Exploring Opuntia ficus-indica as a Strategy to Mitigate High Temperatures Effects in Vineyards: Insights into Physiological and Proteomic Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Place
2.2. Opuntia ficus-indica Extract
2.3. Plant Material and Experimental Design
2.4. Measurements of Selected Physiological Parameters
2.4.1. Stomatal Conductance and Leaf Water Potential
2.4.2. Chlorophyll Content and Relative Water Content
2.5. Proteome Profile Analysis Through Two-Dimensional Electrophoresis (2-DE)
2.5.1. Protein Extraction and Quantification
2.5.2. 2-DE and Gel Image Analysis
2.5.3. Protein Identification by Mass Spectrometry Analysis (MS)
2.6. Statistical Analysis
3. Results
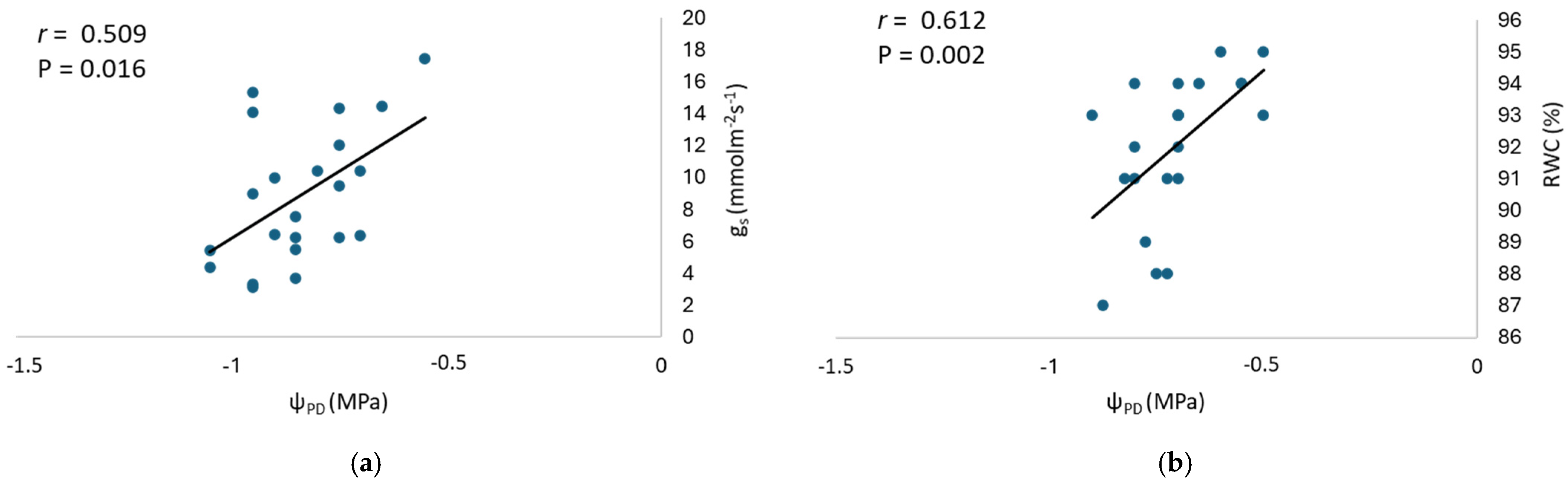
3.1. Physiological Parameters
3.2. Two-Dimensional Protein Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSts | Biostimulants |
| ROS | Reactive Oxygen Species |
| AMF | Arbuscular Mycorrhizal Fungi |
| ABA | Abscisic acid |
| GPX | Guaiacyl peroxidase |
| CAT | Catalase |
| ψ | Leaf water potential |
| gs | Stomatal conductance |
| RWC | Relative water content |
| FW | Fresh weight |
| TW | Turgid weight |
| DW | Dry weight |
| 2-DE | Two-dimensional electrophoresis |
| IEF | Isoelectric focusing |
| SDS | Sodium dodecyl sulfate |
| DTT | Dithiothreitol |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| CBB | Coomassie Brilliant Blue |
| MALDI-TOF | Matrix-Assisted Laser Desorption⁄Ionization Time-Of-Flight Mass Spectrometry |
| CHCA | Alpha-cyano-4-hydroxycinnamic acid |
| MS | Mass spectrometry |
| MW | Molecular weight |
| pI | Isoelectric point |
| RuBisCO | Ribulose bisphosphate carboxylase |
| HSP | Heat Shock Protein |
| CO₂ | Carbon dioxide |
References
- Santos, R.B.; Figueiredo, A. Biotic and Abiotic Stress Management in Grapevine: Recent Advances and Major Breakthroughs. Agronomy 2023, 13, 1584. [Google Scholar] [CrossRef]
- Prasad, P.; Staggenborg, S.; Ristic, Z. Impacts of Drought and/or Heat Stress on Physiological, Developmental, Growth, and Yield Processes of Crop Plants. Resp. Crops Limit. Water Underst. Model. Water Stress Eff. Plant Growth Process. 2008, 1, 301–355. [Google Scholar]
- Essemine, J.; Ammar, S.; Bouzid, S. Impact of Heat Stress on Germination and Growth in Higher Plants: Physiological, Biochemical and Molecular Repercussions and Mechanisms of Defence. J. Biol. Sci. 2010, 10, 565–572. [Google Scholar] [CrossRef]
- Tan, W.; Qing, W.M.; Brestic, M.; Olsovska, K.; Yang, X. Photosynthesis Is Improved by Exogenous Calcium in Heat-Stressed Tobacco Plants. J. Plant Physiol. 2011, 168, 2063–2071. [Google Scholar] [PubMed]
- Rustioni, L.; Rocchi, L.; Guffanti, E.; Cola, G.; Failla, O. Characterization of Grape (Vitis vinifera L.) Berry Sunburn Symptoms by Reflectance. J. Agric. Food Chem. 2014, 62, 3043–3046. [Google Scholar]
- Bhupenchandra, I.; Chongtham, S.K.; Devi, E.L.; Ramesh, R.; Choudhary, A.K.; Salam, M.D.; Sahoo, M.R.; Bhutia, T.L.; Devi, S.H.; Thounaojam, A.S.; et al. Role of Biostimulants in Mitigating the Effects of Climate Change on Crop Performance. Front. Plant Sci. 2022, 13, 967665. [Google Scholar]
- Kauffman, G.L.; Kneivel, D.P.; Watschke, T.L. Effects of a Biostimulant on the Heat Tolerance Associated with Photosynthetic Capacity, Membrane Thermostability, and Polyphenol Production of Perennial Ryegrass. Crop Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar]
- Castiglione, A.M.; Mannino, G.; Contartese, V.; Bertea, C.M.; Ertani, A. Microbial Biostimulants as Response to Modern Agriculture Needs: Composition, Role and Application of These Innovative Products. Plants 2021, 10, 1533. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef]
- Rakkammal, K.; Maharajan, T.; Ceasar, S.A.; Ramesh, M. Biostimulants and Their Role in Improving Plant Growth under Drought and Salinity. Cereal Res. Commun. 2023, 51, 61–74. [Google Scholar] [CrossRef]
- Khurana, A.; Kumar, V. State of Biofertilizers and Organic Fertilizers in India; Centre for Science and Environment: New Delhi, India, 2022. [Google Scholar]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The Role of Biostimulants and Bioeffectors as Alleviators of Abiotic Stress in Crop Plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Monteiro, E.; Gonçalves, B.; Cortez, I.; Castro, I. The Role of Biostimulants as Alleviators of Biotic and Abiotic Stresses in Grapevine: A Review. Plants 2022, 11, 396. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-Based Biostimulants: Sustainable Applications in Agriculture for the Stimulation of Plant Growth, Stress Tolerance, and Disease Management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Mattii, G.B. Biostimulants in Viticulture: A Sustainable Approach against Biotic and Abiotic Stresses. Plants 2022, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Irani, H.; ValizadehKaji, B.; Naeini, M.R. Biostimulant-Induced Drought Tolerance in Grapevine Is Associated with Physiological and Biochemical Changes. Chem. Biol. Technol. Agric. 2021, 8, 5. [Google Scholar] [CrossRef]
- Olavarrieta, C.E.; Sampedro, M.C.; Vallejo, A.; Štefelová, N.; Barrio, R.J.; De Diego, N. Biostimulants as an Alternative to Improve the Wine Quality from Vitis vinifera (Cv. Tempranillo) in La Rioja. Plants 2022, 11, 1594. [Google Scholar] [CrossRef]
- Samuels, L.J.; Setati, M.E.; Blancquaert, E.H. Towards a Better Understanding of the Potential Benefits of Seaweed Based Biostimulants in Vitis vinifera L. Cultivars. Plants 2022, 11, 348. [Google Scholar] [CrossRef]
- Pardo-García, A.I.; Martínez-Gil, A.M.; Cadahía, E.; Pardo, F.; Alonso, G.L.; Salinas, M.R. Oak Extract Application to Grapevines as a Plant Biostimulant to Increase Wine Polyphenols. Food Res. Int. 2014, 55, 150–160. [Google Scholar] [CrossRef]
- Zhang, Q.; White, J.F. Bioprospecting Desert Plants for Endophytic and Biostimulant Microbes: A Strategy for Enhancing Agricultural Production in a Hotter, Drier Future. Biology 2021, 10, 961. [Google Scholar] [CrossRef]
- Bellumori, M.; Innocenti, M.; Andrenelli, L.; Melani, F.; Cecchi, L.; Pandino, G.; Mauromicale, G.; La Malfa, S.; Mulinacci, N. Composition of Discarded Sicilian Fruits of Opuntia ficus indica L.: Phenolic Content, Mineral Profile and Antioxidant Activity in Peel, Seeds and Whole Fruit. Food Chem. 2023, 428, 136756. [Google Scholar]
- Kallel, F.; Chaibi, Z.; Neifar, M.; Chaabouni, S.E. Effect of Enzymatic Treatments and Microfiltration on the Physicochemical Properties and Antioxidant Activities of Two Tunisian Prickly Pear Juices. Process Biochem. 2023, 132, 140–151. [Google Scholar]
- Murta, G.; Rato, A.E.; Martins, M.d.R.; Coelho, R. Application of Opuntia ficus indica Extracts on Grapevine to Mitigate the Effects of Climate Change. In Proceedings of the VIII PhD Students Meeting in Environment and Agriculture, Universidade de Évora, Evora, Portugal, 11–12 December 2023. [Google Scholar]
- Pedroso, V.; Martins, S.; Brites, J.; Lopes, C. Efeito Do Porta-Enxerto No Vigor, Rendimento e Qualidade Do Mosto Da Casta Touriga Nacional Na Região Do Dão. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; de Vitivinicultura do Alentejo, ATEVA/CVRA: Evora, Portugal, 1960. [Google Scholar]
- Wessel, D.; Flügge, U.I. A Method for the Quantitative Recovery of Protein in Dilute Solution in the Presence of Detergents and Lipids. Anal. Biochem. 1984, 138, 141–143. [Google Scholar] [PubMed]
- Hammond, J.B.W.; Kruger, N.J. The Bradford Method for Protein Quantitation. In New Protein Techniques; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 1988; pp. 25–32. ISBN 978-1-59259-490-0. [Google Scholar]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar]
- Levene, H. Robust Tests for Equality of Variances. Contrib. Probab. Stat. 1960, 278–292. [Google Scholar]
- Buckley, T.N. How Do Stomata Respond to Water Status? New Phytol. 2019, 224, 21–36. [Google Scholar]
- Van Zyl, J.L. Diurnal Variation in Grapevine Water Stress as a Function of Changing Soil Water Status and Meteorological Conditions. SAJEV S. Afr. J. Enol. Vitic. 2017, 8, 2314. [Google Scholar] [CrossRef]
- Vaz, M.; Coelho, R.; Rato, A.; Samara-Lima, R.; Silva, L.L.; Campostrini, E.; Mota, J.B. Adaptive Strategies of Two Mediterranean Grapevines Varieties (Aragonez syn. Tempranillo and Trincadeira) Face Drought: Physiological and Structural Responses. Theor. Exp. Plant Physiol. 2016, 28, 205–220. [Google Scholar]
- Marinho, L.B.; Rodrigues, J.J.V.; Soares, J.M.; Santos, I.S.; Brandão, E.O.; Lima Filho, J.M.P. Potencial de água no solo e na folha da videira “Sugraone” sob déficit hídrico. Rev. Bras. Eng. Agrícola Ambient. 2011, 15, 1115–1122. [Google Scholar] [CrossRef]
- Edwards, E.J.; Smithson, L.; Graham, D.C.; Clingeleffer, P.R. Grapevine Canopy Response to a High-Temperature Event during Deficit Irrigation. Aust. J. Grape Wine Res. 2011, 17, 153–161. [Google Scholar] [CrossRef]
- Melo, G.B.; da Silva, A.G.; da Costa, A.C.; Alves da Silva, A.; Rosa, M.; Bessa, L.A.; Rodrigues, C.R.; Castoldi, G.; Vitorino, L.C. Foliar Application of Biostimulant Mitigates Water Stress Effects on Soybean. Agronomy 2024, 14, 414. [Google Scholar] [CrossRef]
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine Responses to Heat Stress and Global Warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef] [PubMed]
- Dubeux, J. Use of Cactus for Livestock Feeding; Universidad Federal Rural de Pernambuco (UFRPE): Pernambuco, Brazil, 2011. [Google Scholar]
- Abbas, E.Y.; Ezzat, M.I.; El Hefnawy, H.M.; Abdel-Sattar, E. An Overview and Update on the Chemical Composition and Potential Health Benefits of Opuntia ficus-indica (L.) Miller. J. Food Biochem. 2022, 46, 14310. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Cactus Stems (Opuntia spp.): A Review on Their Chemistry, Technology, and Uses. Mol. Nutr. Food Res. 2005, 49, 175–194. [Google Scholar] [CrossRef]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction and Characterization of Three Polysaccharides Extracted from Opuntia ficus indica Cladodes. Int. J. Biol. Macromol. 2016, 92, 441–450. [Google Scholar] [CrossRef]
- Bertamini, M.; Zulini, L.; Muthuchelian, K.; Nedunchezhian, N. Effect of Water Deficit on Photosynthetic and Other Physiological Responses in Grapevine (Vitis vinifera L. Cv. Riesling) Plants. Photosynthetica 2006, 44, 151–154. [Google Scholar] [CrossRef]
- Chyliński, W.K.; Łukaszewska, A.J.; Kutnik, K. Drought Response of Two Bedding Plants. Acta Physiol. Plant. 2007, 29, 399–406. [Google Scholar] [CrossRef]
- Mazorra, L.M.; Núñez, M.; Hechavarria, M.; Coll, F.; Sánchez-Blanco, M.J. Influence of Brassinosteroids on Antioxidant Enzymes Activity in Tomato Under Different Temperatures. Biol. Plant. 2002, 45, 593–596. [Google Scholar] [CrossRef]
- Abdi, S.; Abbaspur, N.; Avestan, S.; Barker, A.V. Sana Physiological Responses of Two Grapevine (Vitis vinifera L.) Cultivars to CycocelTM Treatment during Drought. J. Hortic. Sci. Biotechnol. 2016, 91, 211–219. [Google Scholar] [CrossRef]
- Min, Z.; Li, R.; Chen, L.; Zhang, Y.; Li, Z.; Liu, M.; Ju, Y.; Fang, Y. Alleviation of Drought Stress in Grapevine by Foliar-Applied Strigolactones. Plant Physiol. Biochem. 2019, 135, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Fahim, S.; Ghanbari, A.; Naji, A.M.; Shokohian, A.A.; Maleki Lajayer, H.; Gohari, G.; Hano, C. Multivariate Discrimination of Some Grapevine Cultivars under Drought Stress in Iran. Horticulturae 2022, 8, 871. [Google Scholar] [CrossRef]
- Karami, L.; Ghaderi, N.; Javadi, T. Morphological and Physiological Responses of Grapevine (Vitis vinifera L.) to Drought Stress and Dust Pollution. Folia Hortic. 2017, 29, 231–240. [Google Scholar] [CrossRef]
- Tahmaz, H.; Yüksel Küskü, D. Investigation of Some Physiological and Chemical Changes in Shoots and Leaves Caused by UV-C Radiation as an Abiotic Stress Source in Grapevine Cuttings. Sci. Hortic. 2024, 336, 113383. [Google Scholar] [CrossRef]
- Ghaderi, N.; Talaie, A.; Ebadi, A.; Lessani, H. The Physiological Response of Three Iranian Grape Cultivars to Progressive Drought Stress. J. Agr. Sci. Tech 2011, 13, 601–610. [Google Scholar]
- Meggio, F.; Trevisan, S.; Manoli, A.; Ruperti, B.; Quaggiotti, S. Systematic Investigation of the Effects of a Novel Protein Hydrolysate on the Growth, Physiological Parameters, Fruit Development and Yield of Grapevine (Vitis vinifera L., Cv Sauvignon Blanc) under Water Stress Conditions. Agronomy 2020, 10, 1785. [Google Scholar] [CrossRef]
- Sabır, A.; Jalil, O.T.J. Changes in leaf and shoot water statutes of grapevines in response to contrasting water availability and glycine betaine pulverization. Int. J. Agric. Environ. Food Sci. 2017, 1, 20–26. [Google Scholar]
- Wu, J.; Zhong, H.; Ma, Y.; Bai, S.; Yadav, V.; Zhang, C.; Zhang, F.; Shi, W.; Abudureheman, R.; Wang, X. Effects of Different Biostimulants on Growth and Development of Grapevine Seedlings under High-Temperature Stress. Horticulturae 2024, 10, 269. [Google Scholar] [CrossRef]
- Xiao, F.; Yang, Z.Q.; Lee, K.W. Photosynthetic and Physiological Responses to High Temperature in Grapevine (Vitis vinifera L.) Leaves during the Seedling Stage. J. Hortic. Sci. Biotechnol. 2017, 92, 2–10. [Google Scholar] [CrossRef]
- Bavaresco, L.; Lucini, L.; Squeri, C.; Zamboni, M.; Frioni, T. Protein Hydrolysates Modulate Leaf Proteome and Metabolome in Water-Stressed Grapevines. Sci. Hortic. 2020, 270, 109413. [Google Scholar] [CrossRef]
- von Caemmerer, S. Rubisco Carboxylase/Oxygenase: From the Enzyme to the Globe: A Gas Exchange Perspective. J. Plant Physiol. 2020, 252, 153240. [Google Scholar] [CrossRef] [PubMed]
- Andrews, T.J.; Lorimer, G.H. 3—Rubisco: Structure, Mechanisms, and Prospects for Improvement. In Photosynthesis; Hatch, M.D., Boardman, N.K., Eds.; Academic Press: Cambridge, MA, USA, 1987; pp. 131–218. [Google Scholar]
- Liu, G.-T.; Ma, L.; Duan, W.; Wang, B.-C.; Li, J.-H.; Xu, H.-G.; Yan, X.-Q.; Yan, B.-F.; Li, S.-H.; Wang, L.-J. Differential Proteomic Analysis of Grapevine Leaves by iTRAQ Reveals Responses to Heat Stress and Subsequent Recovery. BMC Plant Biol. 2014, 14, 110. [Google Scholar]
- Król, A.; Weidner, S. Changes in the Proteome of Grapevine Leaves (Vitis vinifera L.) during Long-Term Drought Stress. J. Plant Physiol. 2017, 211, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Azri, W.; Cosette, P.; Guillou, C.; Rabhi, M.; Nasr, Z.; Mliki, A. Physiological and Proteomic Responses to Drought Stress in Leaves of Two Wild Grapevines (Vitis sylvestris): A Comparative Study. Plant Growth Regul. 2020, 91, 37–52. [Google Scholar]
- Ilangumaran, G.; Subramanian, S.; Smith, D.L. Soybean Leaf Proteomic Profile Influenced by Rhizobacteria Under Optimal and Salt Stress Conditions. Front. Plant Sci. 2022, 13, 809906. [Google Scholar]
- Goswami, A.K.; Jain, M.K.; Paul, B. α- and β-Amylases in Seed Germination. Biol. Plant. 1977, 19, 469–471. [Google Scholar]
- Vierling, E. The Roles of Heat Shock Proteins in Plants. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1991, 42, 579–620. [Google Scholar]
- Qiu, C.; Sun, J.; Shen, J.; Zhang, S.; Ding, Y.; Gai, Z.; Fan, K.; Song, L.; Chen, B.; Ding, Z.; et al. Fulvic Acid Enhances Drought Resistance in Tea Plants by Regulating the Starch and Sucrose Metabolism and Certain Secondary Metabolism. J. Proteom. 2021, 247, 104337. [Google Scholar]

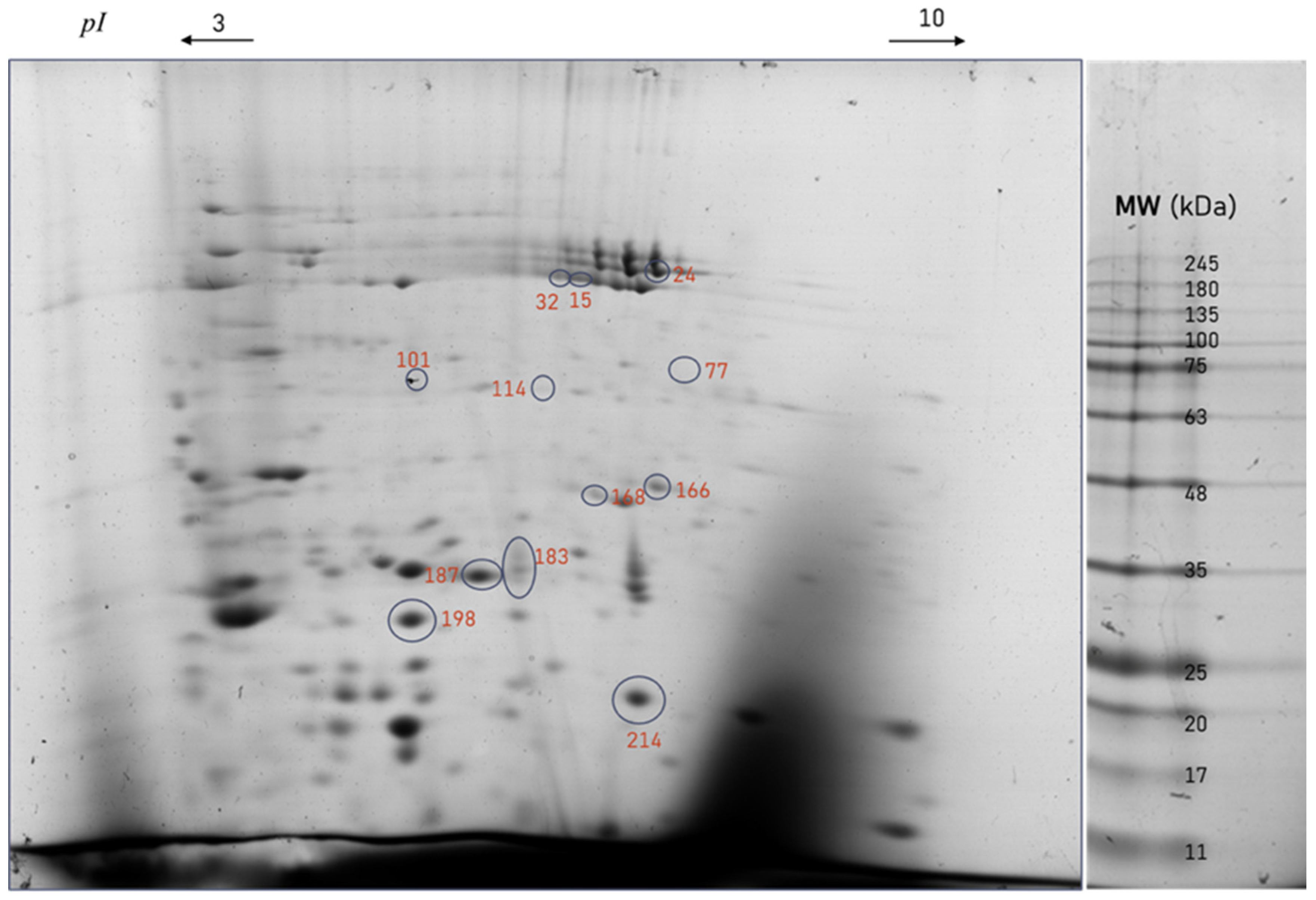
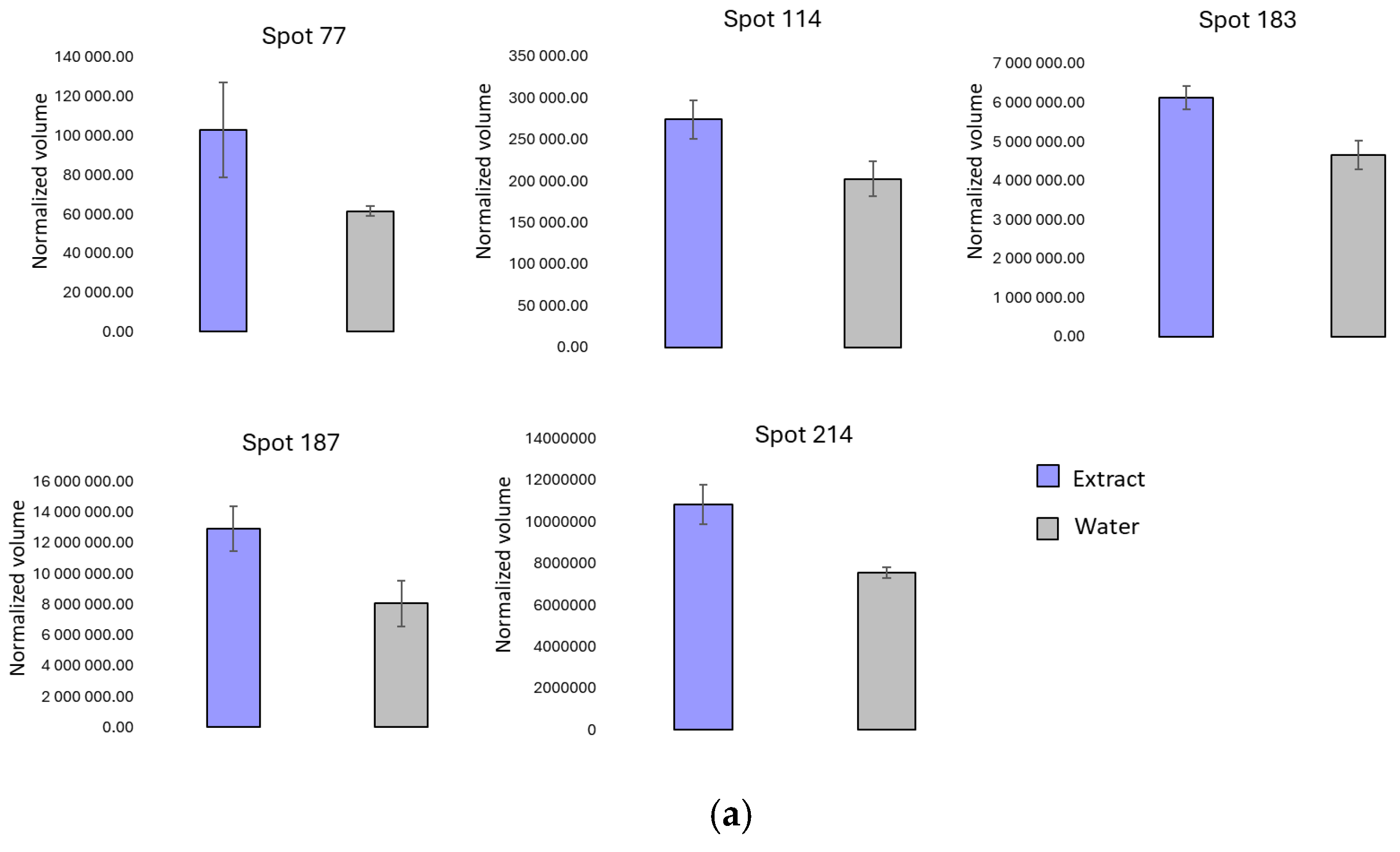
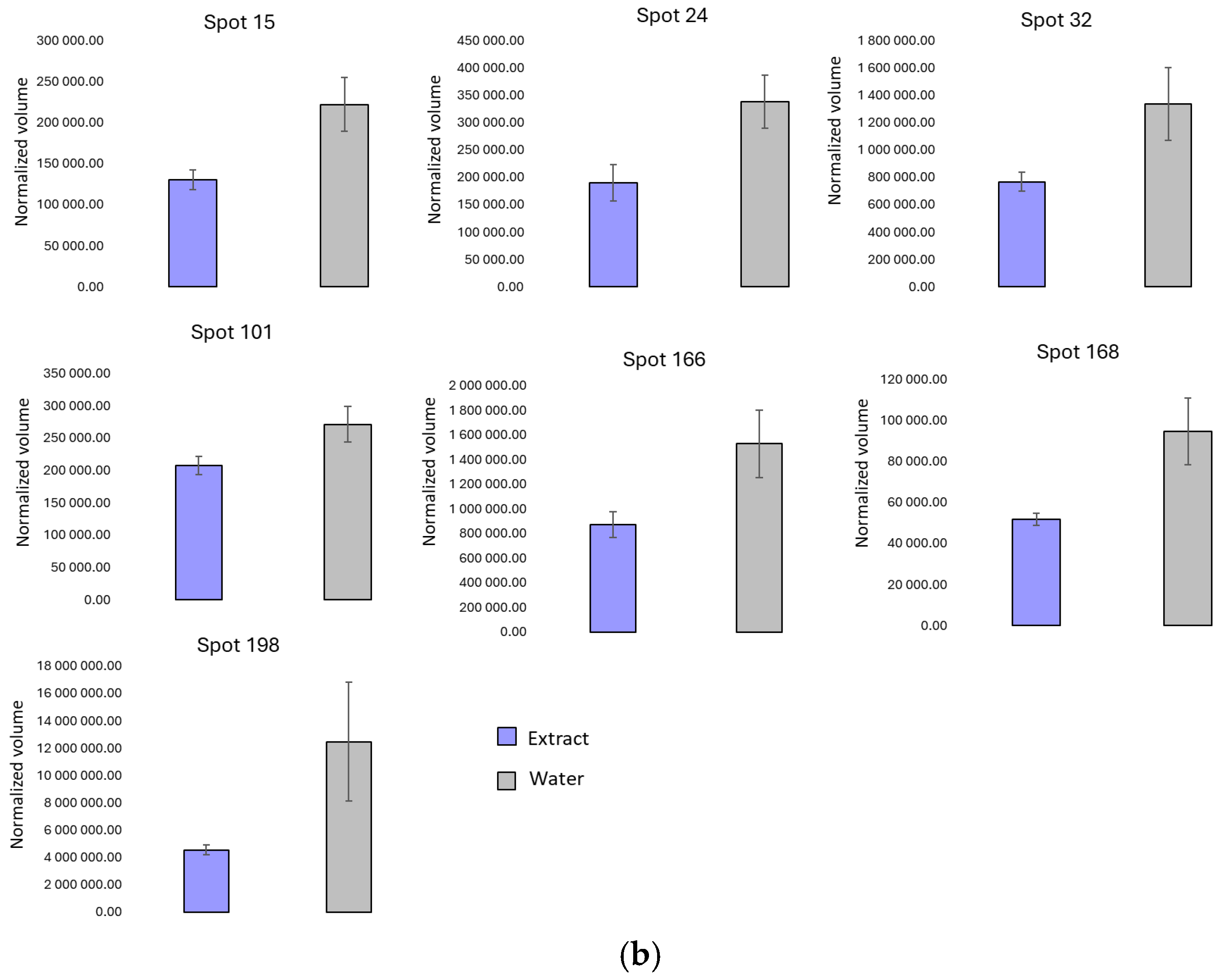
| Spot | Protein | Swissprot/Uniprot Entry Reference | Theoretical MW (kDa) | Theoretical pI | Score ID | Seq. C (%) | N° Peptide Matched | O. ficus-indica Extract Pulverization | Water Pulverization |
|---|---|---|---|---|---|---|---|---|---|
| 24 | α-Amy 1A | P0DUB6 | 58.4 | 9.25 | 221 | 38 | 27 | ↑ | |
| 166 | RBC (large chain) | A0A0K1CWJ6 | 22.3 | 8.33 | 201 | 50 | 17 | ↑ | |
| 168 | RBC small subunit, chloroplastic | A0A438JZ20 | 20.7 | 9.11 | 60 | 21 | 6 | ↑ | |
| 187 | RBC (large chain) | A0A0D4BPD8 | 29.5 | 9.18 | 111 | 31 | 13 | ↑ | |
| 198 | RBC (large chain) | A0A6G8J0R0 | 21.2 | 6.23 | 78 | 15 | 12 | ↑ | |
| 214 | HSP18.1 | F6HNP7 | 18.1 | 6.78 | 264 | 47 | 11 | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, L.; Santana, I.; Coelho, R.; Murta, G.; Cardoso, H.; Campos, C.; Barroso, J.M.; Rato, A.E. Exploring Opuntia ficus-indica as a Strategy to Mitigate High Temperatures Effects in Vineyards: Insights into Physiological and Proteomic Responses. Agronomy 2025, 15, 869. https://doi.org/10.3390/agronomy15040869
Rodrigues L, Santana I, Coelho R, Murta G, Cardoso H, Campos C, Barroso JM, Rato AE. Exploring Opuntia ficus-indica as a Strategy to Mitigate High Temperatures Effects in Vineyards: Insights into Physiological and Proteomic Responses. Agronomy. 2025; 15(4):869. https://doi.org/10.3390/agronomy15040869
Chicago/Turabian StyleRodrigues, Lénia, Inês Santana, Renato Coelho, Gabriela Murta, Hélia Cardoso, Catarina Campos, João Mota Barroso, and Ana Elisa Rato. 2025. "Exploring Opuntia ficus-indica as a Strategy to Mitigate High Temperatures Effects in Vineyards: Insights into Physiological and Proteomic Responses" Agronomy 15, no. 4: 869. https://doi.org/10.3390/agronomy15040869
APA StyleRodrigues, L., Santana, I., Coelho, R., Murta, G., Cardoso, H., Campos, C., Barroso, J. M., & Rato, A. E. (2025). Exploring Opuntia ficus-indica as a Strategy to Mitigate High Temperatures Effects in Vineyards: Insights into Physiological and Proteomic Responses. Agronomy, 15(4), 869. https://doi.org/10.3390/agronomy15040869










