Different Intercropped Soybean Planting Patterns Regulate Leaf Growth and Seed Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
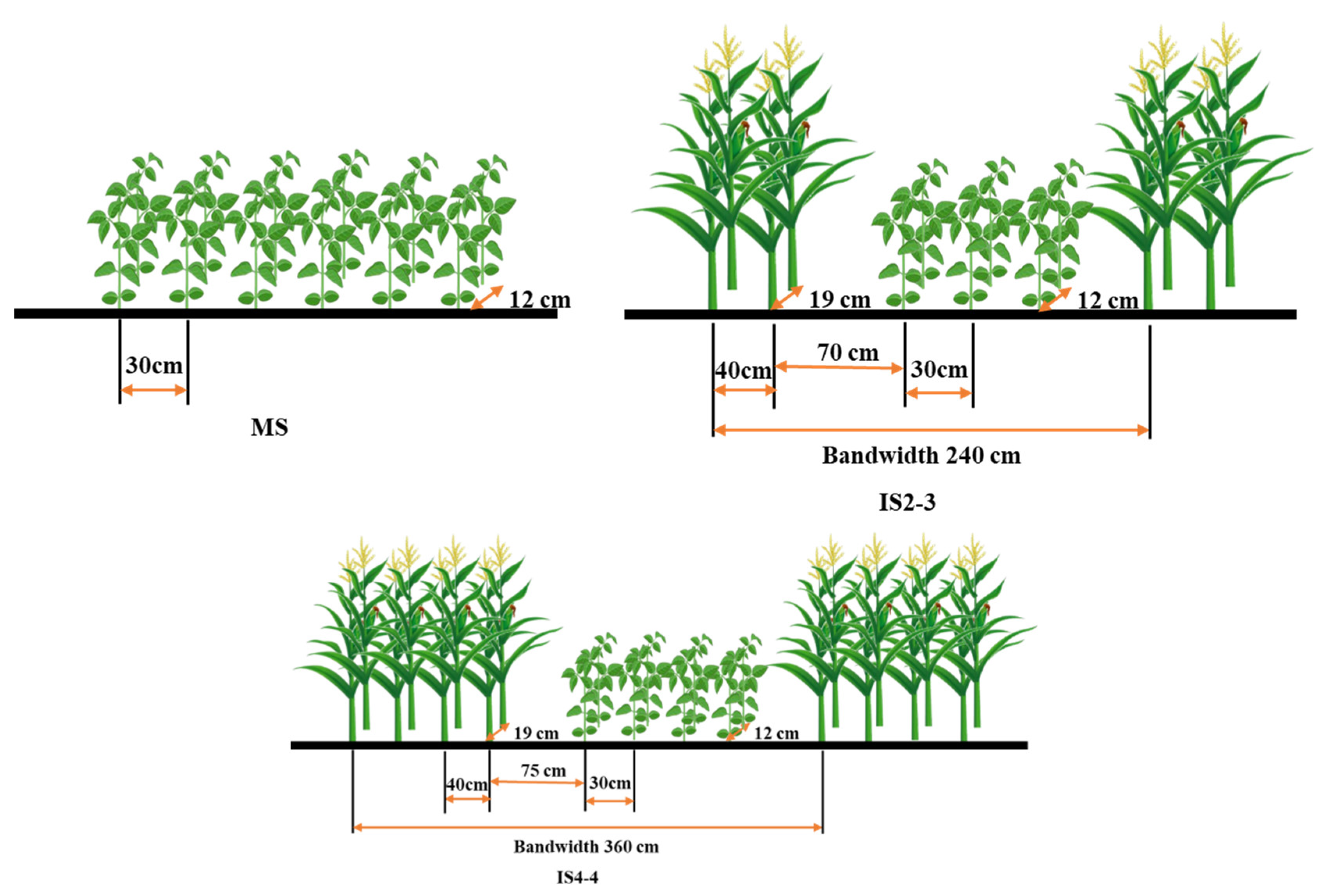
2.2. Experimental Treatments and Design
2.3. Grain Yield and Composition
2.4. Leaf Parameter Measurement
2.5. Soybean Seed Measurement
2.6. Metabolite Extraction, UHPLC-MS Analysis, and Data Preprocessing and Annotation
2.7. DNA Extraction, Sequencing, and Analysis of the Endophytic Bacterial Community in Soybean Leaves
2.8. Statistical Analyses
3. Results
3.1. Soybean Yields Under Different Planting Patterns
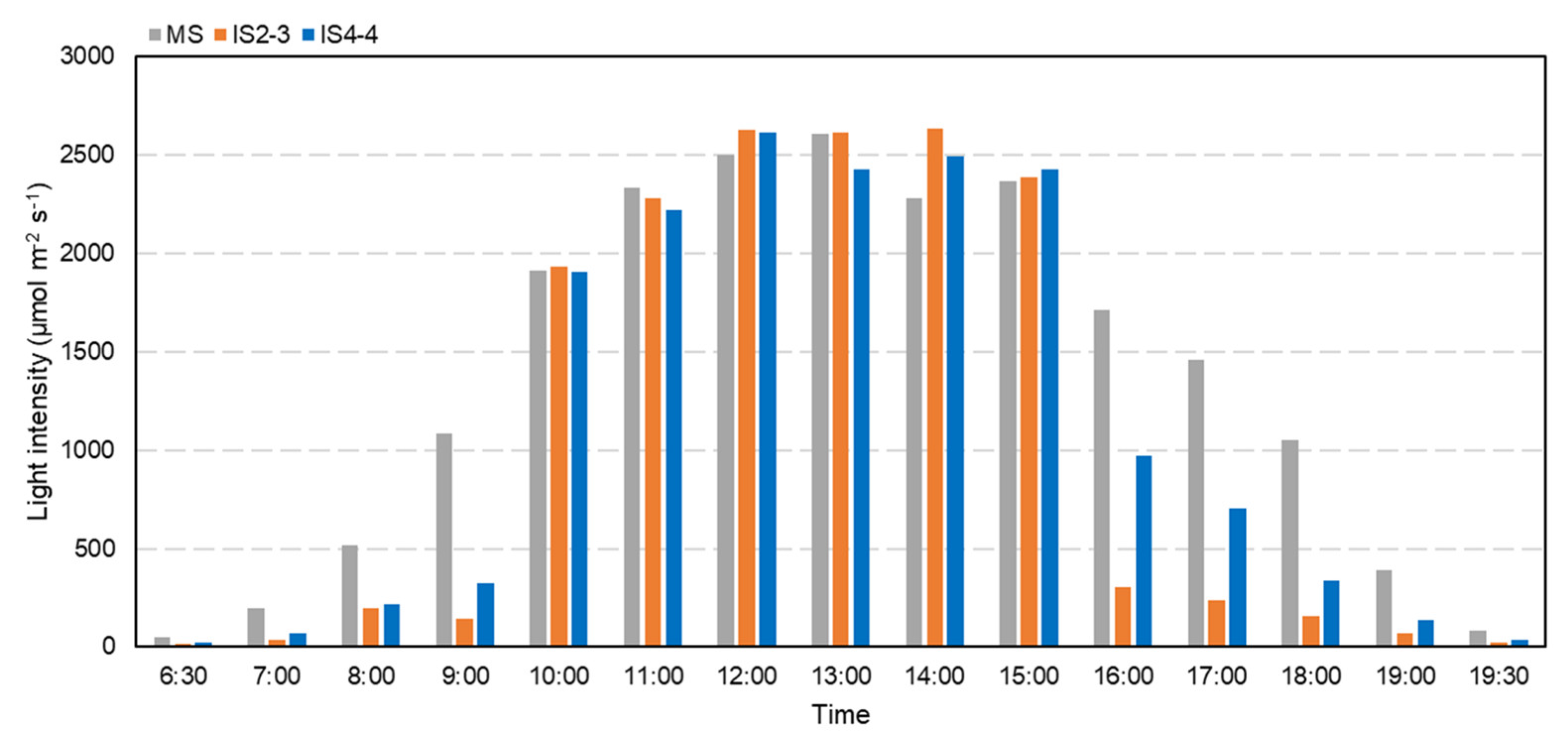
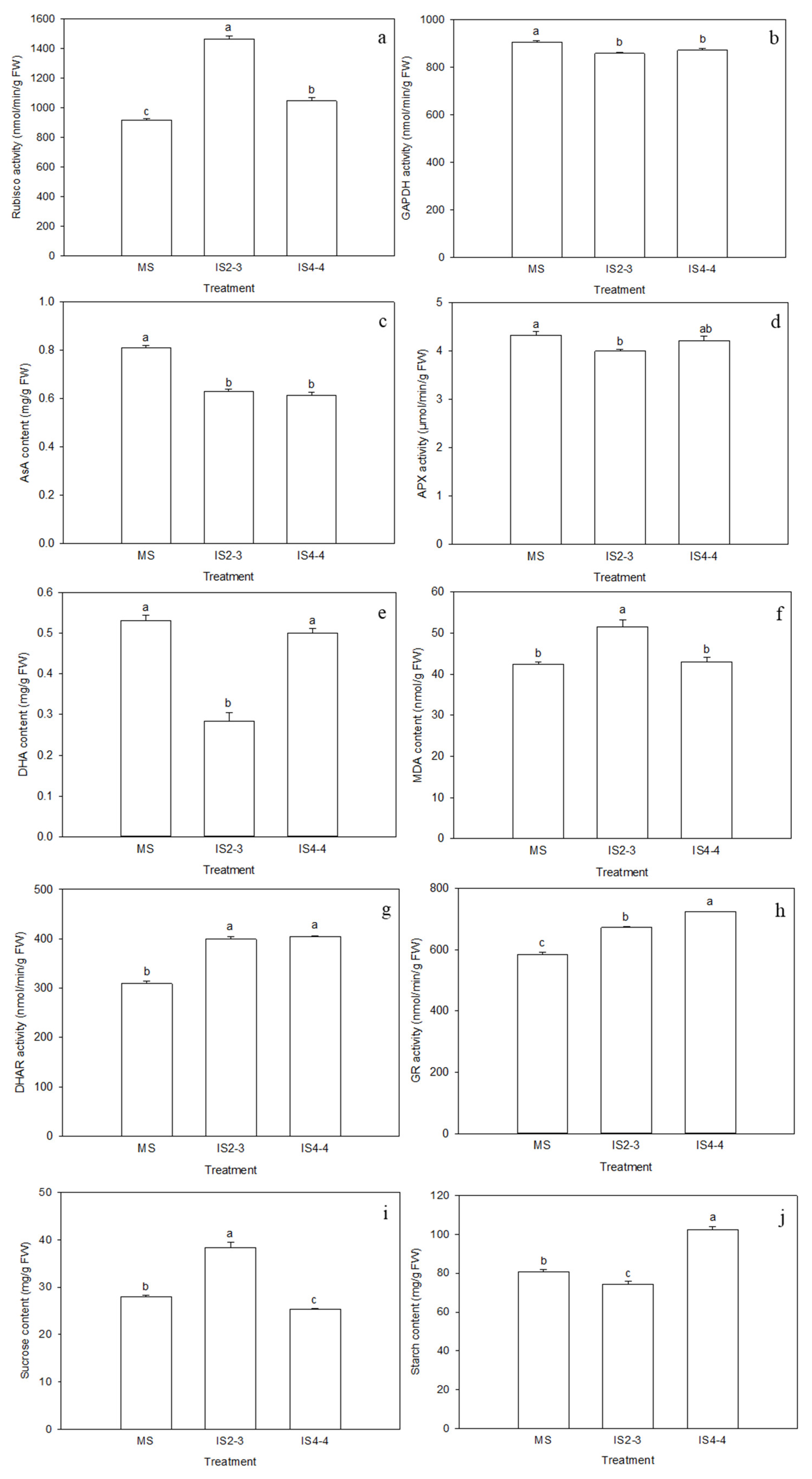
3.2. Soybean Leaf Health Under Different Planting Patterns
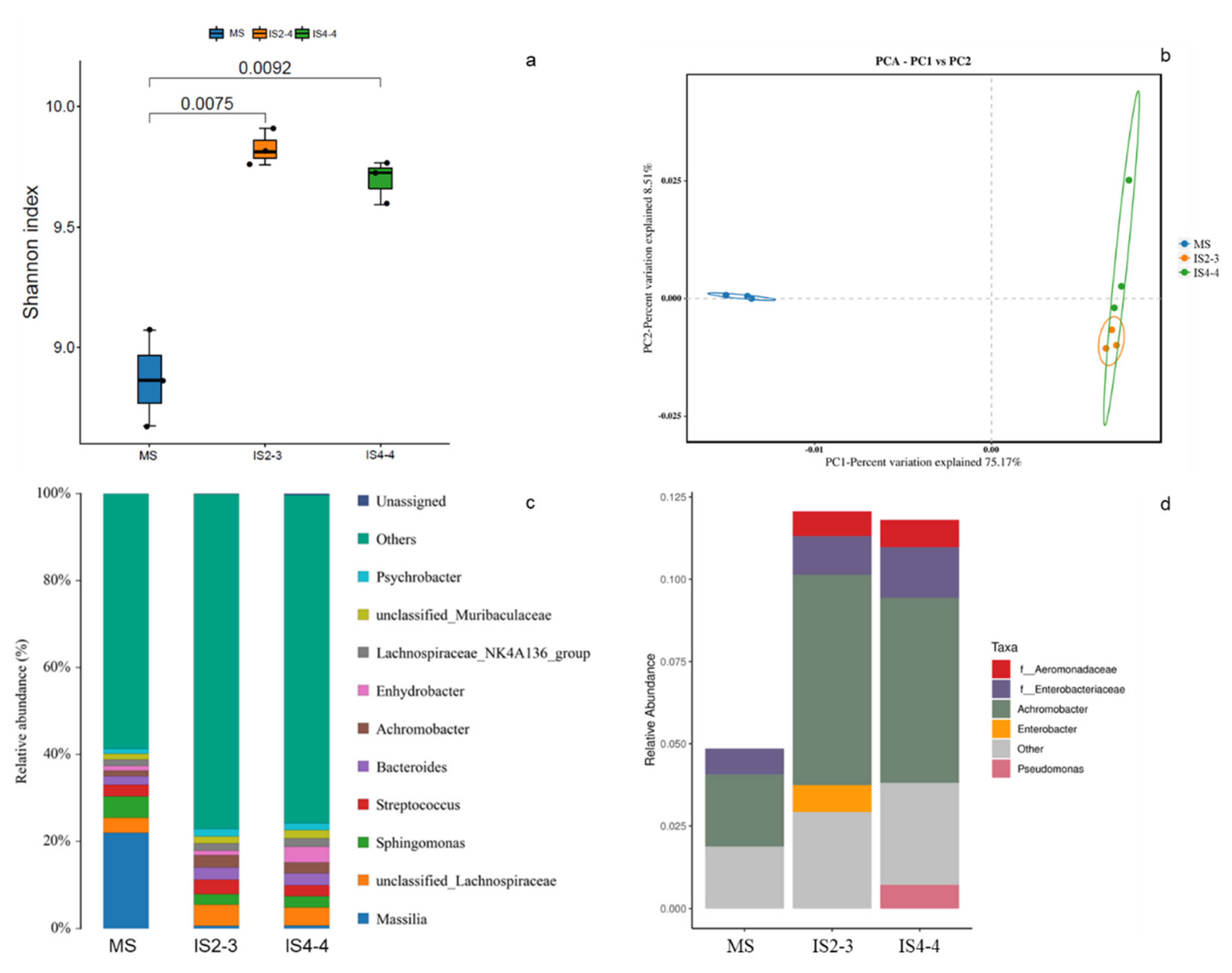
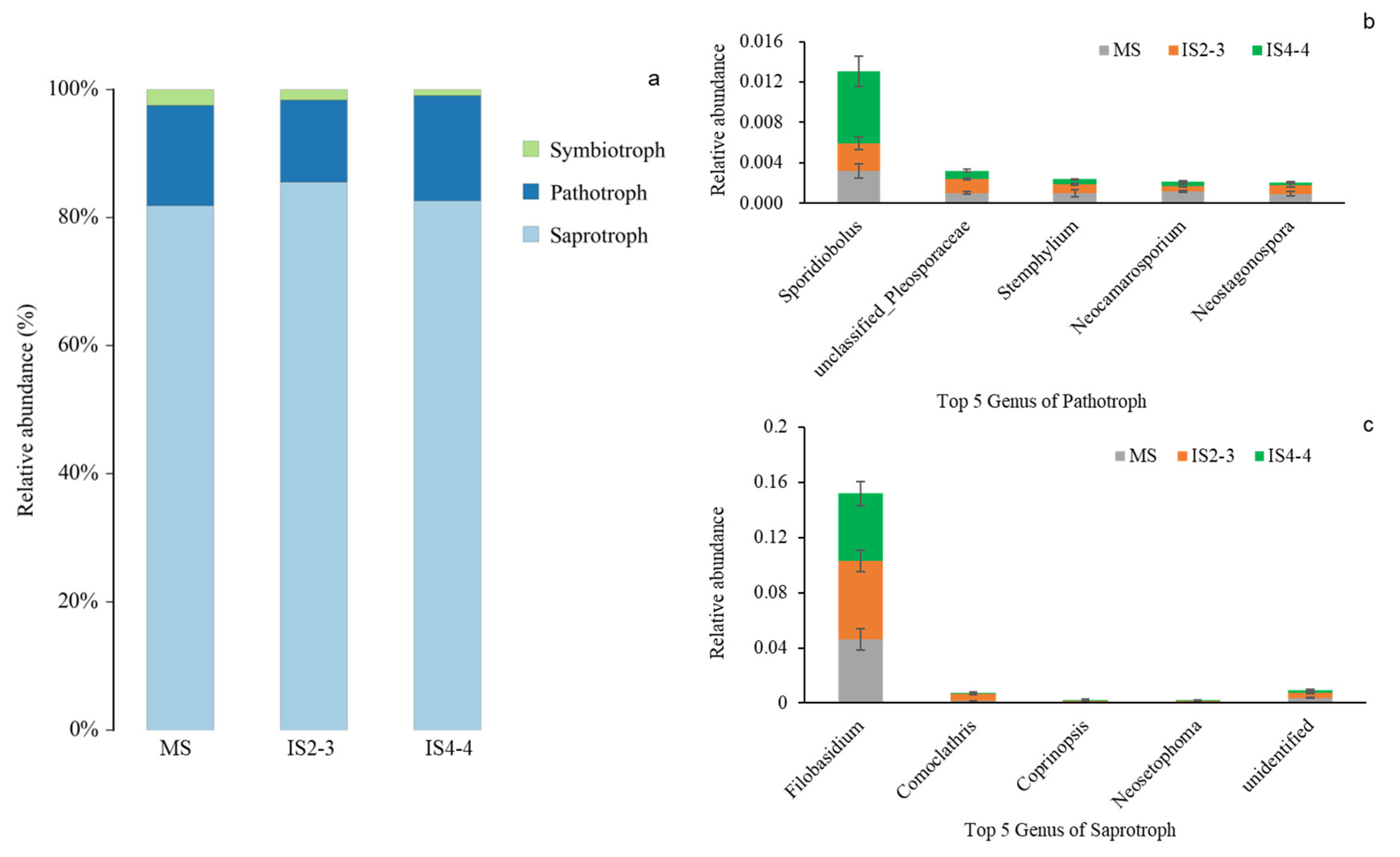
3.3. Microbial Community Composition of Soybean Leaves Under Different Planting Patterns
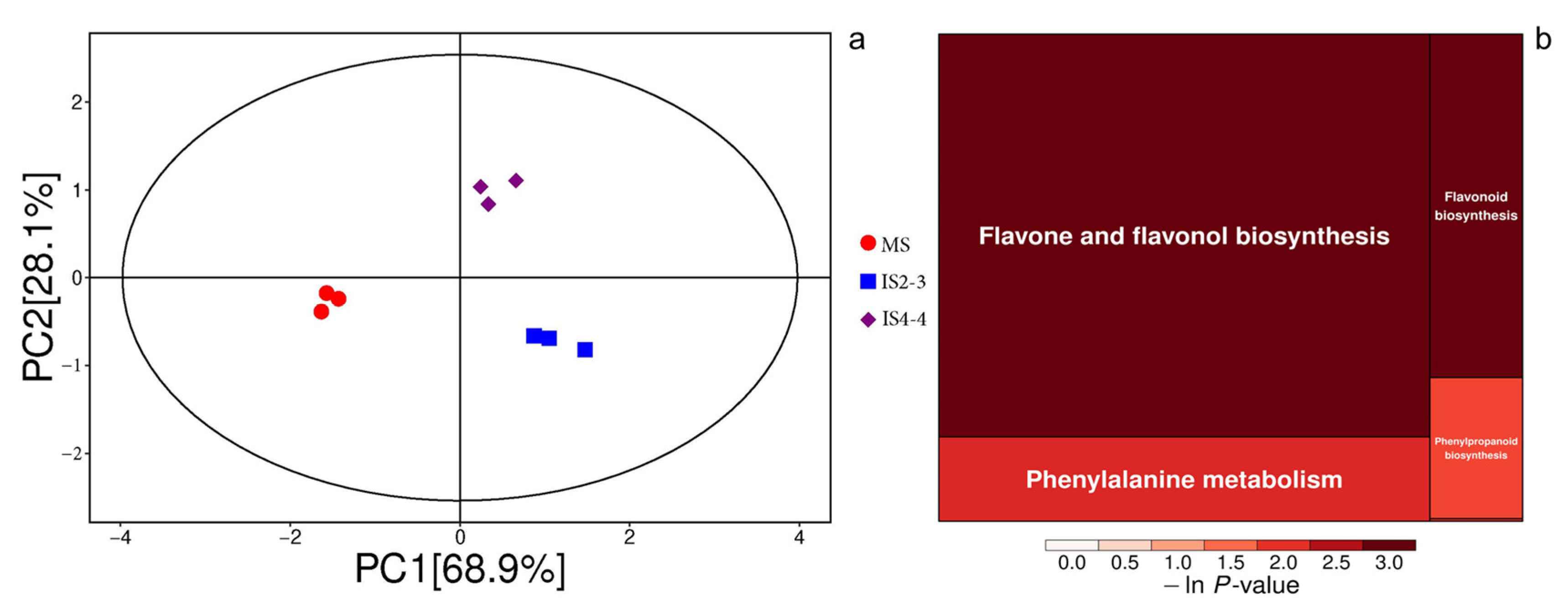
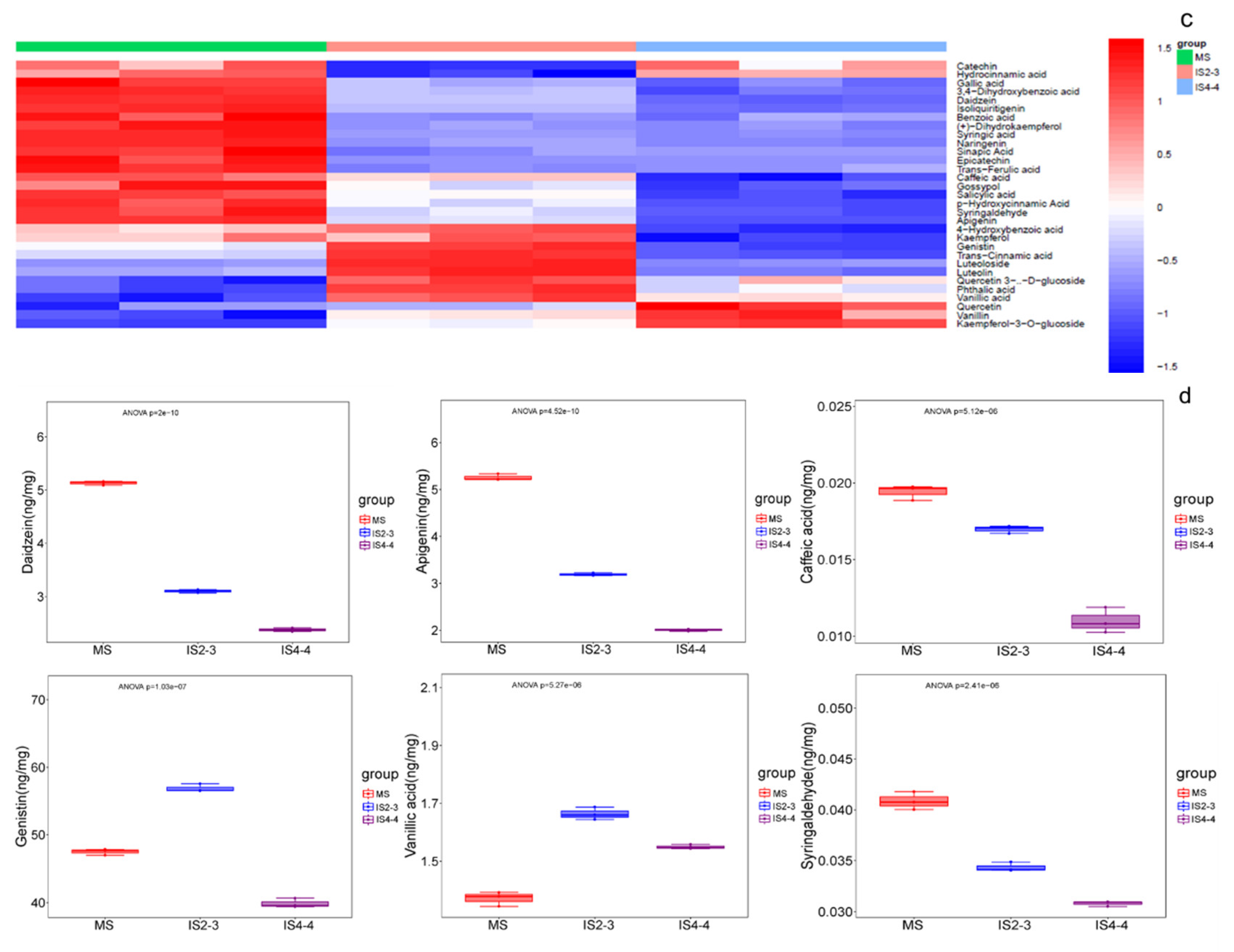
3.4. Soybean Quality Under Different Planting Patterns
4. Discussion
4.1. Soybean Leaf Dynamics Under Different Planting Patterns
4.2. Soybean Quality Under Different Planting Patterns
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hao, W.; Shen, G.H.; Shi, Z.Z.; Hu, X.D. Effects of climate and price on soybean production: Empirical analysis based on panel data of 116 prefecture-level Chinese cities. PLoS ONE 2023, 18, e0273887. [Google Scholar]
- Rotundo, J.L.; Borrás, L.; Westgate, M.E.; Orf, J.H. Relationship between assimilate supply per seed during seed filling and soybean seed composition. Field Crops Res. 2009, 112, 90–96. [Google Scholar]
- Sakuraba, Y. Light-mediated regulation of leaf senescence. Int. J. Mol. Sci. 2021, 22, 3291. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, H.; Zeng, R.; Wang, X.; Huang, L.; Wang, L.; Wang, X.; Zhang, L. Shade effects on peanut yield associate with physiological and expressional regulation on photosynthesis and sucrose metabolism. Int. J. Mol. Sci. 2020, 21, 5284. [Google Scholar] [CrossRef]
- Wu, H.Y.; Liu, L.A.; Shi, L.; Zhang, W.F.; Jiang, C.D. Photosynthetic acclimation during low-light-induced leaf senescence in post-anthesis maize plants. Photosynth. Res. 2021, 150, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.J.; Schenk, P.M. Inner Plant Values: Diversity, Colonization and Benefits from Endophytic. Front. Microbiol. 2017, 8, 2552. [Google Scholar]
- Bianculli, M.L.; Aguirrezabal, L.A.N.; Pereyra Irujo, G.A.; Echarte, M.M. Contribution of incident solar radiation on leaves and pods to soybean seed weight and composition. Eur. J. Agron. 2016, 77, 1–9. [Google Scholar]
- Hernandez-Sebastia, C.; Marsolais, F.; Saravitz, C.; Israel, D.; Dewey, R.E.; Huber, S.C. Free amino acid profiles suggest a possible role for asparagine in the control of storage-product accumulation in developing seeds of low- and high-protein soybean lines. J. Exp. Bot. 2005, 417, 1951–1963. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Pang, T.; Iqbal, N.; Shafiq, I.; Skalicky, M.; Ahmad, A.; Asghar, M.A.; Raza, A.; Allakhverdiev, S.I.; Wang, Y.; et al. Acclimation strategy and plasticity of different soybean genotypes in intercropping. Funct. Plant Biol. 2020, 47, 592–610. [Google Scholar]
- Sobko, O.; Stahl, A.; Hahn, V.; Zikeli, S.; Claupein, W.; Gruber, S. Environmental effects on soybean (Glycine max (L.) Merr) production in central and south Germany. Agronomy 2020, 10, 1847. [Google Scholar] [CrossRef]
- Khalid, M.H.B.; Cui, L.; Abbas, G.; Raza, M.A.; Anwar, A.; Zhmed, Z.; Waheed, A.; Saeed, A.; Ahmed, W.; Babar, M.J.; et al. Effect of row spacing under maize-soybean relay intercropping system on yield, competition, and economic returns. Turk. J. Agric. For. 2023, 47, 390–401. [Google Scholar]
- Xu, Z.; Li, C.; Zhang, C.; Yu, Y.; van der Werf, W.; Zhang, F. Intercropping maize and soybean increases efficiency of land and fertilizer nitrogen use: A meta-analysis. Field Crops Res. 2020, 246, 107661. [Google Scholar]
- Inal, I.; Yucel, D.; Yucel, C.; Hatipoglu, R. Forage yield and quality of soybean -maize intercropping. Fresen. Environ. Bull. 2021, 30, 10463–10473. [Google Scholar]
- Unay, A.; Sabanci, I.; Cinar, V.M. The effect of maize (Zea mays L.) / soybean (Glycine max (L.) Merr.) intercropping and biofertilizer (Azotobacter) on yield, leaf area index and land equivalent ratio. J. Agr. Sci-Tarim Bili. 2021, 27, 76–82. [Google Scholar]
- Pelech, E.A.; Evers, J.B.; Pederson, T.L.; Drag, D.W.; Fu, P.; Bernacchi, C.J. Leaf, plant, to canopy: A mechanistic study on aboveground plasticity and plant density within a maize–soybean intercrop system for the Midwest, USA. Plant Cell Environ. 2022, 46, 405–421. [Google Scholar] [PubMed]
- Baishya, L.K.; Jamir, T.; Walling, N.; Rajkhowa, D.J. Evaluation of maize (Zea mays L.) plus legume intercropping system for productivity, profitability, energy budgeting and soil health in hill terraces of eastern himalayan region. Legume Res. 2021, 44, 1343–1347. [Google Scholar]
- Jin, F. Effects of Herbicide Under Different Soil Moisture on the Efficacy, Phytotoxicity and Recovery in Maize and Soybean Intercropping in Hexi Corridor. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2019. [Google Scholar]
- Yang, J.B. Effects of Nitrogen-Saving Mode of Maize-Soybean Intercropping on Crop Growth and Greenhouse Gas Emission in Farmland. Master’s Thesis, Northwest A&F University, Yangling, China, 2022. [Google Scholar]
- Yang, C.; Fan, Z.; Chai, Q. Agronomic and economic benefits of pea/maize intercropping systems in relation to n fertilizer and maize density. Agronomy 2018, 8, 52. [Google Scholar] [CrossRef]
- He, W.; Pu, M.; Li, J.; Xu, Z.G.; Gan, L. Potato tuber growth and yield under red and blue LEDs in plant factories. J. Plant Growth Regul. 2021, 41, 40–51. [Google Scholar]
- Bi, H.; Dong, X.; Wang, M.; Ai, X. Foliar spray calcium and salicylic acid improve the activities and gene expression of photosynthetic enzymes in cucumber seedlings under low light intensity and suboptimal temperature. Acta Hortic. Sin. 2015, 42, 56–64. [Google Scholar]
- He, W.; Chai, Q.; Zhang, D.; Li, W.; Zhao, C.; Yin, W.; Fan, H.; Yu, A.; Hu, F.; Fan, Z. Beneficial effects of red and blue light on potato leaf antioxidant capacity and tuber bulking. Physiol. Mol. Biol. Plants 2023, 29, 513–523. [Google Scholar]
- GB 5009.5-2016; Determination of Protein in Foods. State Food and Drug Administration: Beijing, China, 2016.
- GB5009.6-2016; Determination of Fat in Foods. State Food and Drug Administration: Beijing, China, 2016.
- GB 5009.4-2016; Determination of Ash in Foods. State Food and Drug Administration: Beijing, China, 2016.
- NY/T 1459-2007; Determination of Acid Detergent Fiber in Feed. Ministry of Agriculture: Beijing, China, 2007.
- GB/T 20806-2006; Determination of Neutral Detergent Fiber in Feed. Ministry of Agriculture: Beijing, China, 2006.
- Ye, Y.; Lin, J.; Yin, J.; He, H. GC/QQQ coupling with metabolomics for selection of predicator of tea fermentation. Food Res. Int. 2023, 173, 113273. [Google Scholar] [CrossRef]
- Feng, Z.; Sun, L.; Dong, M.; Fan, S.; Shi, K.; Qu, Y.; Zhu, L.; Shi, J.; Wang, W.; Liu, Y.; et al. Novel players in organogenesis and flavonoid biosynthesis in cucumber glandular trichomes. Plant Physiol. 2023, 192, 2723–2736. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.; Tautenhahn, R.; Böttcher, C.; Larson, T.R.; Neumann, S. CAMERA: An Integrated Strategy for Compound Spectra Extraction and Annotation of Liquid Chromatography/Mass Spectrometry Data Sets. Anal. Chem. 2012, 84, 283–289. [Google Scholar] [PubMed]
- Zhang, Z.M.; Tong, X.; Peng, Y.; Ma, P.; Zhang, M.J.; Lu, H.M.; Chen, X.Q.; Liang, Y.Z. Multiscale peak detection in wavelet space. Analyst 2015, 140, 7955–7964. [Google Scholar] [CrossRef] [PubMed]
- Walters, W.; Hyde, E.R.; Berglyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems 2016, 1, e00009. [Google Scholar] [CrossRef]
- Karlsson, I.; Friberg, H.; Steinberg, C.; Persson, P. Fungicide effects on fungal community composition in the wheat phyllosphere. PLoS ONE 2014, 9, e111786. [Google Scholar]
- Raza, M.A.; Gul, H.; Hasnain, A.; Bin Khalid, M.H.; Hussain, S.; Abbas, G.; Ahmed, W.; Babar, M.J.; Ahmed, Z.; Saeed, A.; et al. Leaf area regulates the growth rates and seed yield of soybean (Glycine max L. Merr.) in intercropping system. Int. J. Plant Prod. 2022, 16, 639–652. [Google Scholar] [CrossRef]
- Brouwer, B.; Ziolklwska, A.; Bagard, M.; Keech, O.; Gardestrom, P. The impact of light intensity on shade-induced leaf senescence. Plant Cell Environ. 2012, 35, 1084–1098. [Google Scholar] [CrossRef]
- Bodenhausen, N.; Horton, M.W.; Bergelson, J. Bacterial Communities Associated with the Leaves and the Roots of Arabidopsis thaliana. PLoS ONE 2013, 8, e356329. [Google Scholar] [CrossRef]
- Jia, T.; Wang, Y.W.; Chai, B.F. Bacterial community characteristics and enzyme activities in Bothriochloa ischaemum litter over progressive phytoremediation years in a copper tailings dam. Front. Microbiol. 2021, 11, 565806. [Google Scholar]
- Du, C.J.; Li, C.X.; Cao, P.; Li, T.T.; Du, D.D.; Wang, X.J.; Zhao, J.W.; Xiang, W.S. Massilia cellulosiltytica sp. nov., a novel cellulose-degrading bacterium isolated from rhizosphere soil of rice (Oryza sativa L.) and its whole genome analysis. Anton. Leeuw. 2021, 114, 1529–1540. [Google Scholar]
- Zhang, G.H.; Zhao, L.; Li, W.; Yao, H.; Lu, C.H.; Zhao, G.K.; Wu, Y.P.; Li, Y.P.; Kong, G.H. Changes in physicochemical properties and microbial community succession during leaf stacking fermentation. AMB Express 2023, 13, 132. [Google Scholar] [PubMed]
- He, D.; Singh, S.K.; Peng, L.; Kaushal, R.; Vilchez, J.I.; Shao, C.; Wu, X.; Zheng, S.; Morcillo, R.J.L.; Pare, P.W.; et al. Flavonoid-attracted Aeromonas sp. from the Arabidopsis root microbiome enhances plant dehydration resistance. ISME J. 2022, 16, 2622–2632. [Google Scholar] [CrossRef]
- Ramesh, A.; Sharma, S.K.; Sharma, M.P.; Yadav, N.; Joshi, O.P. Plant growth-promoting traits in Enterobacter cloacae subsp. dissolvens MDSR9 isolated from soybean rhizosphere and its impact on growth and nutrition of soybean and wheat upon inoculation. Agr. Res. 2014, 3, 53–66. [Google Scholar] [CrossRef]
- Mohamed, B.F.; Sallam, N.; Alamri, S.A.; Abo-Elyousr, K.A.; Mostafa, Y.S.; Hashem, M. Approving the biocontrol method of potato wilt caused by Ralstonia solanacearum (Smith) using Enterobacter cloacae PS14 and Trichoderma asperellum T34. Egyt. J. Biol. Pest Co. 2020, 30, 61. [Google Scholar]
- Ahmed, W.; Zhou, G.; Yang, J.; Munir, S.; Ahmed, A.; Liu, Q.; Zhao, Z.; Ji, G. Bacillus amyloliquefaciens WS-10 as a potential plant growth-promoter and biocontrol agent for bacterial wilt disease of flue-cured tobacco. Egyt. J. Biol. Pest Co. 2022, 32, 25. [Google Scholar]
- Danish, S.; Zafar-UI-Hye, M.; Hussain, S.; Riaz, M.; Qayyum, M.F. Mitigation of drought stress in maize through inoculation with drought tolerant ACC deaminase containing PGPR under axenic conditions. Pak. J. Bot. 2020, 52, 49–60. [Google Scholar]
- Kour, D.; Khan, S.S.; Kaur, T.; Kour, H.; Singh, G.; Yadav, A.; Yadav, A.N. Drought adaptive microbes as bioinoculants for the horticultural crops. Heliyon 2022, 8, e0949. [Google Scholar]
- Izquierdo, N.G.; Aguirrezabal, L.A.N.; Andrade, F.H.; Geroudet, C.; Valentinuz, O.; Iraola, M.P. Intercepted solar radiation affects oil fatty acid composition in crop species. Field Crops Res. 2009, 114, 66–74. [Google Scholar]
- Han, T.F.; Wang, J.L.; Yang, Q.K.; Gai, J.Y. Effects of post flowering photoperiod on chemical composition of soybeans. Acta Agron. Sin. 1997, 30, 47–53. [Google Scholar]
- Song, W.; Yang, R.; Wu, T.; Wu, C.; Sun, S.; Zhang, S.; Jiang, B.; Tian, S.; Liu, X.; Han, T. Analyzing the Effects of Climate Factors on Soybean Protein, Oil Contents, and Composition by Extensive and High-Density Sampling in China. J. Agric. Food Chem. 2016, 64, 4121–4130. [Google Scholar] [CrossRef] [PubMed]
- Son, N.T.; Thanh, D.T.M.; Trang, N.V. Flavone norartocarpetin and isoflavone 2′-hydroxygenistein: A spectroscopic study for structure, electronic property and antioxidant potential using DFT (density functional theory). J. Mol. Struct. 2019, 1193, 76–88. [Google Scholar] [CrossRef]
- Yamagata, K.; Yamori, Y. Potential Effects of Soy Isoflavones on the Prevention of Metabolic Syndrome. Molecules 2021, 26, 5863. [Google Scholar] [CrossRef]
- Hoeck, J.A.; Fehr, W.R.; Murphy, P.A.; Welke, G.A. Influence of genotype and environment on isoflavone contents of soybean. Crop Sci. 2000, 40, 48–51. [Google Scholar] [CrossRef]
- Tsukamoto, C.; Nawaz, M.A.; Kurosaka, A.; Le, B.; Lee, J.D.; Son, E.; Yang, S.H.; Kurt, C.; Baloch, F.S.; Chung, G. Isoflavone profile diversity in Korean wild soybeans (Glycine soja Sieb. & Zucc.). Turk. J. Agric. For. 2018, 42, 248–261. [Google Scholar]
- Tang, R.; Seguin, P.; Morrison, M.; Smedbol, E. Impact of climatic factors at specific growth stages on soybean soyasaponin I concentration. Can. J. Plant Sci. 2021, 101, 124–128. [Google Scholar] [CrossRef]
- Li, M.; Kong, J.; Chen, Y.; Li, Y.; Xuan, H.; Liu, M.; Zhang, Q. Comparative interaction study of soy protein isolate and three flavonoids (Chrysin, Apigenin and Luteolin) and their potential as natural preservatives. Food Chem. 2021, 414, 135738. [Google Scholar] [CrossRef]
- Olk, D.C.; Dinnes, D.L.; Hatfield, R.D.; Scoresby, J.R.; Darlington, J.W. Variable humic product effects on maize structural biochemistry across annual weather patterns and soil types in two Iowa (USA) production fields. Front. Plant Sci. 2023, 13, 1058141. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yang, E.J.; Son, Y.K.; Yeo, J.H.; Song, K.S. Enhanced anti-oxidative effect of fermented Korean mistletoe is originated from an increase in the contents of caffeic acid and lyoniresinol. Food Funct. 2016, 7, 2270–2277. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Gu, Z.X.; Sun, M.M.; Yang, R.Q. Effects of germination on physio-biochemical metabolism and phenolic acids of soybean seeds. J. Food Compos. Anal. 2022, 112, 104717. [Google Scholar] [CrossRef]
| Month | 2022 | 2023 | 2024 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Precipitation (mm) | Average T (°C) | Total Solar Radiation (MJ m−2) | Total Precipitation (mm) | Average T (°C) | Total Solar Radiation (MJ m−2) | Total Precipitation (mm) | Average T (°C) | Total Solar Radiation (MJ m−2) | |
| May | 12.90 | 17.55 | 623.04 | 14.40 | 16.32 | 579.87 | 9.5 | 20.19 | 575.99 |
| June | 18.00 | 22.57 | 624.56 | 5.70 | 22.37 | 637.51 | 54.4 | 21.30 | 558.12 |
| July | 84.10 | 22.74 | 613.92 | 15.60 | 23.93 | 666.02 | 28.3 | 22.37 | 521.80 |
| August | 56.20 | 20.80 | 498.24 | 6.70 | 22.55 | 583.34 | 75.3 | 21.63 | 530.09 |
| September | 33.40 | 16.42 | 470.00 | 26.60 | 17.60 | 441.64 | 90.3 | 15.36 | 320.49 |
| Years | Treatments | Internode Number | Branch Number per Plant | Pod Number per Plant | Bean Number per Pod | 100 Grains Mass (g) |
|---|---|---|---|---|---|---|
| 2022 | MS | 17.87 ± 0.78 a | 2.27 ± 0.16 a | 40.87 ± 0.48 a | 2.40 ± 0.02 a | 23.02 ± 0.15 a |
| IS2-3 | 18.60 ± 0.30 a | 2.93 ± 0.52 a | 42.47 ± 0.39 a | 2.40 ± 0.05 a | 24.34 ± 0.44 a | |
| IS4-4 | 18.00 ± 0.44 a | 1.97 ± 0.38 a | 33.93 ± 2.90 b | 2.34 ± 0.05 a | 21.80 ± 0.90 a | |
| 2023 | MS | 13.66 ± 0.03 a | 1.50 ± 0.06 a | 32.40 ± 0.78 a | 2.33 ± 0.03 b | 21.42 ± 0.33 a |
| IS2-3 | 12.27 ± 0.15 b | 1.43 ± 0.09 a | 39.97 ± 2.03 a | 2.50 ± 0.01 a | 21.58 ± 0.20 a | |
| IS4-4 | 11.63 ± 0.29 b | 1.47 ± 0.12 a | 39.77 ± 2.10 a | 2.42 ± 0.04 ab | 21.68 ± 0.22 a | |
| 2024 | MS | 19.53 ± 0.20 a | 1.37 ± 0.07 b | 39.63 ± 0.87 b | 2.18 ± 0.05 c | 24.37 ± 0.41 a |
| IS2-3 | 21.17 ± 0.96 a | 2.53 ± 0.33 a | 45.40 ± 1.48 a | 2.51 ± 0.02 a | 24.97 ± 0.07 a | |
| IS4-4 | 19.50 ± 0.46 a | 1.53 ± 0.03 b | 39.63 ± 0.50 b | 2.38 ± 0.04 b | 24.63 ± 0.03 a | |
| F value | Year(Y) | ** | ** | NS | * | NS |
| Treatment(T) | NS | * | ** | ** | ** | |
| Y ∗ T | NS | NS | ** | ** | ** |
| Years | Treatments | Yield (kg ha−1) | LER | Total LER | ||
|---|---|---|---|---|---|---|
| Soybean | Maize | Soybean | Maize | |||
| 2022 | MS | 6271.19 ± 89.54 a | - | - | - | - |
| MM | - | 16,955.95 ± 61.24 a | - | - | - | |
| IS2-3 | 2584.57 ± 96.32 b | 9576.55 ± 45.09 c | 0.41 ± 0.02 a | 0.56 ± 0.01 b | 0.98 ± 0.03 a | |
| IS4-4 | 1590.98 ± 83.11 c | 11,181.01 ± 24.48 b | 0.25 ± 0.01 b | 0.66 ± 0.01 a | 0.91 ± 0.02 a | |
| 2023 | MS | 4498.02 ± 97.49 a | - | - | - | - |
| MM | - | 15,633.28 ± 40.46 a | - | - | - | |
| IS2-3 | 2243.91 ± 104.85 b | 10,257.16 ± 25.12 b | 0.50 ± 0.02 a | 0.66 ± 0.01 a | 1.16 ± 0.02 a | |
| IS4-4 | 1928.79 ± 112.81 b | 10,372.32 ± 16.37 b | 0.43 ± 0.02 a | 0.66 ± 0.02 a | 1.09 ± 0.03 b | |
| 2024 | MS | 5857.44 ± 189.40 a | - | - | - | - |
| MM | - | 17,788.85 ± 32.93 a | - | - | - | |
| IS2-3 | 2959.92 ± 105.30 b | 9251.83 ± 27.50 b | 0.51 ± 0.03 a | 0.53 ± 0.01 a | 1.03 ± 0.03 a | |
| IS4-4 | 2154.70 ± 18.67 c | 9340.06 ± 15.52 b | 0.37 ± 0.01 b | 0.52 ± 0.01 a | 0.89 ± 0.01 b | |
| F value | Year(Y) | ** | NS | ** | ** | ** |
| Treatment(T) | ** | ** | ** | ** | ** | |
| Y ∗ T | ** | ** | NS | ** | NS | |
| Treatments | Crude Protein Content (g/100 gDW) | Crude Fat Content % (DW) | Ash Content % (DW) | Acid Detergent Fiber Content % (DW) | Neutral Detergent Fiber Content % (DW) |
|---|---|---|---|---|---|
| MS | 29.96 ± 0.18 a | 17.40 ± 0.02 a | 12.35 ± 0.31 a | 27.03 ± 0.57 a | 39.97 ± 1.64 a |
| IS2-3 | 31.20 ± 0.74 a | 15.46 ± 0.40 b | 12.81 ± 0.69 a | 27.17 ± 1.06 a | 33.60 ± 1.14 b |
| IS4-4 | 31.22 ± 0.76 a | 16.44 ± 0.50 ab | 12.17 ± 0.53 a | 26.42 ± 0.69 a | 34.45 ± 0.86 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Chai, Q.; Zhao, C.; Yin, W.; Fan, H.; Yu, A.; Fan, Z.; Hu, F.; Sun, Y.; Wang, F. Different Intercropped Soybean Planting Patterns Regulate Leaf Growth and Seed Quality. Agronomy 2025, 15, 880. https://doi.org/10.3390/agronomy15040880
He W, Chai Q, Zhao C, Yin W, Fan H, Yu A, Fan Z, Hu F, Sun Y, Wang F. Different Intercropped Soybean Planting Patterns Regulate Leaf Growth and Seed Quality. Agronomy. 2025; 15(4):880. https://doi.org/10.3390/agronomy15040880
Chicago/Turabian StyleHe, Wei, Qiang Chai, Cai Zhao, Wen Yin, Hong Fan, Aizhong Yu, Zhilong Fan, Falong Hu, Yali Sun, and Feng Wang. 2025. "Different Intercropped Soybean Planting Patterns Regulate Leaf Growth and Seed Quality" Agronomy 15, no. 4: 880. https://doi.org/10.3390/agronomy15040880
APA StyleHe, W., Chai, Q., Zhao, C., Yin, W., Fan, H., Yu, A., Fan, Z., Hu, F., Sun, Y., & Wang, F. (2025). Different Intercropped Soybean Planting Patterns Regulate Leaf Growth and Seed Quality. Agronomy, 15(4), 880. https://doi.org/10.3390/agronomy15040880







