Reduced Precipitation Frequency Decreases the Stability of the Soil Organic Carbon Pool by Altering Microbial Communities in Degraded Grasslands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Site
2.2. Experimental Design
2.3. Experimental Setup
2.4. Soil Sampling and Soil Properties
2.5. Soil Microbial Communities
2.6. Soil Carbon Pool Calculation
2.7. Statistical Analysis
3. Results
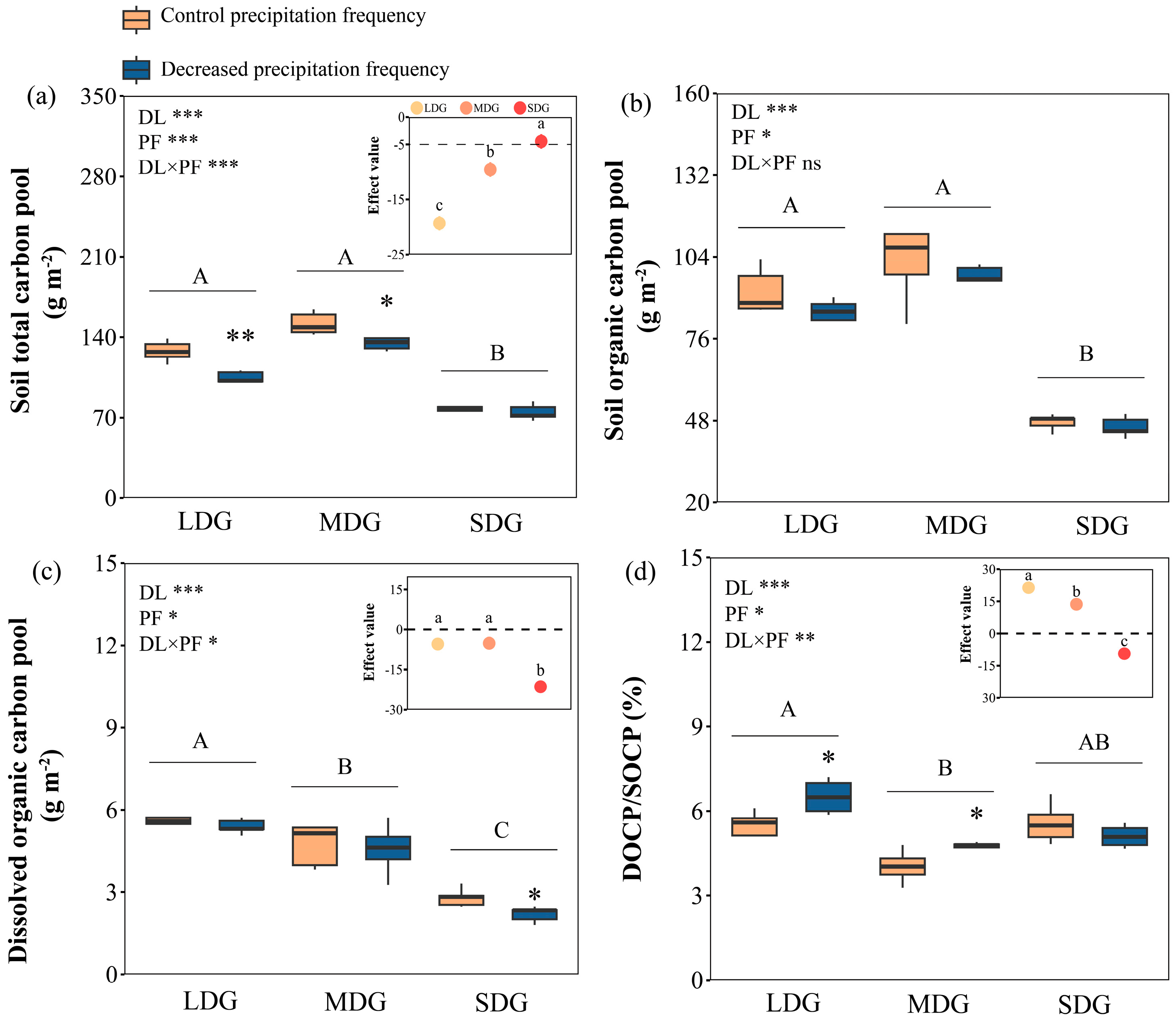
3.1. Soil Carbon Pools in Response to Grassland Degradation and Reduced Precipitation Frequency
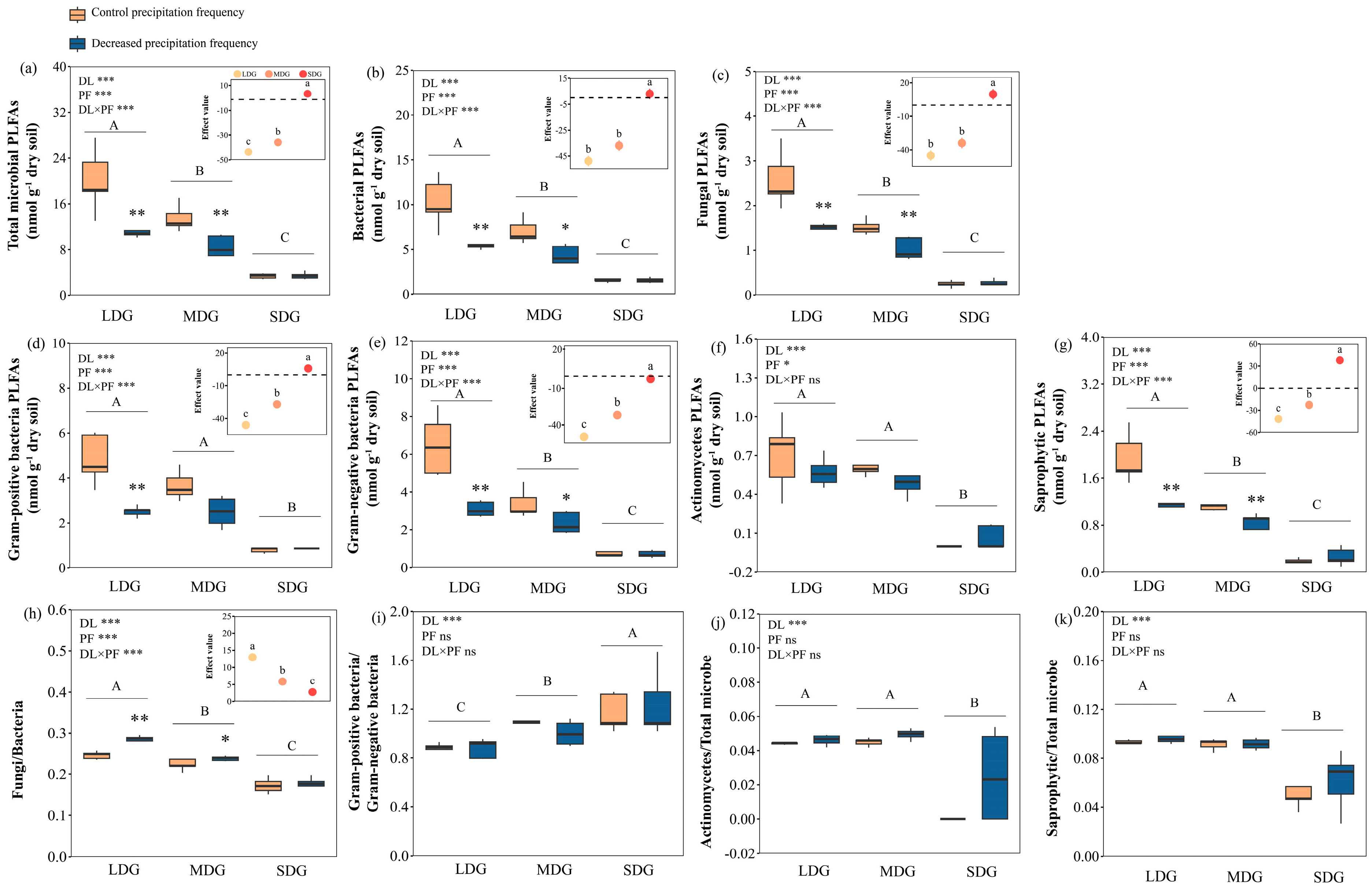
3.2. Effects of Decreased Precipitation Frequency and Grassland Degradation on Microbial Communities
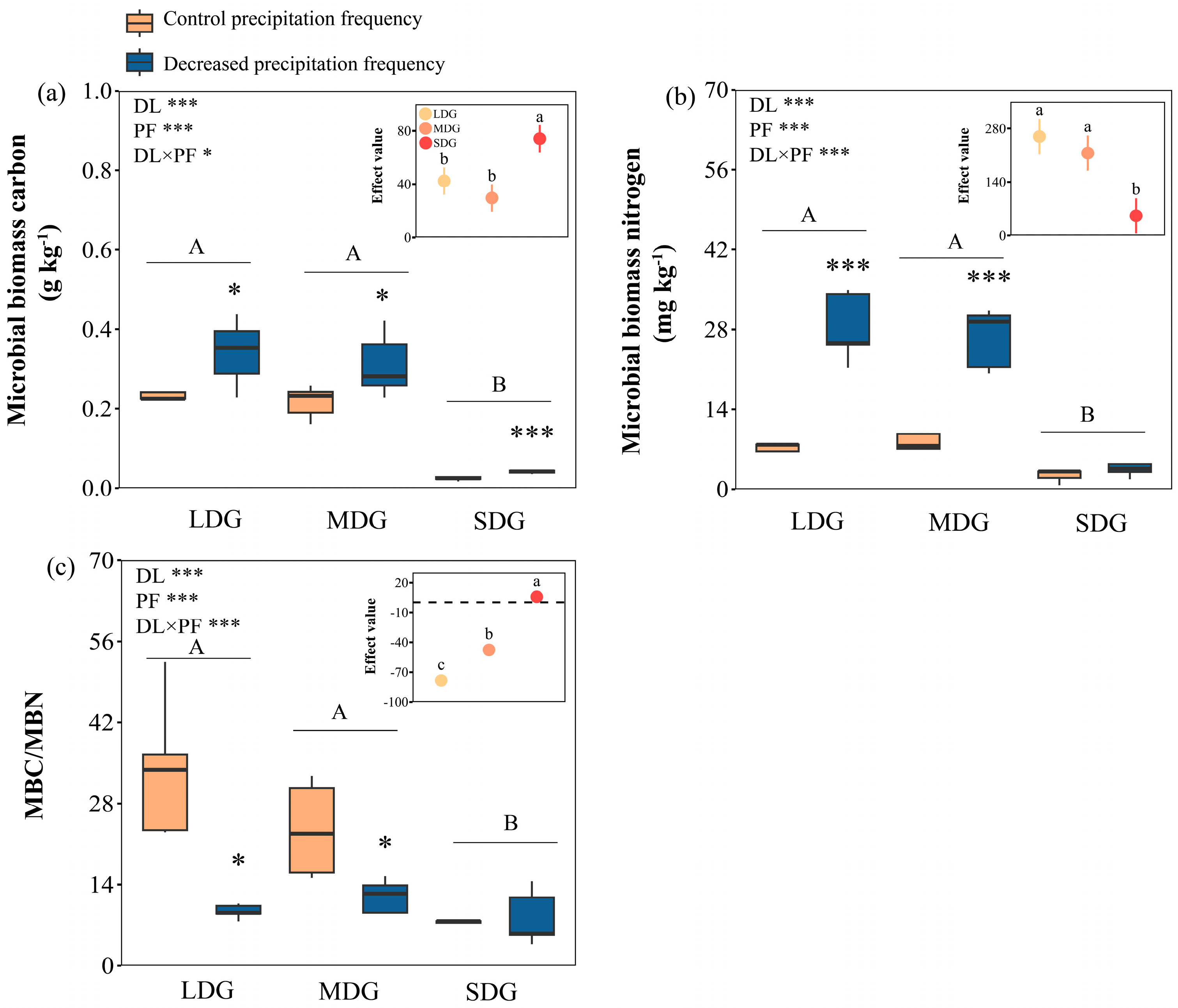
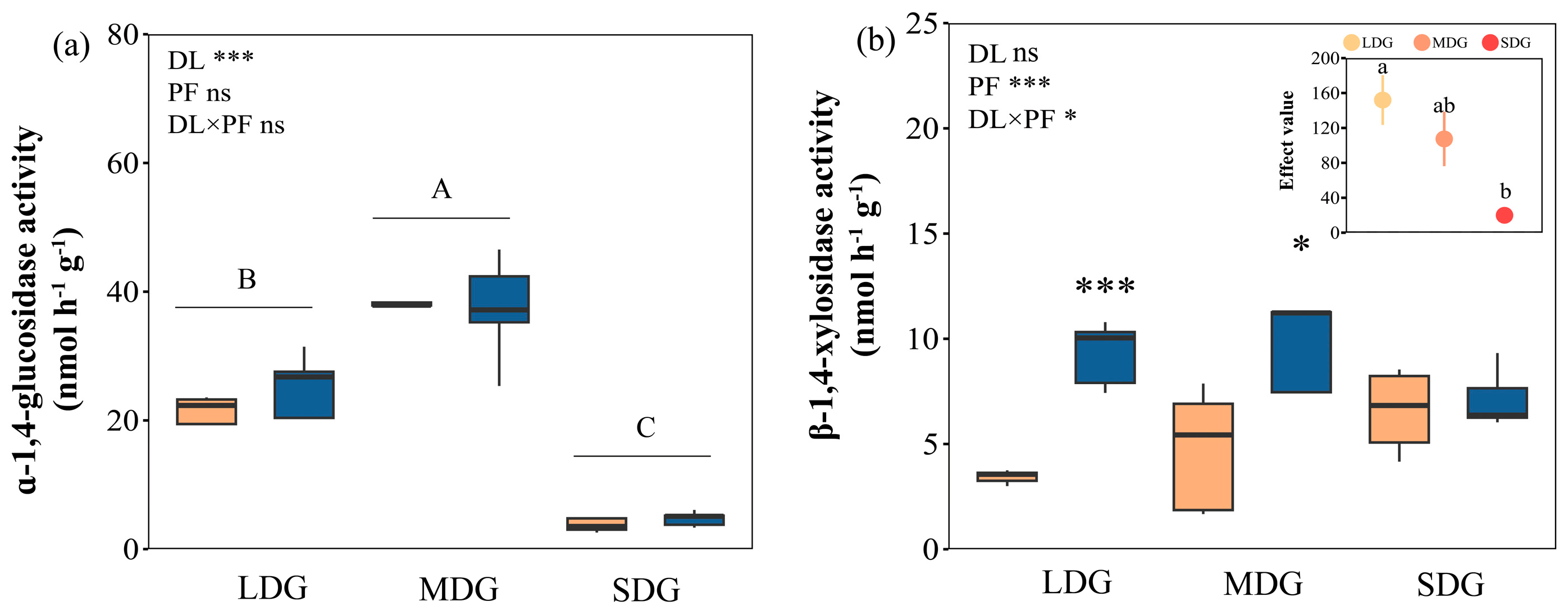
3.3. Responses of Microbial Stoichiometric Characteristics and Enzyme Activity to Precipitation Manipulation and Grassland Degradation
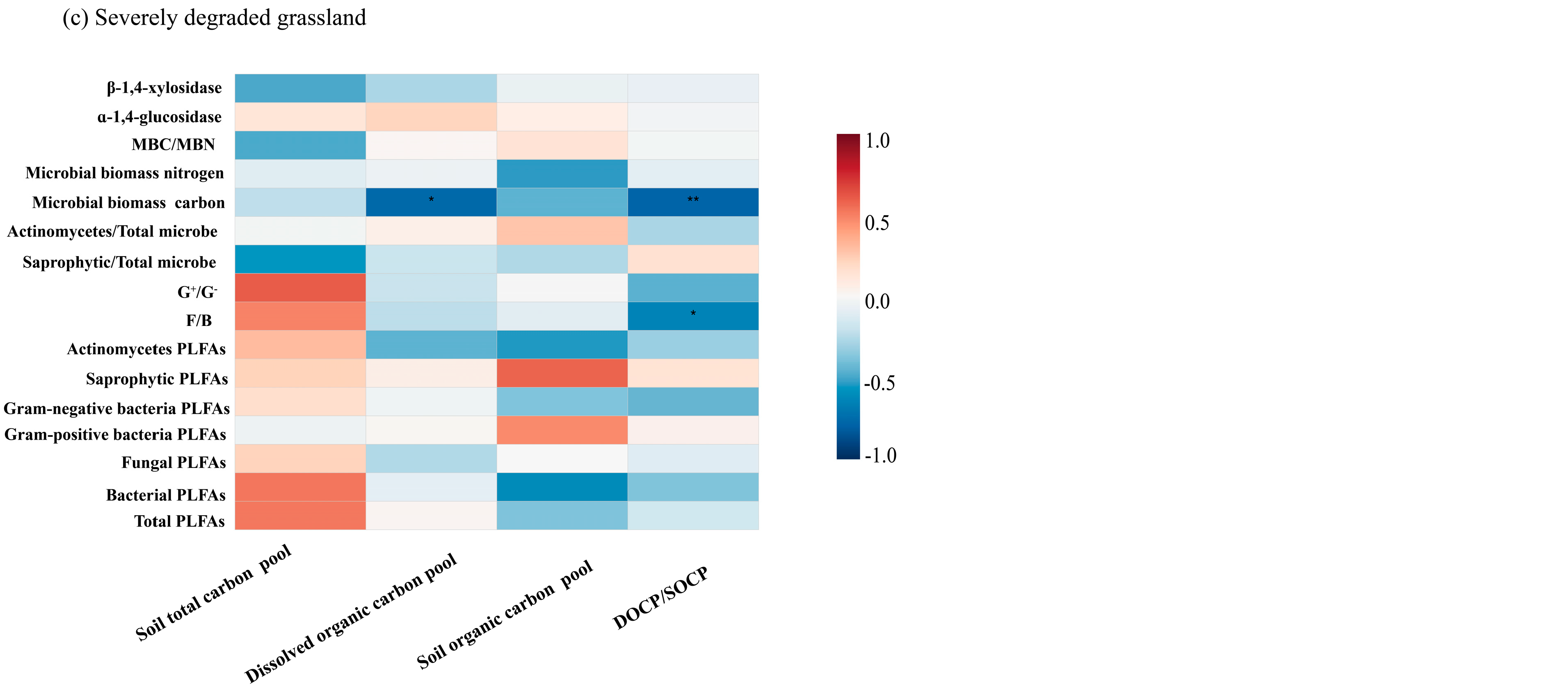
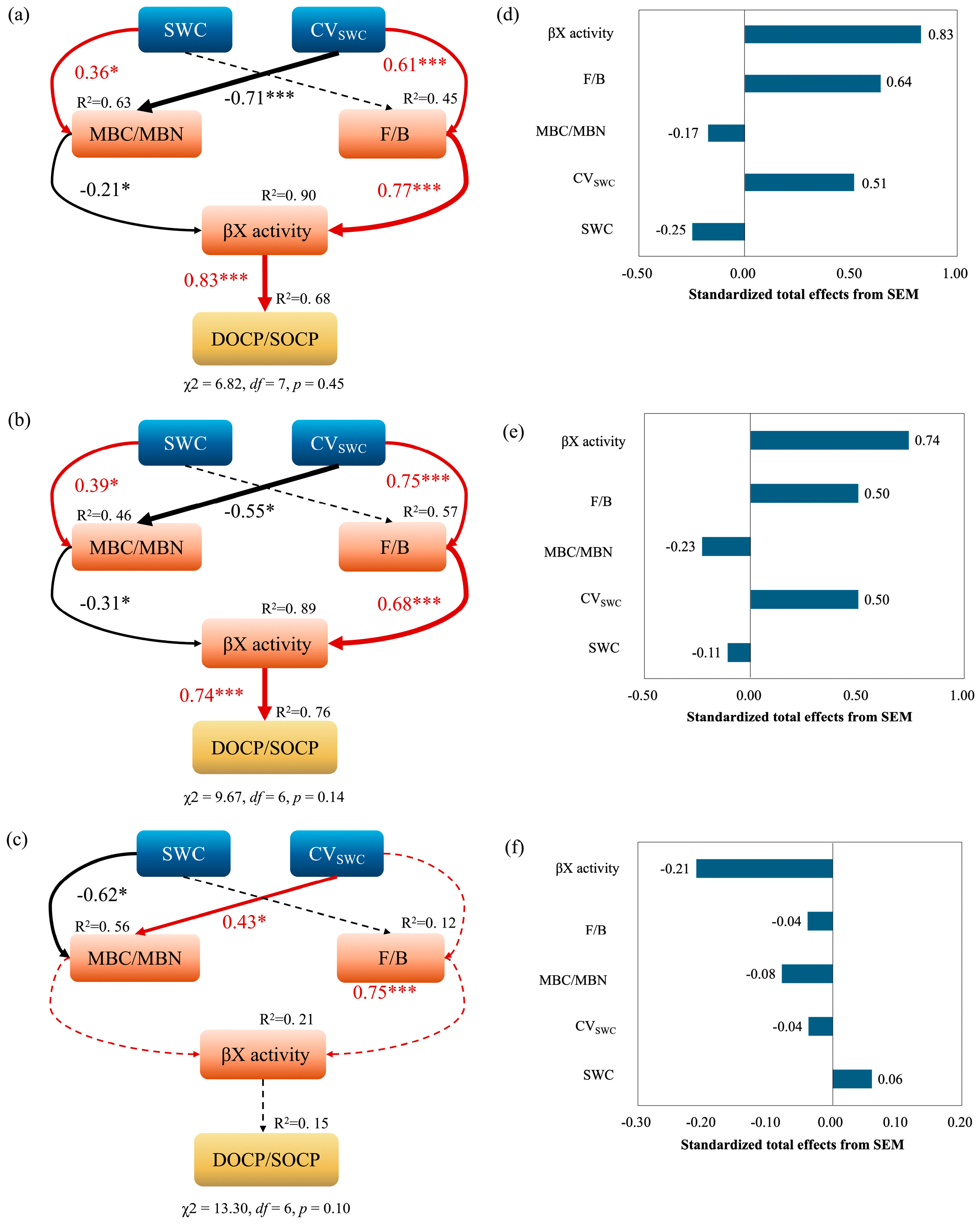
3.4. Relationships Between Microbial Community Properties, the Soil Organic Carbon Pool, and Its Stability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angst, G.; Mueller, K.E.; Castellano, M.J.; Vogel, C.; Wiesmeier, M.; Mueller, C.W. Unlocking complex soil systems as carbon sinks: Multi-pool management as the key. Nat. Commun. 2023, 14, 2967. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Soussana, J.; Angers, D.; Schipper, L.; Chenu, C.; Rasse, D.P.; Batjes, N.H.; Van Egmond, F.; McNeill, S.; Kuhnert, M.; et al. How to measure, Report and verify soil carbon change to realize the potential of soil carbon sequestration for atmospheric greenhouse gas removal. Glob. Chang. Biol. 2020, 26, 219–241. [Google Scholar] [CrossRef]
- Ren, T.; Ukalska-Jaruga, A.; Smreczak, B.; Cai, A. Dissolved organic carbon in cropland soils: A global meta-analysis of management effects. Agric. Ecosyst. Environ. 2024, 371, 109080. [Google Scholar] [CrossRef]
- Kan, Z.; Liu, W.; Liu, W.; Lal, R.; Dang, Y.P.; Zhao, X.; Zhang, H. Mechanisms of Soil organic carbon stability and its response to no-till: A global synthesis and perspective. Glob. Chang. Biol. 2022, 28, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Hansel, C.M.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, M.; Schimel, J.P.; Torn, M.S.; et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Y.; Wang, Y.; Sun, Y.; Chen, Y. High stand density promotes soil organic carbon sequestration in Robinia pseudoacacia plantations in the hilly and gully region of the Loess Plateau in China. Agric. Ecosyst. Environ. 2023, 343, 108256. [Google Scholar] [CrossRef]
- Campos, X.; Germino, M.J.; De Graaff, M.-A. Enhanced Precipitation promotes decomposition and soil C stabilization in semiarid ecosystems, but seasonal timing of wetting matters. Plant Soil 2017, 416, 427–436. [Google Scholar] [CrossRef]
- Yang, T.; Chen, J.; Zhong, X.; Yang, X.; Wang, G.; Yao, Y.; Sternberg, M.; Sun, W. Divergent responses of plant biomass and its allocation to the altered precipitation regimes among different degraded grasslands in China. Plant Soil 2022, 473, 149–166. [Google Scholar] [CrossRef]
- Chen, J.; Yao, Y.; Wang, G.; Zhong, X.; Yang, T.; Sun, W. Decreased precipitation frequency altered abundance, but not community structure, of soil nematodes in degraded grasslands. Ecol. Indic. 2021, 131, 108184. [Google Scholar] [CrossRef]
- Yang, T.; Zhong, X.; Chen, J.; Nielsen, U.N.; Ochoa-Hueso, R.; Qu, Y.; Sui, Y.; Gao, W.; Sun, W. Ecosystem-level decoupling in response to reduced precipitation frequency and degradation in steppe grassland. Funct. Ecol. 2023, 37, 2910–2926. [Google Scholar] [CrossRef]
- Beare, M.H.; Gregorich, E.G.; St-Georges, P. Compaction effects on CO2 and N2O production during drying and rewetting of soil. Soil Biol. Biochem. 2009, 41, 611–621. [Google Scholar] [CrossRef]
- Manici, L.M.; Caputo, F.; De Sabata, D.; Fornasier, F. The enzyme patterns of Ascomycota and Basidiomycota fungi reveal their different functions in soil. Appl. Soil Ecol. 2024, 196, 105323. [Google Scholar] [CrossRef]
- Mayer, M.; Rewald, B.; Matthews, B.; Sandén, H.; Rosinger, C.; Katzensteiner, K.; Gorfer, M.; Berger, H.; Tallian, C.; Berger, T.W.; et al. Soil fertility relates to fungal-mediated decomposition and organic matter turnover in a temperate mountain forest. New Phytol. 2021, 231, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Yang, X.; Zhang, M.; Chen, J.; Sui, Y.; Zhang, X.; Zeng, Y.; Huang, M.; Gao, Y.; Ochoa-Hueso, R.; et al. Bacterial and fungal diversity and species interactions inversely affect ecosystem functions under drought in a semi-arid grassland. Microbiol. Res. 2025, 293, 128075. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, B.S.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 2013, 37, 112–129. [Google Scholar] [CrossRef] [PubMed]
- A’Bear, A.D.; Jones, T.H.; Boddy, L. Size Matters: What have we learnt from microcosm studies of decomposer fungus-invertebrate interactions? Soil Biol. Biochem. 2014, 78, 274–283. [Google Scholar] [CrossRef]
- Ni, X.; Liao, S.; Wu, F.; Groffman, P.M. Microbial biomass in forest soils under altered moisture conditions: A review. Soil Sci. Soc. Am. J. 2022, 86, 358–368. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; Yang, Y.; Zou, J. Effects of drying-rewetting on soil CO2 emissions and the regulatory factors involved: A meta-analysis. Plant Soil 2024, 499, 349–361. [Google Scholar] [CrossRef]
- Munson, S.M.; Benton, T.J.; Lauenroth, W.K.; Burke, I.C. Soil carbon flux following pulse precipitation events in the shortgrass steppe. Ecol. Res. 2010, 25, 205–211. [Google Scholar] [CrossRef]
- Michalk, D.L.; Kemp, D.R.; Badgery, W.B.; Wu, J.; Zhang, Y.; Thomassin, P.J. Sustainability and future food security-A global perspective for livestock production. Land Degrad. Dev. 2019, 30, 561–573. [Google Scholar] [CrossRef]
- Wan, L.; Liu, G.; Su, X. Organic fertilization balances biodiversity maintenance, grass production, soil storage, nutrient cycling and greenhouse gas emissions for sustainable grassland development in China: A meta-analysis. Agric. Ecosyst. Environ. 2025, 381, 109473. [Google Scholar] [CrossRef]
- Luan, J.; Cui, L.; Xiang, C.; Wu, J.; Song, H.; Ma, Q. Soil carbon stocks and quality across intact and degraded alpine wetlands in Zoige, east Qinghai-Tibet Plateau. Wetl. Ecol. Manage. 2014, 22, 427–438. [Google Scholar] [CrossRef]
- Xu, Q.; Wei, Y.; Zhao, X.; Xu, H. Dynamics of soil carbon fractions and carbon stability in relation to grassland degradation in Xinjiang, northwest China. Sustainability 2022, 14, 5860. [Google Scholar] [CrossRef]
- Ao, D.; Wang, B.; Wang, Y.; Chen, Y.; Anum, R.; Feng, C.; Ji, M.; Liang, C.; An, S. Grassland degraded patchiness reduces microbial necromass content but Increases contribution to soil organic carbon accumulation. Sci. Total Environ. 2024, 951, 175717. [Google Scholar] [CrossRef]
- Wei, X.; Van Meerbeek, K.; Yue, K.; Ni, X.; Desie, E.; Heděnec, P.; Yang, J.; Wu, F. Responses of soil C pools to combined warming and altered precipitation regimes: A meta-analysis. Glob. Ecol. Biogeogr. 2023, 32, 1660–1675. [Google Scholar] [CrossRef]
- Prommer, J.; Walker, T.W.N.; Wanek, W.; Braun, J.; Zezula, D.; Hu, Y.; Hofhansl, F.; Richter, A. Increased microbial growth, biomass, and turnover drive soil organic carbon accumulation at higher plant diversity. Glob. Chang. Biol. 2020, 26, 669–681. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, J.; Liu, H.; Wang, W. Soil respiration and its autotrophic and heterotrophic components in response to nitrogen addition among different degraded temperate grasslands. Soil Biol. Biochem. 2018, 124, 255–265. [Google Scholar] [CrossRef]
- Dong, S.; Shang, Z.; Gao, J.; Boone, R.B. Enhancing sustainability of grassland ecosystems through ecological restoration and grazing management in an era of climate change on Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2020, 287, 106684. [Google Scholar] [CrossRef]
- Liu, S.; Zamanian, K.; Schleuss, P.M.; Zarebanadkouki, M.; Kuzyakov, Y. Degradation of Tibetan grasslands: Consequences for carbon and nutrient cycles. Agric. Ecosyst. Environ. 2018, 252, 93–104. [Google Scholar] [CrossRef]
- Heisler-White, J.L.; Knapp, A.K.; Kelly, E.F. Increasing precipitation event size increases above-ground net primary productivity in a semi-arid grassland. Oecologia 2008, 158, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Inubushi, K.; Brookes, P.C.; Jenkinson, D.S. Soil microbial biomass C, N and ninhydrin-N in aerobic and anaerobic soils measured by the fumigation-extraction method. Soil Biol. Biochem. 1991, 23, 737–741. [Google Scholar] [CrossRef]
- Chen, D.; Pan, Q.; Bai, Y.; Hu, S.; Huang, J.; Wang, Q.; Naeem, S.; Elser, J.J.; Wu, J.; Han, X. Effects of plant functional group loss on soil biota and net ecosystem exchange: A plant removal experiment in the Mongolian grassland. J. Ecol. 2016, 104, 734–743. [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2011, 43, 1621–1625. [Google Scholar] [CrossRef]
- Lu, W.; Ding, W.; Zhang, J.; Li, Y.; Luo, J.; Bolan, N.; Xie, Z. Biochar suppressed the decomposition of organic carbon in a cultivated sandy loam soil: A negative priming effect. Soil Biol. Biochem. 2014, 76, 12–21. [Google Scholar] [CrossRef]
- Cai, K. Pyrolysis-assisted transesterification for accurate quantification of phospholipid fatty acids: Application to microbial community analysis in 1000-years paddy soil chronosequence. Geoderma 2022, 406, 115504. [Google Scholar] [CrossRef]
- Denef, K.; Roobroeck, D.; Manimel Wadu, M.C.W.; Lootens, P.; Boeckx, P. Microbial community composition and rhizodeposit-carbon assimilation in differently managed temperate grassland soils. Soil Biol. Biochem. 2009, 41, 144–153. [Google Scholar] [CrossRef]
- Unger, S.; Máguas, C.; Pereira, J.S.; David, T.S.; Werner, C. The Influence of precipitation pulses on soil respiration-Assessing the “Birch Effect” by stable carbon isotopes. Soil Biol. Biochem. 2010, 42, 1800–1810. [Google Scholar] [CrossRef]
- Shahbaz, M.; Kuzyakov, Y.; Sanaullah, M.; Heitkamp, F.; Zelenev, V.; Kumar, A.; Blagodatskaya, E. Microbial decomposition of soil organic matteris mediated by quality and quantity of crop residues: Mechanisms and thresholds. Biol. Fertil. Soils 2017, 53, 287–301. [Google Scholar] [CrossRef]
- Xu, S.; Sayer, E.J.; Eisenhauer, N.; Lu, X.; Wang, J.; Liu, C. Aboveground litter inputs determine carbon storage across soil Profiles: A meta-analysis. Plant Soil 2021, 462, 429–444. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Gao, J.; Rinnan, R.; Jiao, Y.; Liang, J.; Yu, F. Precipitation patterns impact soil aggregates and organic carbon of an alpine wetland on the Qinghai-Tibetan Plateau. Catena 2024, 244, 108249. [Google Scholar] [CrossRef]
- Joly, F.; Kurupas, K.L.; Throop, H.L. Pulse frequency and soil-litter mixing alter the control of cumulative precipitation over litter decomposition. Ecology 2017, 98, 2255–2260. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Hueso, R.; Arca, V.; Delgado-Baquerizo, M.; Hamonts, K.; Piñeiro, J.; Serrano-Grijalva, L.; Shawyer, J.; Power, S.A. Links between soil microbial communities, functioning, and plant nutrition under altered rainfall in Australian grassland. Ecol. Monogr. 2020, 90, e01424. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R.; Collins, S.L.; Delgado-Baquerizo, M.; Hamonts, K.; Pockman, W.T.; Sinsabaugh, R.L.; Smith, M.D.; Knapp, A.K.; Power, S.A. Drought consistently alters the composition of soil fungal and bacterial communities in grasslands from two continents. Glob Change Biol. 2018, 24, 2818–2827. [Google Scholar] [CrossRef]
- Boer, W.D.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a fungal world: Impact of fungi on soil bacterial niche development. FEMS Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhou, H.; Zuo, J.; Chen, P.; She, Y.; Yao, B.; Dong, S.; Wu, J.; Li, F.; Njoroge, D.M.; et al. Responses of soil microbial metabolic activity and community structure to different degraded and restored grassland gradients of the Tibetan Plateau. Front. Plant Sci. 2022, 13, 770315. [Google Scholar] [CrossRef]
- Ma, G.; Wang, X.; Sun, X.; Wang, S.; Du, Y.; Jiang, J. Effects of warming and litter positions on litter decomposition in a boreal peatland. Front. Ecol. Evol. 2022, 10, 1078104. [Google Scholar] [CrossRef]
- Bai, X.; Zhai, G.; Zhai, Y.; Li, H.; An, S.; Rafiq, A.; Liu, J. Mechanism of microbial necromass formation during decomposition of Stipa bungeana above-ground gesidues. Catena 2024, 245, 108283. [Google Scholar] [CrossRef]
- Min, X.; Xiao, L.; Li, Z.; Li, P.; Wang, F.; Liu, X.; Chen, S.; Wang, Z.; Pan, L. Effects of incubation temperature and sludge addition on soil organic carbon and nitrogen mineralization characteristics in degraded grassland soil. Agronomy 2024, 14, 1590. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, T.; Lu, J.; Zhu, J.; Ren, T.; Cong, R.; Lu, Z.; Zhang, Y.; Li, X. Long-term rice-crayfish farming alters soil dissolved organic carbon quality and biodegradability by regulating microbial metabolism and iron oxidation. J. Environ. Manag. 2024, 370, 122777. [Google Scholar] [CrossRef]
- Wang, X.; Azarbad, H.; Leclerc, L.; Dozois, J.; Mukula, E.; Yergeau, É. A drying-rewetting cycle imposes more important shifts on soil microbial communities than does reduced precipitation. mSystems 2022, 7, e00247-22. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Chen, Y.; Liu, Y.; Heděnec, P.; Peng, Y.; Xu, Z.; Tan, B.; Zhang, L.; Guo, L.; Wang, L.; et al. Effects of litter quality diminish and effects of vegetation type develop during litter decomposition of two shrub species in an alpine treeline ecotone. Ecosystems 2021, 24, 197–210. [Google Scholar] [CrossRef]
- De Vries, F.T.; Liiri, M.E.; Bjørnlund, L.; Bowker, M.A.; Christensen, S.; Setälä, H.M.; Bardgett, R.D. Land use alters the resistance and resilience of soil food webs to drought. Nat. Clim. Chang. 2012, 2, 276–280. [Google Scholar] [CrossRef]
- Fuchslueger, L.; Bahn, M.; Hasibeder, R.; Kienzl, S.; Fritz, K.; Schmitt, M.; Watzka, M.; Richter, A. Drought history affects grassland plant and microbial carbon turnover during and after a subsequent drought event. J. Ecol. 2016, 104, 1453–1465. [Google Scholar] [CrossRef]
- Canarini, A.; Fuchslueger, L.; Schnecker, J.; Metze, D.; Nelson, D.B.; Kahmen, A.; Watzka, M.; Pötsch, E.M.; Schaumberger, A.; Bahn, M.; et al. Soil fungi remain active and invest in storage compounds during drought independent of future climate conditions. Nat. Commun. 2024, 15, 10410. [Google Scholar] [CrossRef] [PubMed]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 2014, 117, 101–113. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant-microbial-soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Yuan, X.; Niu, D.; Gherardi, L.A.; Liu, Y.; Wang, Y.; Elser, J.J.; Fu, H. Linkages of stoichiometric imbalances to soil microbial respiration with increasing nitrogen addition: Evidence from a long-term grassland experiment. Soil Biol. Biochem. 2019, 138, 107580. [Google Scholar] [CrossRef]
- Koch, A.L. The macroeconomics of bacterial growth. In Bacteria in their Natural Environments; Fletcher, M., Floodgate, G.D., Eds.; Academic Press: London, UK, 1985; pp. 1–42. [Google Scholar]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Ågren, G.I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Li, X.; Leizeaga, A.; Rousk, J.; Zhou, S.; Hugelius, G.; Manzoni, S. Recovery of soil microbial metabolism after rewetting depends on interacting environmental conditions and changes in functional groups and life history strategies. Glob. Change Biol. 2024, 30, e17522. [Google Scholar] [CrossRef]
- Malik, A.A.; Martiny, J.B.H.; Brodie, E.L.; Martiny, A.C.; Treseder, K.K.; Allison, S.D. Defining trait-based microbial strategies with consequences for soil carbon cycling under climate change. ISME J. 2020, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.A.; Bouskill, N.J. Drought impacts on microbial trait distribution and feedback to soil carbon cycling. Funct. Ecol. 2022, 36, 1442–1456. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Gao, Y.; Zeng, Y.; Huang, M.; Yang, X.; Ochoa-Hueso, R.; Sun, W.; Yang, T. Reduced Precipitation Frequency Decreases the Stability of the Soil Organic Carbon Pool by Altering Microbial Communities in Degraded Grasslands. Agronomy 2025, 15, 977. https://doi.org/10.3390/agronomy15040977
Chen J, Gao Y, Zeng Y, Huang M, Yang X, Ochoa-Hueso R, Sun W, Yang T. Reduced Precipitation Frequency Decreases the Stability of the Soil Organic Carbon Pool by Altering Microbial Communities in Degraded Grasslands. Agronomy. 2025; 15(4):977. https://doi.org/10.3390/agronomy15040977
Chicago/Turabian StyleChen, Junda, Yifan Gao, Yizhu Zeng, Muping Huang, Xuechen Yang, Raúl Ochoa-Hueso, Wei Sun, and Tianxue Yang. 2025. "Reduced Precipitation Frequency Decreases the Stability of the Soil Organic Carbon Pool by Altering Microbial Communities in Degraded Grasslands" Agronomy 15, no. 4: 977. https://doi.org/10.3390/agronomy15040977
APA StyleChen, J., Gao, Y., Zeng, Y., Huang, M., Yang, X., Ochoa-Hueso, R., Sun, W., & Yang, T. (2025). Reduced Precipitation Frequency Decreases the Stability of the Soil Organic Carbon Pool by Altering Microbial Communities in Degraded Grasslands. Agronomy, 15(4), 977. https://doi.org/10.3390/agronomy15040977






