Abstract
In recent years, Cadmium (Cd) pollution in soybean farmland is severe. Therefore, this study focused on whether biochar influences soil physiochemical properties, the Cd content in soil and soybean grains, and the abundance and community structure of the czcA gene. Four doses of rice husk biochar (0, 5, 15, and 25 t·ha−1) were applied under continuous cropping and crop rotation systems, and soil samples were collected after four years of one-time addition. The results indicated that biochar addition significantly increased soil available nitrogen, phosphorus, and soil organic carbon contents under continuous cropping and rotation. Biochar application significantly reduced the total Cd content of soil samples and soybean grains. Additionally, biochar application reduced czcA gene abundance in soybean soils by 14.26–37.88% and 35.96–48.71%, respectively. Correlation analysis revealed that Cd content and the abundance of the czcA gene significantly correlated with soil nutrients and pH. High-throughput sequencing revealed that the relative abundances of several Cd-resistant microorganisms were decreased by biochar addition. In addition, adding biochar significantly affected the Cd-resistant microbial community structure and diversity by influencing soil properties and Cd content. Therefore, this study has important practical significance for improving the soil environment and ensuring the quality and safety of agricultural products.
1. Introduction
With the acceleration of urbanization and industrialization, the pollution of agricultural soil by heavy metals and other pollutants has become a significant global environmental issue [1]. Applying chemical fertilizers, pesticides, sewage sludge, and industrial emissions from farmlands is the primary source of heavy metal accumulation in soil [2]. This accumulation poses a significant problem in agricultural production, as it can negatively impact crop growth and the overall environmental health of soil organisms [3]. Cadmium (Cd) is a significant environmental pollutant that has emerged as a major soil heavy metal pollution issue because of its high toxicity, strong mobility, and widespread contamination worldwide [4]. As the most extensive production and supply base for high-quality soybeans in China, Heilongjiang Province plays a vital role in promoting the revitalization of the soybean industry [5]. Soybeans are sensitive to continuous cropping. Continuous cropping will deteriorate the physical and chemical properties of the soil and cause the accumulation of Cd in the plant. After harvesting, Cd will return to the soil and increase the Cd enrichment [6], which ultimately reduces both yield and quality [7]. However, reasonable crop rotations can change the structure and function of rhizosphere microbial community, prompt some microorganisms to participate in the fixation and transformation of Cd, then reduce the enrichment of heavy metal cadmium, and ultimately benefit the growth and development of soybean [8].
Soil microorganisms are a vital component of terrestrial ecosystems. They play a significant role in the cycling of material and energy [9] and are highly sensitive to ameliorant and heavy metal contamination [10,11]. Research shows soil amendments can enhance microbial diversity and change the microbial community structure [12]. Thus, microbial communities responding to soil amendments are attracting increasing attention. In addition, excessive heavy metals have been reported to reduce the biomass of microorganisms and microbial diversity and alter the microbial community structure [13]. Heavy metals damage the survival and growth of microbial communities and promote the emergence of specific resistant bacteria to reduce the ecological toxicity of pollutants [14]. Metal resistance genes constitute the genetic basis for bacteria to acquire resistance to toxic metals [15]. These resistance genes can persist in the environment and can be enriched in soybeans and will be potentially harmful to human health [16]. Cd is considered as one of the most toxic heavy metals and a persistent poisonous substance, and Cd pollution is irreversible [17,18]. Therefore, a lack of attention to Cd-resistant genes may threaten the ecosystem and public health.
Biochar is a carbon-rich byproduct generated through the pyrolysis of organic materials such as agricultural crop residues or wood waste in an oxygen-depleted environment. It is widely studied as a soil ameliorant globally [19,20]. Biochar provides notable agricultural and environmental benefits, such as enhancing soil health, promoting crop growth and production, and promoting carbon sequestration [21]. This, in turn, increases plant resistance to pathogens, reduces the availability of heavy metals, and enhances the ability of plants to withstand environmental stress [22]. Biochar can immobilize cadmium through its large specific surface area and porous structure, reacting with the heavy metal via processes such as precipitation, surface complexation, ion exchange, physical adsorption, electrostatic interaction, and other comprehensive reactions [23]. Additionally, biochar modifies soil physicochemical properties, thereby reducing the bioavailability and mobility of soil cadmium [24]. Previous studies have shown that biochar can greatly minimize Cd uptake by plants [25]. Wang et al. [26] reported that the Cd content in potato pulp with biochar was reduced by 68.08%. Xing et al. [27] also reported that biochar significantly decreased the amount of Cd in pepper plants in yellow soil. As a result, the use of biochar in agricultural soils has attracted significant attention.
There are few reports on the effects of biochar on the cadmium-resistant bacterial community in agricultural soil under soybean continuous cropping and rotation. Thus, this study conducted a field experiment with four dosages (0, 5, 15, and 25 t·ha−1) of biochar in a soybean continuous cropping field in Heilongjiang Province, China. The present study aimed to (1) investigate the effects of biochar on soil properties and heavy metal Cd content, (2) assess the effects of biochar on soil Cd resistance genes, and (3) evaluate the effects of biochar on microbial diversity and community structure of soil Cd-resistant microorganism. The results of this study would provide novel insights into Cd-resistant microbial diversity in response to different doses of biochar in soybean cropping systems.
2. Materials and Methods
2.1. Field Description and Experimental Design
The field trial was conducted in a soybean field in Anda, southwest of Heilongjiang, China (46°27′ N and 125°18′ E). The region has a typical semiarid temperate continental monsoon climate with a mean annual temperature of 4.2 °C and a mean annual precipitation of 432.5 mm. The soil for the experiment was typical black soil with a soil texture of clay loam, which is classified as Mollisol according to the U.S.’s soil taxonomy. The soil moisture in the experimental area was naturally varied. The biochar was pyrolyzed from rice husk under the preparation temperature range of 400–500 °C. The biochar had a pH of 8.34 and contained total carbon (53.64%), total nitrogen (1.23%), total phosphorus (0.89%), and total potassium (1.56%).
A positioning experiment involving the one-time application of biochar was established in 2021. Biochar was artificially applied with dosages of 0, 5, 15, and 25 t·ha−1 (i.e., 0%, 0.2%, 0.6%, and 1.0% of biochar to 20 cm plough layer soil), then mechanical plowing and ridging were carried out before spring sowing. No more biochar was applied in the following years. Two cropping systems of soybean continuous cropping and corn–soybean rotation were selected in this study. There were eight treatment plots with three replicates (each plot area was 3.9 m × 10 m = 39 m2) in a block design: (1) C0: continuous cropping with a biochar dosage of 0 t·ha−1; (2) C1: continuous cropping with a biochar dosage of 5 t·ha−1; (3) C2: continuous cropping with a biochar dosage of 15 t·ha−1; (4) C3: continuous cropping with a biochar dosage of 25 t·ha−1; (5) R0: rotation with a biochar dosage of 0 t·ha−1; (6) R1: rotation with a biochar dosage of 5 t·ha−1; (7) R2: rotation with a biochar dosage of 15 t·ha−1; and (8) R3: rotation with a biochar dosage of 25 t·ha−1. The soybean cultivar Heinong 86 and the corn cultivar Xianyu 335 were selected for this study. Chemical fertilizers were applied with compound fertilizer. The application rates of compound fertilizer were 45 kg N ha−1, 60 kg P2O5 ha−1, and 30 kg K2O ha−1 for soybean, and 200 kg N ha−1, 100 kg P2O5 ha−1, and 100 kg K2O ha−1 for corn. Field management was conducted following traditional local practices.
2.2. Sample Collection
Soil samples were collected from 2 to 15 cm depths in October (maturation stage) 2024. Each soil sample was composited and homogenized from five soil cores in each plot. All the soil samples were placed in a portable ice box and transported to the laboratory as soon as possible. In the laboratory, debris such as soybean roots and stones was removed from the soil samples, and then the soil was sieved through a 2 mm nylon sieve. A small amount of each fresh soil sample was then taken uniformly and immediately stored at −80 °C for further DNA extraction. The remaining soils were air dried to determine the soil chemical properties and heavy metal cadmium content.
2.3. Analysis of Soil Physicochemical Properties
The soil moisture content (MC) was determined gravimetrically after drying at 105 °C. The soil pH was measured via a pH meter with a soil/water mixture at a w/v ratio of 1:2.5 after shaking for 1 h [28]. The available nitrogen (AN), available phosphorus (AP), and available potassium (AK) contents were determined according to the methods described by Bao [29]. Specifically, the AN content was measured via the alkali hydrolysis diffusion method, the AP content was measured via the sodium bicarbonate extraction molybdenum blue colorimetry method, and the AK content was measured via the ammonium acetate extraction flame photometry method [29]. Soil organic carbon (SOC) was analyzed via the oxidation of potassium dichromate method. The extraction of total Cd from the soil was measured in a graphite furnace with an atomic absorption spectrophotometer (PinAAcle 900 E, Needham, MA, USA) [30].
2.4. Soil DNA Extraction, Quantitative PCR, and High-Throughput Sequencing
The total genomic DNA was extracted from 0.5 g of homogenized soil (fresh weight) via the M5635-02 DNA Isolation Kit according to the manufacturer’s protocol and then stored at −80 °C until use. Here, we quantified the absolute abundances of cadmium resistance genes (czcA) through quantitative real-time PCR (qPCR) via a fluorescence quantifier (LightCycler 480 II, 384, Roche, Basel, Switzerland). The analyses for microbial diversity and community structure of cadmium resistance genes in the soil samples were carried out on an Illumina paired-end (PE-300) platform by Shanghai Personal Biotechnology Co., Ltd (Shanghai, China). The czcA gene was amplified via the primers czcA F (5′-TCGACGGBGCCGTGGTSMTBGTCGAGAA-3′) and czcA R (5′-GTVAWSGCCAKCGGVBGGAACA-3′). The specific analysis process was carried out according to Walker’s methods [31]. The sequences in the FASTQ file were trimmed and filtered via QIIME Pipeline Version 1.8.0, and the chimeric sequences were further optimized via UCHIME algorithm software version 4.2. The representative sequence of each classification unit was clustered and analyzed via a phylogenetic tree with PyNAST (Python Nearest Alignment Speace Termination tool) and FastTree software version 2.1. Finally, all samples were leveled according to the minimum sequencing data 8796 for follow-up. All sequences have been deposited in the GenBank short-read archive SRP567551.
2.5. Statistical Analysis
Statistical analysis was implemented via SPSS (v27.0). One-way analysis of variance (ANOVA), followed by the Warren–Duncan test, was used to determine the statistical significance (p < 0.05) of the soil parameters among the different treatments. Pearson correlation analysis was used to assess the relationships between the biochar addition and the soil physicochemical properties. Alpha diversity (including the Chao, Shannon, and Simpson indices) was calculated using Mothur software version 1.48.2. Beta diversity analysis via NMDS was performed via R software version 3.6.3. All the figures were plotted using GraphPad Prism software version 8.0 and ChiPlot platform.
3. Results
3.1. Soil Properties
The physical and chemical properties of the soils in the investigated treatments are summarized in Table 1. The contents of soil AN, AP, and SOC were increased by biochar addition significantly, especially in the treatment with biochar of 25 t·ha−1. Their contents were all the highest under both continuous cropping and crop rotation. However, the influences of biochar on soil MC and pH were relatively small. When biochar was applied with 25 t·ha−1, the soil MC and pH in crop rotation soils increased by 7.08% and 0.61% compared with R0 treatment, respectively. In addition, when the application amount of biochar was 0 t·ha−1, the contents of AN, AP, AK, and SOC in the soybean continuous cropping soil were lower than those in the crop rotation soil. In contrast, soil MC and pH showed the opposite trend.

Table 1.
Soil physicochemical properties under different treatments.
3.2. Cd Content of Soil and Soybean Grain
The total Cd content of the soil samples under continuous cropping and crop rotation conditions was measured in this study, and the results are shown in Figure 1. Compared with the control (C0) treatment, the total Cd content of soil significantly decreased (p < 0.05) after biochar addition under soybean continuous cropping conditions (Figure 1a). Moreover, when the application dose of biochar increased from 0 t·ha−1 to 15 t·ha−1 in the soil, the Cd content substantially decreased by 11.78% (Figure 1a). Similarly, under the soybean rotation condition, biochar application significantly reduced the Cd contents in the soil (p < 0.05). Compared with that without biochar (R0), the Cd content in the R2 treatment (15 t·ha−1) decreased by 15.12% (Figure 1b).
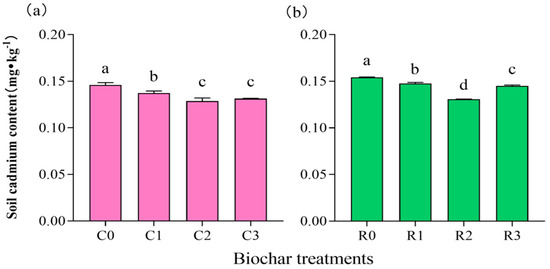
Figure 1.
Soil Cd content of different biochar treatments under continuous cropping (a) and crop rotation (b). Different lowercase letters on the columns indicate significant differences between four biochar treatments (p < 0.05) under the same cropping pattern. The short lines on the bar chart represent standard errors. The same as below.
Additionally, the total Cd content of soybean grains under continuous cropping and crop rotation conditions was measured in this study (Figure 2). In both cropping systems, with the increase in biochar dosage, the Cd content in soybean grains first decreased rapidly but then increased slightly. Compared with the C0 treatment, the total Cd concentration in the soybean grains strongly decreased in the C1, C2, and C3 treatments by 8.91%, 14.64%, and 13.94%, respectively (Figure 2). Similarly, under crop rotation conditions, the total Cd concentrations of soybean grains in the R1, R2, and R3 treatments were firmly lower than that in the R0 treatment by 2.82%, 10.43%, and 6.86%, respectively (Figure 2). Overall, biochar addition decreased the total Cd content of the soil and soybean grains under both continuous cropping and crop rotation conditions.

Figure 2.
Soybean Cd content of different biochar treatments under continuous cropping and crop rotation conditions. The biochar dosages were 0 t·ha−1 (0), 5 t·ha−1 (1), 15 t·ha−1 (2), and 25 t·ha−1 (3), respectively. Different letters between the four treatments indicate significant differences (p < 0.05) under the same cropping pattern. The short lines on the line chart represent standard errors.
3.3. Absolute Abundance of the czcA Gene
The czcA gene abundances ranged from 1.06 × 106 gene copies·g−1 dry soil to 2.28 × 106 gene copies·g−1 dry soil across all the samples (Figure 3). Compared with the C0 treatment, the C1, C2, and C3 treatments significantly reduced the czcA gene abundance in the soil under continuous cropping by 14.26%, 30.20%, and 37.88%, respectively (Figure 3a). Compared with the R0 treatment, the R1, R2, and R3 treatments significantly reduced the czcA gene abundance in the crop rotation soil by 35.96%, 48.71%, and 43.19%, respectively (Figure 3b). Overall, the czcA gene abundances were influenced by biochar addition under both soybean continuous cropping and rotation.

Figure 3.
The absolute abundance of the czcA gene under different biochar treatments under continuous cropping (a) and crop rotations (b). Different lowercase letters on the columns indicate significant differences between four biochar treatments (p < 0.05) under the same cropping pattern. The short lines on the bar chart represent standard errors.
3.4. Correlations Between the Soil Properties, Cd Content, and czcA Abundance
The correlation analysis was conducted among physicochemical properties, abundance of gene czcA, and Cd contents of soil and soybean grain (Figure 4). Under the continuous soybean cropping condition, the soil Cd content, soybean Cd content, and czcA abundance were all negatively correlated with soil AN, AP, AK, and SOC significantly (p < 0.01), whereas pH was positively correlated with soil Cd (Figure 4). In addition, there is a significant positive pairwise correlation between soil Cd content, soybean Cd content, and czcA abundance (p < 0.01), and the same trend was observed for soil AN, AP, AK, and SOC. However, no significant correlation was detected between MC and other indexes in this study under continuous cropping condition (Figure 4).

Figure 4.
Pearson’s correlation analysis of soil properties, Cd content, and czcA abundance under continuous cropping condition. * and ** indicate significant correlation at p < 0.05 and p < 0.01 levels, respectively.
For crop rotation of soybean, soybean Cd content and czcA abundance were all negatively correlated with soil MC, AN, and SOC significantly (p < 0.05), whereas soil Cd was only negatively correlated with SOC significantly (p < 0.01) (Figure 5). Like continuous cropping, the relationship between the three pairs of soil Cd content, soybean Cd content, and czcA abundance positively correlated significantly (p < 0.01) under crop rotation condition. A significant positive correlation was also shown among the physicochemical properties of crop rotation soil.
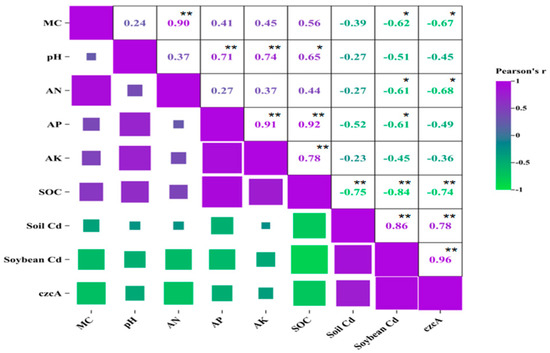
Figure 5.
Pearson’s correlation analysis of soil properties, Cd content, and czcA abundance under crop rotation condition. * and ** indicate significant correlation at p < 0.05 and p < 0.01 levels, respectively.
3.5. Analysis of czcA Gene Microbial Community
3.5.1. Alpha Diversity
The richness indexes (Chao1) and diversity indexes (Shannon and Simpson indexes) of the czcA gene were compared between different treatments in continuous cropping and crop rotation soils (Figure 6). The results showed that biochar addition significantly affected the diversity of bacterial communities. Compared with C0, the Chao 1, Shannon, and Simpson indexes of the czcA gene under continuous cropping increased by biochar with 3.9%, 4.7%, and 0.6% on average, respectively (p < 0.05) (Figure 6a). Compared with R0, the Simpson index of the czcA gene in biochar treatments increased by 0.4% on average (p < 0.05) (Figure 6b). In contrast, the Chao index only decreased in the R3 treatment by 20.1% (p < 0.05), and no significant difference was detected in the Shannon index (Figure 6b).

Figure 6.
Alpha diversity of the czcA gene community under continuous cropping (a) and crop rotation (b). Different lowercase letters on the boxes indicate significant differences between four biochar treatments (p < 0.05).
3.5.2. Beta Diversity
Nonmetric multidimensional scaling (NMDS), which is based on the Bray–Curtis method, was employed to simplify the analysis of Cd antagonistic microorganism structural variations between samples. The results revealed a broad distribution of sample sites across all treatments (Figure 7). The Cd antagonistic bacterial community was clearly divided into two groups according to the soil samples from the different cropping systems (Figure 7), which indicated that the cropping significantly affected the composition of the cadmium antagonistic bacterial community in the bulk soil. However, no significant difference existed among the four biochar treatments under soybean continuous cropping or rotation.

Figure 7.
Non-metric multidimensional scaling of the czcA gene community structure. C, continuous cropping; R, crop rotation; and the biochar dosages were 0 t·ha⁻1 (C0, R0), 5 t·ha⁻1 (C1, R1), 15 t·ha⁻1 (C2, R2), and 25 t·ha⁻1 (C3, R3), respectively.
3.5.3. Relative Abundance of the czcA Gene at Phylum and Genus Levels
Changes in the relative abundances of the top five Cd-antagonistic bacterial phyla (Figure 8) were characterized in the soil samples from the different treatments. The dominant bacterial phyla across all the soil samples were Proteobacteria, Planctomycetes, Actinobacteria, Acidobacteria, and Verrucomicrobia, accounting for more than 55% of the total sequences (Figure 8). Their relative abundances varied from 43.78% to 49.48%, 4.70% to 7.96%, 0.78% to 1.22%, 0.53% to 0.86%, and 0.23% to 0.37% across all the samples, respectively.

Figure 8.
The relative abundance of Cd-antagonistic bacteria at the phylum level under different treatments.
This study selected 12 genera with relative abundances greater than 0.3% to demonstrate the changes in cadmium antagonistic bacterial community composition in continuous cropping and crop rotation soils (Figure 9). The top five dominant bacterial genera across all the soil samples were Planctomyces, Lysobacter, Bradyrhizobium, Sorangium, and Cupriavidus (Figure 9). Under continuous cropping conditions, the relative abundance of Lysobacter in the C1 and C2 treatments decreased by 47.24% and 43.83%, respectively. The relative abundance of Bradyrhizobium in the C1, C2, and C3 treatments decreased by 71.74%, 70.47%, and 59.16%, respectively. However, there was no significant change in the relative abundance of Planctomyces, Sorangium, and Cupriavidus. In the crop rotation soil, the relative abundance of Lysobacter, Bradyrhizobium, and Sorangium remained relatively stable. In contrast, the relative abundances of Planctomyces in the R3 treatment and Cupriavidus in the R2 treatment were significantly decreased and increased by 39.68% and 96.74%, respectively, compared with the R0 treatment.

Figure 9.
The relative abundance of Cd-antagonistic bacteria at the genus level under different treatments. Different letters in the treatments indicate significant differences (p < 0.05).
3.6. Redundancy Analysis of czcA Community Structure and Soil Properties
Redundancy analysis (RDA) was performed to further analyze the effects of the soil physicochemical properties on the bacterial (czcA) community composition. As shown in Figure 10, similar to the NMDS results, the microbial communities of continuous cropping and crop rotation were clearly separated along the RDA2 axis, accounting for 13.57% of the variation. Moreover, under continuous cropping conditions, the biochar-amended treatments and control treatment (C0) were clearly separated along the RDA1 axis, accounting for 75.06% of the variation. This indicates that biochar application had a more significant impact on Cd-resistant gene community structure than the cropping system. The RDA plot showed that among the tested soil properties, the arrows for MC, AN, AP, SOC, and soil Cd content were relatively longer than pH and AK, indicating that they have a more significant impact on microbial communities. This result was further validated and supported by mantel test analysis (Table 2), which showed that MC, AN, AP, SOC, and soil Cd significantly correlated with the czcA gene microbial community structure.

Figure 10.
RDA analysis between the czcA gene community and the soil properties. C, continuous cropping; R, crop rotation; and the biochar dosages were 0 t·ha⁻1 (C0, R0), 5 t·ha⁻1 (C1, R1), 15 t·ha⁻1 (C2, R2), and 25 t·ha⁻1 (C3, R3), respectively.

Table 2.
Relationships between czcA gene community structure and environmental variables as revealed by Mantel.
Under soybean continuous cropping condition, a Pearson’s correlation analysis between the czcA gene microbial community and soil variables indicated that the relative abundance of Bradyrhizobium was significantly positively correlated with the Cd content (p < 0.05) and significantly negatively correlated with the SOC (p < 0.05) and AN (p < 0.05) (Figure 11a), whereas Ralstonia had the opposite trend. For crop rotation of soybean, the relative abundance of Planctomyces was significantly negatively correlated with the AK (p < 0.05) and MC (p < 0.05), while the relative abundance of Sphingopyxis was significantly negatively correlated with the AK (p < 0.05) (Figure 11b). In addition, the relative abundance of Cupriavidus was significantly negatively correlated with the Cd (p < 0.001) (p < 0.05), whereas it was significantly positively correlated with the SOC (p < 0.05) (Figure 11b).

Figure 11.
Pearson’s correlation analysis between the czcA gene microbial community and soil variables under continuous cropping (a) and crop rotation (b). * and ** indicate significant correlation at p < 0.05 and p < 0.01 levels, respectively.
4. Discussion
4.1. Effects of Biochar on Soil Properties
Several studies have shown that the addition of biochar alters the soils physicochemical properties, and its influence depends on the characteristics and amount of added biochar [32,33]. In this study, significant changes in pH, AN, AP, AK, and SOC, whether in continuous cropping soil or rotation soil, were detected after biochar application, except for MC (Table 1). Soil pH is an essential indicator of the changes in soil properties caused by biochar application and directly affects soil fertility, nutrient availability, and microbial activity [32,34]. In many previous studies, biochar was found to increase the soil pH and reduce the content of heavy metals [35,36,37]. Nevertheless, these studies were carried out in acidic soil, and the high pH of biochar directly led to an increase in the soil pH. In this study, we found that applying biochar to continuous cropping soil significantly reduced the soil pH in the C2 treatment with a suitable amount of biochar added (15 t·ha−1) (Table 1). Our result was similar to those of Xiu et al. [38], who reported that the soil pH significantly decreased over two years when the soil was amended with wheat straw biochar. The reason may be that applying biochar increases the oxalic acid and acetic acid content in the soil, reducing the soil pH [39]. In addition, we found that the application of biochar to rotation soil significantly increased the soil pH in the C2 treatment with a suitable amount of biochar added (15 t·ha−1) (Table 1), which corresponds with the results of Fan et al. [34], who reported that soil pH increased approximately 0.44 units after the application of corn straw biochar.
Soil available nutrients are important factors affecting crop yield and quality [40]. In this study, the contents of AN and AP increased continuously with increasing biochar addition to the continuous cropping soil (Table 1) by 7.57–18.61% and 26.10–142.69%, respectively. A similar result of AN was shown in the rotation soil. Our results are consistent with the finding of Huang et al. [41], who reported that biochar addition significantly increased the AN and AP contents in phragmites rhizosphere soil, implying that biochar might be a source of N and P. Furthermore, as a valuable indicator of soil health and fertility [42], the content of SOC increased with increasing biochar in both the continuous cropping and crop rotation soils (Table 1). These results indicate that the application of biochar can improve soil fertility.
4.2. Effects of Biochar on Cd Contents of Soil and Soybean
The heavy metal Cd naturally exists in soil but may be enhanced by anthropogenic activities such as agricultural practices. The bioaccumulation of the heavy metal Cd in the food chain, following its uptake by plants, can increase the ecotoxicological risk associated with the remediation of contaminated soils via plants [43,44]. In our study, whether continuous cropping or rotation, the Cd content of the soil was decreased by biochar addition (Figure 1). Comparable findings were presented by Han et al. [45], who reported that the application of biochar significantly reduced the amount of Cd in soil. Lang et al. [46] also reported that CaO-modified hydrochar dramatically reduced the soil Cd content by 8.19–16.2%. Furthermore, Wang et al. [47] revealed that the application of sulfur-modified woody peat compost significantly reduced Cd concentrations, with only 4.01 mg/kg found. In addition, we found that when the application amount of biochar was 25 t·ha−1, the Cd content in the soil was greater than that of 15 t·ha−1, both under continuous cropping and rotation. This is because during the preparation of biochar, the organic components were released in the form of volatile gases such as CO2, CH4, and H2O, but the heavy metals did not decompose and volatilize like them. Therefore, the biochar itself carried relatively more enriched cadmium into the soil when applied at 25 t·ha−1 [48,49]. It was, therefore, reasonable that the application of too much biochar resulted in a relative increase in the total heavy metal content in soil (Figure 1).
With the application of biochar, the changing trend of the Cd content in the soybean grains was consistent with that in the soil (Figure 2). Correlation analysis revealed that soybean Cd was positively correlated with soil Cd (p < 0.01) (Figure 4 and Figure 5). The addition of biochar could increase the soil adsorption capacity due to its large surface area, which might cause high retention of Cd in biochar-amended soils [50]. Therefore, the addition of biochar is beneficial for decreasing the cadmium content in soybean grains, but it cannot be applied excessively, and the optimal application amount is 15 t·ha−1. Biochar has a porous structure, a large specific surface area, and abundant surface functional groups, which makes it have a strong adsorption effect on heavy metal pollutants. Alternatively, it may be that biochar changes the soil acid–alkali environment, reducing the effectiveness of heavy metals, and the harm of heavy metals [35]. Overall, our results indicate that biochar at higher dosages will relatively increase the cadmium content in the soil, which has potential negative impacts on soil cadmium pollution. However, the application of an appropriate dosage of biochar can reduce the Cd content in both the soil and soybean grains to a certain extent and reduce the risk of Cd being transferred from the soil to the food chain [51,52].
4.3. Effects of Biochar on the Expression of the czcA Gene
Identifying the metal resistance genes in soil is helpful for understanding the potential environmental risks of microorganisms and their ecological functions in the metal biogeochemical cycle [53,54]. The czc series of resistance genes are the most distinctive Cd ion efflux genes, and their products form complex cation efflux pumps, which endow many gram-negative bacteria with resistance to cadmium [55]. These systems are generally composed of four parts: czcA, czcB, czcC, and czcD. czcA is a chemically isomeric cation/H reverse transporter protein and RND-driven export system [53].
The accumulation of heavy metals in the soil promotes heavy metal resistance. In our study, the application of biochar reduced the abundance of the czcA gene in both the continuous cropping and crop rotation soils (Figure 3). Moreover, we observed that the abundance of the czcA gene was positively correlated with the concentration of Cd content (p < 0.05) (Figure 4 and Figure 5), indicating the role of heavy metal in inducing the expression of the Cd resistance gene in soybean soil, which is consistent with the experimental results of Liu et al. [56]. They reported that there was a significant correlation between the metal resistance genes level and exchangeable heavy metal content. Therefore, our research shows that the application of biochar indirectly reduces the abundance of the czcA gene by reducing the cadmium content in soil.
4.4. Effects of Biochar on Soil Microbial Community Structure of czcA Gene
The α diversity index of cadmium-resistant bacteria reflects the richness and distribution of the soil cadmium-resistant microbial community. In addition, a higher index indicates that the soil Cd-resistant microbial diversity is richer and that the microbial ecosystem function is more complex and stable [57]. The Chao index reflects the actual number of species in a community. Generally, a higher Chao index indicates greater community richness. The results revealed that the Chao1 index increased by biochar with 2.82% on average in continuous cropping soil compared with the non-biochar treatment (Figure 6a), indicating that biochar significantly increased the richness of the soil Cd-antibiotic bacterial community. This is due to the unique porous structure of biochar, which can provide suitable habitats for microorganisms [58]. In contrast, under crop rotation conditions, when the amount of biochar applied was 25 t·ha−1, the Chao1 index of the soil microorganisms decreased significantly (Figure 6b). Our results are consistent with those of Marris [59], who reported that biochar can reduce the richness of microorganisms, possibly because microorganisms can directly use the nutrient of biochar, but these contents are limited. The Shannon index and Simpson index reflect the richness and evenness of community species. We found that after biochar addition, the Shannon index increased significantly in continuous cropping soil, but there was no significant difference in crop rotation soil (Figure 6), indicating that agricultural practices affected the changes of the bacterial community to some extent. In addition, the Simpson index increased in all the treatments after biochar application. Comparable findings were presented by Yan et al. [60], who reported that the application of biochar significantly increased the Shannon index and Simpson index of soil microorganisms (p < 0.05). This is primarily due to the fact that biochar directly offers a habitat for microorganisms and indirectly improves the living environment by influencing soil structure and nutrient availability. The results of bacterial community β diversity indicated that the cropping system had a significant effect on the composition of the bacterial community (Figure 7), which was consistent with the results of Wang et al. [61]. In summary, our results show that the cropping system affects the soil bacterial community structure. Biochar addition increases the diversity of soil bacteria, which play a vital role in maintaining soil stability.
The application of biochar changed the soil environment and further affected the composition of the bacterial community. Changes in these communities serve as a key indicator for assessing soil health [62]. In this study, Proteobacteria, Planctomycetes, Actinobacteria, Acidobacteria, and Verrucomicrobia were the five dominant phyla in the bacterial community across all the soils (Figure 8). Our results are consistent with the report of applying straw biochar in sandy loam [63]. In addition, the application of biochar reduced the relative abundance of Proteobacteria, Planctomycetes, and Actinobacteria. At the genus level, after the application of biochar, the relative abundance of Lysobacter and Bradyrhizobium in the continuous cropping soil decreased significantly, while the relative abundance of Planctomyces and Cupriavidu in the crop rotation soil decreased significantly (Figure 9). This reduction might be attributed to the alterations in soil pH and nutrient content triggered by the addition of biochar [64]. Moreover, RDA results revealed that biochar application indirectly changed the czcA gene microbial community structure by altering the soil characteristics (Figure 10, Table 2). This result has been confirmed by a large number of studies [35,65]. Based on Pearson’s correlation analysis, soil Cd content was significantly correlated with the major czcA gene microbial communities (Bradyrhizobium, Ralstonia, and Cupriavidus) in continuous cropping and crop rotation soils, as shown in Figure 11. Xu et al. [66] reported that the introduction of Cd increased the abundance of resistant species and decreased the abundance of sensitive species, which led to the readjustment of microbial communities. This finding suggested that the application of biochar affected the content of Cd and then indirectly changed the microbial communities.
5. Conclusions
This study revealed that the addition of biochar significantly increased the contents of AN, AP, AK, and SOC. The Cd content in soybean kernels could be reduced effectively when the application amount of biochar was 15 t·ha−1. Furthermore, the application of biochar reduced the abundance of the czcA gene in both continuous and rotational cropping soils and altered the diversity and community structure of Cd-resistant microorganisms. Correlation analysis indicated that soil Cd, soybean Cd, and the czcA gene were negatively correlated with AN, AP, AK, and SOC. The study also found that the relative abundances of dominant bacterial phyla (Proteobacteria, Planctomycetes, Actinobacteria, Acidobacteria, and Verrucomicrobia) and dominant genus (Planctomyces, Lysobacter, Bradyrhizobium, Sorangium, and Cupriavidus) were influenced by the addition of biochar. The RDA analysis revealed that MC, AN, AP, SOC, and Cd are the primary environmental parameters affecting the composition of soil Cd-resistant bacterial community. Therefore, this study provides theoretical support and reference for the application of biochar in the microbial remediation of Cd-contaminated soil, which holds significant practical implications and application value.
Author Contributions
Conceptualization, Q.Y. and C.Z.; methodology, S.Y., X.Z. and G.H.; software, Y.Z., Y.L. and Y.T.; validation, J.S.; formal analysis, G.H. and C.Z.; investigation, G.H. and Y.Z.; resources, X.Z., Y.L. and Y.W.; data curation, Y.S.; writing—original draft preparation, Q.Y.; writing—review and editing, G.H.; visualization, Y.W. and Y.T.; project administration, J.S. and Y.G.; funding acquisition, Q.Y. and Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
The Construction Project of Double First-Class Initiative in Heilongjiang Province “Green and Low-Carbon of Grain Crops” (LJGXCG2022-107), Heilongjiang Provincial Natural Science Foundation of China (LH2023C075), Heilongjiang Bayi Agricultural University Support Program for San Heng San Zong (ZRCQC202001), Research Project on Ecological Environment Protection in Heilongjiang Province (HST2024TR011, HST2024TR012), Talent Introduction Project of Heilongjiang Bayi Agricultural University (XYB202005), Innovative scientific research project for postgraduates of Heilongjiang Bayi Agricultural University (YJSCX2023-Y15), Heilongjiang Provincial Natural Science Foundation of China (LH2022D002), Heilongjiang Provincial Natural Science Foundation Joint Guidance Project (LH2023C074).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-ur-Rehman, M.; Qayyum, M.F.; Abbas, F.; Hannan, F.; Rinklebe, J.; Ok, Y.S. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Yang, Y.; Li, B.; Wu, Y.; Sun, H.; Yang, Y. Cadmium accumulation characteristics in turnip landraces from China and assessment of their phytoremediation potential for contaminated soils. Front. Plant Sci. 2016, 7, 1862. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ma, Y.; Luo, S. Spatiotemporal evolution and influencing factors of soybean production in Heilongjiang province, China. Land 2023, 12, 2090. [Google Scholar] [CrossRef]
- Wang, X. Characteristic and environmental risk assessment of heavy metals in farmland soil of based on specia-tion analysis. Inform. Manag. Sci. I 2013, 204, 213–220. [Google Scholar] [CrossRef]
- Xu, L.; Jin, S.; Su, Y.; Lyu, X.; Yan, S.; Wang, C.; Cao, L.; Yan, C.; Ma, C. Combined metagenomics and metabolomic analysis of microbial community structure and metabolic function in continuous soybean crop soils of Songnen Plain, China. Chem. Biol. Technol. Agric. 2024, 11, 46. [Google Scholar] [CrossRef]
- Aschi, A.; Aubert, M.; Riah-Anglet, W.; Nélieu, S.; Dubois, C.; Akpa-Vincentas, M.; Trinsoutrot-Gattin, I. Introduction of Faba bean in crop rotation: Impacts on soil chemical and biological characteristics. Appl. Soil Ecol. 2017, 120, 219–228. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Zhang, H.; Shi, W.; Liu, Y. Bacterial heavy-metal and antibiotic resistance genes in a copper tailing dam area in northern China. Front. Microbiol. 2019, 10, 1916. [Google Scholar] [CrossRef]
- Geisseler, D.; Linquist, B.A.; Lazicki, P.A. Effect of fertilization on soil microorganisms in paddy rice systems–a meta-analysis. Soil Biol. Biochem. 2017, 115, 452–460. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, M.; Wei, L.; Gao, Y.; Ruan, Y.; Wang, Q.; Zhang, Z. Changes in soil chemical properties and rhizosphere bacterial community induced by soil amendments associated with reduction in cadmium accumulation by rice. Agronomy 2023, 13, 3051. [Google Scholar] [CrossRef]
- Guo, J.; Yan, C.; Luo, Z.; Fang, H.; Hu, S.; Cao, Y. Synthesis of a novel ternary HA/Fe-Mn oxides-loaded biochar composite and its application in cadmium(II) and arsenic(V) adsorption. J. Environ. Sci. 2019, 85, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, R.; Sun, X.; Han, F.; Xiao, E.; Chen, L.; Qiu, L.; Sun, W. Microbiome–environment interactions in antimony-contaminated rice paddies and the correlation of core microbiome with arsenic and antimony contamination. Chemosphere 2021, 263, 128227. [Google Scholar] [CrossRef] [PubMed]
- Gillard, B.; Chatzievangelou, D.; Thomsen, L.; Ullrich, M.S. Heavy-metal-resistant microorganisms in deep-sea sediments disturbed by mining activity: An application toward the development of experimental in vitro systems. Front. Mar. Sci. 2019, 6, 462. [Google Scholar] [CrossRef]
- Xie, X.; Yuan, K.; Chen, X.; Zhao, Z.; Huang, Y.; Hu, L.; Liu, H.; Luan, T.; Chen, B. Characterization of metal resistance genes carried by waterborne free-living and particle-attached bacteria in the Pearl River estuary. Environ. Pollut. 2023, 327, 121547. [Google Scholar] [CrossRef]
- Hu, H. Faculty opinions recommendation of Antibiotic resistomes in plant microbiomes. Fac. Opin.—Post-Publ. Peer Rev. Biomed. Lit. 2019, 24, 530–554. [Google Scholar] [CrossRef]
- Bruins, M.R.; Kapil, S.; Oehme, F.W. Microbial resistance to metals in the environment. Ecotoxicol. Environ. Saf. 2000, 45, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiu, J.; Wang, X.; Zhong, Y.; Lan, C.; Shu, W. Cadmium contamination in orchard soils and fruit trees and its potential health risk in Guangzhou, China. Environ. Sci. Pollut. R. 2006, 143, 159–165. [Google Scholar] [CrossRef]
- Visconti, D.; Álvarez-Robles, M.J.; Fiorentino, N.; Fagnano, M.; Clemente, R. Use of Brassica juncea and Dactylis glomerata for the phytostabilization of mine soils amended with compost or biochar. Chemosphere 2020, 260, 127661. [Google Scholar] [CrossRef]
- Singh, R.; Babu, J.N.; Kumar, R.; Srivastava, P.; Singh, P.; Raghubanshi, A.S. Multifaceted application of crop residue biochar as a tool for sustainable agriculture: An ecological perspective. Ecol. Eng. 2015, 77, 324–347. [Google Scholar] [CrossRef]
- Mustafa, A.; Brtnicky, M.; Hammerschmiedt, T.; Kucerik, J.; Kintl, A.; Chorazy, T.; Naveed, M.; Skarpa, P.; Baltazar, T.; Malicek, O.; et al. Food and agricultural wastes-derived biochars in combination with mineral fertilizer as sustainable soil amendments to enhance soil microbiological activity, nutrient cycling and crop production. Front. Plant Sci. 2022, 13, 1028101. [Google Scholar] [CrossRef] [PubMed]
- Amalina, F.; Abd Razak, A.S.; Zularisam, A.W.; Aziz, M.A.A.; Krishnan, S.; Nasrullah, M. Comprehensive assessment of biochar integration in agricultural soil conditioning: Advantages, drawbacks, and future prospectives. Phys. Chem. Earth Parts A/B/C 2023, 132, 103508. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, X.; Zhao, L.; Arellano, E. Biochar- and phosphate-induced immobilization of heavy metals in contaminated soil and water: Implication on simultaneous remediation of contaminated soil and groundwater. Environ. Sci. Pollut. Res. 2014, 21, 4665–4674. [Google Scholar] [CrossRef] [PubMed]
- Abou Jaoude, L.; Castaldi, P.; Nassif, N.; Pinna, M.V.; Garau, G. Biochar and compost as gentle remediation options for the recovery of trace elements-contaminated soils. Sci. Total. Environ. 2020, 711, 134511. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ni, X.; Huang, Q.; Liu, D.; Ye, Z. Effect of bamboo biochar on reducing grain cadmium content in two contrasting wheat genotypes. Environ. Sci. Pollut. Res. 2021, 28, 17405–17416. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; Yang, K.; Er Ze, L.; Lu, Z.; Li, Y.; Mu, L.; Zhang, N. Reducing Cd and Pb accumulation in potatoes: The role of soil passivators in contaminated mining soils. Life 2024, 14, 1615. [Google Scholar] [CrossRef]
- Xing, D.; Cheng, H.; Ning, Z.; Liu, Y.; Lin, S.; Li, Y.; Wang, X.; Hill, P.; Chadwick, D.; Jones, D.L. Field aging declines the regulatory effects of biochar on cadmium uptake by pepper in the soil. J. Environ. Manag. 2022, 321, 115832. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; He, L.; Lin, X.; Che, L.; Ye, Z.; et al. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. 2016, 23, 974–984. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Yuan, H.M.; Xue, W.; Roiloa, S.; Yao, J.; Yu, F.H. Increasing biochar diversity promotes the impacts of plant diversity on remediating cadmium in soil. J. Plant Ecol. 2024, 17, rtae068. [Google Scholar] [CrossRef]
- Walker, N.J. Real-time and quantitative PCR: Applications to mechanism-based toxicology. J. biochem. Mol. Toxicol. 2001, 15, 121–127. [Google Scholar] [CrossRef]
- Hou, X.; He, W.; Zhang, Y.; Zhang, N.; Yan, J.; Chen, Y. Maize-straw biochar enhances soil properties and grain yield of foxtail millet in a newly reclaimed land. Agronomy 2024, 14, 2465. [Google Scholar] [CrossRef]
- Malghani, S.; Kim, J.; Lee, S.-H.; Yoo, G.-y.; Kang, H. Application of two contrasting rice-residue-based biochars triggered gaseous loss of nitrogen under denitrification-favoring conditions: A short-term study based on acetylene inhibition technique. Appl. Soil Ecol. 2018, 127, 112–119. [Google Scholar] [CrossRef]
- Fan, S.; Zuo, J.; Dong, H. Changes in soil properties and bacterial community composition with biochar amendment after six years. Agronomy 2020, 10, 746. [Google Scholar] [CrossRef]
- Houssou, A.A.; Jeyakumar, P.; Niazi, N.K.; Van Zwieten, L.; Li, X.; Huang, L.; Wei, L.; Zheng, X.; Huang, Q.; Huang, Y.; et al. Biochar and soil properties limit the phytoavailability of lead and cadmium by Brassica chinensis L. in contaminated soils. Biochar 2022, 4, 5. [Google Scholar] [CrossRef]
- Oladele, S.O. Changes in physicochemical properties and quality index of an Alfisol after three years of rice husk biochar amendment in rainfed rice—Maize cropping sequence. Geoderma 2019, 353, 359–371. [Google Scholar] [CrossRef]
- Futa, B.; Oleszczuk, P.; Andruszczak, S.; Kwiecińska-Poppe, E.; Kraska, P. Effect of natural aging of biochar on soil enzymatic activity and physicochemical properties in long-term field experiment. Agronomy 2020, 10, 449. [Google Scholar] [CrossRef]
- Xiu, L.; Zhang, W.; Sun, Y.; Wu, D.; Meng, J.; Chen, W. Effects of biochar and straw returning on the key cultivation limitations of Albic soil and soybean growth over 2 years. Catena 2019, 173, 481–493. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Z.; Liu, Z.; Xie, G.; Rookes, J.; An, F. Root secretion of oxalic and malic acids mitigates the rubber tree aluminum toxicity. J. Rubber Res. 2021, 24, 381–390. [Google Scholar] [CrossRef]
- Yu, F.; Zhao, S.; Zhao, Y.; Wang, Y.; Zhai, C.; Zhong, R.; Zhang, J.; Meng, Q. Long-term cattle manure application to saline-sodic soil increases maize yield by decreasing key obstacle factors in the black soil region of Northeastern China. Int. J. Agric. Biol. Eng. 2023, 16, 176–183. [Google Scholar] [CrossRef]
- Huang, X.F.; Li, S.Q.; Li, S.Y.; Ye, G.Y.; Lu, L.J.; Zhang, L.; Yang, L.Y.; Qian, X.; Liu, J. The effects of biochar and dredged sediments on soil structure and fertility promote the growth, photosynthetic and rhizosphere microbial diversity of Phragmites communis (Cav.) Trin. ex Steud. Sci. Total. Environ. 2019, 697, 134073. [Google Scholar] [CrossRef]
- Naeem, M.B.; Jahan, S.; Rashid, A.; Shah, A.A.; Raja, V.; El-Sheikh, M.A. Improving maize yield and drought tolerance in field conditions through activated biochar application. Sci. Rep. 2024, 14, 25000. [Google Scholar] [CrossRef] [PubMed]
- Lemanowicz, J.; Bartkowiak, A.; Lamparski, R.; Wojewódzki, P.; Pobereżny, J.; Wszelaczyńska, E.; Szczepanek, M. Physicochemical and enzymatic soil properties influenced by cropping of primary wheat under organic and conventional farming systems. Agronomy 2020, 10, 1652. [Google Scholar] [CrossRef]
- Haddad, S.A.; Lemanowicz, J. Benefits of corn-cob biochar to the microbial and enzymatic activity of soybean plants grown in soils contaminated with heavy metals. Energies 2021, 14, 5763. [Google Scholar] [CrossRef]
- Han, J.; Wu, D.; Yang, J.; Shi, Y.; Abid, G.; Wang, L.; Li, Z. A biochar-based amendment improved cadmium (Cd) immobilization, reduced its bioaccumulation, and increased rice yield. Front. Env. Sci. 2024, 12, 1487190. [Google Scholar] [CrossRef]
- Lang, Q.; Xia, Y.; Li, Y.; Wang, C.; Liu, Z.; Zou, G.; Sun, Q. CaO-modified hydrochar reduces soil cadmium bioavailability by altering soil properties, shifting bacterial community, and promoting microbial metabolisms. Environ. Technol. Innov. 2024, 35, 103698. [Google Scholar] [CrossRef]
- Wang, X.; Ding, S.; Cheng, T.; Wang, S.; Qing, Z.; Hu, J.; Wang, X. Mechanism of Cd adsorption by sulphur modified biochar and its application in Cd-contaminated soil. Pol. J. Environ. Stud. 2024, 33, 5901–5912. [Google Scholar] [CrossRef]
- Tian, Y.; Cui, L.; Lin, Q.; Li, G.; Zhao, X. The sewage sludge biochar at low pyrolysis temperature had better improvement in urban soil and turf grass. Agronomy 2019, 9, 156. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef]
- Mohamed, I.; Zhang, G.S.; Li, Z.G.; Liu, Y.; Chen, F.; Dai, K. Ecological restoration of an acidic Cd contaminated soil using bamboo biochar application. Ecol. Eng. 2015, 84, 67–76. [Google Scholar] [CrossRef]
- Irfan, M.; Mudassir, M.; Khan, M.J.; Dawar, K.M.; Muhammad, D.; Mian, I.A.; Ali, W.; Fahad, S.; Saud, S.; Hayat, Z.; et al. Heavy metals immobilization and improvement in maize (Zea mays L.) growth amended with biochar and compost. Sci. Rep. 2021, 11, 18416. [Google Scholar] [CrossRef]
- Haider, F.U.; Coulter, J.A.; Cheema, S.A.; Farooq, M.; Wu, J.; Zhang, R.; Guo, S.J.; Cai, L.Q. Co-application of biochar and microorganisms improves soybean performance and remediate cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2021, 214, 112112. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhou, M.; Zhang, H.; Yuan, L.; Lv, P.; Wang, L.; Zhang, J. Changes in Cd forms and Cd resistance genes in municipal sludge during coupled earthworm and biochar composting. Ecotoxicol. Environ. Saf. 2024, 286, 117–179. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Xue, X.M.; Kappler, A.; Rosen, B.P.; Meharg, A.A. Linking genes to microbial biogeochemical cycling: Lessons from arsenic. Environ. Sci. Technol. 2017, 51, 7326–7339. [Google Scholar] [CrossRef]
- Khan, Z.; Nisar, M.A.; Hussain, S.Z.; Arshad, M.N.; Rehman, A. Cadmium resistance mechanism in Escherichia coli P4 and its potential use to bioremediate environmental cadmium. Appl. Microbiol. Biotechnol. 2015, 99, 10745–10757. [Google Scholar] [CrossRef]
- Liu, K.; Sun, M.; Ye, M.; Chao, H.; Zhao, Y.; Xia, B.; Jiao, W.; Feng, Y.; Zheng, X.; Liu, M.; et al. Coexistence and association between heavy metals, tetracycline and corresponding resistance genes in vermicomposts originating from different substrates. Environ. Pollut. 2019, 244, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; He, L.; Chen, J.; Lyu, H.; Wang, Y.; Yang, L.; Yang, S.; Liu, Y. Long-term successive biochar amendments alter the composition and α-diversity of bacterial community of paddy soil in rice-wheat rotation. Front. Environ. Sci. 2022, 10, 921766. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Marris, E. Putting the carbon back: Black is the new green. Nature 2006, 442, 7103. [Google Scholar] [CrossRef]
- Yan, T.; Xue, J.; Zhou, Z.; Wu, Y. Biochar-based fertilizer amendments improve the soil microbial community structure in a karst mountainous area. Sci. Total Environ. 2021, 794, 148757. [Google Scholar] [CrossRef]
- Wang, P.; Yan, S.; Zhang, W.; Xie, X.; Li, M.; Ren, T.; Gu, L.; Zhang, Z. Effect of soil management systems on the rhizosphere bacterial community structure of tobacco: Continuous cropping vs. paddy-upland rotation. Ecol. Eng. 2022, 13, 996858. [Google Scholar] [CrossRef]
- Semenov, M.V.; Zhelezova, A.D.; Ksenofontova, N.A.; Ivanova, E.A.; Nikitin, D.A.; Semenov, V.M. Microbiological indicators for assessing the effects of agricultural practices on soil health: A review. Agronom. 2025, 15, 335. [Google Scholar] [CrossRef]
- Gao, W.; Gao, K.; Guo, Z.; Liu, Y.; Jiang, L.; Liu, C.; Liu, X.; Wang, G. Different responses of soil bacterial and fungal communities to 3 years of biochar amendment in an alkaline soybean soil. Front. Microbiol. 2021, 12, 630418. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Yuan, P.; Ullah, S.; Iqbal, A.; Zhao, Q.; Liang, H.; Khan, A.; Imran; Zhang, H.; Wu, X.; et al. Biochar amendment and nitrogen fertilizer contribute to the changes in soil properties and microbial communities in a paddy field. Front. Microbiol. 2022, 13, 834751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, G.; Xue, S.; Wang, G. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Xu, H.; Huang, Y.; Xiong, X.; Zhu, H.; Lin, J.; Shi, J.; Tang, C.; Xu, J. Changes in soil Cd contents and microbial communities following Cd-containing straw return. Environ. Pollut. 2023, 330, 121753. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).