Inorganic and Organic Fertilization Effects on the Growth, Nutrient Uptake, Chlorophyll Fluorescence and Fruit Quality in Solanum melongena L. Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Experimental Design and Conditions of the Experimental Greenhouse
2.2. Fertilization Treatments
2.3. Soil Sampling and Lab Analyses
2.4. Plant Growth
2.5. Tissue Nutrient Concentrations and Total Plant Nutrient Uptake
2.6. Chlorophyll Fluorescence and Chlorophyll Content
2.7. Total Phenοlic Content (TPC), Total Flavonoid Content (TFC) and Antioxidant Activity (DPPH) in Fruits
2.8. Statistical Analysis
3. Results
3.1. Soil Fertility
3.2. Plant Growth
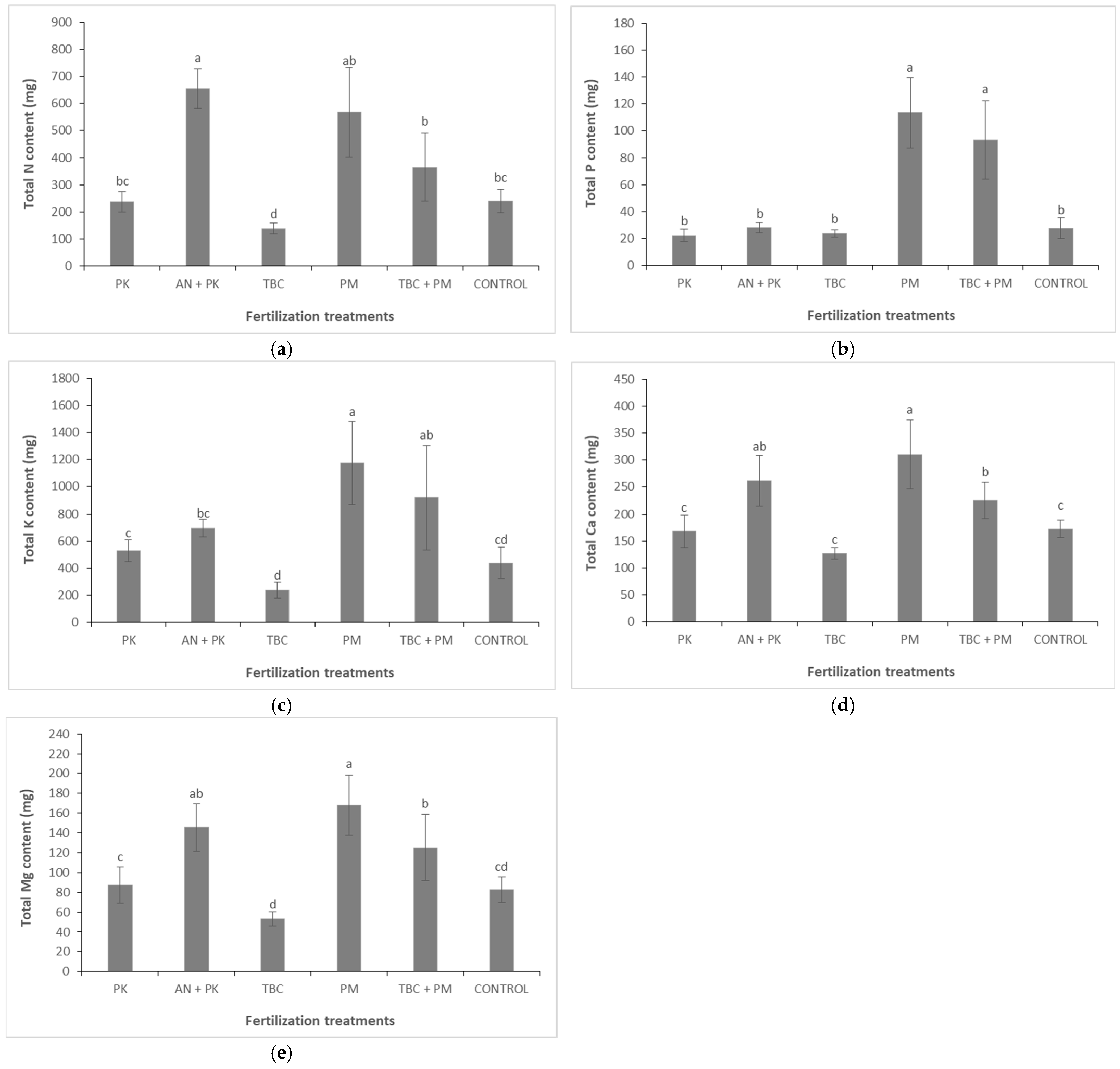
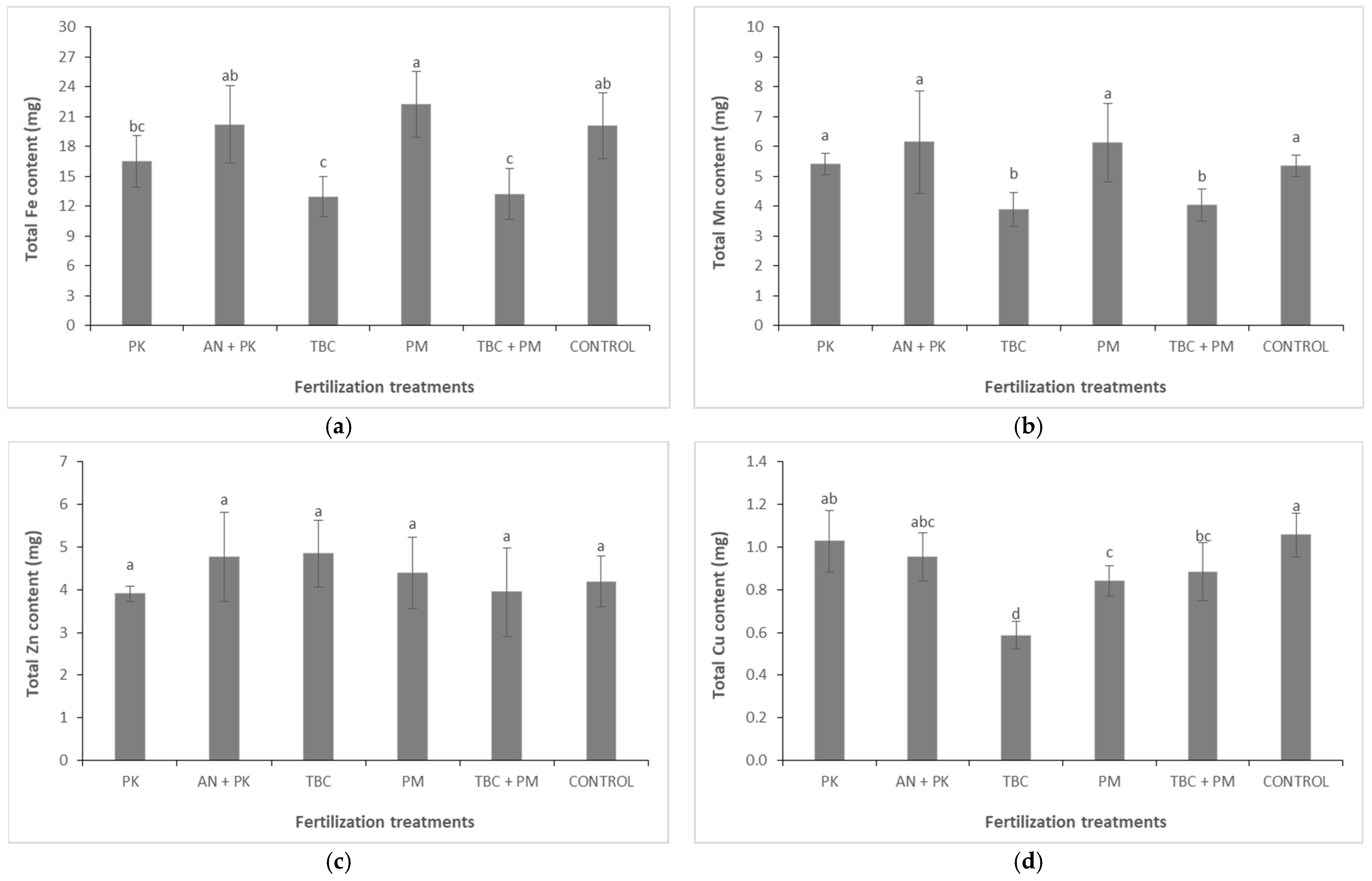
3.3. Tissue Nutrient Concentrations and Total Plant Nutrient Uptake
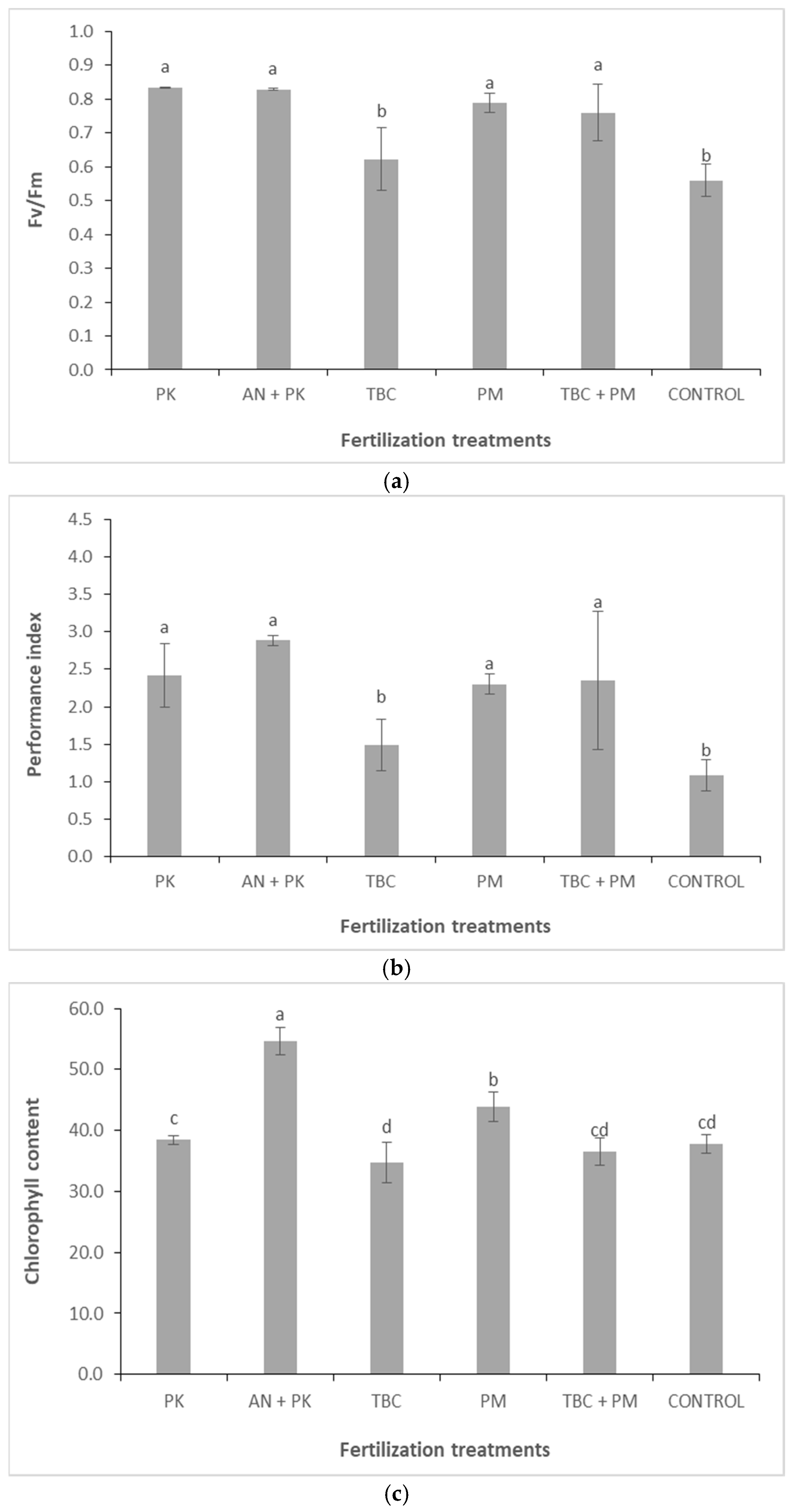
3.4. Chlorophyll Fluorescence Parameters and Chlorophyll Content
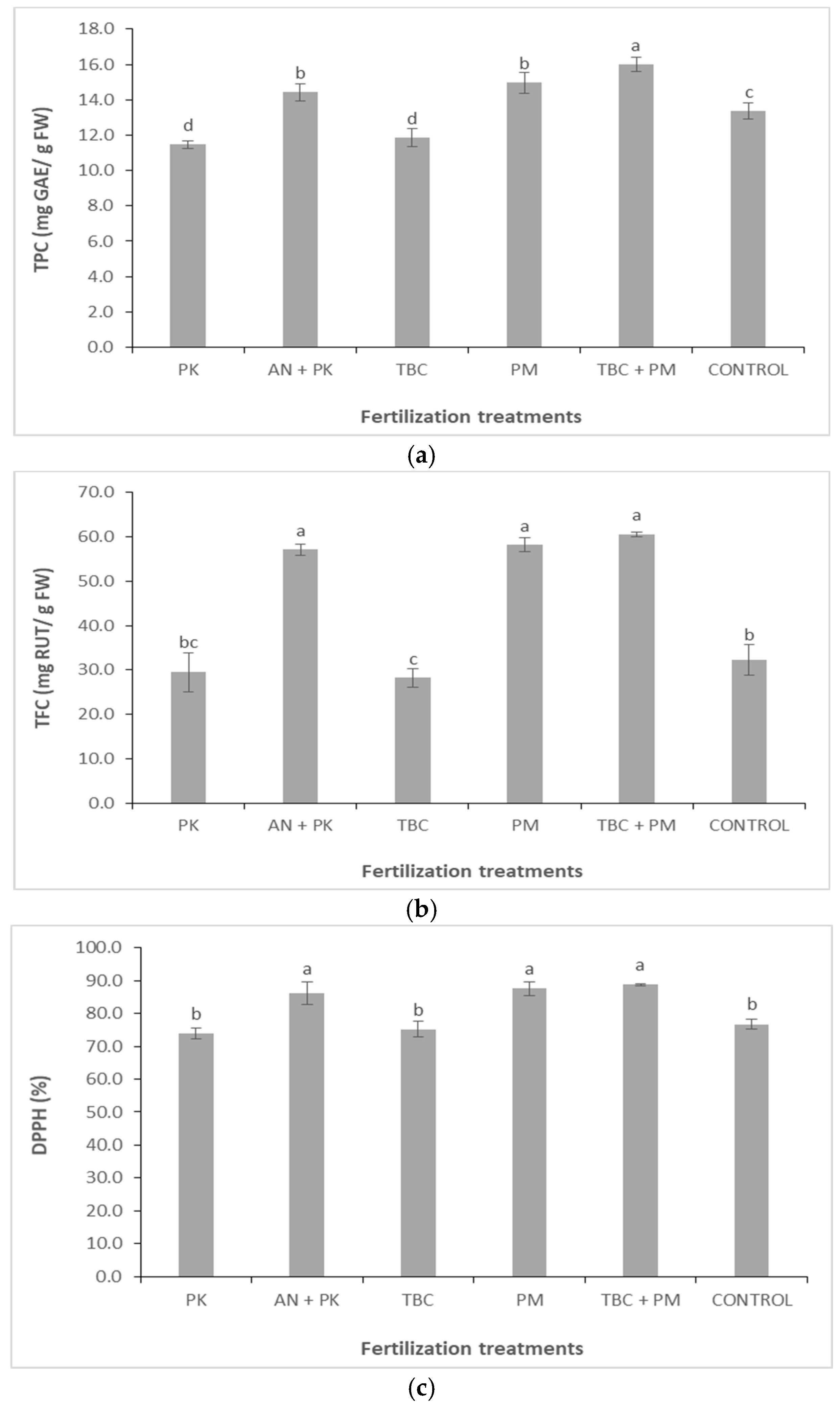
3.5. Total Phenolic Content (TPC), Total Flavonoid Content (TFC) and Antioxidant Activity—DPPH Method in Fruits of Solanum melongena L. Plants Under Different Fertilization Treatments
4. Discussion
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). FAOSTAT Database; FAO: Rome, Italy, 2014; Available online: http://faostat.fao.org/default.aspx (accessed on 28 March 2025).
- Food and Agriculture Organization. Corporate Statistical Database. FAOSTAT. 2021. Available online: http://www.fao.org/faostat/en/#data/QCFAO (accessed on 10 March 2021).
- Taher, D.; Solberg, S.; Prohens, J.; Chou, Y.; Rakha, M.; Wu, T. World vegetable center eggplant collection: Origin, composition, seed dissemination and utilization in breeding. Front. Plant Sci. 2017, 8, 1484. [Google Scholar] [CrossRef]
- Akanitapichat, P.; Phraibung, K.; Nuchklang, K.; Prompitakkul, S. Antioxidant and hepatoprotective activities of five eggplant varieties. Food Chem. Toxicol. 2010, 48, 3017–3021. [Google Scholar] [CrossRef] [PubMed]
- Nayanathara, A.R.; Anu, M.; Aalolam, K.P.; Reshma, J.K. Evaluation of total phenol, flavonoid and anthocyanin content in different varieties of eggplant. Emergent Life Sci. Res. 2016, 2, 63–65. Available online: https://www.emergentresearch.org/uploads/38/1809_pdf.pdf (accessed on 3 February 2025).
- Niño-Medina, G.; Urías-Orona, V.; Muy-Rangel, M.D.; Heredia, J.B. Structure and content of phenolics in eggplant (Solanum melongena)—A review. S. Afr. J. Bot. 2017, 111, 161–169. [Google Scholar] [CrossRef]
- Zenia, M.; Halina, B. Content of microelements in eggplant fruits depending on nitrogen fertilization and plant training method. J. Elementol. 2008, 13, 269–274. [Google Scholar] [CrossRef]
- Naeem, M.Y.; Ugur, S. Nutritional content and health benefits of eggplant. Turk. J. Agric. Food Sci. Technol. 2019, 7, 31–36. [Google Scholar] [CrossRef]
- Kasyap, S.; Kumar, S.; Maji, S.; Kumar, D. Effect of organic manures and inorganic fertilizers on growth, yield and quality of brinjal (Solanum melongena L.) cv. Pant Rituraj. Int. J. Agric. Sci. 2014, 10, 305–308. [Google Scholar]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- National Diabetes Education Program (NIH); National Institutes of Health, USA. 2008. Available online: www.ndep.nih.gov (accessed on 30 November 2024).
- American Diabetes Association (ADA). 2015. Available online: http://vgs.diabetes.org (accessed on 30 December 2024).
- Adamczewska-Sowińska, K.; Krygier, M. Yield quantity and quality of field cultivated eggplant in relation to its cultivar and the degree of fruit maturity. Acta Sci. Pol. Hortorum Cultus 2013, 12, 13–23. Available online: https://czasopisma.up.lublin.pl/asphc/article/view/2854/1989 (accessed on 24 February 2025).
- Abbas, M.A.; Elamin, S.D.M.; Elamin, E.A.M. Effects of chicken manure as component of organic production on yield and quality of eggplant (Solanum melongena L.) fruits. J. Sci. Technol. 2011, 12, 1–8. Available online: https://search.emarefa.net/detail/BIM-388590 (accessed on 24 February 2025).
- Prusty, M.; Panda, N.; Dash, A.K.; Mishra, N. Nutrient management modules for eggplant (Solanum melongena L.): Yield, quality, economics, nutrient uptake and post-harvest soil properties. J. Indian Soc. Coast. Agric. Res. 2022, 40, 46–53. [Google Scholar] [CrossRef]
- Umalaxmi, T.; Dipa, K.; Rubina, K.; Dipa, M.; Victor, T. Integrated nutrient management in brinjal—A review study. Agric. Res. Technol. 2016, 1, 555–562. [Google Scholar] [CrossRef]
- Suravaiya, S.N.; Patel, N.B.; Ahir, M.P.; Patel, N.M.; Desai, K.D.; Patel, J.B. Integrated nutrient management (INM) approaches for brinjal (Solanum melongena L.) and other solanacious vegetables—A review. Agric. Rev. 2010, 31, 79–92. Available online: https://arccarticles.s3.amazonaws.com/webArticle/articles/ar312001.pdf (accessed on 3 February 2025).
- Zaidi, A.; Ahmad, E.; Khan, M.S.; Saif, S.; Rizvi, A. Role of plant growth promoting rhizobacteria in sustainable production of vegetables: Current perspective. Sci. Hortic. 2015, 193, 231–239. [Google Scholar] [CrossRef]
- Shivay, Y.S.; Pooniya, V.; Madan, P.; Ghasil, P.C.; Bana, R.S.; Jat, S.L. Coated urea materials for improving yields, profitability and nutrient use efficiencies of aromatic rice. Glob. Chall. 2019, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brennan, R.F.; Penrose, B.; Bell, R.W. Micronutrients limiting pasture production in Australia. Crop Pasture Sci. 2019, 70, 1053–1064. [Google Scholar] [CrossRef]
- Torquato, L.S.; Ivo dos Santos Aguiar, F.; dos Santos Matos, S.; Costa, R.M.; dos Santos Reis, C.; Parra-Serrano, L.J. Use of alternative fertilization in eggplant production. Sci. Electron. Arch. 2023, 16, 41–46. [Google Scholar] [CrossRef]
- Fahrurrozi, F.; Sariasih, Y.; Muktamar, Z.; Setyowati, N.; Chozin, M.; Sudjatmiko, S. Identification of nutrient contents in six potential green biomasses for developing liquid organic fertilizer in closed agricultural production system. Int. J. Adv. Sci. Eng. Inf. Technol. 2017, 7, 559–565. [Google Scholar] [CrossRef]
- Muktamar, Z.; Fahrurrozi, F.; Sudjatmiko, S.; Setyowati, N.; Chozin, M. Nitrogen, phosphorous, and potassium uptakes of organically grown sweet corn on coastal Entisols. Int. J. Agric. Technol. 2021, 17, 213–226. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20210228457 (accessed on 24 February 2025).
- Muktamar, Z.; Sudjatmiko, S.; Fahrurrozi, F.; Setyowati, N.; Chozin, M. Soil chemical improvement under application of liquid organic fertilizer in closed agriculture system. Int. J. Agric. Technol. 2017, 13, 1715–1727. Available online: http://www.aatsea.org/images/conference_publications/pdf/v13_n7_2_2017_December/19_IJAT_13(7.2)_2017_Muktamar%20Zainal_Environmental%20Science,%20Soil%20and%20Water%20Conservation.pdf (accessed on 24 February 2025).
- Muktamar, Z.; Fahrurrozi, F.; Sudjatmiko, S.; Chozin, M.; Setyowati, N. Quality of enriched liquid organic fertilizer from dairy cattle wastes on closed agriculture system. Int. J. Adv. Sci. Eng. Inf. Technol. 2020, 10, 1682–1687. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Kavvadias, V.; Sotiropoulos, T.; Papadakis, I.E. Organic fertilization and tree orchards. Agriculture 2021, 11, 692. [Google Scholar] [CrossRef]
- Vijaya, K.S.; Seethalakshmi, S. Response of eggplant (Solanum melongena L.) to integrated nutrient management amended soil. Int. J. Sci. Eng. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Christo, I.E.; Okorie, H.A.; Njoku, C.C.I.E.; Okorie, H.A.; Chikere, N.C. Performance of eggplant (Solenum gilo L.) as affected by manure types and rates. Glob. Res. J. Sci. 2011, 1, 43–47. [Google Scholar]
- Chatzistathis, T.; Papadakis, I.E.; Papaioannou, A.; Chatzissavvidis, C.; Giannakoula, A. Comparative study effects between manure application and a controlled release fertilizer on the growth, nutrient uptake, photosystem II activity and photosynthetic rate of Olea europaea L. (cv. ‘Koroneiki’). Sci. Hortic. 2020, 264, 109176. [Google Scholar] [CrossRef]
- Ahmad, C.A.; Akhter, A.; Haider, M.S.; Abbas, M.T.; Hashem, A.; Avila-Quezada, G.D.; Abd_Allah, E.F. Demonstration of the synergistic effect of biochar and Trichoderma harzianum on the development of Ralstonia solanacearum in eggplant. Front. Microbiol. 2024, 15, 1360703. [Google Scholar] [CrossRef]
- Sarhan, T.Z.; Mohammad, G.H.; Teli, J.A. Effects of humic acid and bread yeast on growth and yield of eggplant (Solanum melongena L.). J. Agric. Sci. Technol. 2011, 1, 1091–1096. [Google Scholar]
- Agbo, C.U.; Chukwudi, P.U.; Ogbu, A.N. Effects of rates and frequency of application of organic manure on growth, yield and biochemical composition of Solanum melongena L. (cv. ‘Ngwa local’) fruits. J. Anim. Plant Sci. 2012, 14, 1952–1960. Available online: https://www.m.elewa.org/JAPS/2012/14.2/4.pdf (accessed on 3 February 2025).
- Rehman, A.; Shahid, M.; Malik, A.A.; Khan, S.; Zakaria, S. Effect of organic and inorganic fertilizers on Brinjal cultivars under the agro-climatic conditions of Mansehra. J. Biol. Agric. Healthc. 2015, 5, 14–19. Available online: https://www.iiste.org/Journals/index.php/JBAH/article/viewFile/23228/23684 (accessed on 6 February 2025).
- Chatzistathis, T.; Tsaniklidis, G.; Papaioannou, A.; Giannakoula, A.; Koukounaras, A. Comparative approach on the effects of soil amendments and controlled-release fertilizer application on the growth, nutrient uptake, physiological performance and fruit quality of pepper (Capsicum annuum L.) plants. Agronomy 2022, 12, 1935. [Google Scholar] [CrossRef]
- Kouassi, A.B.; Kouassi, K.B.A.; Sylla, Z.; Plazas, M.; Fonseka, R.M.; Kouassi, A.; Prohens, J. Genetic parameters of drought tolerance for agromorphological traits in eggplant, wild relatives, and inter specific hybrids. Crop Sci. 2021, 61, 55–68. [Google Scholar] [CrossRef]
- Bana, R.S.; Grover, M.; Kumar, V.; Jat, G.S.; Kuri, B.R.; Singh, D.; Kumar, H.; Bamboriya, S.D. Multi-micronutrient foliar fertilization in eggplant under diverse fertility scenarios: Effects on productivity, nutrient biofortification and soil microbial activity. Sci. Hortic. 2022, 294, 110781. [Google Scholar] [CrossRef]
- Bana, R.S.; Jat, G.S.; Grover, M.; Bamboriya, S.D.; Singh, D.; Bansal, R.; Choudhary, A.K.; Kumar, V.; Laing, A.M.; Godara, S.; et al. Foliar nutrient supplementation with micronutrient-embedded fertilizer increases biofortification, soil biological activity and productivity of eggplant. Sci. Rep. 2022, 12, 5146. [Google Scholar] [CrossRef]
- McLean, E. Soil pH and lime requirement. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph; American Society of Agronomy (ASA), Crop Science Society of America (CSSA), and Soil Science Society of America: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar] [CrossRef]
- Gee, G.; Bauder, J. Particle-size analysis. In Methods of Soil Analysis, Part 1: Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; American Society of Agronomy (ASA), Crop Science Society of America (CSSA), and Soil Science Society of America: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph; American Society of Agronomy (ASA), Crop Science Society of America (CSSA), and Soil Science Society of America: Madison, WI, USA, 1982; pp. 539–547. [Google Scholar] [CrossRef]
- Hood-Nowotny, R.; Umana, N.H.N.; Inselbacher, E.; Oswald-Lachouani, P.; Wanek, W. Alternative methods for measuring inorganic, organic, and total dissolved nitrogen. Soil Sci. Soc. Am. J. 2010, 74, 1018–1027. [Google Scholar] [CrossRef]
- Olsen, S.; Sommers, L. Phosphorus. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph; American Society of Agronomy (ASA), Crop Science Society of America (CSSA), and Soil Science Society of America: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar] [CrossRef]
- Thomas, G.W. Exchangeable cations methods of soil analysis. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph; American Society of Agronomy (ASA), Crop Science Society of America (CSSA), and Soil Science Society of America: Madison, WI, USA, 1982; pp. 159–166. [Google Scholar] [CrossRef]
- Wolf, B. The determination of boron in soil extracts, plant materials, composts, manures, water and nutrient solutions. Commun. Soil Sci. Plant Anal. 1971, 2, 363–374. [Google Scholar] [CrossRef]
- Hansen, T.H.; De Bang, T.C.; Laursen, K.H.; Pedas, P.; Husted, S.; Schjoerring, J.K. Multielement plant tissue analysis using ICP spectrometry. In Plant Mineral Nutrients. Methods in Molecular Biology (Methods and Protocols); Maathuis, F., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 953, pp. 121–141. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soils, Plants and Waters; University of California Division of Agricultural Sciences, Office of Agricultural Publications: Riverside, CA, USA, 1961; p. 309. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G., Govindjee, G., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Lucini, L.; Miras-Moreno, B.; Rouphael, Y.; Cardarelli, M.; Colla, G. Combining molecular weight fractionation and metabolomics to elucidate the bioactivity of vegetal protein hydrolysates in tomato plants. Front. Plant Sci. 2020, 11, 976. [Google Scholar] [CrossRef]
- Scalbert, A.; Monties, B.; Janin, G. Tannins in wood: Comparison of different estimation methods. J. Agric. Food Chem. 1989, 37, 1324–1329. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Dichala, O.; Giannakoula, A.E.; Therios, I. Effect of salinity on physiological and biochemical parameters of leaves in three pomegranate (Punica granatum L.) cultivars. Appl. Sci. 2022, 12, 8675. [Google Scholar] [CrossRef]
- Su, C.; Li, T.; Wang, Y.; Ge, Z.; Xiao, J.; Shi, X.; Wang, B. Comparison of phenolic composition, vitamin c, antioxidant activity, and aromatic components in apricots from Xinjiang. J. Food Sci. 2021, 87, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Shahein, M.M.; El Sayed, S.F.; Hassan, H.A.; Abou-El-Hassan, S. Producing sweet pepper organically using different sources of organic fertilizers under plastic house conditions. In Proceedings of the International Conference on Advances in Agricultural, Biological & Environmental Sciences (AABES-2015), London, UK, 22–23 July 2015. [Google Scholar]
- Therios, I. Mineral Nutrition of Plants; Dedousi Publications: Thessaloniki, Greece, 1996. (In Greek) [Google Scholar]
- Chatzistathis, T.; Therios, I. How soil nutrient availability influences plant biomass and how biomass stimulation alleviates heavy metal toxicity in soils: The cases of nutrient use efficient genotypes and phytoremediators, respectively. In Biomass Now—Cultivation and Utilization; Matovic, M.D., Ed.; IntechOpen: Rijeka, Croatia, 2013; Chapter 18; pp. 427–448. [Google Scholar] [CrossRef]
- Therios, I. Olives; CAB International: Oxfordshire, UK, 2009. [Google Scholar]
- Al Ali, M.; Gencoglan, C.; Gençoğlan, S. The effects of organic and inorganic fertilizer applications on yield and plant vegetative growth of eggplant (Solanum melongena L.). Int. J. Plant Soil Sci. 2019, 29, 1–9. [Google Scholar] [CrossRef]
- Al-Bayati, J.M.; Hamdoon, D.H. Response of eggplant Solanum melongena L. to soil mulching, organic and inorganic fertilizers on vegetative growth traits and yield grown under unheated plastic house. IOP Conf. Ser. Earth Environ. Sci. 2019, 388, 012075. [Google Scholar] [CrossRef]
- Antonious, G.F.; Turley, E.; Mishra, B.; Heist, Q.; Upadhyaya, Y.; Trivette, T.; Nkuwi, L. Characterization of eggplant grown in animal manure amended soil. Int. J. Environ. Health Res. 2019, 30, 492–503. [Google Scholar] [CrossRef]
- Sanni, K.O.; Okeowo, T.A. Growth, yield performance and cost benefit of eggplant (Solanum melongena) production using goat and pig manure in Ikorodu Lagos Nigeria. Int. J. Sci. Res. Eng. Stud. 2016, 3, 22–26. [Google Scholar]
- Swarnam, T.P.; Velmurugan, A.; Jaisankar, I.; Roy, N. Effect of foliar application of panchagavya on yield and quality characteristics of eggplant (Solanum melongena L.). Adv. Life Sci. 2016, 5, 2636–2639. Available online: https://www.researchgate.net/publication/314153540_Effect_of_Foliar_Application_of_Panchagavya_on_Yield_and_Quality_Characteristics_of_Eggplant_Solanum_melongena_L (accessed on 28 March 2025).
- Rego, A.F.; Nabais, C.N.; Viegas, E. The influence of dosing of chicken manure and water washing rice against growth and crop yield purple eggplant (Solanum melongena L.) on dry land. Int. J. Curr. Res. 2019, 10, 373–378. [Google Scholar] [CrossRef]
- Mahamad, N.I.A.; Samah, S.N.A.A.; Khidzir, M.N.A.M. Effects of different organic fertilizers on growth and yield potential of Solanum melongena (eggplant) in Malaysia. IOP Conf. Ser. Earth Environ. Sci. 2022, 1114, 012083. [Google Scholar] [CrossRef]
- Bhuvaneshwari, R.; Nayana, N.L.; Srinivasan, S.; Karthikeyan, P.; Suganthi, S. Response of humic acid and zinc fertilization on the yield characters of brinjal (Solanum melongena L.). Plant Arch. 2020, 20, 1332–1334. Available online: https://plantarchives.org/SPECIAL%20ISSUE%2020-1/1332-1334%20(563).pdf (accessed on 3 March 2025).
- Setyowati, N.; Darmina; Chozin, M.; Muktamar, Z. Application of organic fertilizer to increase growth and yield of organic eggplant (Solanum melongena L.) in Ultisols. Int. J. Eng. Sci. Invent. 2021, 10, 15–21. [Google Scholar] [CrossRef]
- Mahmood, O.H.; Jaafar, H.S. Effect of decomposed palm residues and foliar application of Marva parviflora L. leaf extract on eggplant growth and yield. Sabrao J. Breed. Genet. 2024, 56, 370–378. [Google Scholar] [CrossRef]
- Saha, B.; Saha, S.; Roy, P.D.; Fatima, A.; Sahoo, S.K.; Solankey, S.S.; Singh, H.K.; Basak, P.; Basak, N. Scheduling of Zn and B fertilization for brinjal (Solanum melongena L.): Impact on yield, nutrient use efficiency, and fruit quality. Com. Soil Sci. Plant Anal. 2023, 54, 2551–2562. [Google Scholar] [CrossRef]
- Nisar, S.; Mavi, M.S.; Singh, J.; Srivastava, S.; Dey, P. Optimizing nutrient management strategies to achieve higher productivity, greater nutrient use efficiency, in eggplant and maintenance of soil health. J. Plant Nutr. 2025. [Google Scholar] [CrossRef]
- Li, X.T.; Cao, P.; Wang, X.J.; Cao, M.J.; Yu, H.Q. Comparison of gas exchange and chlorophyll fluorescence of low-potassium-tolerant and -sensitive soybean (Glycine max L. Merr.) cultivars under low-potassium condition. Photosynthetica 2011, 49, 633–636. [Google Scholar] [CrossRef]
- Tak, H.I.; Babalola, O.O.; Huyser, M.H.; Inam, A. Urban wastewater irrigationand its effects on growth, photosynthesis and yield of chickpea under differentdoses of potassium. Soil Sci. Plant Nutr. 2013, 59, 155–167. [Google Scholar] [CrossRef]
- De Souza, A.H.C.; Rezende, R.; Lorenzoni, M.Z.; Seron, C.C.; Hachmann, T.L.; Lozano, C.S. Response of eggplant crop fertigated with doses of nitrogen and potassium. Rev. Bras. Eng. Agric. Ambient. 2017, 21, 21–26. [Google Scholar] [CrossRef]
- Cao, F.L.; Wang, G.B.; Yu, W.W. Oriented cultivation techniques for leaf-harvest plantation of Ginkgo biloba L. J. Nanjing For. Univ. 2014, 38, 146–152. [Google Scholar] [CrossRef]
- Aminifard, M.; Aroiee, H.; Azizi, M.; Nemati, H.; Jaafar, H. Effect of compost on antioxidant components and fruit quality of sweet pepper (Capsicum annuum L.). J. Cent. Eur. Agric. 2013, 14, 47–56. [Google Scholar] [CrossRef]
- Jagessar, R.C.; Chester, L.; Blair, T. Investigating the antioxidant content of sweet pepper in response to fertilisers. J. Agric. Sci. Food Technol. 2020, 6, 34–43. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Celletti, S.; Maresca, V.; Munzi, S.; Cruz, C.; Alexandrov, D.; Guarnieri, M.; Loppi, S. Foliar application of wood distillate boosts plant yield and nutritional parameters of chickpea. Ann. Appl. Biol. 2022, 182, 57–64. [Google Scholar] [CrossRef]
- Ye, Y.; Hongwei, S.; Yue, W.; Zisong, X.; Shixin, H.; Guoqiang, H.; Kuide, Y.; Zhang, H. Wood vinegar alleviates photosynthetic inhibition and oxidative damage caused by Pseudomonas syringae pv. tabaci (Pst) infection in tobacco leaves. J. Plant Interact. 2022, 17, 801–811. [Google Scholar] [CrossRef]
- González-Coria, J.; Lozano-Castellón, J.; Jaime-Rodríguez, C.; Olmo-Cunillera, A.; Laveriano-Santos, E.P.; Pérez, M.; Lamuela-Raventós, R.M.; Puig, J.; Vallverdú-Queralt, A.; Romanyà, J. The effects of differentiated organic fertilization on tomato production and phenolic content in traditional and high-yielding varieties. Antioxidants 2022, 11, 2127. [Google Scholar] [CrossRef] [PubMed]
- Bhat, B.A.; Islam, S.T.; Ali, A.; Sheikh, B.A.; Tariq, L.; Islam, S.U.; Hassan, T.U. Role of micronutrients in secondary metabolism of plants. In Plant Micronutrients; Aftab, T., Hakeem, K.R., Eds.; Springer: Cham, Switzerland, 2020; pp. 311–329. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil. 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Song, Q.; Wang, S.; Wang, Y.; Ge, Y. Root proteomics reveals the effects of wood vinegar on wheat growth and subsequent tolerance to drought stress. Int. J. Mol. Sci. 2019, 20, 943. [Google Scholar] [CrossRef] [PubMed]

| Soil Type | Soil Texture | pH | Organic Matter (%) | CaCO3 (%) | NO3-N | P | K | Mg | Ca | Fe | Zn | Mn | Cu | B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | ||||||||||||||
| GNEISS (CONTROL) | L | 6.47 | 2.94 | 0 | 32.98 | 5.83 | 72 | 287 | 1921 | 7.96 | 1.0 | 14.31 | 2.28 | 0.9 |
| PK | L | 6.49 | 2.90 | 0 | 33.27 | 5.59 | 90 | 377 | 1960 | 6.36 | 1.0 | 12.20 | 1.98 | 0.9 |
| AN + PK | L | 6.62 | 2.85 | 0.1 | 37.78 | 6.02 | 86 | 358 | 2021 | 6.90 | 1.1 | 12.85 | 2.06 | 0.8 |
| TBC | L | 6.39 | 3.01 | 0.0 | 33.05 | 5.68 | 69 | 299 | 1897 | 7.71 | 0.9 | 12.45 | 1.95 | 0.9 |
| PM | L | 6.80 | 3.09 | 0.1 | 38.36 | 5.57 | 96 | 283 | 1886 | 8.04 | 1.1 | 14.46 | 2.09 | 1.0 |
| TBC + PM | L | 6.68 | 3.05 | 0.1 | 36.46 | 6.00 | 92 | 297 | 2045 | 7.62 | 0.9 | 13.35 | 1.99 | 0.8 |
| Optimum range | 5.5–7.5 | >4 | <10 | 20–40 | 15–25 | 140–200 | 50–100 | 300–750 | 5–25 | 1–2.5 | 12–25 | 0.6–1.5 | 0.5 | |
| (a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Macronutrient Concentration (% D.W.) | Micronutrient Concentration (mg kg−1 D.W.) | |||||||||
| Organic Material | N | P | K | Ca | Mg | B | Mn | Zn | Fe | Cu |
| Poultry Manure | 2.31 | 1.38 | 2.51 | 12.78 | 1.38 | 32.67 | 298.35 | 159.1 | 4200.5 | 51.6 |
| Tree Branch Chips | 1.66 | 0.14 | 0.08 | 2.23 | 0.27 | 29.66 | 60.38 | 35.55 | 833.05 | 11.98 |
| (b) | ||||||||||
| Nutrient Content (Nutrient Units, %) | ||||||||||
| Organic Material | N | P | K | Ca | Mg | S | Mn | Zn | Fe | B |
| Patent Kali | 0 | 0 | 30 | 0 | 10 | 42.5 | 0 | 0 | 0 | 0 |
| Ammonium Nitrate | 34.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fertilization/Soil Amendment Treatment | Plant Height (cm) | ||||||
|---|---|---|---|---|---|---|---|
| 17 May (Initial) | 30 May | 9 June | 19 June | 29 June | 9 July | 19 July (Final) | |
| PK | 20.5 ± 2.0 a | 27.8 ± 2.3 a | 33.0 ± 3.0 a | 37.0 ± 1.4 ab | 38.4 ± 1.5 a | 38.4 ± 1.5 a | 38.6 ± 1.8 a |
| AN + PK | 19.3 ± 3.5 a | 29.0 ± 4.24 a | 34.3 ± 3.0 a | 37.0 ± 2.2 ab | 40.0 ± 3.4 a | 40.5 ± 3.4 a | 41.5 ± 3.4 a |
| TBC | 17.7 ± 1.8 a | 21.3 ± 2.8 bc | 24.0 ± 1.6 b | 30.0 ± 3.9 c | 34.8 ± 2.5 a | 36.8 ± 3.3 a | 37.5 ± 3.6 a |
| PM | 18.6 ± 2.9 a | 25.2 ± 3.0 ab | 33.2 ± 2.4 a | 39.2 ± 2.8 a | 41.0 ± 4.2 a | 41.8 ± 4.4 a | 42.2 ± 4.8 a |
| TBC + PM | 17.8 ± 2.9 a | 20.2 ± 3.6 c | 28.4 ± 2.1 c | 34.6 ± 3.6 b | 36.8 ± 3.1 a | 37.8 ± 3.6 a | 38.2 ± 3.4 a |
| CONTROL | 18.6 ± 2.2 a | 27.5 ± 2.7 a | 32.3 ± 1.5 a | 37.0 ± 1.9 ab | 38.3 ± 2.2 a | 38.5 ± 2.1 a | 38.8 ± 2.1 a |
| p-values | 0.115 ns | 0.000 *** | 0.000 *** | 0.001 ** | 0.052 ns | 0.178 ns | 0.180 ns |
| Fertilization Treatment | Main Shoot Elongation Rate (cm) per Day | |||||
|---|---|---|---|---|---|---|
| 17–30 May (1–13th day) | 30 May–9 June (13–23th day) | 9–19 June (23–33th day) | 19–29 June (33–43th day) | 29 June–9 July (43–53th day) | 9–19 July (53–63th day) | |
| PK | 0.64 ± 0.09 ab | 0.60 ± 0.07 b | 0.30 ± 0.10 bc | 0.14 ± 0.05 c | 0.00 ± 0.00 d | 0.02 ± 0.004 c |
| AN + PK | 0.75 ± 0.23 a | 0.60 ± 0.07 b | 0.28 ± 0.16 c | 0.30 ± 0.07 b | 0.05 ± 0.01 bc | 0.10 ± 0.03 a |
| TBC | 0.48 ± 0.08 bc | 0.17 ± 0.05 c | 0.65 ± 0.11 a | 0.57 ± 0.11 a | 0.20 ± 0.06 a | 0.08 ± 0.02 ab |
| PM | 0.51 ± 0.09 bc | 0.80 ± 0.14 a | 0.60 ± 0.12 a | 0.18 ± 0.06 bc | 0.08 ± 0.02 b | 0.04 ± 0.01 b |
| TBC + PM | 0.37 ± 0.06 c | 0.82 ± 0.19 a | 0.63 ± 0.04 a | 0.22 ± 0.04 b | 0.10 ± 0.03 b | 0.04 ± 0.01 b |
| CONTROL | 0.68 ± 0.08 a | 0.48 ± 0.13 b | 0.48 ± 0.11 ab | 0.13 ± 0.03 c | 0.03 ± 0.005 c | 0.03 ± 0.003 bc |
| p-values | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.016 * |
| Treatment | Biomass | ||
|---|---|---|---|
| Plant Tissue Type | Fertilization Treatment | FW (g) | DW (g) |
| Leaves | PK | 29.89 ± 6.12 ijkl B (c) | 3.66 ± 0.64 def C (b) |
| AN + PK | 64.53 ± 16.08 fghi A (b) | 9.12 ± 2.11 cd A (b) | |
| TBC | 25.35 ± 2.92 jkl B (c) | 3.56 ± 0.54 def C (b) | |
| PM | 53.30 ± 8.71 fghijkl A (b) | 6.46 ± 0.69 cdef B (b) | |
| TBC + PM | 37.48 ± 8.25 ghijkl B (bc) | 4.71 ± 0.98 cdef C (b) | |
| CONTROL | 27.55 ± 5.00 jkl B (c) | 3.37 ± 0.48 ef C (b) | |
| Shoots | PK | 23.69 ± 1.87 kl C (c) | 5.51 ± 0.57 cdef BC (ab) |
| AN + PK | 32.40 ± 3.92 hijkl AB (b) | 7.27 ± 1.50 cdef A (b) | |
| TBC | 19.59 ± 2.66 l C (d) | 4.36 ± 0.69 def C (a) | |
| PM | 36.60 ± 3.47 ghijkl A(b) | 8.08 ± 1.09 cde A (b) | |
| TBC + PM | 30.45 ± 6.29 hijkl B (c) | 6.00 ± 0.99 cdef B (b) | |
| CONTROL | 22.86 ± 1.83 l C (c) | 5.39 ± 0.60 cdef BC (ab) | |
| Roots | PK | 67.13 ± 14.80 fgh B (b) | 7.34 ± 1.63 cdef B (a) |
| AN + PK | 60.13 ± 20.91 fghijk B (b) | 7.37 ± 3.39 cdef B (b) | |
| TBC | 37.34 ± 2.68 ghijkl C (b) | 4.04 ± 0.17 def C (ab) | |
| PM | 85.86 ± 3.83 ef A (b) | 9.97 ± 1.01 c A (b) | |
| TBC + PM | 71.01 ± 9.64 fg AB (b) | 6.21 ± 1.23 cdef BC (b) | |
| CONTROL | 64.84 ± 10.92 fghi B (b) | 6.18 ± 0.77 cdef BC (ab) | |
| Fruits | PK | 129.43 ± 27.26 d B (a) | 7.14 ± 2.36 cdef BC (a) |
| AN + PK | 248.52 ± 55.03 b A (a) | 14.61 ± 2.34 b AB (a) | |
| TBC | 61.62 ± 0.00 fghij C (a) | 2.40 ± 0.00 f C (c) | |
| PM | 291.70 ± 77.61 a A (a) | 23.64 ± 11.08 a A (a) | |
| TBC + PM | 175.53 ± 48.77 c B (a) | 15.41 ± 11.48 b AB (a) | |
| CONTROL | 115.99 ± 30.52 de BC (a) | 7.72 ± 4.38 cdef BC (a) | |
| Treatment | Macronutrient Concentrations | |||
|---|---|---|---|---|
| Plant Tissue Type | Fertilization Treatment | N | P (% D.W.) | K |
| Leaves | PK | 1.53 ± 0.11 c C (a) | 0.09 ± 0.01 jk C (b) | 1.77 ± 0.27 jkl BC (c) |
| AN + PK | 2.33 ± 0.32 a A (a) | 0.06 ± 0.01 lm D (c) | 1.87 ± 0.15 ijk ABC (b) | |
| TBC | 1.55 ± 0.12 c C (a) | 0.29 ± 0.01 c B (a) | 1.57 ± 0.23 l C (c) | |
| PM | 1.68 ± 0.22 c C (a) | 0.29 ± 0.02 c B (a) | 2.15 ± 0.28 fghi A (b) | |
| TBC + PM | 2.03 ± 0.22 b B (a) | 0.39 ± 0.01 a A (a) | 2.09 ± 0.36 ghi AB (c) | |
| CONTROL | 1.63 ± 0.10 c C (a) | 0.10 ± 0.01 ij C (b) | 1.52 ± 0.26 l C (c) | |
| Shoots | PK | 0.52 ± 0.09 g B (c) | 0.05 ± 0.01 mn D (c) | 2.06 ± 0.16 ghi BC (bc) |
| AN + PK | 1.00 ± 0.16 f A (c) | 0.04 ± 0.01 n D (d) | 1.52 ± 0.17 l D (c) | |
| TBC | 0.54 ± 0.05 g B (c) | 0.10 ± 0.01 ij C (d) | 1.87 ± 0.22 ijk C (b) | |
| PM | 0.59 ± 0.09 g B (c) | 0.12 ± 0.02 h B (c) | 2.26 ±0.12 efg AB (b) | |
| TBC + PM | 0.50 ± 0.07 g B (d) | 0.19 ± 0.03 f A (c) | 2.38 ± 0.19 ef A (c) | |
| CONTROL | 0.57 ± 0.11 g B (c) | 0.05 ± 0.00 mn D (c) | 1.93 ± 0.06 hij C (b) | |
| Roots | PK | 1.13 ± 0.15 def B (b) | 0.10 ± 0.00 ij D (b) | 2.20 ± 0.14 efgh B (b) |
| AN + PK | 1.70 ± 0.11 c A (b) | 0.08 ± 0.01 kl E (b) | 0.97 ± 0.13 m E (d) | |
| TBC | 1.23 ± 0.11 d B (b) | 0.19 ± 0.01 f C (c) | 1.65 ± 0.14 jkl D (bc) | |
| PM | 1.14 ± 0.09 def B (b) | 0.25 ± 0.02 de B (b) | 1.93 ± 0.32 hij C (b) | |
| TBC + PM | 1.25 ± 0.07 def B (b) | 0.37 ± 0.01 b A (a) | 2.89 ± 0.09 c A (b) | |
| CONTROL | 1.19 ± 0.07 def B (b) | 0.11 ± 0.01 hi D (b) | 1.59 ± 0.27 kl D (c) | |
| Fruits | PK | 1.02 ± 0.14 ef C (b) | 0.17 ± 0.02 g D (a) | 2.70 ± 0.30 cd B (a) |
| AN + PK | 1.72 ± 0.12 c A (b) | 0.10 ± 0.01 ij E (a) | 2.48 ± 0.18 de BC (a) | |
| TBC | 1.18 ± 0.00 def BC (b) | 0.25 ± 0.00 de AB (b) | 2.61 ± 0.00 a B (a) | |
| PM | 1.21 ± 0.17 de B (b) | 0.25 ± 0.02 e B (b) | 2.75 ± 0.21 c B (a) | |
| TBC + PM | 1.02 ± 0.11 ef C (c) | 0.27 ± 0.01 d A (b) | 3.25 ± 0.16 b A (a) | |
| CONTROL | 1.09 ± 0.13 def BC (b) | 0.19 ± 0.01 f C (a) | 2.45 ± 0.06 de C (a) | |
| Treatment | Ca, Mg and Na Concentrations | |||
|---|---|---|---|---|
| Plant Tissue Type | Fertilization Treatment | Ca (% D.W.) | Mg (% D.W.) | Na (% D.W.) |
| Leaves | PK | 1.86 ± 0.11 c C (a) | 0.59 ± 0.05 c C (a) | 0.15 ± 0.02 j ABC (c) |
| AN + PK | 1.50 ± 0.25 d D (a) | 0.76 ± 0.09 a A (a) | 0.18 ± 0.02 hij A (b) | |
| TBC | 2.09 ± 0.07 b BC (a) | 0.67 ± 0.03 b B (a) | 0.13 ± 0.01 j BCD (d) | |
| PM | 2.25 ± 0.25 a AB (a) | 0.79 ± 0.05 a A (a) | 0.11 ± 0.05 j D (c) | |
| TBC + PM | 2.32 ± 0.11 a A (a) | 0.80 ± 0.02 a A (a) | 0.13 ± 0.01 j CD (c) | |
| CONTROL | 1.92 ± 0.08 c C (a) | 0.64 ± 0.04 bc BC (a) | 0.16 ± 0.02 j AB (c) | |
| Shoots | PK | 0.70 ± 0.07 e B (b) | 0.26 ± 0.04 ghi BC (c) | 0.25 ± 0.01 fghi B (b) |
| AN + PK | 0.69 ± 0.06 e B (b) | 0.29 ± 0.01 gh AB (c) | 0.29 ± 0.02 f A (b) | |
| TBC | 0.70 ± 0.08 e B (b) | 0.21 ± 0.02 i D (c) | 0.27 ± 0.02 fg AB (b) | |
| PM | 0.80 ± 0.04 e A (b) | 0.22 ± 0.01 i CD (c) | 0.24 ± 0.02 fghi B (b) | |
| TBC + PM | 0.78 ± 0.05 e AB (b) | 0.28 ± 0.05 gh AB (c) | 0.25 ± 0.03 fghi B (b) | |
| CONTROL | 0.76 ± 0.06 e AB (b) | 0.31 ± 0.03 g A (c) | 0.26 ± 0.02 fgh B (b) | |
| Roots | PK | 0.48 ± 0.03 fg B (b) | 0.44 ± 0.07 cd AB (b) | 0.56 ± 0.10 d D (a) |
| AN + PK | 0.57 ± 0.04 f A (b) | 0.39 ± 0.02 ef BC (b) | 0.96 ± 0.17 b B (a) | |
| TBC | 0.47 ± 0.03 fg B (c) | 0.43 ± 0.03 def ABC (b) | 0.72 ± 0.08 c C (a) | |
| PM | 0.48 ± 0.01 fg B (c) | 0.40 ± 0.04 ef BC (b) | 0.43 ± 0.11 e D (a) | |
| TBC + PM | 0.49 ± 0.06 fg B (c) | 0.47 ± 0.03 d A (b) | 0.44 ± 0.08 e D (a) | |
| CONTROL | 0.51 ± 0.01 fg B (c) | 0.38 ± 0.01 f C (b) | 1.12 ± 0.09 a A (a) | |
| Fruits | PK | 0.29 ± 0.04 hi B (b) | 0.27 ± 0.05 ghi BC (c) | 0.15 ± 0.03 j BC (c) |
| AN + PK | 0.26 ± 0.07 i B (c) | 0.24 ± 0.02 hi C (c) | 0.17 ± 0.02 ij B (b) | |
| TBC | 0.46 ± 0.00 fg B (c) | 0.43 ± 0.00 def A (b) | 0.19 ± 0.00 ghij A (c) | |
| PM | 0.23 ± 0.05 i B (d) | 0.25 ± 0.01 hi BC (c) | 0.15 ± 0.01 j B (c) | |
| TBC + PM | 0.23 ± 0.05 i B (d) | 0.26 ± 0.01 ghi BC (c) | 0.17 ± 0.01 ij B (c) | |
| CONTROL | 0.40 ± 0.05 gh A (d) | 0.28 ± 0.03 gh B (c) | 0.13 ± 0.01 j C (c) | |
| Treatment | Micronutrient Concentrations (μg g−1 D.W.) | ||||
|---|---|---|---|---|---|
| Plant Tissue | Fertilization | Fe | Mn | Cu | Zn |
| Leaves | PK | 247.4 ± 11.1 fgh C (b) | 97.4 ± 6.2 bc B (a) | 10.2 ± 1.3 e A (b) | 48.9 ± 1.9 ghi A (c) |
| AN + PK | 300.6 ± 34.7 def AB (b) | 132.3 ± 52.6 a A (a) | 8.4 ± 1.0 e B (b) | 51.5 ± 11.4 ghi A (c) | |
| TBC | 266.7 ± 25.1 efg BC (a) | 80.7 ± 5.0 cd B (a) | 7.6 ± 0.5 e B (c) | 44.7 ± 4.3 hi A (c) | |
| PM | 346.4 ± 39.3 cd A (b) | 107.2 ± 15.8 b AB (a) | 8.1 ± 0.9 e B (b) | 47.9 ± 9.6 ghi A (a) | |
| TBC + PM | 224.2 ± 7.9 ghi C (b) | 78.2 ± 6.5 d B (a) | 8.4 ± 1.1 e B (b) | 44.5 ± 7.9 hi A (c) | |
| CONTROL | 306.5 ± 60.9 de AB (b) | 91.4 ± 4.2 bcd B (a) | 9.9 ± 1.1 e A (b) | 52.3 ± 4.8 ghi A (c) | |
| Shoots | PK | 205.5 ± 22.9 hij BC (c) | 26.3 ± 4.4 f B (c) | 7.5 ± 1.0 e BC (b) | 83.0 ± 10.5 e B (b) |
| AN + PK | 174.2 ± 9.6 ij D (c) | 34.7 ± 5.6 ef A (bc) | 6.3 ± 0.5 e CD (b) | 128.2 ± 16.8 a A (a) | |
| TBC | 208.2 ± 18.9 hij BC (b) | 22.8 ± 1.4 f BC (c) | 9.1 ± 1.4 e A (bc) | 96.7 ± 6.1 bcd B (a) | |
| PM | 216.5 ± 18.0 ghij AB (c) | 21.8 ± 1.7 f BC (c) | 6.0 ± 0.9 e D (b) | 58.7 ± 5.7 fg C (a) | |
| TBC + PM | 167.7 ± 32.0 ij D (b) | 19.3 ± 1.6 f C (c) | 9.0 ± 0.7 e A (b) | 91.2 ±11.5 cde B (b) | |
| CONTROL | 248.2 ± 44.8 fgh A (c) | 31.3 ± 5.0 ef A (b) | 8.3 ± 1.1 e AB (b) | 89.6 ± 9.1 de B (b) | |
| Roots | PK | 394.1 ± 34.1 bc A (a) | 47.4 ± 20.8 e B (b) | 58.6 ± 12.0 b AB (a) | 102.3 ± 11.9 bc A (a) |
| AN + PK | 409.0 ± 113.5 b A (a) | 73.0 ± 24.9 d A (b) | 62.3 ± 3.2 ab A (a) | 68.0 ± 6.5 f B (b) | |
| TBC | 202.9 ± 53.7 hij B (bc) | 37.0 ± 2.9 ef B (b) | 48.7 ± 6.2 c B (a) | 98.5 ± 7.3 bcd A (a) | |
| PM | 443.6 ± 60.4 ab A (a) | 33.8 ± 4.6 ef B (b) | 22.6 ± 3.6 d C (a) | 58.4 ± 5.1 fg B (a) | |
| TBC + PM | 478.1 ± 81.5 a A (a) | 33.2 ± 5.3 ef B (b) | 57.4 ± 12.5 b AB (a) | 105.3 ± 10.5 b A (a) | |
| CONTROL | 415.3 ± 27.7 b A (a) | 36.1 ± 2.5 ef B (b) | 66.6 ± 5.6 a A (a) | 102.4 ± 10.9 bc A (a) | |
| Fruits | PK | 40.2 ± 13.0 k D (d) | 24.1 ± 5.8 f B (c) | 8.1 ± 0.7 e C (b) | 42.7 ± 2.9 i B (c) |
| AN + PK | 75.3 ± 14.8 k B (d) | 22.5 ± 2.3 f BC (c) | 7.6 ± 1.0 e C (b) | 40.5 ± 5.3 i B (c) | |
| TBC | 164.4 ± 0.0 j A (c) | 35.6 ± 0.0 ef A (b) | 12.3 ± 0.0 e A (b) | 55.9 ± 0.0 gh A (b) | |
| PM | 44.7 ± 8.2 k CD (d) | 19.1 ± 2.1 f CD (c) | 7.7 ± 1.2 e C (b) | 41.4 ± 4.7 i B (b) | |
| TBC + PM | 30.8 ± 3.5 k D (c) | 17.2 ± 0.7 f D (c) | 7.3 ± 0.6 e C (b) | 44.6 ± 5.8 hi B (c) | |
| CONTROL | 58.2 ± 19.6 k C (d) | 24.5 ± 1.8 f B (c) | 10.2 ± 1.8 e B (b) | 42.5 ± 5.1 i B (c) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzistathis, T.; Sarropoulou, V.; Papaioannou, E.; Giannakoula, A. Inorganic and Organic Fertilization Effects on the Growth, Nutrient Uptake, Chlorophyll Fluorescence and Fruit Quality in Solanum melongena L. Plants. Agronomy 2025, 15, 872. https://doi.org/10.3390/agronomy15040872
Chatzistathis T, Sarropoulou V, Papaioannou E, Giannakoula A. Inorganic and Organic Fertilization Effects on the Growth, Nutrient Uptake, Chlorophyll Fluorescence and Fruit Quality in Solanum melongena L. Plants. Agronomy. 2025; 15(4):872. https://doi.org/10.3390/agronomy15040872
Chicago/Turabian StyleChatzistathis, Theocharis, Virginia Sarropoulou, Evgenia Papaioannou, and Anastasia Giannakoula. 2025. "Inorganic and Organic Fertilization Effects on the Growth, Nutrient Uptake, Chlorophyll Fluorescence and Fruit Quality in Solanum melongena L. Plants" Agronomy 15, no. 4: 872. https://doi.org/10.3390/agronomy15040872
APA StyleChatzistathis, T., Sarropoulou, V., Papaioannou, E., & Giannakoula, A. (2025). Inorganic and Organic Fertilization Effects on the Growth, Nutrient Uptake, Chlorophyll Fluorescence and Fruit Quality in Solanum melongena L. Plants. Agronomy, 15(4), 872. https://doi.org/10.3390/agronomy15040872









