Promoted Growth of Sweet Potato Root by Bacillus amyloliquefaciens HN11 and Enhanced Uptake of Fosthiazate
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Bacterial Strains
2.2. Chemicals and Reagents
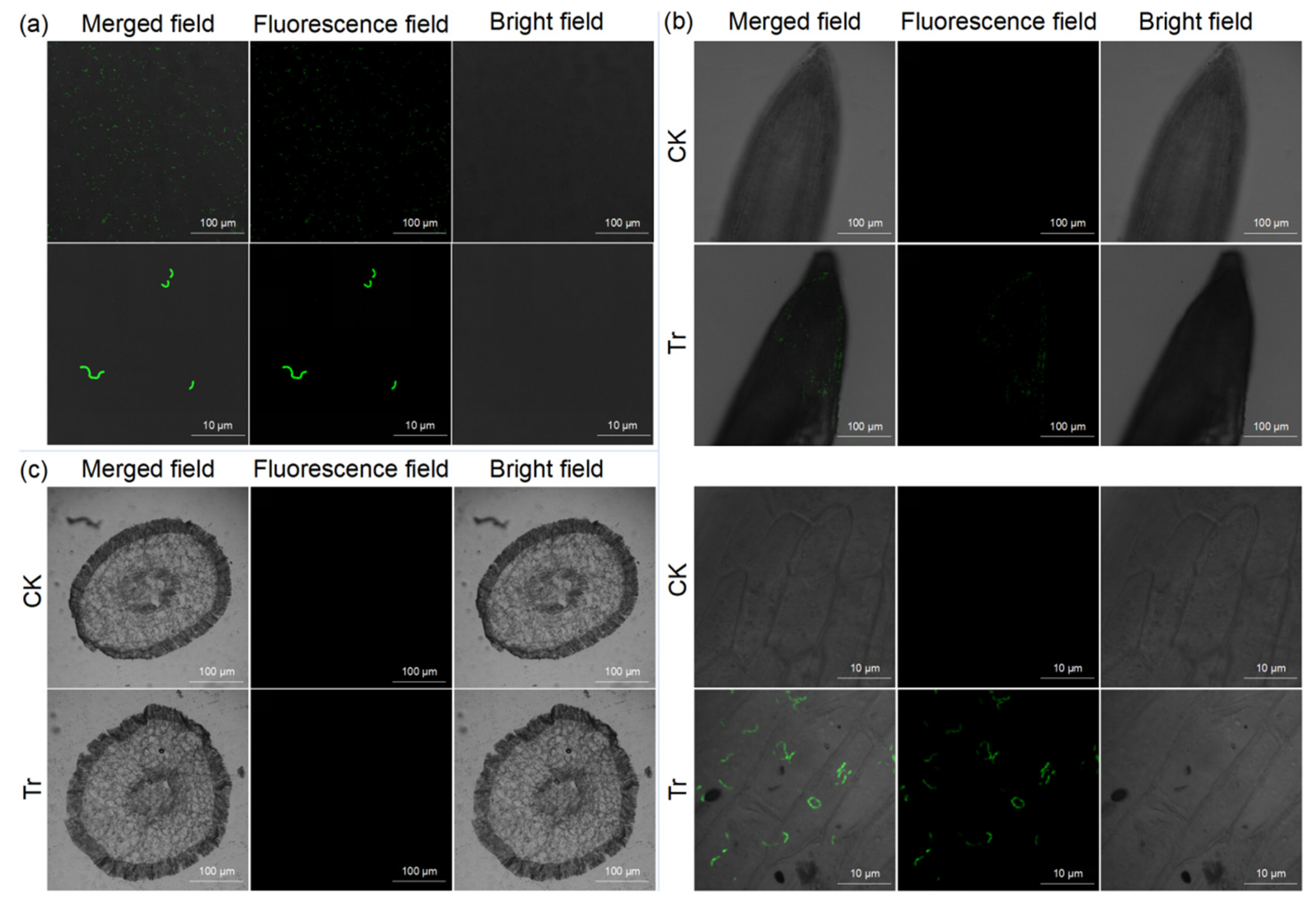
2.3. Observation of Colonization of B. amyloliquefaciens HN11-GFP in Sweet Potato Roots
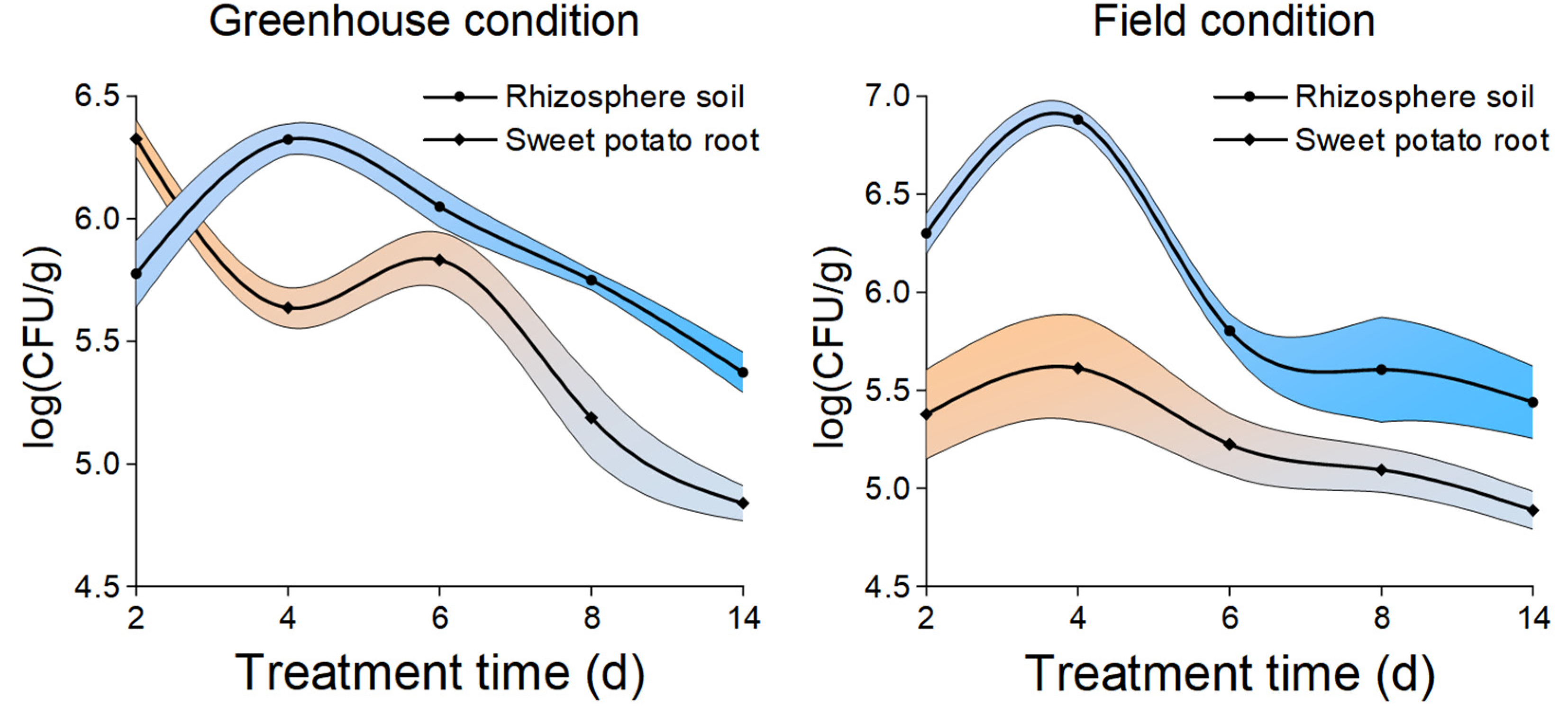
2.4. Colonization Dynamics of B. amyloliquefaciens HN11-GFP in Sweet Potato Roots and Rhizosphere Soil
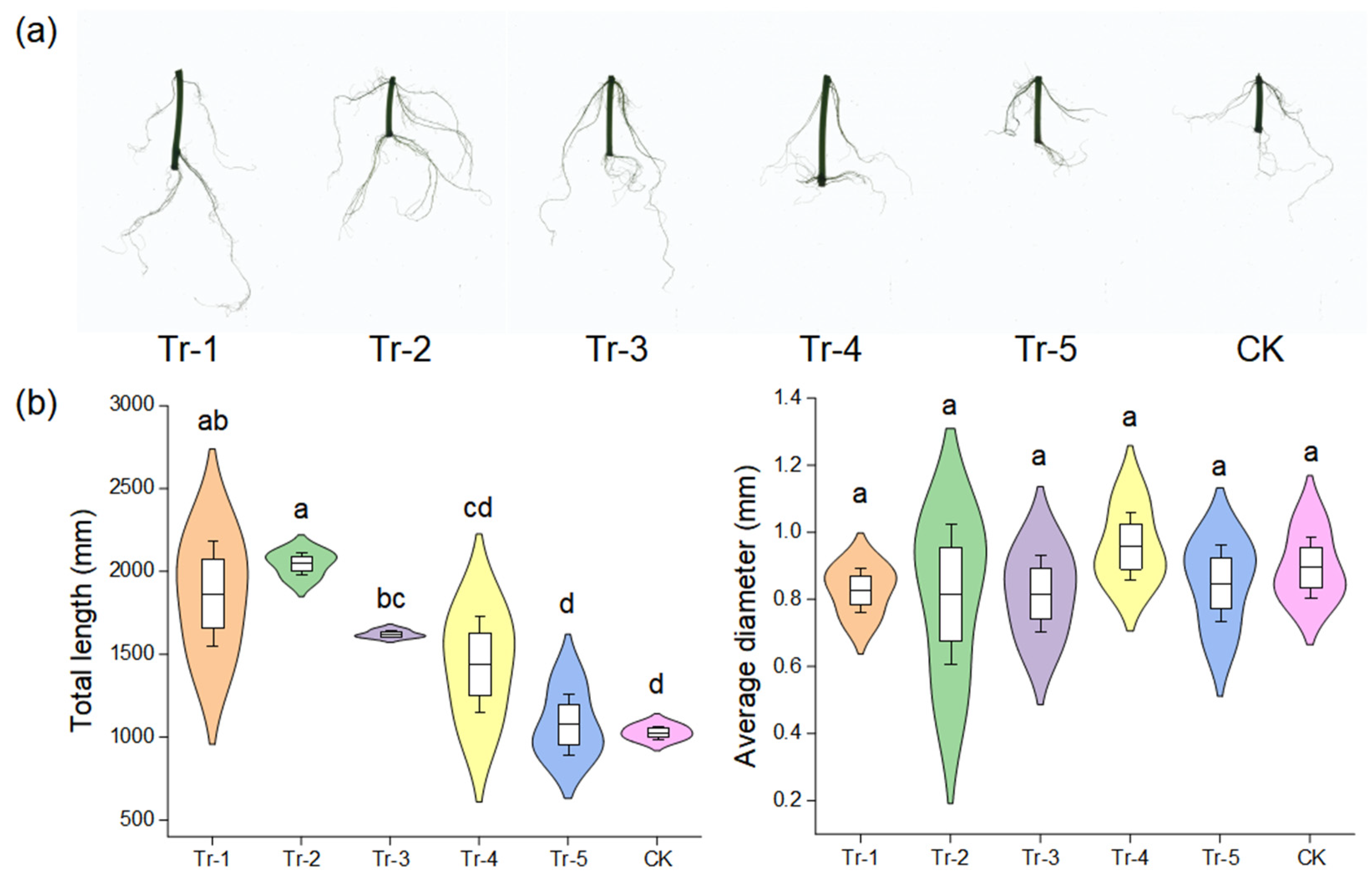
2.5. Effect of B. amyloliquefaciens HN11 on the Growth of Sweet Potatoes Under Greenhouse Condition
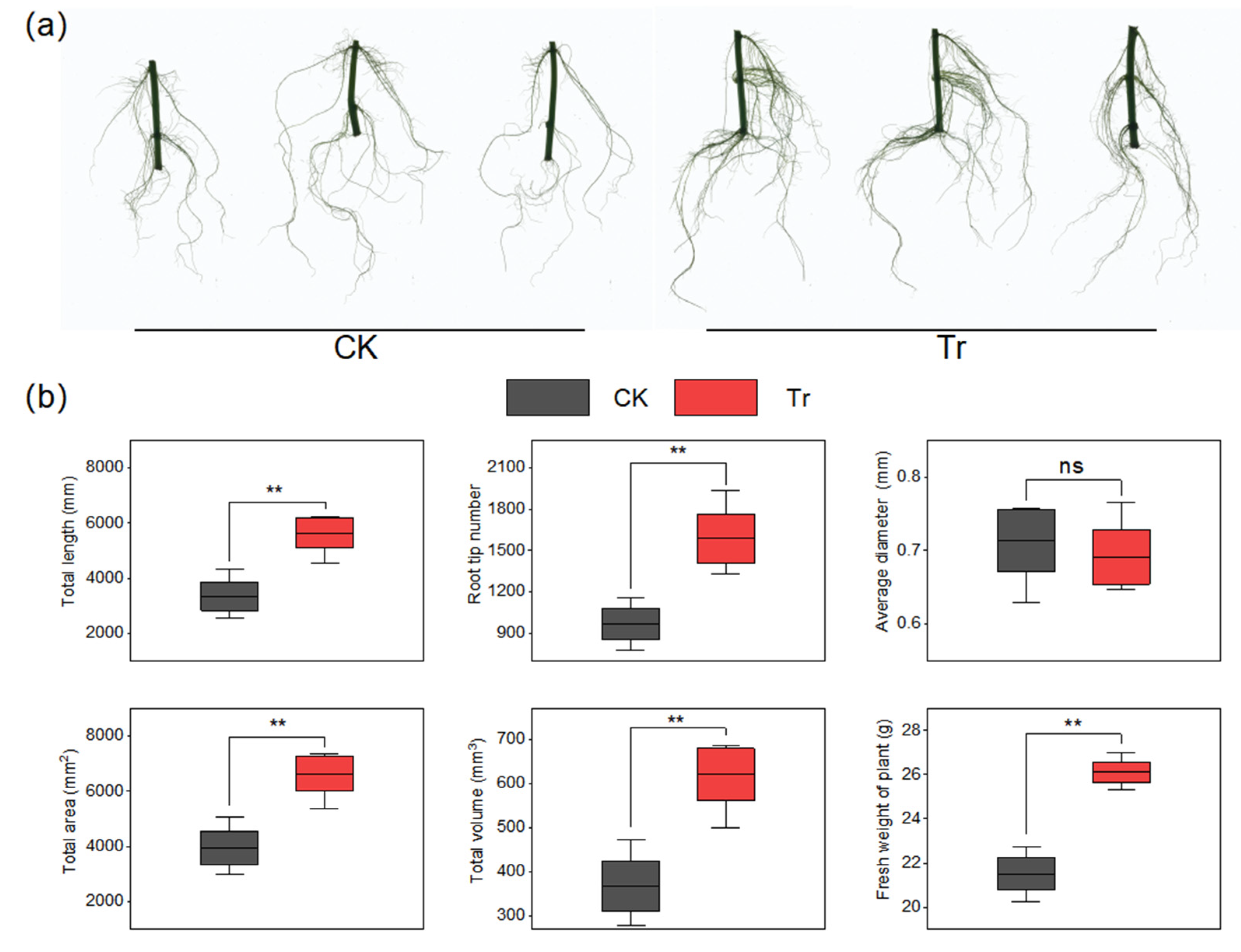
2.6. Effect of B. amyloliquefaciens HN11 on the Growth of Sweet Potatoes Under Field Condition
2.7. Effect of B. amyloliquefaciens HN11 on Fosthiazate Uptake in Sweet Potato Roots
2.8. Effect of B. amyloliquefaciens HN11 on the Control Efficiency to Root Knot Nematode of Sweet Potato
3. Results
3.1. Colonization of B. amyloliquefaciens HN11-GFP in Sweet Potato Rhizosphere
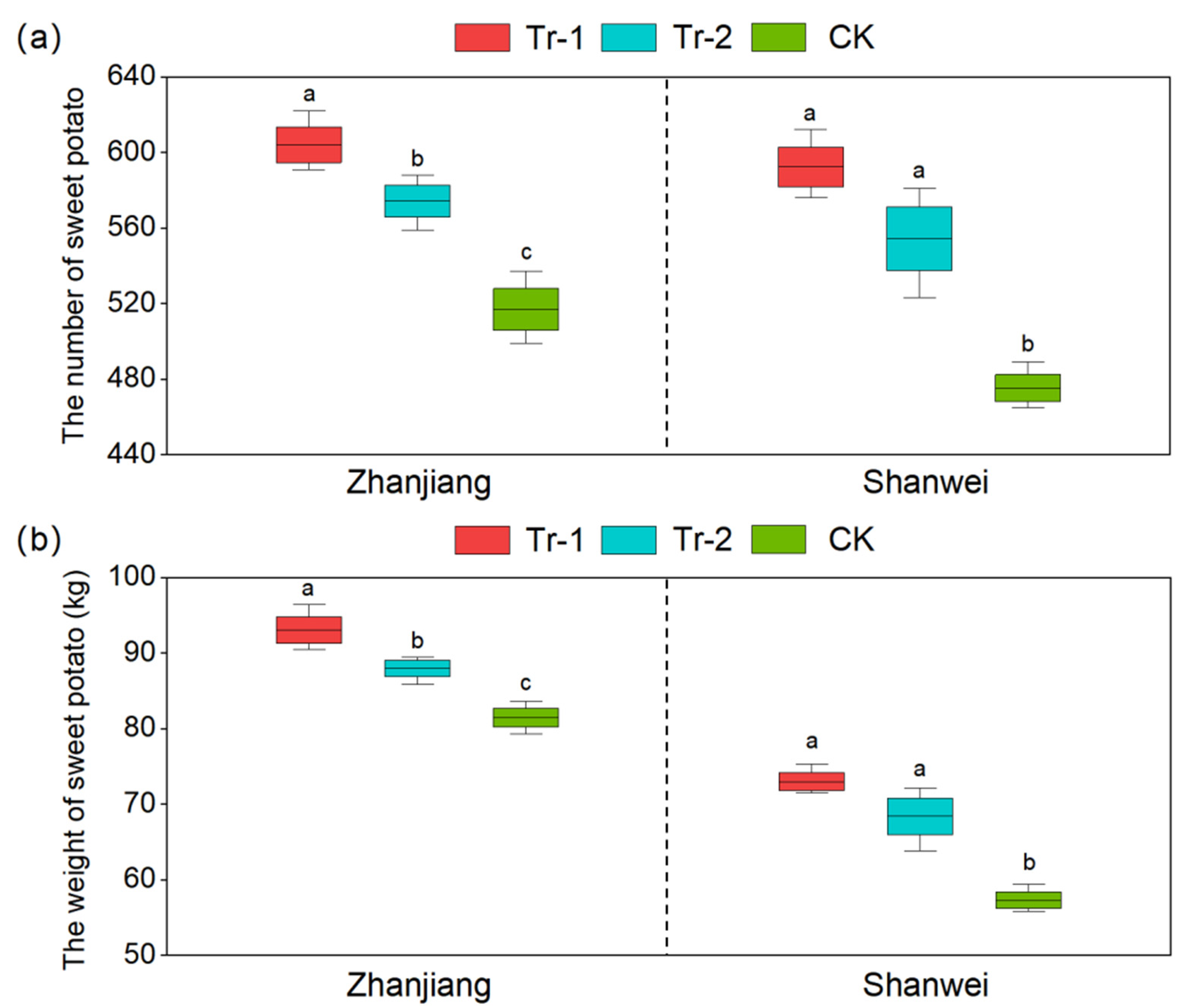
3.2. Effect of B. amyloliquefaciens HN11 on the Growth of Sweet Potatoes
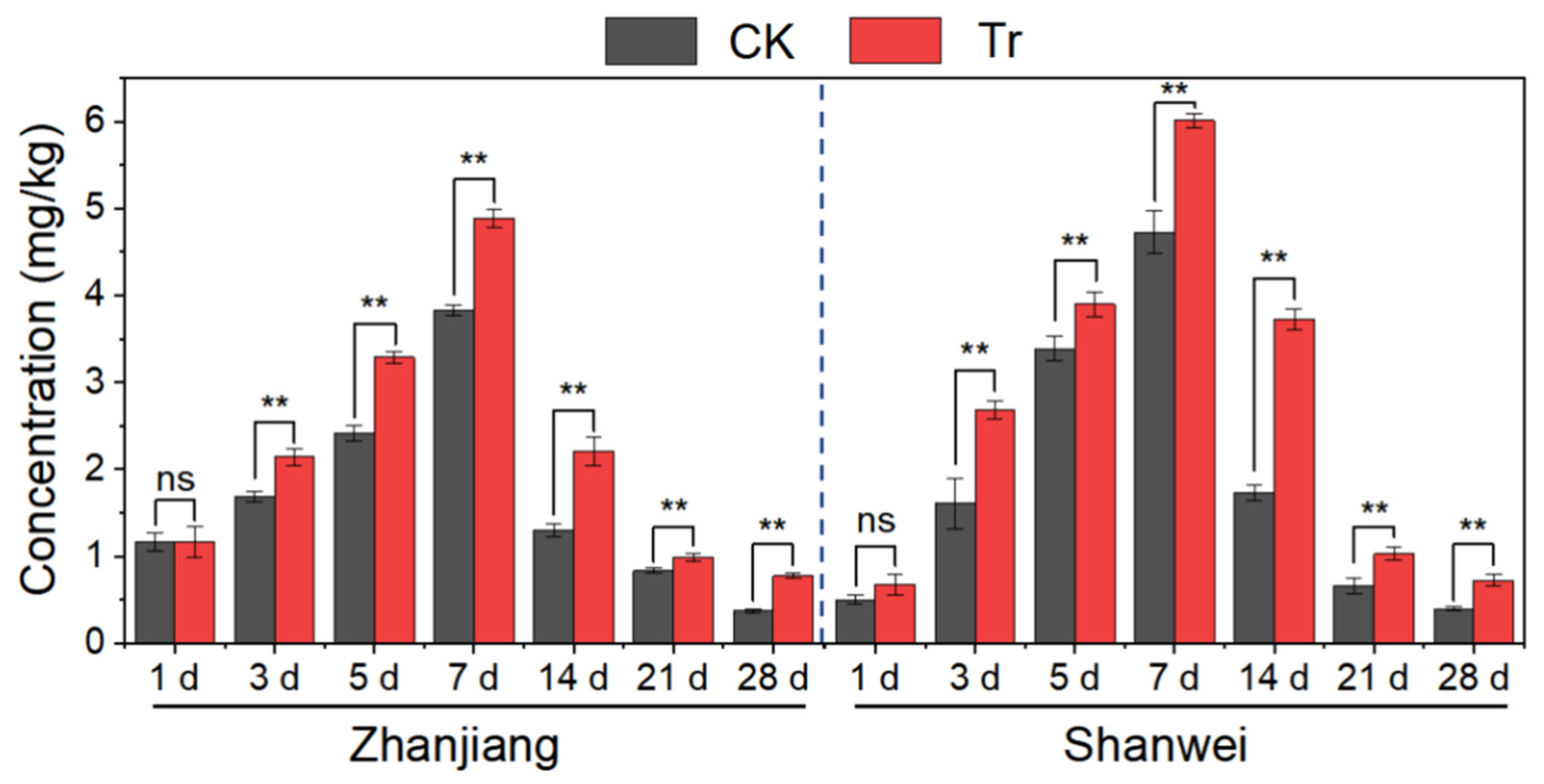
3.3. Effect of B. amyloliquefaciens HN11 on Fosthiazate Uptake in Sweet Potato Roots
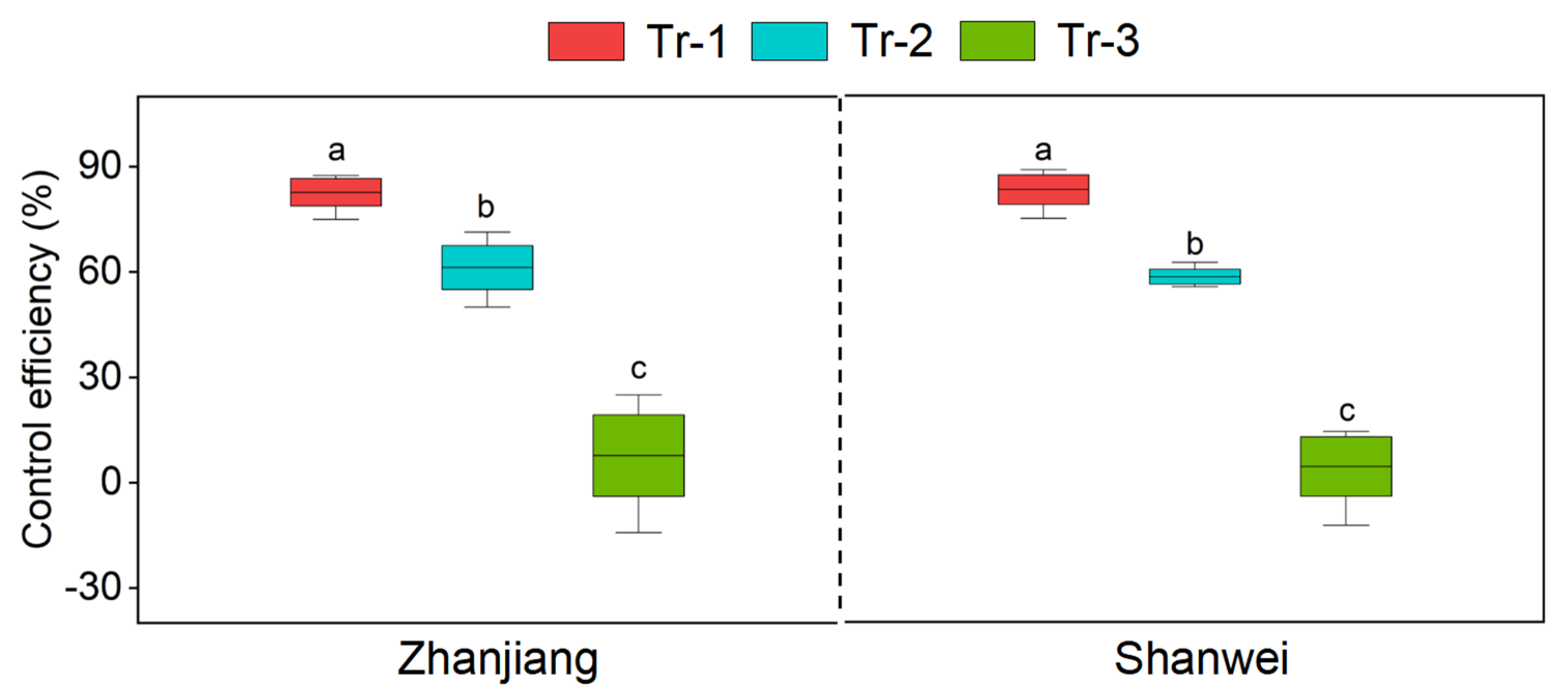
3.4. Effect of B. amyloliquefaciens HN11 on the Control Efficiency to Root Knot Nematode of Sweet Potato
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, M.X.; Nie, H.Z.; Wang, Y.Z.; Wang, X.Y.; Jarret, R.; Zhao, J.M.; Wang, H.X.; Yang, J. Exploring and exploiting genetics and genomics for sweet potato improvement: Status and perspectives. Plant Commun. 2022, 3, 100332. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Pan, Z.J.; Yang, C.X.; Jia, Z.Z.; Guo, X.B. Comparative assessment of phenolic profiles, cellular antioxidant and antiproliferative activities in ten varieties of sweet potato (Ipomoea Batatas) storage roots. Molecules 2019, 24, 4476. [Google Scholar] [CrossRef]
- de Albuquerque, T.M.R.; Sampaio, K.B.; de Souza, E.L. Sweet potato roots: Unrevealing an old food as a source of health promoting bioactive compounds—A review. Trends Food Sci. Tech. 2019, 85, 277–286. [Google Scholar] [CrossRef]
- Alam, M.K. A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- Chen, S.P.; Kuo, Y.W.; Lin, J.S. Review: Defense responses in sweet potato (Ipomoea batatas L.) against biotic stress. Plant Sci. 2023, 337, 111893. [Google Scholar] [CrossRef]
- Palomares-Rius, J.E.; Kikuchi, T. -Omics fields of study related to plant-parasitic nematodes. J. Integr. Omics 2013, 3, 1–10. [Google Scholar] [CrossRef]
- Wu, H.Y.; Luo, M.; Zhang, L.Y.; Zhou, X.B. Nematicidal activity of fosthiazate against soybean cyst nematode Heterodera glycines. J. Nematol. 2019, 51, 1–9. [Google Scholar] [CrossRef]
- Wang, C.; Yao, X.; Li, X.; Wang, Q.; Jiang, N.; Hu, X.; Lv, H.; Mu, B.; Wang, J. Fosthiazate, a soil-applied nematicide, induces oxidative stress, neurotoxicity and transcriptome aberrations in earthworm (Eisenia fetida). J. Hazard. Mater. 2023, 463, 132865. [Google Scholar] [CrossRef]
- Li, H.D.; Du, H.X.; Fang, L.P.; Dong, Z.; Guan, S.; Fan, W.J.; Chen, Z.L. Residues and dissipation kinetics of carbendazim and diethofencarb in tomato (Lycopersicon esculentum Mill.) and intake risk assessment. Regul. Toxicol. Pharmacol. 2016, 77, 200–205. [Google Scholar] [CrossRef]
- Qin, S.J.; Gan, J.Y.; Liu, W.P.; Becker, J.O. Degradation and adsorption of fosthiazate in soil. J. Agric. Food Chem. 2004, 52, 6239–6242. [Google Scholar] [CrossRef]
- Cheng, D.M.; Lin, S.K.; Liu, G.Q.; Zhou, Y.; Zhang, Z.X. Residue and distribution of fosthiazate in cucumber (Cucumis sativus L.) and dietary intake risk assessment for various populations. Int. J. Environ. Anal. Chem. 2021, 103, 6804–6815. [Google Scholar] [CrossRef]
- Lin, S.K.; Zhou, Y.; Wu, J.Y.Z.; Zhang, Z.X.; Cheng, D.M. Dissipation and residue of fosthiazate in tomato and cherry tomato and a risk assessment of dietary intake. Environ. Sci. Pollut. Res. 2022, 29, 9248–9256. [Google Scholar] [CrossRef]
- Bagheri, A.; Emami, N.; Damalas, C.A. Farmers’ behavior towards safe pesticide handling: An analysis with the theory of planned behavior. Sci. Total Environ. 2021, 751, 141709. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, K.; Suyal, D.C.; Kumar, S.; Singh, K.; Goswami, P. New insights into engineered plant-microbe interactions for pesticide removal. Chemosphere 2022, 309, 136635. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.; Del Cid, C.; Hnasko, R.; Gorski, L.; McGarvey, J.A. Bacillus amyloliquefaciens ALB65 inhibits the growth of Listeria monocytogenes on cantaloupe melons. Appl. Environ. Microbiol. 2020, 87, e01926-20. [Google Scholar] [CrossRef]
- Wang, S.Y.; Herrera-Balandrano, D.D.; Wang, Y.X.; Shi, X.C.; Chen, X.; Jin, Y.; Liu, F.Q.; Laborda, P. Biocontrol ability of the Bacillus amyloliquefaciens group, B. amyloliquefaciens, B. velezensis, B. nakamurai, and B. siamensis, for the management of fungal postharvest diseases: A review. J. Agric. Food Chem. 2022, 70, 6591–6616. [Google Scholar]
- Lin, F.; Liu, N.; Lai, D.; Kang, X.H.; Pang, N.W.; Jiang, H.K.; Xu, H.H. A formulation of neem cake seeded with Bacillus sp. provides control over tomato Fusarium crown and root rot. Biocontrol Sci. Techn 2017, 27, 393–407. [Google Scholar] [CrossRef]
- de Boer, A.S.; Diderichsen, B. On the safety of Bacillus subtilis and B. amyloliquefaciens: A review. Appl. Microbiol. Biotechnol. 1991, 36, 1–4. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, K.; He, X.; Li, S.Q.; Zhang, Z.H.; Shen, B.; Yang, X.M.; Zhang, R.F.; Huang, Q.W.; Shen, Q.R. A new bioorganic fertilizer can effectively control banana wilt by strong colonization with Bacillus subtilis N11. Plant Soil 2011, 344, 87–97. [Google Scholar] [CrossRef]
- NY/T 788-2018; Guideline for the Testing of Pesticide Residues in Crops. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2018.
- Tian, X.L.; Yao, Y.R.; Chen, G.H.; Mao, Z.C.; Wang, X.T.; Xie, B.Y. Suppression of Meloidogyne incognita by the endophytic fungus Acremonium implicatum from tomato root galls. Int. J. Pest. Manag. 2014, 60, 239–245. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Xu, Z.H.; Chen, L.; Xun, W.B.; Shu, X.; Chen, Y.; Sun, X.L.; Wang, Z.Q.; Ren, Y.; Shen, Q.R.; et al. Root colonization by beneficial rhizobacteria. FEMS Microbiol. Rev. 2024, 48, fuad066. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.J.; Dekkers, L.; Bloemberg, G.V. Molecular determinants of rhizosphere colonization by Pseudomonas. Annu. Rev. Phytopathol. 2001, 39, 461–490. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Zalila-Kolsi, I.; Ben-Mahmoud, A.; Al-Barazie, R. Bacillus amyloliquefaciens: Harnessing its potential for industrial, medical, and agricultural applications-a comprehensive review. Microorganisms 2023, 11, 2215. [Google Scholar] [CrossRef]
- Escobar-Puentes, A.A.; Palomo, I.; Rodríguez, L.; Fuentes, E.; Villegas-Ochoa, M.A.; González-Aguilar, G.A.; Olivas-Aguirre, F.J.; Wall-Medrano, A. Sweet potato (Ipomoea batatas L.) phenotypes: From agroindustry to health effects. Foods 2022, 11, 1058. [Google Scholar] [CrossRef]
- Taoheed, A.M.; Ateka, E.M.; Losenge, T. Arbuscular mycorrhiza fungi promotes growth of tomato seedings in the absence of phosphate in nutrient solution. Asian J. Nat. Appl. Sci. 2018, 7, 1–9. [Google Scholar]
- Lin, J.X.; Wang, Y.N.; Sun, S.N.; Mu, C.S.; Yan, X.F. Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci. Total Environ. 2017, 576, 234–241. [Google Scholar] [CrossRef]
- Jiang, H.; Tian, Y.Q.; Chen, J.J.; Zhang, Z.Z.; Xu, H.H. Enhanced uptake of drip-pplied flonicamid by arbuscular mycorrhizal fungi and improved control of cotton aphid. Pest Manag. Sci. 2020, 76, 4222–4230. [Google Scholar] [CrossRef]
- Xiao, T.J.; Chen, F.; Ghao, C.; Zhao, Q.Y.; Shen, Q.R.; Wei, R. Bacilus cereus X5 enhanced bio-organic fertilizers effectively control root-knot nematodes (Meloidogyne sp.). Pedosphere 2013, 23, 160–168. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, C.K.; Ma, L.; Zhang, K.Q.; Duan, C.Q.; Mo, M.H. Characterisation of volatiles produced from Bacillus megaterium YfM3.25 and their nematicidal activity against Meloidogyne incognita. Eur. J. Plant Pathol. 2010, 126, 417–422. [Google Scholar] [CrossRef]
- Xie, S.S.; Jiang, L.; Wu, Q.; Wan, W.K.; Gan, Y.T.; Zhao, L.L.; Wen, J.J. Maize root exudates recruit Bacillus amyloliquefaciens OR2-30 to inhibit Fusarium graminearum infection. Phytopathology 2022, 112, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Hu, X.W.; He, H.L.; Zhang, X.Z.; Guo, J.H.; Bai, J.; Cheng, Y.Q. Digital gene expression profiling of the transcriptional response to Sclerotinia sclerotiorum and its antagonistic bacterium Bacillus amyloliquefaciens in soybean. Front. Microbiol. 2022, 13, 1025771. [Google Scholar] [CrossRef]
- Arrington, A.E.; Kennedy, G.G.; Abney, M.R. Applying insecticides through drip irrigation to reduce wireworm (Coleptera: Elateridae) feeding damage in sweet potato. Pest Manag. Sci. 2016, 72, 1133–1140. [Google Scholar] [CrossRef]
- Boina, D.R.; Bloomquist, J.R. Chemical control of the Asian citrus psyllid and of huanglongbing disease in citrus. Pest Manag. Sci. 2015, 71, 808–823. [Google Scholar] [CrossRef]
- Ghidiu, G.; Kuhar, T.P.; Palumbo, J.C.; Schuster, D. Drip chemigation of insecticides as a pest management tool in vegetable production. J. Integr. Pest Manag. 2012, 3, E1–E5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Hu, X.; Ye, X.; Zhang, Z.; Xu, H. Promoted Growth of Sweet Potato Root by Bacillus amyloliquefaciens HN11 and Enhanced Uptake of Fosthiazate. Agronomy 2025, 15, 1098. https://doi.org/10.3390/agronomy15051098
Lin S, Hu X, Ye X, Zhang Z, Xu H. Promoted Growth of Sweet Potato Root by Bacillus amyloliquefaciens HN11 and Enhanced Uptake of Fosthiazate. Agronomy. 2025; 15(5):1098. https://doi.org/10.3390/agronomy15051098
Chicago/Turabian StyleLin, Sukun, Xin Hu, Xulang Ye, Zhixiang Zhang, and Hanhong Xu. 2025. "Promoted Growth of Sweet Potato Root by Bacillus amyloliquefaciens HN11 and Enhanced Uptake of Fosthiazate" Agronomy 15, no. 5: 1098. https://doi.org/10.3390/agronomy15051098
APA StyleLin, S., Hu, X., Ye, X., Zhang, Z., & Xu, H. (2025). Promoted Growth of Sweet Potato Root by Bacillus amyloliquefaciens HN11 and Enhanced Uptake of Fosthiazate. Agronomy, 15(5), 1098. https://doi.org/10.3390/agronomy15051098







