Biochar Supplementation of Recycled Manure Solids: Impact on Their Characteristics and Greenhouse Gas Emissions During Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. RMS Treatment
- (1)
- RMS without supplementation, serving as the negative control (C−);
- (2)
- RMS supplemented with 10% H2SO4 serving as the positive control (C+); acidification was performed by the addition of 20 mL of 10% H2SO4 to one kg of RMS to reach a final pH of 5. The 10% H2SO4 was obtained by diluting the concentrated H2SO4 (98% w/w) with distilled water.
- (3)
- RMS with 2.5% biochar (2.5B);
- (4)
- RMS with 10% biochar (10B).
| Treatment | pH | EC | DM | TOC | |
|---|---|---|---|---|---|
| mS cm−1 | g kg−1 (DM) | ||||
| Day 0 | C− | 9.30 a ± 0.05 | 0.466 c ± 0.01 | 287.60 b ± 14.20 | 150.80 ± 7.39 |
| C+ | 3.81 b ± 1.52 | 3.452 a ± 1.81 | 272.80 b ± 4.79 | 142.00 ± 2.20 | |
| 2.5B | 9.15 a ± 0.08 | 0.488 bc ± 0.00 | 294.40 ab ± 24.49 | 148.00 ± 7.11 | |
| 10B | 9.17 a ± 0.05 | 0.526 b ± 0.00 | 322.50 a ± 16.59 | 150.00 ± 3.92 | |
| Day 30 | C− | 8.22 a ± 0.31 | 0.143 c ± 0.05 | 444.70 a ± 1.67 | 227.90 a ± 0.55 |
| C+ | 5.06 b ± 0.16 | 0.770 a ± 0.12 | 314.30 c ± 0.05 | 160.00 c ± 0.63 | |
| 2.5B | 8.66 a ± 0.21 | 0.610 ab ± 0.05 | 383.50 b ± 20.20 | 212.40 b ± 11.16 | |
| 10B | 8.58 a ± 0.13 | 0.497 bc ± 0.03 | 280.40 d ± 8.41 | 131.00 d ± 6.97 | |
| Biochar | 9.69 ± 0.03 | 0.518 ± 0.02 | 852.57 ± 180.47 | 245.10 ± 69.77 | |
| Treatment | N-NH4+ | Ntotal | Na | K | Ca | Mg | P | S | |
|---|---|---|---|---|---|---|---|---|---|
| g kg−1 (DM) | |||||||||
| Day 0 | C− | 1.64 ± 0.03 | 20.67 a ± 3.56 | 0.96 a ± 0.00 | 5.53 b ± 0.01 | 10.25 a ± 0.20 | 3.58 a ± 0.12 | 2.51 a ± 0.02 | 3.39 b ± 0.07 |
| C+ | 1.79 ± 0.12 | 18.95 ab ± 0.88 | 0.85 b ± 0.01 | 4.82 c ± 0.06 | 8.80 c ± 0.12 | 2.76 b ± 0.58 | 2.23 b ± 0.03 | 13.83 a ± 0.57 | |
| 2.5B | 1.93 ± 0.62 | 16.41 bc ± 1.33 | 0.85 b ± 0.02 | 5.14 c ± 0.04 | 9.39 b ± 0.09 | 3.28 b ± 0.00 | 2.18 b ± 0.05 | 2.89 c ± 0.00 | |
| 10B | 0.97 ± 0.11 | 13.76 c ± 1.11 | 0.70 c ± 0.00 | 5.73 a ± 0.16 | 9.70 b ± 0.29 | 3.55 a ± 0.21 | 1.69 c ± 0.06 | 2.15 c ± 0.02 | |
| Day 30 | C− | 0.82 a ± 0.17 | 21.20 ± 1.43 | 1.12 a ± 0.07 | 6.57 b ± 0.00 | 11.34 b ± 0.14 | 4.00 c ± 0.00 | 2.67 a ± 0.03 | 3.85 b ± 0.04 |
| C+ | 0.85 a ± 0.08 | 21.60 ± 4.09 | 1.02 b ± 0.01 | 5.79 c ± 0.05 | 10.35 c ± 0.90 | 3.64 d ± 0.04 | 2.60 b ± 0.05 | 11.75 a ± 0.70 | |
| 2.5B | 0.72 ab ± 0.09 | 20.00 ± 0.68 | 1.01 b ± 0.02 | 6.72 b ± 0.15 | 11.51 b ± 0.01 | 4.23 b ± 0.10 | 2.35 c ± 0.03 | 3.30 c ± 0.10 | |
| 10B | 0.67 b ± 0.04 | 18.20 ± 1.50 | 0.84 c ± 0.02 | 6.84 a ± 0.08 | 12.70 a ± 0.12 | 4.68 a ± 0.06 | 2.01 d ± 0.04 | 2.41 d ± 0.03 | |
| Biochar | 0.18 ± 0.10 | 2.28 ± 0.04 | 0.52 ± 0.05 | 7.60 ± 1.09 | 16.06 ± 1.39 | 5.95 ± 0.68 | 1.22 ± 0.36 | 0.91 ± 0.30 | |
| Treatment | Fe | Cu | Zn | Mn | B | Mo | Cr | Ni | Cd | Pb | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 (DM) | |||||||||||
| Day 0 | C− | 1386 c ± 75.61 | 25 ± 1.62 | 139 ± 0.96 | 106 c ± 0.42 | 39 b ± 2.14 | 2.06 a ± 0.07 | 4.66 ± 0.74 | 3.19 ± 0.40 | 0.25 ± 0.02 | 0.15 b ± 0.16 |
| C+ | 1131 d ± 13.96 | 23 ± 0.26 | 129 ± 0.10 | 95 d ± 0.82 | 33 c ± 0.74 | 1.82 a ± 0.02 | 4.02 ± 0.01 | 2.57 ± 0.30 | 0.24 ± 0.03 | 0.36 b ± 0.03 | |
| 2.5B | 1699 b ± 213.06 | 22 ± 0.06 | 128 ± 2.12 | 148 b ± 5.24 | 39 b ± 2.53 | 1.51 b ± 0.21 | 5.00 ± 0.36 | 2.82 ± 0.88 | 0.26 ± 0.02 | 1.22 b ± 0.05 | |
| 10B | 7111 a ± 176.59 | 22 ± 1.79 | 127 ± 11.26 | 449 a ± 100.80 | 75 a ± 14.51 | 1.12 c ± 0.08 | 15.29 ± 0.75 | 3.20 ± 0.46 | 0.61 ± 0.05 | 2.69 a ± 0.30 | |
| Day 30 | C− | 1409 c ± 68.85 | 28 b ± 0.07 | 153 ± 6.60 | 124 c ± 2.95 | 44 c ± 0.59 | 2.29 a ± 0.20 | 4.54 c ± 0.21 | 2.61 b ± 0.14 | 0.25 c ± 0.01 | 0.16 c ± 0.26 |
| C+ | 1259 d ± 18.46 | 24 c ± 0.56 | 137 ± 10.57 | 121 c ± 6.59 | 35 d ± 4.61 | 2.13 a ± 0.10 | 4.29 c ± 0.10 | 2.58 b ± 0.15 | 0.24 c ± 0.01 | 0.02 c ± 0.01 | |
| 2.5B | 2784 b ± 100.02 | 27 b ± 0.13 | 158 ± 0.78 | 271 b ± 14.10 | 53 b ± 0.31 | 1.93 a ± 0.02 | 9.71 b ± 0.26 | 2.97 b ± 0.28 | 0.39 b ± 0.03 | 1.28 b ± 0.22 | |
| 10B | 6331 a ± 17.10 | 29 a ± 0.85 | 152 ± 10.36 | 573 a ± 5.92 | 84 a ± 0.02 | 1.50 b ± 0.22 | 15.39 a ± 0.43 | 3.78 a ± 0.11 | 0.74 a ± 0.03 | 2.90 a ± 0.49 | |
| Biochar | 14,691.56 ± 2294.31 | 27.75 ± 3.54 | 149.97 ± 47.65 | 1181.33 ± 95.46 | 121.03 ± 51.61 | 1.95 ± 2.53 | 27.28 ± 9.87 | 38.15 ± 57.88 | 1.02 ± 0.28 | 7.66 ± 2.59 | |
2.2. Small-Scale Experiment
2.3. Chemical Analysis of RMS and Biochar
2.4. GHG Emissions Monitoring
2.5. Calculation and Data Analysis
- G is the gas concentration in mg/m3 from the Innova system;
- F is the airflow rate, set at 2.2 L/min;
- The value 1440 is the number of minutes in a day, which is used to convert the reading to daily emissions;
- RMS’s weight is the weight of the RMS sample in kilograms.
3. Results
3.1. RMS Characteristics
3.2. GHG Emissions and GWP
3.2.1. Continuous Sampling
3.2.2. Intermittent Sampling
4. Discussion
4.1. Impact of Biochar Supplementation on RMS Characteristics
4.2. Impact of Biochar Supplementation on GHG Emissions and GWP
4.2.1. Continuous Sampling
4.2.2. Intermittent Sampling
4.2.3. Sampling Methods Comparison
4.3. Biochar Carbon Footprint: Balancing Production Emissions and RMS GHG Reduction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Commission. The European Green Deal; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- European Commission. A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- United States Environmental Protection Agency. Global Anthropogenic Non-CO2 Greenhouse Gas Emissions: 1990–2020; United States Environmental Protection Agency: Washington, DC, USA, 2006. [Google Scholar]
- da Silva, T.E.; Cabrera, V.E. The DairyPrint Model: A Decision-Support Model to Help Dairy Farmers and Other Stakeholders Towards Improved Sustainability. J. Dairy Sci. 2024, 107, 10998–11015. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, D.; Pereira, J.L.S.; Fraga, I.; Surgy, S.; Vasconcelos, E.; Coutinho, J. Band Application of Acidified Slurry as an Alternative to Slurry Injection in a Mediterranean Double Cropping System: Agronomic Effect and Gaseous Emissions. Agric. Ecosyst. Environ. 2018, 267, 87–99. [Google Scholar] [CrossRef]
- Yan, X.; Ying, Y.; Li, K.; Zhang, Q.; Wang, K. A Review of Mitigation Technologies and Management Strategies for Greenhouse Gas and Air Pollutant Emissions in Livestock Production. J. Environ. Manag. 2024, 352, 120028. [Google Scholar] [CrossRef] [PubMed]
- Prado, J.; Chieppe, J.; Raymundo, A.; Fangueiro, D. Bio-Acidification and Enhanced Crusting as an Alternative to Sulphuric Acid Addition to Slurry to Mitigate Ammonia and Greenhouse Gases Emissions During Short Term Storage. J. Clean. Prod. 2020, 263, 121443. [Google Scholar] [CrossRef]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of Animal Slurry—A Review. J. Environ. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef]
- Regueiro, I.; Coutinho, J.; Gioelli, F.; Balsari, P.; Dinuccio, E.; Fangueiro, D. Acidification of Raw and Co-Digested Pig Slurries with Alum before Mechanical Separation Reduces Gaseous Emission during Storage of Solid and Liquid Fractions. Agric. Ecosyst. Environ. 2016, 227, 42–51. [Google Scholar] [CrossRef]
- Ma, S.; Shen, Y.; Ding, J.; Cheng, H.; Zhou, H.; Ge, M.; Wang, J.; Cheng, Q.; Zhang, D.; Zhang, Y.; et al. Effects of Biochar and Volcanic Rock Addition on Humification and Microbial Community during Aerobic Composting of Cow Manure. Bioresour. Technol. 2024, 391, 129973. [Google Scholar] [CrossRef]
- Fangueiro, D.; Pereira, J.L.S.; Macedo, S.; Trindade, H.; Vasconcelos, E.; Coutinho, J. Surface Application of Acidified Cattle Slurry Compared to Slurry Injection: Impact on NH3, N2O, CO2 and CH4 Emissions and Crop Uptake. Geoderma 2017, 306, 160–166. [Google Scholar] [CrossRef]
- Sapkota, S.; Ghimire, R.; Bista, P.; Hartmann, D.; Rahman, T.; Adhikari, S. Greenhouse Gas Mitigation and Soil Carbon Stabilization Potential of Forest Biochar Varied with Biochar Type and Characteristics. Sci. Total Environ. 2024, 931, 172942. [Google Scholar] [CrossRef]
- Bello, A.; Deng, L.; Sheng, S.; Jiang, X.; Yang, W.; Meng, Q.; Wu, X.; Han, Y.; Zhu, H.; Xu, X. Biochar Reduces Nutrient Loss and Improves Microbial Biomass of Composted Cattle Manure and Maize Straw. Biotechnol. Appl. Biochem. 2020, 67, 799–811. [Google Scholar] [CrossRef]
- Du, L.; Ahmad, S.; Liu, L.; Wang, L.; Tang, J. A Review of Antibiotics and Antibiotic Resistance Genes (ARGs) Adsorption by Biochar and Modified Biochar in Water. Sci. Total Environ. 2023, 858, 159815. [Google Scholar] [CrossRef]
- Schmidt, H.P. European Biochar Certificate (EBC)—Guidelines, Version 6.1; European Biochar Certificate: Arbaz, Switzerland, 2015. [Google Scholar]
- Meyer, S.; Genesio, L.; Vogel, I.; Schmidt, H.-P.; Soja, G.; Someus, E.; Shackley, S.; Verheijen, F.G.A.; Glaser, B. Biochar Standardization and Legislation Harmonization. J. Environ. Eng. Landsc. Manag. 2017, 25, 175–191. [Google Scholar] [CrossRef]
- Shrestha, R.K.; Jacinthe, P.-A.; Lal, R.; Lorenz, K.; Singh, M.P.; Demyan, S.M.; Ren, W.; Lindsey, L.E. Biochar as a Negative Emission Technology: A Synthesis of Field Research on Greenhouse Gas Emissions. J. Environ. Qual. 2023, 52, 769–798. [Google Scholar] [CrossRef]
- Burrell, L.D.; Zehetner, F.; Rampazzo, N.; Wimmer, B.; Soja, G. Long-Term Effects of Biochar on Soil Physical Properties. Geoderma 2016, 282, 96–102. [Google Scholar] [CrossRef]
- Bruun, E.W.; Müller-Stöver, D.; Ambus, P.; Hauggaard-Nielsen, H. Application of Biochar to Soil and N2O Emissions: Potential Effects of Blending Fast-Pyrolysis Biochar with Anaerobically Digested Slurry. Eur. J. Soil Sci. 2011, 62, 581–589. [Google Scholar] [CrossRef]
- Guerreiro, J.F.; Pires, A.J.; Nunes, M.; Esteves, A.; Chambel, L.; Pascoal, P.; Pereira, M.; Fangueiro, D.; Tavares, L.; Dias, R.; et al. Biochar Supplementation Affects the Microbiome of Recycled Manure Solids for Cow Bedding: A Metagenomic Analysis. J. Dairy Sci. 2025, 108, 2620–2631. [Google Scholar] [CrossRef]
- Pires, A.J.; Esteves, A.F.; Pereira, G.; Geraldes, C.; Guerreiro, J.F.; Abreu, R.; Magalhães, R.; Chambel, L.; Silva, E.; Fangueiro, D.; et al. Potential of Pine Biochar to Mitigate Bacterial Hazards Present in Recycled Manure Solids from Dairy Cows. Vet. Sci. 2025, 12, 43. [Google Scholar] [CrossRef]
- Horneck, D.A.; Miller, R.O. Determination of total nitrogen in plant tissue. In Handbook of Reference Methods for Plant Analysis; CRC Press LLC: Boca Raton, FL, USA, 1998; pp. 73–85. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2021—The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2023. [Google Scholar]
- Sørensen, P.; Eriksen, J. Effects of Slurry Acidification with Sulphuric Acid Combined with Aeration on the Turnover and Plant Availability of Nitrogen. Agric. Ecosyst. Environ. 2009, 131, 240–246. [Google Scholar] [CrossRef]
- Eriksen, J.; Sørensen, P.; Elsgaard, L. The Fate of Sulfate in Acidified Pig Slurry during Storage and Following Application to Cropped Soil. J. Environ. Qual. 2008, 37, 280–286. [Google Scholar] [CrossRef]
- Busch, F.; Leal, O.d.A.; Siebers, N.; Brüggemann, N. Biochar Captures Ammonium and Nitrate in Easily Extractable and Strongly Retained form without Stimulating Greenhouse Gas Emissions during Composting. J. Environ. Qual. 2024, 53, 1099–1115. [Google Scholar] [CrossRef]
- Vieira Firmino, M.; Trémier, A.; Couvert, A.; Szymczyk, A. New Insights into Biochar Ammoniacal Nitrogen Adsorption and Its Correlation to Aerobic Degradation Ammonia Emissions. Waste Manag. 2024, 178, 257–266. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, Y.; Hu, R.; Xu, X.; Xian, J.; Yang, Y.; Liu, L.; Cheng, Z. Effect of Rice Straw and Swine Manure Biochar on N2O Emission from Paddy Soil. Sci. Rep. 2020, 10, 10843. [Google Scholar] [CrossRef]
- Liu, M.; Liu, C.; Liao, W.; Xie, J.; Zhang, X.; Gao, Z. Impact of Biochar Application on Gas Emissions from Liquid Pig Manure Storage. Sci. Total Environ. 2021, 771, 145454. [Google Scholar] [CrossRef]
- Overmeyer, V.; Trimborn, M.; Clemens, J.; Hölscher, R.; Büscher, W. Acidification of Slurry to Reduce Ammonia and Methane Emissions: Deployment of a Retrofittable System in Fattening Pig Barns. J. Environ. Manag. 2023, 331, 117263. [Google Scholar] [CrossRef]
- Schmidt, H.-P.; Kammann, C.; Hagemann, N.; Leifeld, J.; Bucheli, T.D.; Sánchez Monedero, M.A.; Cayuela, M.L. Biochar in Agriculture—A Systematic Review of 26 Global Meta-Analyses. GCB Bioenergy 2021, 13, 1708–1730. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, W.; Zhong, X.; Wang, Y.; Li, S.; Zhou, Y.; Wang, C. The Impact of Combined Application of Biochar and Fertilizer on the Biochemical Properties of Soil in Soybean Fields. PeerJ 2024, 12, e18172. [Google Scholar] [CrossRef]
- Meyer, S.; Glaser, B.; Quicker, P. Technical, Economical, and Climate-Related Aspects of Biochar Production Technologies: A Literature Review. Environ. Sci. Technol. 2011, 45, 9473–9483. [Google Scholar] [CrossRef]
- Handiso, B.; Pääkkönen, T.; Wilson, B.P. Effect of Pyrolysis Temperature on the Physical and Chemical Characteristics of Pine Wood Biochar. Waste Manag. Bull. 2024, 2, 281–287. [Google Scholar] [CrossRef]
- Wu, G.; Tham, P.E.; Chew, K.W.; Munawaroh, H.S.H.; Tan, I.S.; Wan-Mohtar, W.A.A.Q.I.; Sriariyanun, M.; Show, P.L. Net Zero Emission in Circular Bioeconomy from Microalgae Biochar Production: A Renewed Possibility. Bioresour. Technol. 2023, 388, 129748. [Google Scholar] [CrossRef]
- Li, H.; Lin, L.; Peng, Y.; Hao, Y.; Li, Z.; Li, J.; Yu, M.; Li, X.; Lu, Y.; Gu, W.; et al. Biochar’s Dual Role in Greenhouse Gas Emissions: Nitrogen Fertilization Dependency and Mitigation Potential. Sci. Total Environ. 2024, 917, 170293. [Google Scholar] [CrossRef]
- Patwa, D.; Bordoloi, U.; Dubey, A.A.; Ravi, K.; Sekharan, S.; Kalita, P. Energy-Efficient Biochar Production for Thermal Backfill Applications. Sci. Total Environ. 2022, 833, 155253. [Google Scholar] [CrossRef] [PubMed]
- Zireeni, Y.; Jones, D.L.; Chadwick, D.R.; Mäenpää, M.I.; Sørensen, P. Low-Dose Acidification of Cattle Slurry with Sulfuric Acid Enhances Oilseed-Rape Yield But Risks Elevated Sulfide Formation during Prolonged Storage. Sci. Total Environ. 2025, 970, 179014. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, B.; Nguyen, D.D.; Chaudhary, D.K.; Chang, S.W.; Kim, J.; Lee, S.R.; Shin, J.; Jeon, B.-H.; Chung, S.; Lee, J. Influence of Biochar on Physico-Chemical and Microbial Community during Swine Manure Composting Process. J. Environ. Manag. 2019, 232, 592–599. [Google Scholar] [CrossRef]
- Chung, W.J.; Chang, S.W.; Chaudhary, D.K.; Shin, J.; Kim, H.; Karmegam, N.; Govarthanan, M.; Chandrasekaran, M.; Ravindran, B. Effect of Biochar Amendment on Compost Quality, Gaseous Emissions and Pathogen Reduction during in-Vessel Composting of Chicken Manure. Chemosphere 2021, 283, 131129. [Google Scholar] [CrossRef] [PubMed]
- Kizito, S.; Jjagwe, J.; Mdondo, S.W.; Nagawa, C.B.; Bah, H.; Tumutegyereize, P. Synergetic Effects of Biochar Addition on Mesophilic and High Total Solids Anaerobic Digestion of Chicken Manure. J. Environ. Manag. 2022, 315, 115192. [Google Scholar] [CrossRef]
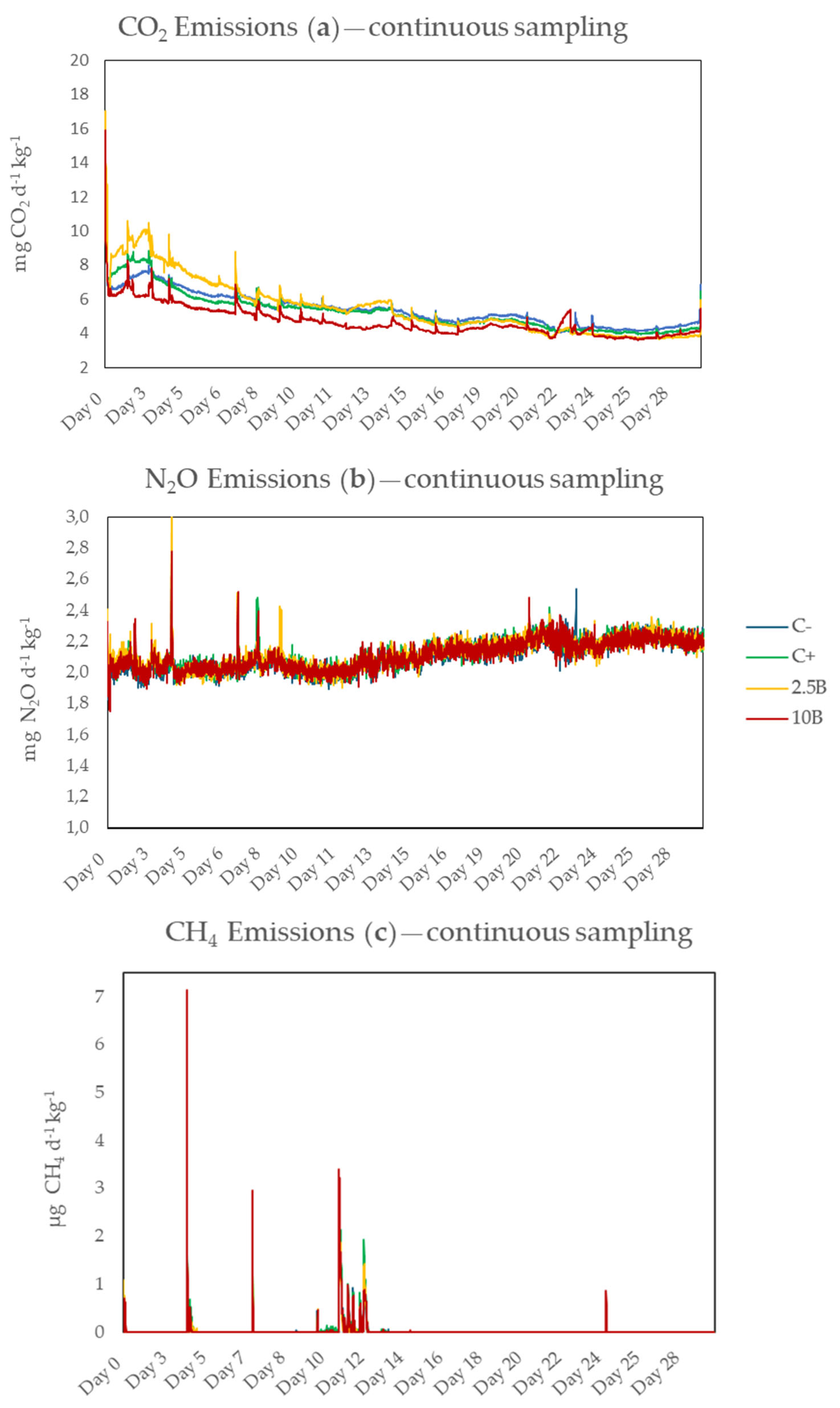
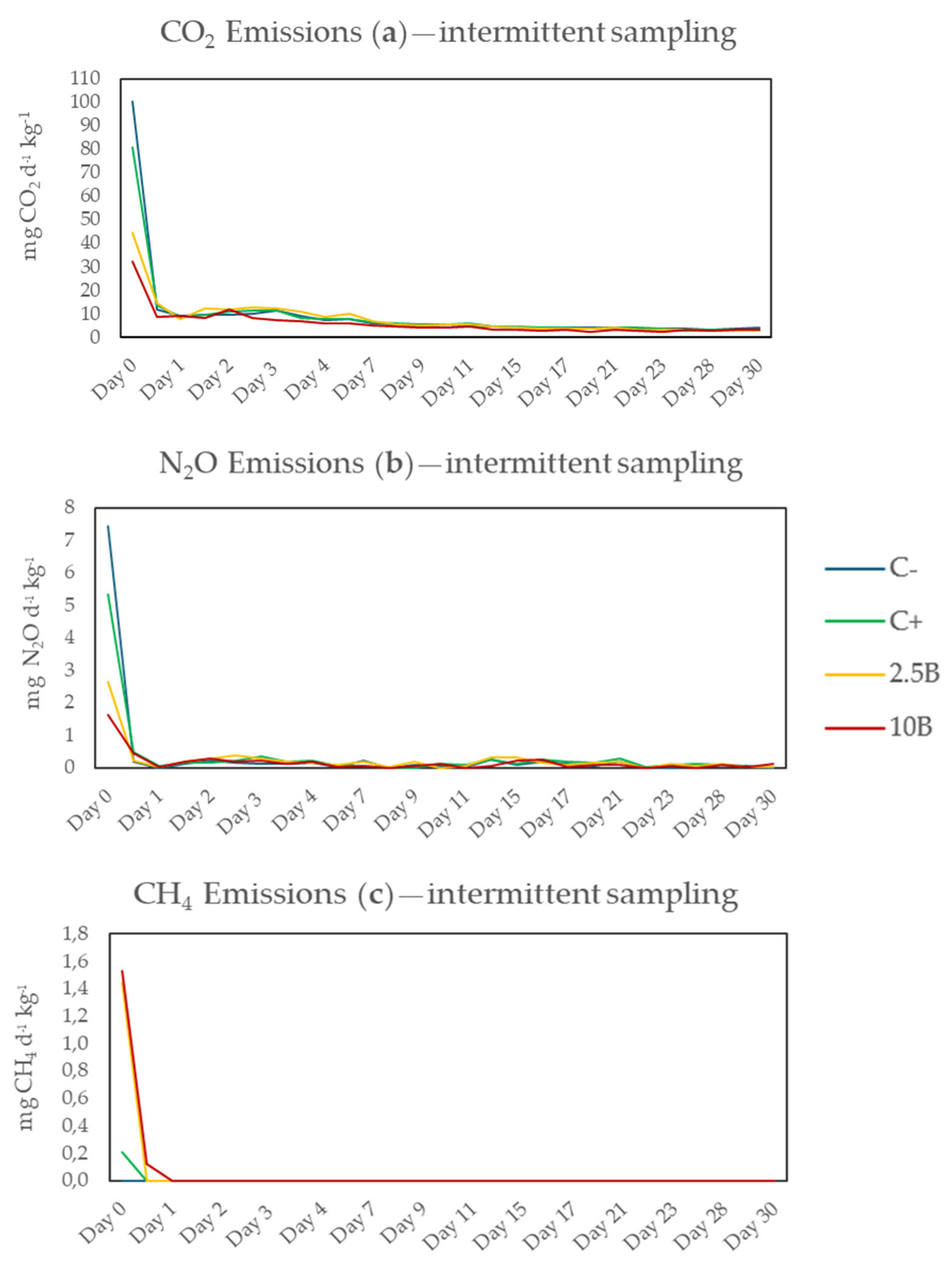
| Treatment | CO2 | N2O | CH4 | GWP |
|---|---|---|---|---|
| g kg−1 | mg kg−1 | g CO2-eq kg−1 | ||
| C− | 249.61 ab ± 14.85 | 96.73 ± 0.52 | 1.39 b ± 0.24 | 275.77 ab ± 14.99 |
| C+ | 243.91 ab ± 13.42 | 97.64 ± 0.51 | 1.60 b ± 0.02 | 270.37 ab ± 13.55 |
| 2.5B | 260.77 a ± 15.60 | 97.59 ± 0.32 | 1.66 ab ± 0.04 | 287.23 a ± 15.56 |
| 10B | 217.40 b ± 4.19 | 97.02 ± 0.33 | 1.73 a ± 0.04 | 243.61 b ± 4.16 |
| Treatment | CO2 | N2O | CH4 | GWP |
|---|---|---|---|---|
| g kg−1 | mg kg−1 | g CO2-eq kg−1 | ||
| C− | 415.60 a ± 4.27 | 13.53 a ± 2.49 | 0.00 ± 0.00 | 419.29 a ± 3.62 |
| C+ | 402.68 ab ± 15.51 | 13.39 a ± 2.04 | 0.23 ± 0.39 | 406.34 ab ± 15.93 |
| 2.5B | 372.75 bc ± 15.95 | 10.94 a ± 1.07 | 1.56 ± 2.70 | 375.78 bc ± 15.89 |
| 10B | 282.17 c ± 21.70 | 7.19 b ± 1.25 | 1.84 ± 2.93 | 284.18 c ± 21.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, A.J.; Esteves, C.; Bexiga, R.; Oliveira, M.; Fangueiro, D. Biochar Supplementation of Recycled Manure Solids: Impact on Their Characteristics and Greenhouse Gas Emissions During Storage. Agronomy 2025, 15, 973. https://doi.org/10.3390/agronomy15040973
Pires AJ, Esteves C, Bexiga R, Oliveira M, Fangueiro D. Biochar Supplementation of Recycled Manure Solids: Impact on Their Characteristics and Greenhouse Gas Emissions During Storage. Agronomy. 2025; 15(4):973. https://doi.org/10.3390/agronomy15040973
Chicago/Turabian StylePires, Ana José, Catarina Esteves, Ricardo Bexiga, Manuela Oliveira, and David Fangueiro. 2025. "Biochar Supplementation of Recycled Manure Solids: Impact on Their Characteristics and Greenhouse Gas Emissions During Storage" Agronomy 15, no. 4: 973. https://doi.org/10.3390/agronomy15040973
APA StylePires, A. J., Esteves, C., Bexiga, R., Oliveira, M., & Fangueiro, D. (2025). Biochar Supplementation of Recycled Manure Solids: Impact on Their Characteristics and Greenhouse Gas Emissions During Storage. Agronomy, 15(4), 973. https://doi.org/10.3390/agronomy15040973






