Abstract
This study aimed to evaluate the effects of different crop cultivation practices on soil chemical properties and microbial communities in the Mu Us Desert, with the goal of optimizing land management and promoting ecological restoration. A one-way randomized block design was used to establish experimental plots for a cereal (Setaria italica, SI), a legume (Glycine max, GM), and a control group with no crops (CK) in the central Mu Us Desert. Soil samples were collected to assess physicochemical properties and to analyze microbial community structures via high-throughput 16S rRNA gene sequencing. Results showed that crop cultivation decreased soil pH while increasing soil organic carbon (SOC), total nitrogen (TN), and available phosphorus (AP), indicating improved soil fertility and reduced soil alkalinity. The composition of soil bacterial communities varied significantly among treatments. Both SI and GM treatments increased the number of operational taxonomic units (OTUs), enhancing bacterial richness and diversity. Proteobacteria and Actinobacteria increased with crop cultivation, whereas Chloroflexi declined. These shifts were largely attributed to changes in pH and nutrient availability. Notably, SI treatment had a stronger positive effect on bacterial richness. Correlation analyses between soil chemical properties and microbial community composition highlighted the potential of crop cultivation to influence soil ecosystem services. These findings provide a scientific basis for sustainable agricultural practices and ecological restoration in arid regions such as the Mu Us Desert. Further studies are warranted to investigate the functional roles of microbial communities under different cropping patterns.
1. Introduction
The Mu Us Desert is a vast arid region. In recent years, large-scale ecological management and afforestation efforts have significantly improved the local ecological environment, offering considerable potential for land development and utilization [1,2,3]. Under a comprehensive regional land improvement strategy [4], large areas of grassland in the eastern Mu Us Desert have been converted into arable farmland [5], greatly enhancing regional food production [6], and contributing to food security. Furthermore, crop cultivation has improved soil structure, increased land productivity [7,8], and optimized the soil ecological environment. Despite these advancements, the dominant soil type in the region remains sandy soil [9,10], characterized by poor fertility and loose structure, along with unique physicochemical properties [11]. Soil microorganisms play a central role in such ecosystems, being key drivers of nutrient cycling, organic matter decomposition, and plant growth [12,13,14]. In this fragile environment, microbial communities help maintain soil fertility, facilitate nutrient turnover, enhance plant productivity, and provide natural pest control [15,16]. In particular, black beans (Glycine max) and cereals such as foxtail millet (Setaria italica), which are commonly grown in the region, interact closely with soil microbes, influencing crop yield and health [17,18]. As drought- and nutrient deficiency-tolerant crops, their root exudates nourish microbial communities, promoting microbial development, improving soil quality, and boosting productivity [19]. Therefore, studying the soil microbial community under different crop cultivation regimes in the Mu Us Desert is essential for guiding agricultural production. Understanding the role of soil bacteria can inform practices such as the addition of beneficial strains to enhance soil fertility, improve crop performance, and promote sustainable agriculture [20].
With the advent of molecular biology and high-throughput sequencing technologies, numerous studies have examined soil microbial communities in this region [21,22,23]. Using platforms like Illumina MiSeq, researchers have explored microbial diversity and community structure in soils cultivated with various crops [24,25,26]. Crop cultivation was found to significantly influence microbial diversity and composition, which notably increased microbial abundance and diversity [27,28,29]. Comparative studies demonstrated the selective effects of crop root exudates on microbial composition and their positive impact on nutrient cycling and plant growth [30,31,32,33]. Moreover, key soil properties such as pH, organic matter, available potassium, and phosphorus were closely linked to microbial community dynamics [34,35,36]. These findings have deepened our understanding of microbial ecological functions and have provided a scientific basis for soil management and crop production strategies [37,38].
However, current research mainly focuses on vegetation restoration areas or remediation of contaminated soils in mining zones [39,40]. There is limited research on soil microbial diversity and function in croplands growing the region’s specialty crops [41,42]. Moreover, most existing studies prioritize soil physicochemical properties, while relatively few address microbiological aspects [43,44]. Although soil harbors a vast number of microbial species, including bacteria, fungi, and oomycetes, bacteria are the most abundant and functionally diverse group [45]. Thus, this study focuses on bacterial communities, aiming to elucidate their roles in shaping soil and crop outcomes.
The objective of this study is to systematically investigate the diversity and structure of soil bacterial communities in croplands cultivated with Setaria italica and Glycine max, and to explore their relationships with soil physicochemical properties. Experimental plots were established in multiple locations across the Mu Us Desert. Soil samples were collected from fields with different crop types and analyzed using high-throughput 16S rRNA sequencing, combined with traditional soil property measurements. The study seeks to uncover how crop cultivation alters bacterial community structures and how microbial communities respond to changes in soil physicochemical conditions. Additionally, correlation and regression analyses were performed to explore interactions between microbial diversity and soil properties and to evaluate the effects of crop cultivation on soil health. We hypothesized that: (1) cereal and legume cultivation enhances soil fertility and reduces soil alkalinity; (2) cereal and legume cultivation significantly alters soil microbial diversity and community structure by modifying soil pH and increasing nutrient availability. This study provides critical insights into how different crop cultivation systems impact inter-root soil chemical properties and microbial community structure in the Mu Us Desert, an ecologically important region in northern China.
2. Materials and Methods
2.1. Design of the Experiment
The experimental site is located in the core region of the Mu Us Desert (Figure 1), specifically at a field monitoring station in Yulin City, China (coordinates: 109°29′ E, 38°26′ N). This area exhibits a typical mesothermal semi-arid continental monsoon climate, characterized by uneven precipitation distribution and a generally arid environment [46]. The mean annual temperature is 8.1 °C, with an average frost-free period of 154 days, annual precipitation of 413.9 mm, annual sunshine duration of 2879 h, a sunshine percentage of 65%, and total annual solar radiation of 145.2 kcal·cm−2 [47]. The dominant soil type in the region is aeolian sandy soil. The land use types within the study area include agricultural land, woodland, and grassland, which are spatially intermixed.
Figure 1.
Location of test plots.
2.2. Experimental Design and Sample Collection
In March 2022, a one-way randomized block design was implemented for two commonly cultivated specialty crops: cereal (Setaria italica) and legume (Glycine max). The plots were categorized as follows: CK (unplanted control, 109°29′52″ E, 38°26′11″ N), SI (Setaria italica, 109°29′45″ E,38°26′12″ N), and GM (Glycine max, 109°29′40″ E,38°26′13″ N). All plots were regularly irrigated to maintain soil moisture at 75–80% of field capacity. The crops were harvested in late August 2023. Each treatment included three replicates, and soil physicochemical and microbial analyses were conducted after harvest. Soil samples were collected at crop maturity. Rhizosphere soil (within 1 mm of the root surface) was obtained by carefully brushing soil from fine roots excavated from the 0–30 cm depth layer. For the CK treatment, soil samples were collected from the same depth. A five-point sampling method was applied in each plot to obtain composite samples, and this procedure was repeated three times per treatment [48]. Samples were sieved through a 2 mm mesh to remove debris and stones, sealed in sterile bags, placed in iceboxes, and transported to the laboratory within one hour. All samples were then stored at –80 °C for subsequent analyses.
2.3. Determination of Soil Chemical Properties
Soil samples were air-dried and sieved through 1 mm and 0.149 mm meshes. Soil pH was measured using a pH meter (PHS-3D, Shanghai Sanxin Instrument Co., Shanghai, China). Soil organic carbon (SOC) content was determined via the potassium dichromate volumetric method. Total nitrogen (TN) was quantified using the Kjeldahl method, and available potassium (AK) content was assessed using the ammonium acetate extraction–flame photometry method [49].
2.4. DNA Extraction, PCR Amplification, and Sequencing
Soil microbial diversity was analyzed using DNA extraction and high-throughput sequencing techniques [50]. Genomic DNA was extracted using the CTAB method, and its quality was assessed via 1% agarose gel electrophoresis. DNA was diluted to 1 ng/µL with sterile water for PCR amplification of the V4 region using primers 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′). Reactions were performed using 15 μL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs, Ipswich, MA, USA), 2 μM of each primer, and approximately 10 ng of template DNA. The PCR products were mixed at equal concentrations and purified using a Qiagen Gel Extraction Kit (Qiagen, Hilden, Germany). Sequencing libraries were generated using the TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, San Diego, CA, USA), following the manufacturer’s instructions, and index codes were added. Library quality was assessed on a Qubit@ 2.0 fluorometer (Thermo Scientific, Carlsbad, CA, USA), and the library was sequenced on the Illumina NovaSeq platform to generate 250 bp paired-end reads [51,52]. Taxonomic classification was performed using the QIIME2 feature-classifier plugin and a pre-trained GREENGENES 13_8 99% database, trimmed to the V3–V4 region [53]. Mitochondrial and chloroplast sequences were removed using QIIME2’s feature table plugin.
2.5. Statistical Analysis of Data
Differentially abundant taxa across groups were identified using ANCOM, ANOVA, Kruskal–Wallis, LEfSe, and DESeq2 methods [54]. Operational taxonomic units (OTUs) were defined based on 16S rRNA gene sequence clustering. Statistical analyses were conducted using SPSS 21.0, with multiple comparisons performed using Duncan’s test (p < 0.05). Additional statistical analysis and visualization were conducted in R version 3.3.2. Alpha diversity was evaluated using Shannon and Simpson indices for diversity, and Chao1 and ACE indices for richness estimation [55,56]. Principal coordinate analysis (PCoA) based on Bray–Curtis dissimilarity was employed to assess differences in microbial community structure. PCoA plots were used to visualize sample similarity patterns, where inter-point distances represent compositional differences [57,58]. Pearson correlation coefficients were calculated to assess relationships among soil physicochemical and microbial indicators [59,60].
3. Results
3.1. Inter-Root Soil Chemistry of Different Crops
The inter-root soil, located immediately around the plant root system, is a biologically active zone where chemical and microbial properties are influenced by root secretions. This zone plays a crucial role in nutrient uptake and plant growth. In this study, we conducted a comparative analysis of the inter-root soil chemical properties among three cropping treatments (Table 1). The results showed a general reduction in soil pH following crop cultivation compared to the uncultivated control (CK). The most pronounced pH decline occurred under the SI treatment, with a decrease of 0.13 units, while a smaller reduction of 0.07 units was observed in the GM treatment. Regarding soil organic carbon (SOC), the CK plot exhibited the highest SOC content (13.58 g·kg−1), with lower values recorded in both SI and GM treatments. For total nitrogen (TN), the GM treatment had the highest content (0.55 g·kg−1), followed by SI (0.44 g·kg−1). Available phosphorus (AP) was highest under SI (326.9 g·kg−1), followed by GM (253.2 g·kg−1), while CK had the lowest TN and AP contents.

Table 1.
Inter-root soil chemistry of different crops treatments.
3.2. Venn Diagram Analysis of Inter-Root Soil Bacterial Composition
Venn diagrams visually represent the overlap and uniqueness of operational taxonomic units (OTUs) among different samples, highlighting similarities and distinctions in microbial community composition. In this study, OTUs were defined based on a 97% similarity threshold (Figure 2). The total number of rarefied OTUs under CK, GM, and SI treatments were 769, 642, and 755, respectively, with 1897 OTUs shared among all three treatments. The GM treatment exhibited the highest number of unique OTUs, while CK had comparatively fewer. These findings indicate that different cropping systems significantly influence microbial diversity in the soil. Additionally, the high overlap in OTUs between GM and CK treatments suggests a strong similarity in bacterial community structure between these two conditions.
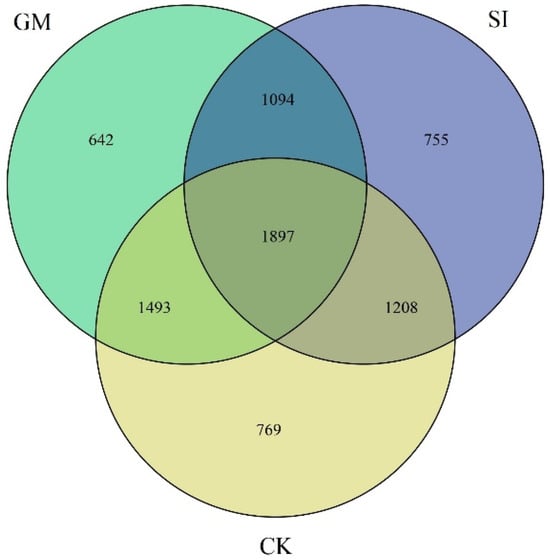
Figure 2.
Venn diagram of inter-root soil bacterial operational taxonomic units (OTUs) under different crop treatments. CK (unplanted control), SI (Setaria italica), GM (Glycine max).
3.3. Bacterial Richness and Diversity in Inter-Root Soil
Alpha diversity is a key indicator of soil bacterial diversity. In this study, the Chao1 and ACE indices were used to assess species richness, while Shannon and Simpson indices measured species diversity (Table 2). The SI treatment showed the highest richness values, with Chao1 and ACE indices at 2693.8 and 3068.3, respectively, followed by GM and then CK. Regarding diversity, Shannon index values ranged from 9.76 to 10.07 across treatments, indicating high microbial diversity in all plots.

Table 2.
Soil bacterial richness and diversity indices under different shrub crops.
3.4. Differential Analysis of Inter-Root Soil Bacterial Community Composition
Bar charts provide a clear visualization of microbial community composition and species abundance at various taxonomic levels. Here, we focused on the phylum level to analyze bacterial community composition across treatments (Figure 3). In the CK treatment, the dominant bacterial phyla were Chloroflexi, Proteobacteria, and Actinobacteria, with firmicutes also showing high abundance. The GM treatment showed a similar phylum distribution. However, in the SI treatment, Acidobacteria replaced Chloroflexi as a dominant phylum, while Actinobacteria and Proteobacteria remained dominant. These results suggest that crop cultivation alters soil bacterial community structure. The increase in Proteobacteria and Actinobacteria, and the decline in Chloroflexi, indicate that changes in soil physical and chemical properties—such as increased carbon sources from root exudates—may promote bacterial groups better suited to such environments. Notably, the CK plots, located in the Mu Us Desert, had sparse vegetation due to harsh conditions (low rainfall and high temperatures). Limited organic matter input under natural conditions likely constrained microbial colonization and diversity.
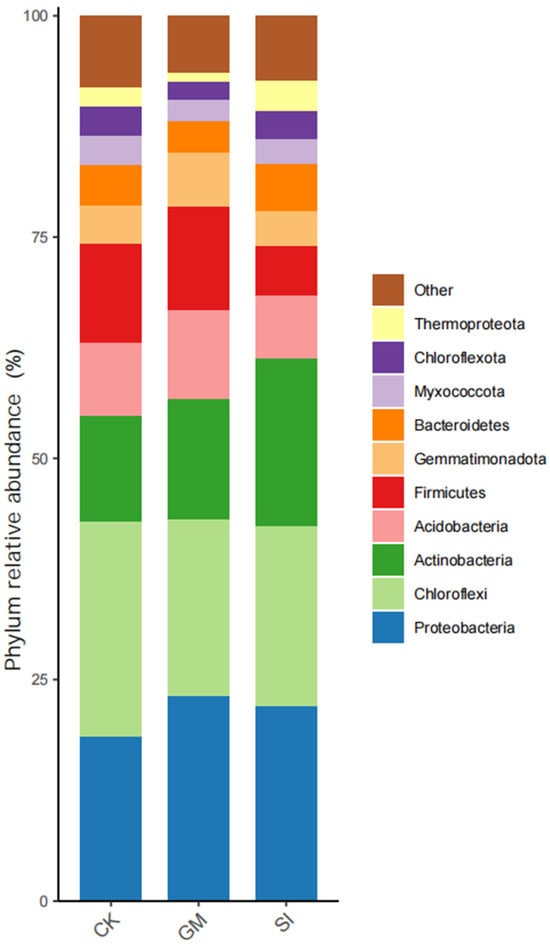
Figure 3.
Bacterial community composition at the phylum level across crop treatments.
3.5. Analysis of Inter-Root Soil β-Diversity and Intergroup Variability
In this study, β-diversity analysis was conducted using weighted UniFrac distances to evaluate differences in bacterial community composition among the inter-root soils of different crops. The results were visualized through principal coordinate analysis (PCoA) (Figure 4). The weighted UniFrac method considers both phylogenetic relationships and the relative abundance of microbial taxa, providing a comprehensive assessment of community structure differences. In the PCoA plot, the first principal coordinate (PCoA1) accounted for 30.7% of the variation, while the second principal coordinate (PCoA2) explained 23.7%. Together, these two axes captured 53.7% of the total variability in bacterial community composition. Along the PCoA1 axis, the bacterial communities of the SI and GM treatments clustered closely, indicating a higher similarity in their community structures. In contrast, both treatments were clearly separated from the CK, suggesting significant differences in microbial community composition between planted and unplanted soils.
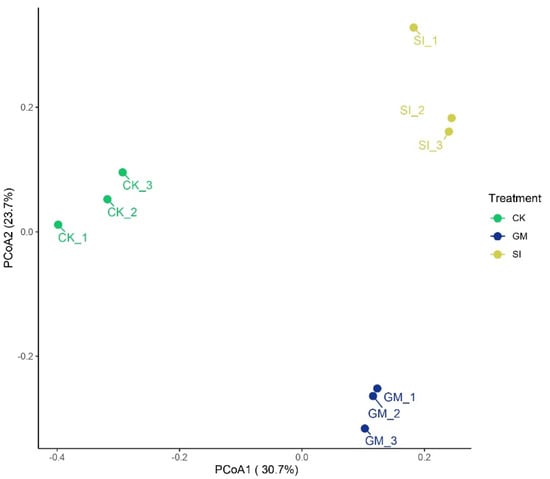
Figure 4.
PCoA analysis of inter-root soil bacterial communities under different crop treatments.
3.6. Correlation Between Soil Chemistry and Microbial Structure and Diversity
Pearson correlation analyses were conducted to explore the relationships between soil chemical properties (pH, SOC, TN, and AP) and the three most abundant bacterial phyla: Chloroflexi, Proteobacteria, and Actinobacteria (Figure 5). Chloroflexi showed generally negative correlations with all chemical indicators, with a significant negative correlation observed with SOC. In contrast, Proteobacteria was positively correlated with all soil factors, showing significant relationships with pH and SOC. Actinobacteria also exhibited positive correlations with SOC and TN, though its association with AP was not significant. These results suggest that pH, SOC, and TN are key drivers of microbial community structure under different cropping systems.
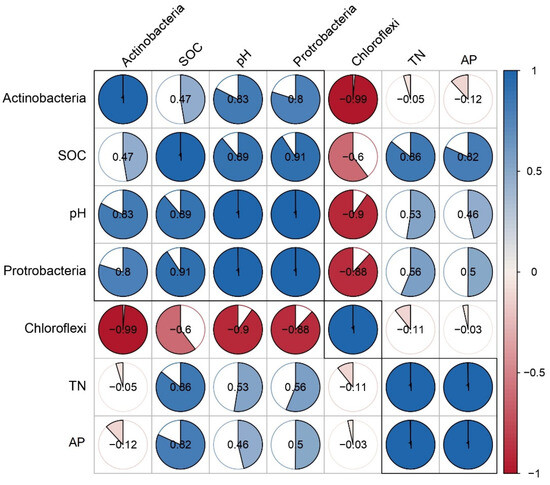
Figure 5.
Correlation between soil chemical properties and dominant bacterial phyla.
In addition, Pearson correlations were calculated between soil properties and bacterial diversity indices (Chao1, ACE, Shannon, and Simpson) (Figure 6). Both Chao1 and ACE were significantly negatively correlated with pH and SOC, and positively correlated with AP. The Shannon index was negatively correlated with pH and SOC, and positively with TN and AP. The Simpson index showed a significant negative correlation with TN only. These findings indicate that pH, SOC, and TN are critical factors influencing microbial diversity in inter-root soils.
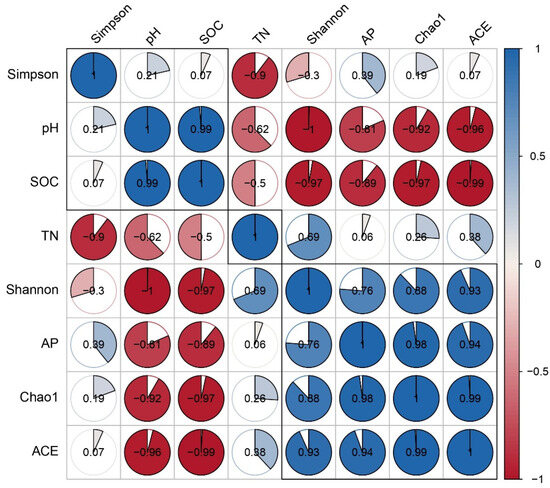
Figure 6.
Correlation between soil chemical properties and bacterial diversity indices.
4. Discussion
The primary objective of this study was to evaluate how different crop cultivation practices influence the chemical properties and microbial community structure of inter-root soil. The findings demonstrated a general decline in soil pH in cultivated plots compared to uncultivated control plots, likely due to crop uptake of alkaline ions and the release of organic acids through root exudates [61]. Notably, the most pronounced pH reduction occurred under the SI (cereal cropping) treatment, possibly linked to the composition of SI crop root secretions that may promote soil acidification.
Crop cultivation significantly enhanced soil fertility indicators, including total nitrogen (TN) and available phosphorus (AP). Despite this, the highest soil organic carbon (SOC) content was observed in the control (CK) treatment, likely due to natural organic matter accumulation [62,63]. The TN and AP contents were greatest in the GM and SI treatments, respectively. These results suggest that different crops exhibit distinct nutrient uptake and utilization patterns, influenced by their root systems and their regulatory effects on soil nutrient dynamics.
Venn diagram and OTU analyses revealed that crop cultivation significantly promoted microbial diversity in the soil. The GM treatment exhibited the highest number of OTUs, possibly due to the favorable influence of GM crop root structures and secretions on microbial habitats [64,65]. Moreover, the similarity in microbial community composition between the GM and CK treatments may indicate overlapping ecological niches.
The analysis of bacterial α-diversity showed that richness was highest under the SI treatment, suggesting that cereal crops may enhance the soil’s biological environment [66,67]. Variations in the Chao1 and ACE indices further underscored differences in microbial richness across crop types, highlighting the potential of optimized cropping patterns to support microbial biodiversity [30,68]. Despite these differences in richness, the Simpson index values (0.9961–0.9983) varied minimally, indicating overall stability in the dominant bacterial communities regardless of crop type.
At the phylum level, crop cultivation significantly altered microbial community composition. The relative abundance of Proteobacteria and Actinobacteria increased, whereas Chloroflexi decreased. These shifts may be driven by changes in soil structure and nutrient availability induced by crop cultivation [69,70]. Additionally, increased availability of carbon substrates (e.g., polysaccharides, readily decomposable carbohydrates) likely favored the proliferation of certain bacterial taxa such as Acidobacteria and Proteobacteria. It is important to note that the CK plots were located in the Mu Us Desert, where natural vegetation is sparse due to harsh climatic conditions, including low rainfall and high temperatures. These environmental constraints limit organic matter input (e.g., plant litter), which negatively affects soil microbial colonization and diversity.
Pearson correlation analysis highlighted strong associations between soil chemical properties and microbial community composition. Specifically, Chloroflexi showed a significant negative correlation with SOC, while Proteobacteria was positively correlated with both SOC and pH. These results underscore the central role of soil physicochemical conditions in shaping microbial diversity and composition [71,72]. Among them, pH, SOC, and TN emerged as key factors influencing the diversity of inter-root bacterial communities.
5. Conclusions
This study provides critical insights into how different crop cultivation systems impact inter-root soil chemical properties and microbial community structure in the Mu Us Desert, an ecologically important region in northern China. Cultivation generally reduced soil pH, potentially due to root-secreted organic acids, which may enhance nutrient solubility but also suppress certain pH-sensitive microbial groups. In terms of fertility, cultivation increased TN and AP levels, while the highest SOC content remained in the uncultivated soils, reflecting organic matter accumulation under natural conditions. Microbial diversity improved under cultivation, particularly under the Glycine max, which supported the highest OTU richness, likely due to favorable root architecture and exudation patterns. Additionally, Setaria italica significantly enhanced bacterial richness indices, suggesting the positive role of cereal crops in improving soil microbial habitats. Cultivation led to compositional shifts, with increases in Proteobacteria and Actinobacteria, and a decrease in Chloroflexi. These changes reflect the microbial community’s response to altered nutrient availability and physical soil conditions. Pearson correlation analysis further confirmed that soil pH, SOC, and TN are critical determinants of microbial community diversity. Overall, the findings demonstrate that crop type plays a significant role in shaping inter-root soil environments and microbial communities. These results provide novel insights into the ecological roles of bacterial communities in the Mu Us Desert and offer a scientific foundation for optimizing crop management strategies to enhance soil fertility, microbial diversity, and long-term ecosystem sustainability. Future research should explore the functional roles of microbial taxa in different cropping systems and their contributions to soil ecosystem services.
Author Contributions
L.H., L.S. and G.L. designed the study. L.H. and L.S. performed experiments and chemical analyses of the soil. L.H. and Y.W. performed the data analysis and graphics. L.H. and L.S. drafted the first version with significant input from G.L., G.L. and G.W. contributed to the sequent manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Natural Science Basic Research Plan in the Shaanxi Province of China (No. 2024JC-YBQN-0329) and the Key Program of the National Natural Science Foundation of China (No. 42130717).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
Author Lei Shi was employed by the company Shaanxi Provincial Land Engineering Construction Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Runnström, M.C. Rangeland Development of the Mu Us Sandy Land in Semiarid China: An Analysis Using Landsat and NOAA Remote Sensing Data. Land Degrad. Dev. 2003, 14, 189–202. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.-H.; Dong, Z.; Xia, D. Evolution of the Southern Mu US Desert in North China over the Past 50 Years: An Analysis Using Proxies of Human Activity and Climate Parameters. Land Degrad. Dev. 2005, 16, 351–366. [Google Scholar] [CrossRef]
- Wang, N.; Xie, J.; Han, J. A Sand Control and Development Model in Sandy Land Based on Mixed Experiments of Arsenic Sandstone and Sand: A Case Study in Mu Us Sandy Land in China. Chin. Geogr. Sci. 2013, 23, 700–707. [Google Scholar] [CrossRef]
- Han, X.; Jia, G.; Yang, G.; Wang, N.; Liu, F.; Chen, H.; Guo, X.; Yang, W.; Liu, J. Spatiotemporal Dynamic Evolution and Driving Factors of Desertification in the Mu Us Sandy Land in 30 Years. Sci. Rep. 2020, 10, 21734. [Google Scholar] [CrossRef]
- Zhang, D.; Deng, H. Historical Human Activities Accelerated Climate-Driven Desertification in China’s Mu Us Desert. Sci. Total Environ. 2020, 708, 134771. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yan, C.; Wang, T.; Du, H. Monitoring Grassland Reclamation in the Mu Us Desert Using Remote Sensing from 2010 to 2015. Envorin. Earth Sci. 2019, 78, 311. [Google Scholar] [CrossRef]
- Horn, R.; Domżżał, H.; Słowińska-Jurkiewicz, A.; van Ouwerkerk, C. Soil Compaction Processes and Their Effects on the Structure of Arable Soils and the Environment. Soil Tillage Res. 1995, 35, 23–36. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, B.-Y.; Liu, S.-L.; Qi, J.-Y.; Wang, X.; Pu, C.; Li, S.-S.; Zhang, X.-Z.; Yang, X.-G.; Lal, R.; et al. Sustaining Crop Production in China’s Cropland by Crop Residue Retention: A Meta-Analysis. Land Degrad. Dev. 2020, 31, 694–709. [Google Scholar] [CrossRef]
- Bethany, J.; Giraldo-Silva, A.; Nelson, C.; Barger, N.N.; Garcia-Pichel, F. Optimizing the Production of Nursery-Based Biological Soil Crusts for Restoration of Arid Land Soils. Appl. Environ. Microbiol. 2019, 85, e00735-19. [Google Scholar] [CrossRef]
- Naorem, A.; Jayaraman, S.; Dang, Y.P.; Dalal, R.C.; Sinha, N.K.; Rao, C.S.; Patra, A.K. Soil Constraints in an Arid Environment—Challenges, Prospects, and Implications. Agronomy 2023, 13, 220. [Google Scholar] [CrossRef]
- Dasgupta, D.; Brahmaprakash, G.P. Soil Microbes Are Shaped by Soil Physico-Chemical Properties: A Brief Review of Existing Literature. Int. J. Plant Soil Sci. 2021, 33, 59–71. [Google Scholar] [CrossRef]
- Soong, J.L.; Fuchslueger, L.; Marañon-Jimenez, S.; Torn, M.S.; Janssens, I.A.; Penuelas, J.; Richter, A. Microbial Carbon Limitation: The Need for Integrating Microorganisms into Our Understanding of Ecosystem Carbon Cycling. Glob. Change Biol. 2020, 26, 1953–1961. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.; Dikilitas, M.; Abdel-Azeem, A.M.; Ahluwalia, A.S.; Saxena, A.K. Biodiversity, and Biotechnological Contribution of Beneficial Soil Microbiomes for Nutrient Cycling, Plant Growth Improvement and Nutrient Uptake. Biocatal. Agric. Biotechnol. 2021, 33, 102009. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Ros, G.H.; Furtak, K.; Iqbal, H.M.N.; Parra-Saldívar, R. Soil Carbon Sequestration—An Interplay between Soil Microbial Community and Soil Organic Matter Dynamics. Sci. Total Environ. 2022, 815, 152928. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, K.L.; Zhao, X.Q.; Zhang, H.Q.; Li, D.; Li, J.J.; Shen, R.F. Balanced Fertilization over Four Decades Has Sustained Soil Microbial Communities and Improved Soil Fertility and Rice Productivity in Red Paddy Soil. Sci. Total Environ. 2021, 793, 148664. [Google Scholar] [CrossRef]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and Soil Microbial Community: A Review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Ek, L. Replenishment of Essential Natural Resources: An Assessment of the Ability of Freshwater Algae to Enhance Crop Quality and Soil Health. Ph.D. Thesis, University of South Carolina, Columbia, SC, USA, 2023. [Google Scholar]
- Adedayo, A.A.; Babalola, O.O. The Potential of Biostimulants on Soil Microbial Community: A Review. Front. Ind. Microbiol. 2023, 1, 1308641. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Sun, C. Differences in Carbon Sequestration Ability of Diverse Tartary Buckwheat Genotypes in Barren Soil Caused by Microbial Action. Int. J. Environ. Res. Public Health 2023, 20, 959. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of Arbuscular Mycorrhizal Fungi in Regulating Growth, Enhancing Productivity, and Potentially Influencing Ecosystems under Abiotic and Biotic Stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Q.; Ju, M.; Yan, S.; Zhang, Q.; Gu, P. The Endophytic Fungi Diversity, Community Structure, and Ecological Function Prediction of Sophora Alopecuroides in Ningxia, China. Microorganisms 2022, 10, 2099. [Google Scholar] [CrossRef]
- Tang, K.; Liang, Y.; Yuan, B.; Meng, J.; Feng, F. Spatial Distribution and Core Community of Diazotrophs in Biological Soil Crusts and Subsoils in Temperate Semi-Arid and Arid Deserts of China. Front. Microbiol. 2023, 14, 1074855. [Google Scholar] [CrossRef]
- Islam, W.; Zeng, F.; Alotaibi, M.O.; Khan, K.A. Unlocking the Potential of Soil Microbes for Sustainable Desertification Management. Earth-Sci. Rev. 2024, 252, 104738. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, W.; Shao, Y.; Li, Y.-J.; Lin, L.-A.; Zhang, Y.-J.; Han, H.; Chen, Z.-J. Miscanthus Cultivation Shapes Rhizosphere Microbial Community Structure and Function as Assessed by Illumina MiSeq Sequencing Combined with PICRUSt and FUNGUIld Analyses. Arch. Microbiol. 2020, 202, 1157–1171. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, K.; Shah, M.P.; Singh, A.K.; Kumar, A.; Kumar, Y. Chapter 1—Application of Omics Technologies for Microbial Community Structure and Function Analysis in Contaminated Environment. In Wastewater Treatment; Shah, M.P., Sarkar, A., Mandal, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–40. ISBN 978-0-12-821881-5. [Google Scholar]
- Jiang, J.; Hu, X.; Ji, X.; Chen, H. High Throughput Sequencing Technology Facility Research of Genomic Modification Crop Cultivation Influencing Soil Microbe. Front. Plant Sci. 2023, 14, 1208111. [Google Scholar] [CrossRef]
- Yang, P.; Luo, Y.; Gao, Y.; Gao, X.; Gao, J.; Wang, P.; Feng, B. Soil Properties, Bacterial and Fungal Community Compositions and the Key Factors after 5-Year Continuous Monocropping of Three Minor Crops. PLoS ONE 2020, 15, e0237164. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Lupwayi, N.; Marc, S.-A.; Siddique, K.H.M.; Bainard, L.D. Anthropogenic Drivers of Soil Microbial Communities and Impacts on Soil Biological Functions in Agroecosystems. Glob. Ecol. Conserv. 2021, 27, e01521. [Google Scholar] [CrossRef]
- Khmelevtsova, L.E.; Sazykin, I.S.; Azhogina, T.N.; Sazykina, M.A. Influence of Agricultural Practices on Bacterial Community of Cultivated Soils. Agriculture 2022, 12, 371. [Google Scholar] [CrossRef]
- Chamberlain, L.A.; Bolton, M.L.; Cox, M.S.; Suen, G.; Conley, S.P.; Ané, J.-M. Crop Rotation, but Not Cover Crops, Influenced Soil Bacterial Community Composition in a Corn-Soybean System in Southern Wisconsin. Appl. Soil Ecol. 2020, 154, 103603. [Google Scholar] [CrossRef]
- Town, J.R.; Gregorich, E.G.; Drury, C.F.; Lemke, R.; Phillips, L.A.; Helgason, B.L. Diverse Crop Rotations Influence the Bacterial and Fungal Communities in Root, Rhizosphere and Soil and Impact Soil Microbial Processes. Appl. Soil Ecol. 2022, 169, 104241. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, Y.; Xu, H.; Xie, Z.; Guo, J.; Qi, Z.; Jiang, H. Soil Metabolomics and Bacterial Functional Traits Revealed the Responses of Rhizosphere Soil Bacterial Community to Long-Term Continuous Cropping of Tibetan Barley. PeerJ 2022, 10, e13254. [Google Scholar] [CrossRef]
- Gong, X.; Feng, Y.; Dang, K.; Jiang, Y.; Qi, H.; Feng, B. Linkages of Microbial Community Structure and Root Exudates: Evidence from Microbial Nitrogen Limitation in Soils of Crop Families. Sci. Total Environ. 2023, 881, 163536. [Google Scholar] [CrossRef]
- Ji, L.; Si, H.; He, J.; Fan, L.; Li, L. The Shifts of Maize Soil Microbial Community and Networks Are Related to Soil Properties under Different Organic Fertilizers. Rhizosphere 2021, 19, 100388. [Google Scholar] [CrossRef]
- Xu, C.; Li, Y.; Hu, X.; Zang, Q.; Zhuang, H.; Huang, L. The Influence of Organic and Conventional Cultivation Patterns on Physicochemical Property, Enzyme Activity and Microbial Community Characteristics of Paddy Soil. Agriculture 2022, 12, 121. [Google Scholar] [CrossRef]
- Wongkiew, S.; Chaikaew, P.; Takrattanasaran, N.; Khamkajorn, T. Evaluation of Nutrient Characteristics and Bacterial Community in Agricultural Soil Groups for Sustainable Land Management. Sci. Rep. 2022, 12, 7368. [Google Scholar] [CrossRef] [PubMed]
- Zhouchang, Y.; Wei, Z.; Liyun, Z.; Shihai, M.; Lin, S.; Yushu, L.; Ying, Z.; AHejiang, S.; Jiayi, S.; Ling, C.; et al. Efficient Vegetation Restoration in Mu Us Desert Reduces Microbial Diversity Due to the Transformation of Nutrient Requirements. Ecol. Indic. 2023, 154, 110758. [Google Scholar] [CrossRef]
- Wang, L.; Li, X. Soil Microbial Community and Their Relationship with Soil Properties across Various Landscapes in the Mu Us Desert. Forests 2023, 14, 2152. [Google Scholar] [CrossRef]
- Song, D.; Hu, Z.; Zeng, J.; Sun, H. Influence of Mining on Vegetation in Semi-Arid Areas of Western China Based on the Coupling of above Ground and below Ground—A Case Study of Daliuta Coalfield. Ecol. Indic. 2024, 161, 111964. [Google Scholar] [CrossRef]
- Xiao, N.; Wang, Y.; Guo, Z.; Shao, T.; Dong, Z.; Xing, B. Tire Plastic and Road-Wear Particles on Yujing Expressway in the Restoration Area of Mu Us Sandy Land: Occurrence Characteristics and Ecological Risk Screening. J. Hazard. Mater. 2024, 468, 133860. [Google Scholar] [CrossRef]
- Rossini, F.; Virga, G.; Loreti, P.; Iacuzzi, N.; Ruggeri, R.; Provenzano, M.E. Hops (Humulus lupulus L.) as a Novel Multipurpose Crop for the Mediterranean Region of Europe: Challenges and Opportunities of Their Cultivation. Agriculture 2021, 11, 484. [Google Scholar] [CrossRef]
- Gebretsadikan, T.; Munro, P.; Forge, T.A.; Jones, M.D.; Nelson, L.M. Mulching Improved Soil Fertility, Plant Growth and Productivity, and Postharvest Deficit Irrigation Reduced Water Use in Sweet Cherry Orchards in a Semi-Arid Region. Arch. Agron. Soil Sci. 2023, 69, 1419–1436. [Google Scholar] [CrossRef]
- Jing, X.; Chen, X.; Fang, J.; Ji, C.; Shen, H.; Zheng, C.; Zhu, B. Soil Microbial Carbon and Nutrient Constraints Are Driven More by Climate and Soil Physicochemical Properties than by Nutrient Addition in Forest Ecosystems. Soil Biol. Biochem. 2020, 141, 107657. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Feng, Y. The Effects of Biochar Addition on Soil Physicochemical Properties: A Review. Catena 2021, 202, 105284. [Google Scholar] [CrossRef]
- Matteoli, F.P.; Silva, A.M.M.; Feiler, H.P.; de Araújo, V.L.V.P.; Cardoso, E.J.B.N. Predicting soil farming system and attributes based on soil bacterial community. Appl. Soil Ecol. 2022, 171, 104335. [Google Scholar] [CrossRef]
- Ding, Z.; Lu, R.; Wang, L.; Yu, L.; Liu, X.; Liu, Y.; Liu, L.; Bai, M.; Wang, S. Early-Mid Holocene Climatic Changes Inferred from Colors of Eolian Deposits in the Mu Us Desert. Geoderma 2021, 401, 115172. [Google Scholar] [CrossRef]
- Guo, Z.; Li, J.; Zhang, Y.; Wang, H.; Li, W. The Soft Rock Can Promote the Improvement of Aeolian Sandy Soil in Mu Us Sandy Land, China. Sci. Rep. 2023, 13, 11813. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, T.; Shi, L.; Wang, W.; Niu, Z.; Guo, W.; Ma, X. Combining Spectral and Texture Features of UAV Hyperspectral Images for Leaf Nitrogen Content Monitoring in Winter Wheat. Int. J. Remote Sens. 2022, 43, 2335–2356. [Google Scholar] [CrossRef]
- Xu, Y.; Pu, L.; Zhang, R.; Zhu, M.; Zhang, M.; Bu, X.; Xie, X.; Wang, Y. Effects of Agricultural Reclamation on Soil Physicochemical Properties in the Mid-Eastern Coastal Area of China. Land 2021, 10, 142. [Google Scholar] [CrossRef]
- van der Heyde, M.; Bunce, M.; Dixon, K.; Wardell-Johnson, G.; White, N.E.; Nevill, P. Changes in Soil Microbial Communities in Post Mine Ecological Restoration: Implications for Monitoring Using High Throughput DNA Sequencing. Sci. Total Environ. 2020, 749, 142262. [Google Scholar] [CrossRef]
- Yin, D.; Li, H.; Wang, H.; Guo, X.; Wang, Z.; Lv, Y.; Ding, G.; Jin, L.; Lan, Y. Impact of Different Biochars on Microbial Community Structure in the Rhizospheric Soil of Rice Grown in Albic Soil. Molecules 2021, 26, 4783. [Google Scholar] [CrossRef]
- Iturbe-Espinoza, P.; Brandt, B.W.; Braster, M.; Bonte, M.; Brown, D.M.; van Spanning, R.J.M. Effects of DNA Preservation Solution and DNA Extraction Methods on Microbial Community Profiling of Soil. Folia Microbiol. 2021, 66, 597–606. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Salehi, F.; Inanloodoghouz, M.; Ghazvineh, S. Influence of Microwave Pretreatment on the Total Phenolics, Antioxidant Activity, Moisture Diffusivity, and Rehydration Rate of Dried Sweet Cherry. Food Sci. Nutr. 2023, 11, 7870–7876. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhou, Y. Effects of Land Use Patterns on the Bacterial Community Structure and Diversity of Wetland Soils in the Sanjiang Plain. J. Soil Sci. Plant Nutr. 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Wang, Z.; Bai, Y.; Hou, J.; Li, F.; Li, X.; Cao, R.; Deng, Y.; Wang, H.; Jiang, Y.; Yang, W. The Changes in Soil Microbial Communities across a Subalpine Forest Successional Series. Forests 2022, 13, 289. [Google Scholar] [CrossRef]
- Jiang, R.; Wang, M.; Chen, W.; Li, X.; Balseiro-Romero, M. Changes in the Integrated Functional Stability of Microbial Community under Chemical Stresses and the Impacting Factors in Field Soils. Ecol. Indic. 2020, 110, 105919. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, L.; Zhang, Y.-G.; Tu, T.-Y.; Wang, S.-T.; Shen, C.-H.; Yuan, H.-W.; Zhong, X.-Z. A Comparison of Microbial Communities and Volatile Compounds in Wheat Qu from Different Geographic Locations. LWT 2021, 148, 111752. [Google Scholar] [CrossRef]
- Siddiqui, A.U.; Jain, M.K.; Masto, R.E. Pollution Evaluation, Spatial Distribution, and Source Apportionment of Trace Metals around Coal Mines Soil: The Case Study of Eastern India. Environ. Sci. Pollut. Res. 2020, 27, 10822–10834. [Google Scholar] [CrossRef]
- Xu, H.; Croot, P.; Zhang, C. Exploration of the Spatially Varying Relationships between Lead and Aluminium Concentrations in the Topsoil of Northern Half of Ireland Using Geographically Weighted Pearson Correlation Coefficient. Geoderma 2022, 409, 115640. [Google Scholar] [CrossRef]
- Thepbandit, W.; Athinuwat, D. Rhizosphere Microorganisms Supply Availability of Soil Nutrients and Induce Plant Defense. Microorganisms 2024, 12, 558. [Google Scholar] [CrossRef]
- Liang, Y.; Al-Kaisi, M.; Yuan, J.; Liu, J.; Zhang, H.; Wang, L.; Cai, H.; Ren, J. Effect of Chemical Fertilizer and Straw-Derived Organic Amendments on Continuous Maize Yield, Soil Carbon Sequestration and Soil Quality in a Chinese Mollisol. Agric. Ecosyst. Environ. 2021, 314, 107403. [Google Scholar] [CrossRef]
- Tang, H.; Cheng, K.; Shi, L.; Li, C.; Wen, L.; Li, W.; Sun, M.; Sun, G.; Long, Z. Effects of Long-Term Organic Matter Application on Soil Carbon Accumulation and Nitrogen Use Efficiency in a Double-Cropping Rice Field. Environ. Res. 2022, 213, 113700. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Pan, Y.; Wang, P.; Ran, L.; Qin, G.; Li, Q.; Kang, P. Response of Phyllosphere and Rhizosphere Microbial Communities to Salt Stress of Tamarix Chinensis. Plants 2024, 13, 1091. [Google Scholar] [CrossRef]
- Kuang, W.; Chen, W.; Lei, C.; Dai, Y.; Tian, X.; Tang, S.; Qian, Q.; Zhang, C.; Fu, L.; Zhou, G.; et al. Diversity of Endophytic Bacterial Community in Rice Roots and Their Roles in Phosphate Solubilization and Plant Growth. Rhizosphere 2024, 30, 100877. [Google Scholar] [CrossRef]
- Dellagi, A.; Quillere, I.; Hirel, B. Beneficial Soil-Borne Bacteria and Fungi: A Promising Way to Improve Plant Nitrogen Acquisition. J. Exp. Bot. 2020, 71, 4469–4479. [Google Scholar] [CrossRef]
- Scavo, A.; Fontanazza, S.; Restuccia, A.; Pesce, G.R.; Abbate, C.; Mauromicale, G. The Role of Cover Crops in Improving Soil Fertility and Plant Nutritional Status in Temperate Climates. A Review. Agron. Sustain. Dev. 2022, 42, 1–25. [Google Scholar] [CrossRef]
- Zimmermann, B.; Claß-Mahler, I.; von Cossel, M.; Lewandowski, I.; Weik, J.; Spiller, A.; Nitzko, S.; Lippert, C.; Krimly, T.; Pergner, I.; et al. Mineral-Ecological Cropping Systems—A New Approach to Improve Ecosystem Services by Farming without Chemical Synthetic Plant Protection. Agronomy 2021, 11, 1710. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, G.; Ding, H.; Ci, D.; Dai, L.; Zhang, Z. Influence of Salt Stress on the Rhizosphere Soil Bacterial Community Structure and Growth Performance of Groundnut (Arachis hypogaea L.). Int. Microbiol. 2020, 23, 453–465. [Google Scholar] [CrossRef]
- Li, Y.; Chi, J.; Ao, J.; Gao, X.; Liu, X.; Sun, Y.; Zhu, W. Effects of Different Continuous Cropping Years on Bacterial Community and Diversity of Cucumber Rhizosphere Soil in Solar-Greenhouse. Curr. Microbiol. 2021, 78, 2380–2390. [Google Scholar] [CrossRef]
- Guo, J.; Wu, Y.; Wu, X.; Ren, Z.; Wang, G. Soil Bacterial Community Composition and Diversity Response to Land Conversion Is Depth-Dependent. Glob. Ecol. Conserv. 2021, 32, e01923. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, R.; Wang, X.; Xu, X.; Ai, C.; He, P.; Liang, G.; Zhou, W.; Zhu, P. Effect of High Soil C/N Ratio and Nitrogen Limitation Caused by the Long-Term Combined Organic-Inorganic Fertilization on the Soil Microbial Community Structure and Its Dominated SOC Decomposition. J. Environ. Manag. 2022, 303, 114155. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).