Qi, G.; Yang, M.; Jiao, Y.; Li, W.; Wenjing, S.; Xianghui, Q. Bacillus subtilis Spore Surface Display Technology: A Review of Its Development and Applications. J. Microbiol. Biotechnol. 2019, 29, 179–190. [Google Scholar] [CrossRef]
- van Tilburg, A.Y.; Cao, H.; van der Meulen, S.B.; Solopova, A.; Kuipers, O.P. Metabolic engineering and synthetic biology employing Lactococcus lactis and Bacillus subtilis cell factories. Curr. Opin. Biotechnol. 2019, 59, 1–7. [Google Scholar] [CrossRef]
- Vieira Gomes, A.M.; Souza Carmo, T.; Silva Carvalho, L.; Mendonça Bahia, F.; Parachin, N.S. Comparison of Yeasts as Hosts for Recombinant Protein Production. Microorganisms 2018, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Celik, E.; Calık, P. Production of recombinant proteins by yeast cells. Biotechnol. Adv. 2012, 30, 1108–1118. [Google Scholar] [CrossRef]
- Wagner, J.M.; Alper, H.S. Synthetic biology and molecular genetics in non-conventional yeasts: Current tools and future advances. Fungal Genet. Biol. 2016, 89, 126–136. [Google Scholar] [CrossRef]
- Wang, G.; Huang, M.; Nielsen, J. Exploring the potential of Saccharomyces cerevisiae for biopharmaceutical protein production. Curr. Opin. Biotechnol. 2017, 48, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Farajnia, S.; Rajabibazl, M.; Ghasemi, Y.; Mafi, A.; Hoseinpoor, R.; Rahbarnia, L.; Aria, M. Yeast expression systems: Overview and recent advances. Mol. Biotechnol. 2019, 61, 365–384. [Google Scholar] [CrossRef]
- Lian, J.; Mishra, S.; Zhao, H. Recent advances in metabolic engineering of Saccharomyces cerevisiae: New tools and their applications. Metab. Eng. 2018, 50, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Nandy, S.K.; Srivastava, R.K. A review on sustainable yeast biotechnological processes and applications. Microbiol. Res. 2018, 207, 83–90. [Google Scholar] [CrossRef]
- Chen, B.; Lee, H.L.; Heng, Y.C.; Chua, N.; Teo, W.S.; Choi, W.J.; Leong, S.S.J.; Foo, J.L.; Chang, M.W. Synthetic biology toolkits and applications in Saccharomyces cerevisiae. Biotechnol. Adv. 2018, 36, 1870–1881. [Google Scholar] [CrossRef]
- Domínguez, A.; Fermiñán, E.; Sánchez, M.; González, F.J.; Pérez-Campo, F.M.; García, S.; Herrero, A.B.; San Vicente, A.; Cabello, J.; Prado, M.; et al. Non-conventional yeasts as hosts for heterologous protein production. Int. Microbiol. 1998, 1, 131–142. [Google Scholar]
- Mattanovich, D.; Branduardi, P.; Dato, L.; Gasser, B.; Sauer, M.; Porro, D. Recombinant protein production in yeasts. Recomb. Gene Expr. 2012, 824, 329–358. [Google Scholar]
- Looser, V.; Bruhlmann, B.; Bumbak, F.; Stenger, C.; Costa, M.; Camattari, A.; Fotiadis, D.; Kovar, K. Cultivation strategies to enhance productivity of Pichia pastoris: A review. Biotechnol. Adv. 2015, 33, 1177–1193. [Google Scholar] [CrossRef]
- Vieira, S.M.; da Rocha, S.L.G.; Neves-Ferreira, A.; Almeida, R.V.; Perales, J. Heterologous expression of the antimyotoxic protein DM64 in Pichia pastoris. PLoS Neglected Trop. Dis. 2017, 11, e0005829. [Google Scholar] [CrossRef]
- Capone, S.; Horvat, J.; Herwig, C.; Spadiut, O. Development of a mixed feed strategy for a recombinant Pichia pastoris strain producing with a de-repression promoter. Microb. Cell Factories 2015, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Hirz, M.; Pichler, H.; Schwab, H. Protein expression in Pichia pastoris: Recent achievements and perspectives for heterologous protein production. Appl. Microbiol. Biotechnol. 2014, 98, 5301–5317. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.; Hearn, M.T. Expression of heterologous proteins in Pichia pastoris: A useful experimental tool in protein engineering and production. J. Mol. Recognit. 2005, 18, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Kuddus, M.R.; Rumi, F.; Tsutsumi, M.; Takahashi, R.; Yamano, M.; Kamiya, M.; Kikukawa, T.; Demura, M.; Aizawa, T. Expression, purification and characterization of the recombinant cysteine-rich antimicrobial peptide snakin-1 in Pichia pastoris. Protein Expr. Purif. 2016, 122, 15–22. [Google Scholar] [CrossRef]
- Mulder, K.C.; de Lima, L.A.; Aguiar, P.S.; Carneiro, F.C.; Franco, O.L.; Dias, S.C.; Parachin, N.S. Production of a modified peptide clavanin in Pichia pastoris: Cloning, expression, purification and in vitro activities. AMB Express 2015, 5, 129. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.X.; Zhao, X.F.; Kang, C.J.; Liu, N.; Xiang, J.H.; Li, F.H.; Sueda, S.; Kondo, H. High level expression, purification, and characterization of the shrimp antimicrobial peptide, Ch-penaeidin, in Pichia pastoris. Protein Expr. Purif. 2005, 39, 144–151. [Google Scholar] [CrossRef]
- Peng, H.; Liu, H.P.; Chen, B.; Hao, H.; Wang, K.J. Optimized production of scygonadin in Pichia pastoris and analysis of its antimicrobial and antiviral activities. Protein Expr. Purif. 2012, 82, 37–44. [Google Scholar] [CrossRef]
- Meng, D.M.; Lv, Y.J.; Zhao, J.F.; Liu, Q.Y.; Shi, L.Y.; Wang, J.P.; Yang, Y.H.; Fan, Z.C. Efficient production of a recombinant Venerupis philippinarum defensin (VpDef) in Pichia pastoris and characterization of its antibacterial activity and stability. Protein Expr. Purif. 2018, 147, 78–84. [Google Scholar] [CrossRef]
- Mao, R.; Teng, D.; Wang, X.; Zhang, Y.; Jiao, J.; Cao, X.; Wang, J. Optimization of expression conditions for a novel NZ2114-derived antimicrobial peptide-MP1102 under the control of the GAP promoter in Pichia pastoris X-33. BMC Microbiol. 2015, 15, 57. [Google Scholar] [CrossRef]
- Wan, J.; Li, Y.; Chen, D.; Yu, B.; Zheng, P.; Mao, X.; Yu, J.; He, J. Expression of a Tandemly Arrayed Plectasin Gene from Pseudoplectania nigrella in Pichia pastoris and its Antimicrobial Activity. J. Microbiol. Biotechnol. 2016, 26, 461–468. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, W.; Wang, J.; Ma, Y.; Wei, D. Comparison of expression of monomeric and multimeric adenoregulin genes in Escherichia coli and Pichia pastorias. Protein Pept. Lett. 2005, 12, 349–355. [Google Scholar] [CrossRef]
- Tiwari, I.; Bhojiya, A.A.; Jain, D.; Kothari, S.L.; El-Sheikh, M.A.; Porwal, S. Managing tomato bacterial wilt through pathogen suppression and host resistance augmentation using microbial peptide. Front. Microbiol. 2024, 15, 1494054. [Google Scholar] [CrossRef]
- Breen, S.; Solomon, P.S.; Bedon, F.; Vincent, D. Surveying the potential of secreted antimicrobial peptides to enhance plant disease resistance. Front. Plant Sci. 2015, 6, 900. [Google Scholar] [CrossRef]
- Puig, M.; Moragrega, C.; Ruz, L.; Montesinos, E.; Llorente, I. Controlling brown spot of pear by a synthetic antimicrobial peptide under field conditions. Plant Dis. 2015, 99, 1816–1822. [Google Scholar] [CrossRef]
- Maximiano, M.R.; Rios, T.B.; Campos, M.L.; Prado, G.S.; Dias, S.C.; Franco, O.L. Nanoparticles in association with antimicrobial peptides (NanoAMPs) as a promising combination for agriculture development. Front. Mol. Biosci. 2022, 9, 890654. [Google Scholar] [CrossRef]
- Gao, A.G.; Hakimi, S.M.; Mittanck, C.A.; Wu, Y.; Woerner, B.M.; Stark, D.M.; Shah, D.M.; Liang, J.; Rommens, C.M. Fungal pathogen protection in potato by expression of a plant defensin peptide. Nat. Biotechnol. 2000, 18, 1307–1310. [Google Scholar] [CrossRef]
- Elfstrand, M.; Fossdal, C.; Swedjemark, G.; Clapham, D.; Olsson, O.; Sitbon, F.; Sharma, P.; Lönneborg, A.; Arnold, S.v. Identification of candidate genes for use in molecular breeding-a case study with the Norway spruce defensin-like gene, spi 1. Silvae Genet. 2001, 50, 75–81. [Google Scholar]
- Wang, Y.; Nowak, G.; Culley, D.; Hadwiger, L.A.; Fristensky, B. Constitutive expression of pea defense gene DRR206 confers resistance to blackleg (Leptosphaeria maculans) disease in transgenic canola (Brassica napus). Mol. Plant-Microbe Interact. 1999, 12, 410–418. [Google Scholar] [CrossRef]
- Park, H.C.; Hwan Kang, Y.; Jin Chun, H.; Choon Koo, J.; Hwa Cheong, Y.; Young Kim, C.; Chul Kim, M.; Sik Chung, W.; Cheol Kim, J.; Hyuk Yoo, J. Characterization of a stamen-specific cDNA encoding a novel plant defensin in Chinese cabbage. Plant Mol. Biol. 2002, 50, 57–68. [Google Scholar] [CrossRef]
- Kanzaki, H.; Nirasawa, S.; Saitoh, H.; Ito, M.; Nishihara, M.; Terauchi, R.; Nakamura, I. Overexpression of the wasabi defensin gene confers enhanced resistance to blast fungus (Magnaporthe grisea) in transgenic rice. Theor. Appl. Genet. 2002, 105, 809–814. [Google Scholar] [CrossRef]
- Turrini, A.; Sbrana, C.; Pitto, L.; Ruffini Castiglione, M.; Giorgetti, L.; Briganti, R.; Bracci, T.; Evangelista, M.; Nuti, M.; Giovannetti, M. The antifungal Dm-AMP1 protein from Dahlia merckii expressed in Solanum melongena is released in root exudates and differentially affects pathogenic fungi and mycorrhizal symbiosis. New Phytol. 2004, 163, 393–403. [Google Scholar] [CrossRef]
- Schaefer, S.C.; Gasic, K.; Cammue, B.; Broekaert, W.; Van Damme, E.J.; Peumans, W.J.; Korban, S.S. Enhanced resistance to early blight in transgenic tomato lines expressing heterologous plant defense genes. Planta 2005, 222, 858–866. [Google Scholar] [CrossRef]
- Choon Koo, J.; Jin Chun, H.; Cheol Park, H.; Chul Kim, M.; Duck Koo, Y.; Cheol Koo, S.; Mi Ok, H.; Jeong Park, S.; Lee, S.-H.; Yun, D.-J. Over-expression of a seed specific hevein-like antimicrobial peptide from Pharbitis nil enhances resistance to a fungal pathogen in transgenic tobacco plants. Plant Mol. Biol. 2002, 50, 441–452. [Google Scholar] [CrossRef]
- Inui Kishi, R.N.; Stach-Machado, D.; Singulani, J.d.L.; Dos Santos, C.T.; Fusco-Almeida, A.M.; Cilli, E.M.; Freitas-Astúa, J.; Picchi, S.C.; Machado, M.A. Evaluation of cytotoxicity features of antimicrobial peptides with potential to control bacterial diseases of citrus. PLoS ONE 2018, 13, e0203451. [Google Scholar] [CrossRef]

| No. | Database | Introduction | URL | AMP Count (as of 2024) | Ref |
|---|---|---|---|---|---|
| 1 | APD3 | Includes AMP sequences, sources, classifications, targets, and antimicrobial spectrum. Offers tools for analysis, classification, and AMP design. | https://aps.unmc.edu/AP/ (accessed on 22 April 2025) | >3000 | [54] |
| 2 | CAMPR3 | Includes both natural and synthetic AMP sequences. Supports predictions of antimicrobial activity and analysis of physicochemical properties. | http://www.camp3.bicnirrh.res.in (accessed on 22 April 2025) | >10,000 | [81] |
| 3 | DBAASP | Includes AMP sequences, structures, and bioactivities. Offers tools for analyzing sequence–activity relationships, facilitating peptide optimization. | https://dbaasp.org (accessed on 22 April 2025) | >20,000 | [82] |
| 4 | DRAMP | Includes detailed information on natural, synthetic, and modified AMPs. Provides data on peptide sequences, activity, and physicochemical properties. | http://dramp.cpu-bioinfor.org/ (accessed on 22 April 2025) | >30,000 | [83] |
| 5 | YADAMP | Includes AMP sequences and detailed information on source species. Provides sequence search and target matching tools. | http://yadamp.unisa.it/ (accessed on 22 April 2025) | >10,000 | [84] |
| 6 | SATPdb | Contains AMPs from various databases and allows searches for different peptide properties based on a query. | http://crdd.osdd.net/raghava/satpdb/ (accessed on 22 April 2025) | >8000 | [85] |
| 7 | dbAMP | An integrated system for identifying AMPs and their functional types based on high-throughput transcriptomic and proteomic data. | https://awi.cuhk.edu.cn/dbAMP/index.php (accessed on 22 April 2025) | >10,000 | [86] |
| No. | Nanoparticle | Description | Ref |
|---|---|---|---|
| 1 | Silver nanoparticles | In vitro assays showed reduced AgNP cytotoxicity, enhanced antimicrobial activity, and improved stability in aqueous solutions. | [109] |
| 2 | Cubosomes | AMPs can be loaded into cubosomes with various formulations, and peptide loading efficiency depends on cubosome properties like lipid structure and curvature. | [110] |
| 3 | Microemulsions | In vitro assays evaluated a microemulsion with essential oils for encapsulating nisin and enhancing its antimicrobial activity on lettuce leaves. | [111] |
| 4 | Nanoparticle self-assemble | In planta assays showed a reduction in citrus canker lesion development, inhibition of biofilm formation, membrane damage, and altered cell membrane permeability. | [112] |
| 5 | Cu-Ag nanoparticles; multiwalled carbon nanotubes | The nanocomposites showed broad-spectrum antibacterial activity against Gram-positive and Gram-negative pathogens, with glasshouse trials confirming their efficacy in protecting rice and tomato. | [113] |
| No. | Name | Source | Target | Mechanism | Application |
|---|---|---|---|---|---|
| 1 | MaSAMP | Microcitrus australasica | Candidatus Liberibacter asiaticus | Antimicrobial activity and immunity induction | Spray |
| 2 | APP3-14 | Artificially designed | Candidatus Liberibacter asiaticus | Antimicrobial activity and immunity induction | Injection |
| 3 | BP15 | Artificially designed | Stemphylium vesicarium | Antimicrobial activity | Spray |
| 4 | pro-SmAMP2 | Stellaria media | Alternaria and Fusarium | Antimicrobial activity | Tranformation |
| 5 | LL-37 | Human | Fusarium oxysporum | Antimicrobial activity and immunity induction | Tranformation |
| 6 | Rs-AFP | Raphanus sativus | Alternaria longipes | Antimicrobial activity | Tranformation |
| 7 | DrsB1-CBD | Artificially designed | Alternaria, Fusarium, Pythium | Antimicrobial activity | Tranformation |
| 8 | AlfAFP | Medicago sativa | Verticillium dahliae | Antimicrobial activity | Tranformation |
| 9 | BSD1 | Brassica campestris | Phytophthora parasitica | Antimicrobial activity | Tranformation |
| 10 | Dm-AMP1 | Dahlia merckii | Botrytis cinerea | Antimicrobial activity | Tranformation |
| 11 | Pn-AMP | Pharbatis nil | Phytophthora parasitica | Antimicrobial activity | Tranformation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Jia, Z.; Zhu, J.; Liu, J.; Chen, Y.; Xu, Z.; Ma, H. Antimicrobial Peptides and Their Potential Applications in Plant Protection. Agronomy 2025, 15, 1113. https://doi.org/10.3390/agronomy15051113
Sun D, Jia Z, Zhu J, Liu J, Chen Y, Xu Z, Ma H. Antimicrobial Peptides and Their Potential Applications in Plant Protection. Agronomy. 2025; 15(5):1113. https://doi.org/10.3390/agronomy15051113
Chicago/Turabian StyleSun, Deming, Zhaohui Jia, Junjie Zhu, Jinhua Liu, Yichao Chen, Zhi Xu, and Haijie Ma. 2025. "Antimicrobial Peptides and Their Potential Applications in Plant Protection" Agronomy 15, no. 5: 1113. https://doi.org/10.3390/agronomy15051113
APA StyleSun, D., Jia, Z., Zhu, J., Liu, J., Chen, Y., Xu, Z., & Ma, H. (2025). Antimicrobial Peptides and Their Potential Applications in Plant Protection. Agronomy, 15(5), 1113. https://doi.org/10.3390/agronomy15051113






