Screening of Positive Regulatory Stimuli for Stomatal Opening in Chinese Cabbage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
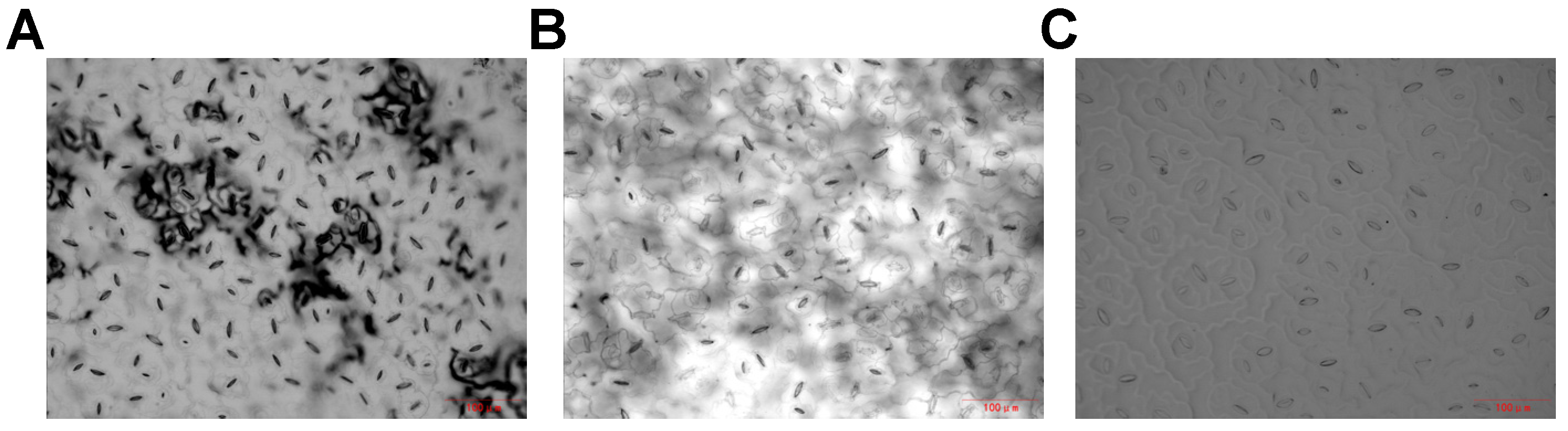
2.2. Development of an Optimal Method for Observing Stomatal Aperture in Chinese Cabbage
2.3. Regulation of the Stomatal Aperture by Environmental Stimuli
2.4. Data Statistics and Plotting
3. Results
3.1. Optimal Stomatal Aperture Observation Method in Chinese Cabbage
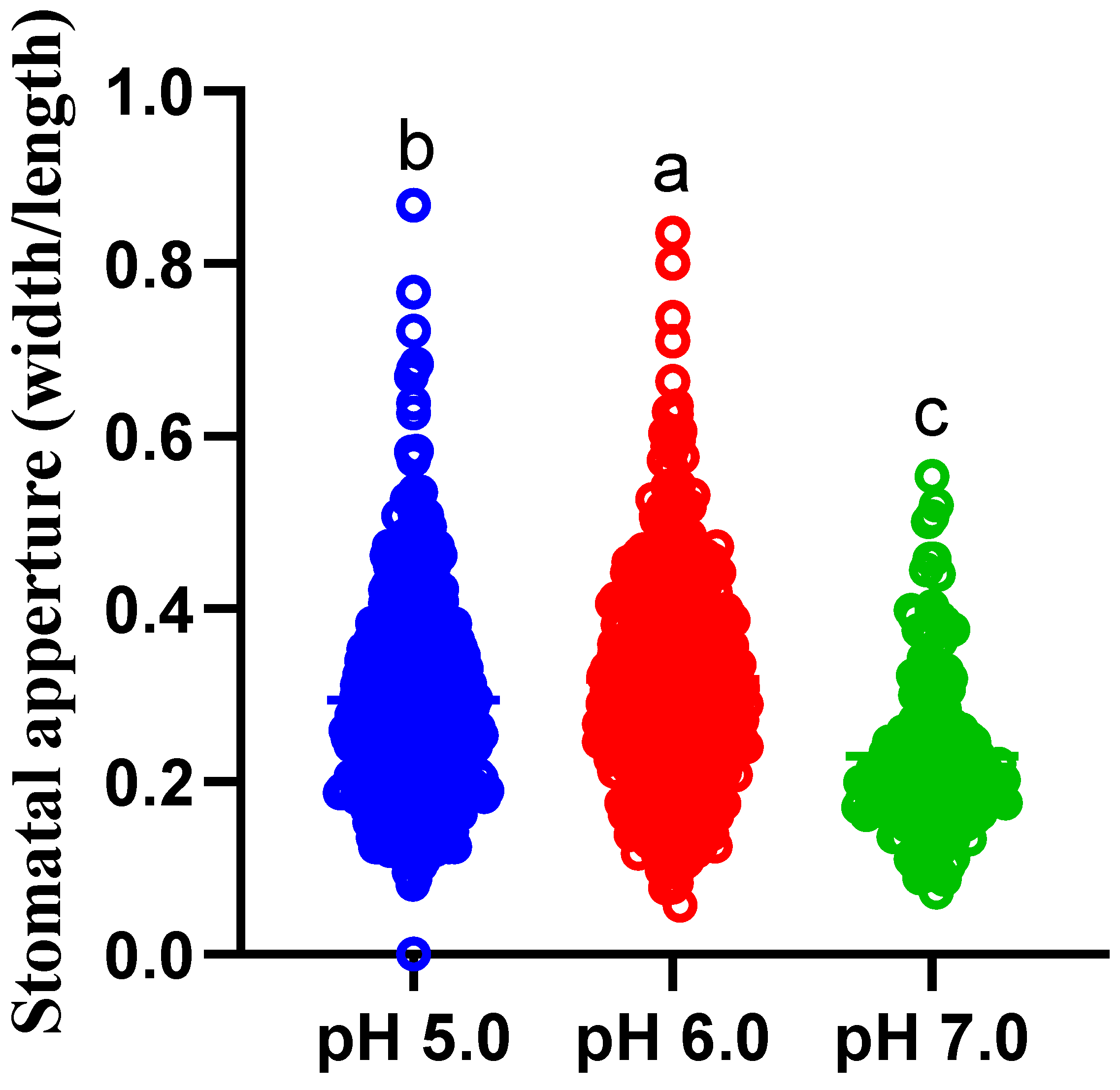
3.2. Dependence of the Stomatal Aperture on Medium pH
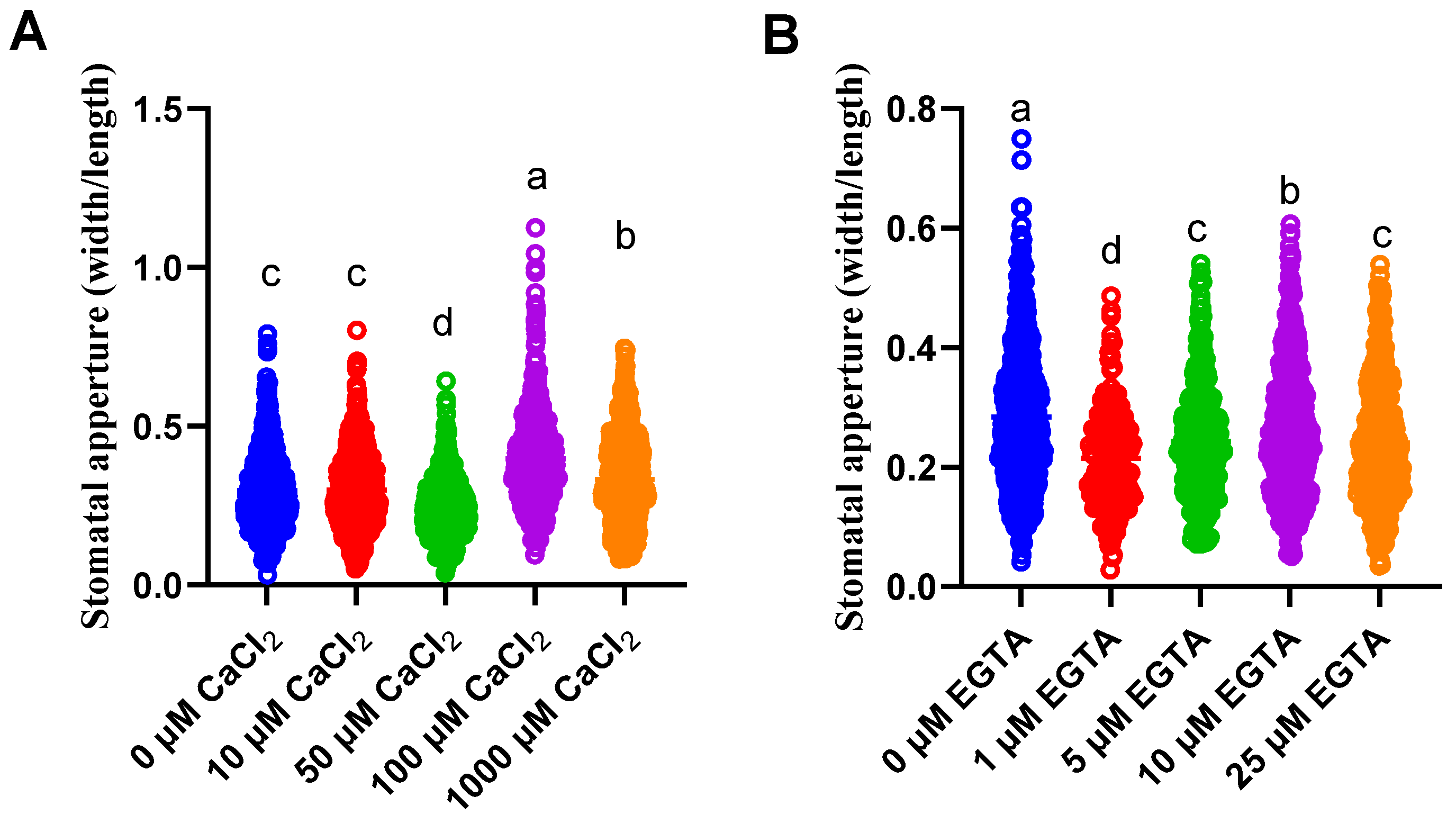
3.3. Regulation of the Stomatal Aperture by Ca2+ and EGTA
3.4. Regulation of the Stomatal Aperture by H2O2, ABA, and MT
3.5. Regulation of the Stomatal Aperture by SA, MJ, and BR
3.6. Regulation of the Stomatal Aperture by GA, IAA, and CTK
4. Discussion
4.1. Establishment of an Optimal Method for Observing Stomatal Aperture in Chinese Cabbage
4.2. Identification of Multiple Environmental Stimuli Positively Regulating Stomatal Opening
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zelitch, I. Improving the Efficiency of Photosynthesis. Science 1975, 188, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.M.; Sun, J.Z.; Liu, H.Q.; Qu, C.M.; Wang, K.J.; Guo, R.J.; Bai, K.Z.; Gao, L.M.; Kuang, T.Y. Changes in the rate of photosynthesis accompanying the yield increase in wheat cultivars released in the past 50 years. J. Plant Res. 2003, 116, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Maschler, J.; Bialic-Murphy, L.; Wan, J.; Andresen, L.C.; Zohner, C.M.; Reich, P.B.; Luscher, A.; Schneider, M.K.; Muller, C.; Moser, G.; et al. Links across ecological scales: Plant biomass responses to elevated CO2. Glob. Change Biol. 2022, 28, 6115–6134. [Google Scholar] [CrossRef] [PubMed]
- Terrer, C.; Jackson, R.B.; Prentice, I.C.; Keenan, T.F.; Kaiser, C.; Vicca, S.; Fisher, J.B.; Reich, P.B.; Stocker, B.D.; Hungate, B.A.; et al. Nitrogen and phosphorus constrain the CO2 fertilization of global plant biomass. Nat. Clim. Change 2019, 9, 684–689. [Google Scholar] [CrossRef]
- Usui, Y.; Sakai, H.; Tokida, T.; Nakamura, H.; Nakagawa, H.; Hasegawa, T. Rice grain yield and quality responses to free-air CO2 enrichment combined with soil and water warming. Glob. Change Biol. 2016, 22, 1256–1270. [Google Scholar] [CrossRef]
- Dier, M.; Meinen, R.; Erbs, M.; Kollhorst, L.; Baillie, C.K.; Kaufholdt, D.; Kucke, M.; Weigel, H.J.; Zorb, C.; Hansch, R.; et al. Effects of free air carbon dioxide enrichment (FACE) on nitrogen assimilation and growth of winter wheat under nitrate and ammonium fertilization. Glob. Change Biol. 2018, 24, e40–e54. [Google Scholar] [CrossRef]
- Dong, J.; Gruda, N.; Li, X.; Tang, Y.; Zhang, P.; Duan, Z. Sustainable vegetable production under changing climate: The impact of elevated CO2 on yield of vegetables and the interactions with environments—A review. J. Clean. Prod. 2020, 253, 119920. [Google Scholar] [CrossRef]
- Kollist, H.; Zandalinas, S.I.; Sengupta, S.; Nuhkat, M.; Kangasjarvi, J.; Mittler, R. Rapid Responses to Abiotic Stress: Priming the Landscape for the Signal Transduction Network. Trends Plant Sci. 2019, 24, 25–37. [Google Scholar] [CrossRef]
- Jiang, Y.; Ye, J.; Niinemets, U. Dose-dependent methyl jasmonate effects on photosynthetic traits and volatile emissions: Biphasic kinetics and stomatal regulation. Plant Signal. Behav. 2021, 16, 1917169. [Google Scholar] [CrossRef]
- Zhu, M.; Geng, S.; Chakravorty, D.; Guan, Q.; Chen, S.; Assmann, S.M. Metabolomics of red-light-induced stomatal opening in Arabidopsis thaliana: Coupling with abscisic acid and jasmonic acid metabolism. Plant J. Cell Mol. Biol. 2020, 101, 1331–1348. [Google Scholar] [CrossRef]
- Eisenach, C.; Chen, Z.H.; Grefen, C.; Blatt, M.R. The trafficking protein SYP121 of Arabidopsis connects programmed stomatal closure and K+ channel activity with vegetative growth. Plant J. Cell Mol. Biol. 2012, 69, 241–251. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Wu, W.H.; Wang, Y. The K+ channel KZM2 is involved in stomatal movement by modulating inward K+ currents in maize guard cells. Plant J. Cell Mol. Biol. 2017, 92, 662–675. [Google Scholar] [CrossRef]
- Wei, W.; Liang, D.W.; Bian, X.H.; Shen, M.; Xiao, J.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Lv, J.; Chen, X.; et al. GmWRKY54 improves drought tolerance through activating genes in abscisic acid and Ca2+ signaling pathways in transgenic soybean. Plant J. Cell Mol. Biol. 2019, 100, 384–398. [Google Scholar] [CrossRef]
- Pathoumthong, P.; Zhang, Z.; Roy, S.J.; El Habti, A. Rapid non-destructive method to phenotype stomatal traits. Plant Methods 2023, 19, 36. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, H.; Wang, J.; Wang, X.; Jia, X.; Wang, L.; Xu, Z.; Li, R.; Jiang, K.; Chen, Z.; et al. AIM1-dependent high basal salicylic acid accumulation modulates stomatal aperture in rice. New Phytol. 2023, 238, 1420–1430. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Zhang, L.; Kong, X.; Zhang, J.; Wang, X.; Pei, Y.; Jin, Z. H2S-mediated balance regulation of stomatal and non-stomatal factors responding to drought stress in Chinese cabbage. Hortic. Res. 2023, 10, uhac284. [Google Scholar] [CrossRef]
- Hao, D.L.; Zhou, J.Y.; Qu, J.; Lu, H.L.; Li, L.; Yao, X.; Chen, J.B.; Liu, J.X.; Guo, H.L.; Zong, J.Q. Screening of environmental stimuli for the positive regulation of stomatal aperture in centipedegrass. Plant Physiol. Biochem. PPB 2024, 213, 108838. [Google Scholar] [CrossRef]
- Po, R.P.; Tari, I. Regulation of stomatal movement and photosynthetic activity in guard cells of tomato abaxial epidermal peels by salicylic acid. Funct. Plant Biol. FPB 2012, 39, 1028–1037. [Google Scholar] [CrossRef]
- An, Y.; Feng, X.; Liu, L.; Xiong, L.; Wang, L. ALA-Induced Flavonols Accumulation in Guard Cells Is Involved in Scavenging H2O2 and Inhibiting Stomatal Closure in Arabidopsis Cotyledons. Front. Plant Sci. 2016, 7, 1713. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, W.; Xian, Z.; Hu, N.; Lin, D.; Ren, H.; Chen, J.; Su, D.; Li, Z. Overexpression of SlGRAS40 in Tomato Enhances Tolerance to Abiotic Stresses and Influences Auxin and Gibberellin Signaling. Front. Plant Sci. 2017, 8, 1659. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L. ALA Upregulates MdPTPA Expression to Increase the PP2A Activity and Promote Stomatal Opening in Apple Leaves. Plant Sci. Int. J. Exp. Plant Biol. 2022, 325, 111490. [Google Scholar] [CrossRef]
- Hao, D.-L.; Zhou, J.-Y.; Li, L.; Qu, J.; Li, X.-H.; Chen, R.-R.; Kong, W.-Y.; Li, D.-D.; Li, J.-J.; Guo, H.-L.; et al. An appropriate ammonium: Nitrate ratio promotes the growth of centipedegrass: Insight from physiological and micromorphological analyses. Front. Plant Sci. 2023, 14, 1324820. [Google Scholar] [CrossRef]
- Ibata, H.; Nagatani, A.; Mochizuki, N. Perforated-tape Epidermal Detachment (PED): A simple and rapid method for isolating epidermal peels from specific areas of Arabidopsis leaves. Plant Biotechnol. 2013, 30, 497–502. [Google Scholar] [CrossRef]
- Hassidim, M.; Dakhiya, Y.; Turjeman, A.; Hussien, D.; Shor, E.; Anidjar, A.; Goldberg, K.; Green, R.M. CIRCADIAN CLOCK ASSOCIATED1 (CCA1) and the Circadian Control of Stomatal Aperture. Plant Physiol. 2017, 175, 1864–1877. [Google Scholar] [CrossRef]
- De Silva, D.L.R.; Honour, S.J.; Mansfield, T.A. Estimations of apoplastic concentrations of K+ and Ca2+ in the vicinity of stomatal guard cells. New Phytol. 2006, 134, 463–469. [Google Scholar] [CrossRef]
- Hiyama, A.; Takemiya, A.; Munemasa, S.; Okuma, E.; Sugiyama, N.; Tada, Y.; Murata, Y.; Shimazaki, K.I. Blue light and CO2 signals converge to regulate light-induced stomatal opening. Nat. Commun. 2017, 8, 1284. [Google Scholar] [CrossRef]
- Zhang, L.; Li, D.; Yao, Y.; Zhang, S. H2O2, Ca2+, and K+ in subsidiary cells of maize leaves are involved in regulatory signaling of stomatal movement. Plant Physiol. Biochem. PPB 2020, 152, 243–251. [Google Scholar] [CrossRef]
- Haubrick, L.L.; Torsethaugen, G.; Assmann, S.M. Effect of brassinolide, alone and in concert with abscisic acid, on control of stomatal aperture and potassium currents of Vicia faba guard cell protoplasts. Physiol. Plant. 2006, 128, 134–143. [Google Scholar] [CrossRef]
- Xia, X.J.; Gao, C.J.; Song, L.X.; Zhou, Y.H.; Shi, K.; Yu, J.Q. Role of H2O2 dynamics in brassinosteroid-induced stomatal closure and opening in Solanum lycopersicum. Plant Cell Environ. 2014, 37, 2036–2050. [Google Scholar] [CrossRef]
- Li, J.G.; Fan, M.; Hua, W.; Tian, Y.; Chen, L.G.; Sun, Y.; Bai, M.Y. Brassinosteroid and Hydrogen Peroxide Interdependently Induce Stomatal Opening by Promoting Guard Cell Starch Degradation. Plant Cell 2020, 32, 984–999. [Google Scholar] [CrossRef]
- Ha, S.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S. Cytokinins: Metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci. 2012, 17, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, Z.; Huang, B. Effects of Cytokinin and Potassium on Stomatal and Photosynthetic Recovery of Kentucky Bluegrass from Drought Stress. Crop Sci. 2013, 53, 221–231. [Google Scholar] [CrossRef]
- Kostaki, K.I.; Coupel-Ledru, A.; Bonnell, V.C.; Gustavsson, M.; Sun, P.; McLaughlin, F.J.; Fraser, D.P.; McLachlan, D.H.; Hetherington, A.M.; Dodd, A.N.; et al. Guard Cells Integrate Light and Temperature Signals to Control Stomatal Aperture. Plant Physiol. 2020, 182, 1404–1419. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A. Role of Ca2+ and EGTA on Stomatal Movements in Commelina communis L. Plant Physiol. 1985, 79, 1003–1005. [Google Scholar] [CrossRef]
- Weinl, S.; Held, K.; Schlucking, K.; Steinhorst, L.; Kuhlgert, S.; Hippler, M.; Kudla, J. A plastid protein crucial for Ca2+-regulated stomatal responses. New Phytol. 2008, 179, 675–686. [Google Scholar] [CrossRef]
- Vetrano, F.; Moncada, A.; Miceli, A. Use of Gibberellic Acid to Increase the Salt Tolerance of Leaf Lettuce and Rocket Grown in a Floating System. Agronomy 2020, 10, 505. [Google Scholar] [CrossRef]
- Acharya, B.R.; Assmann, S.M. Hormone interactions in stomatal function. Plant Mol. Biol. 2009, 69, 451–462. [Google Scholar] [CrossRef]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Meng, Y.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biol. 2021, 21, 331. [Google Scholar] [CrossRef]
- Lajeunesse, G.; Roussin-Leveillee, C.; Boutin, S.; Fortin, E.; Laforest-Lapointe, I.; Moffett, P. Light prevents pathogen-induced aqueous microenvironments via potentiation of salicylic acid signaling. Nat. Commun. 2023, 14, 713. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.-Y.; Hao, D.-L.; Gu, Z.-C. Screening of Positive Regulatory Stimuli for Stomatal Opening in Chinese Cabbage. Agronomy 2025, 15, 914. https://doi.org/10.3390/agronomy15040914
Zhou J-Y, Hao D-L, Gu Z-C. Screening of Positive Regulatory Stimuli for Stomatal Opening in Chinese Cabbage. Agronomy. 2025; 15(4):914. https://doi.org/10.3390/agronomy15040914
Chicago/Turabian StyleZhou, Jin-Yan, Dong-Li Hao, and Ze-Chen Gu. 2025. "Screening of Positive Regulatory Stimuli for Stomatal Opening in Chinese Cabbage" Agronomy 15, no. 4: 914. https://doi.org/10.3390/agronomy15040914
APA StyleZhou, J.-Y., Hao, D.-L., & Gu, Z.-C. (2025). Screening of Positive Regulatory Stimuli for Stomatal Opening in Chinese Cabbage. Agronomy, 15(4), 914. https://doi.org/10.3390/agronomy15040914




