Russian Sage Revealed: Exploring Biology, Cultivation, and Chemical Dimensions of Salvia yangii
Abstract
1. Introduction
2. Materials and Methods
3. Results
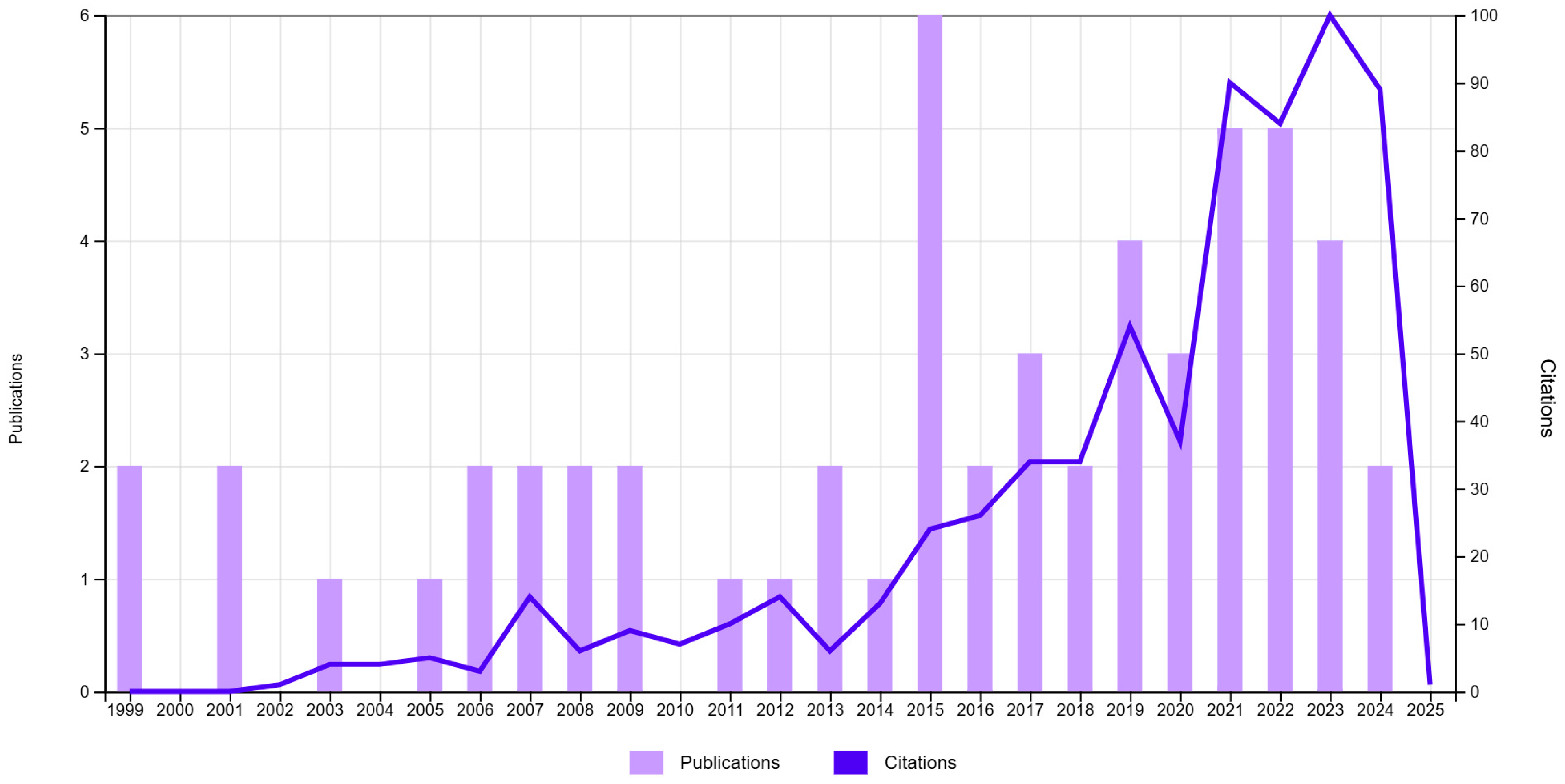
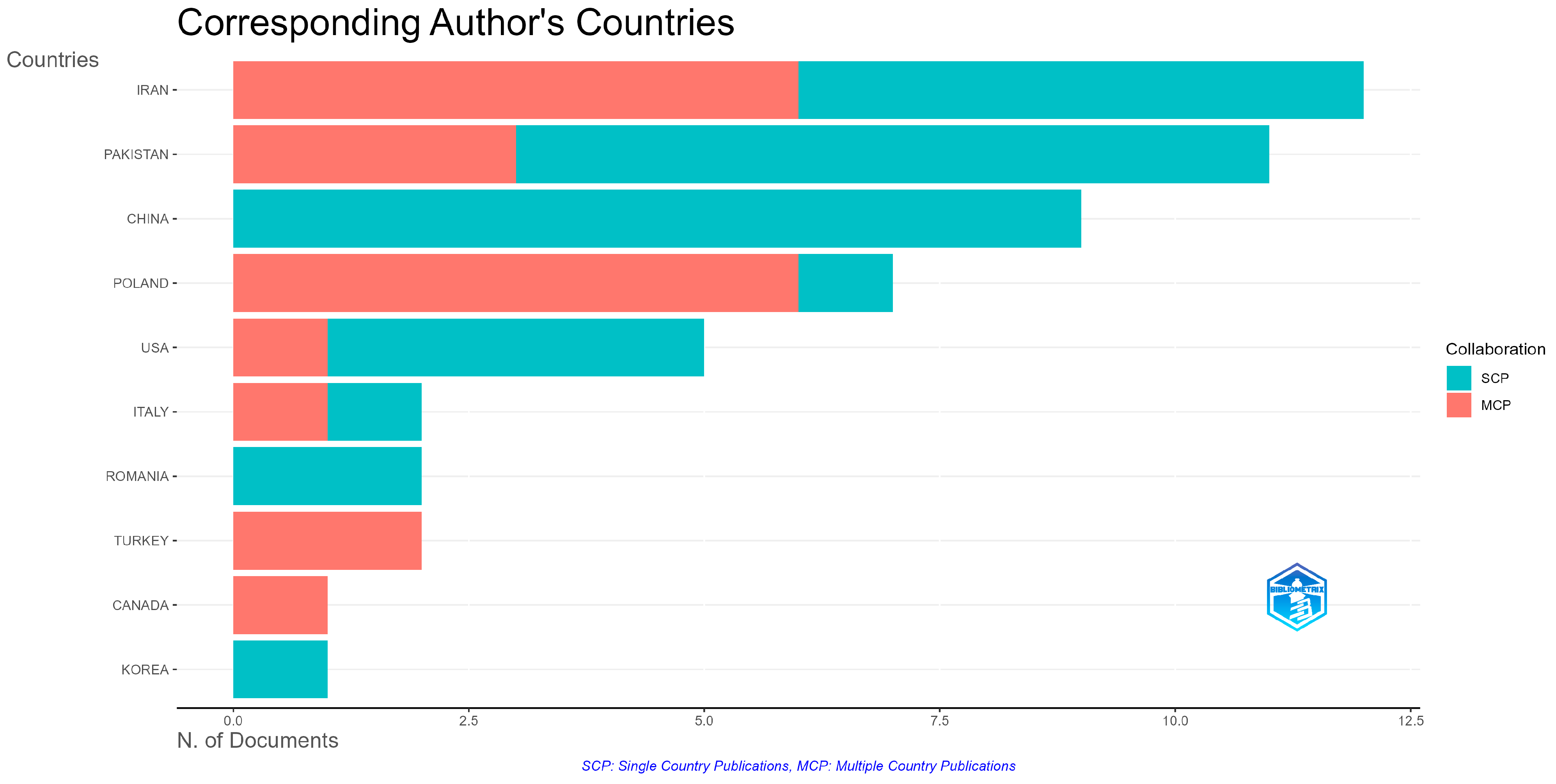
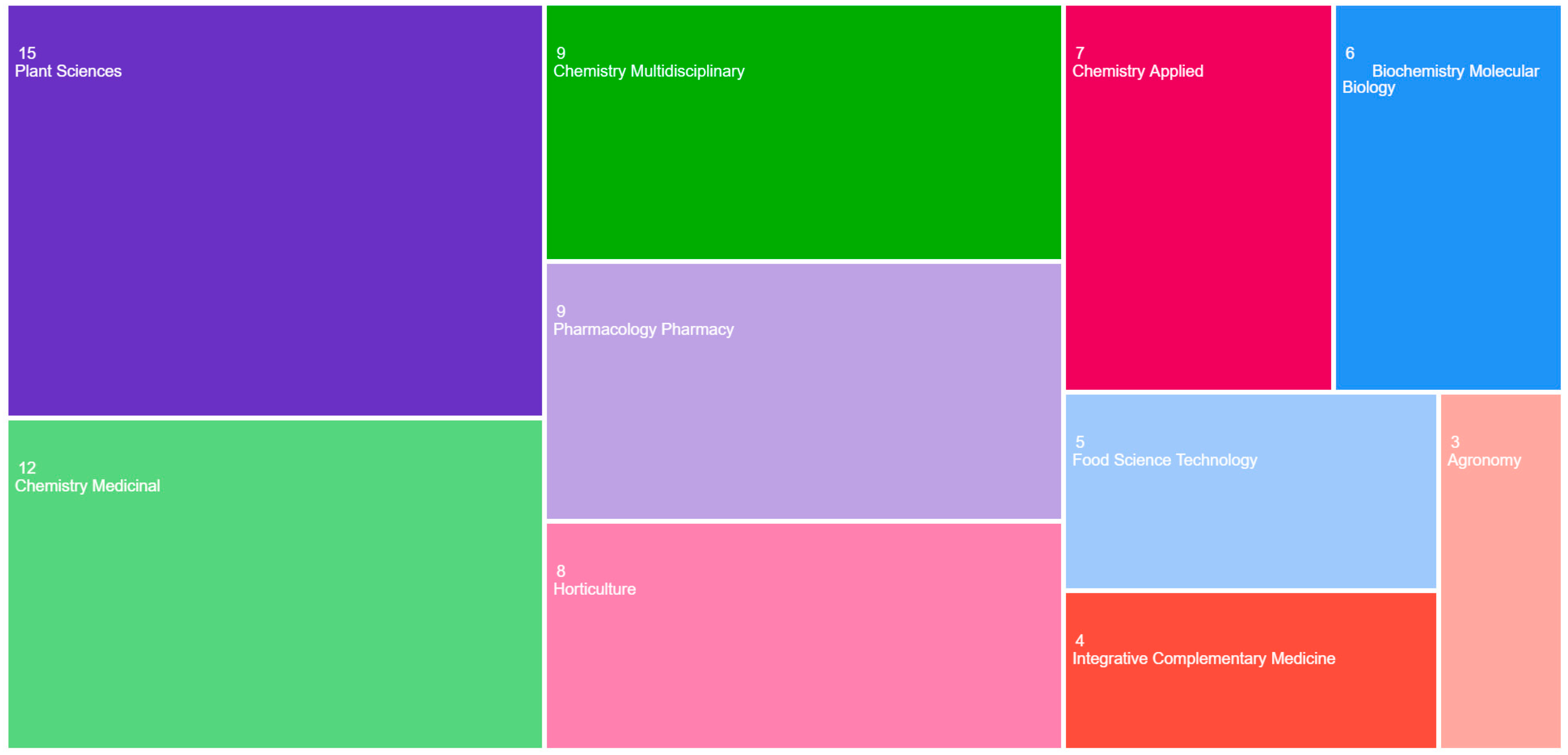
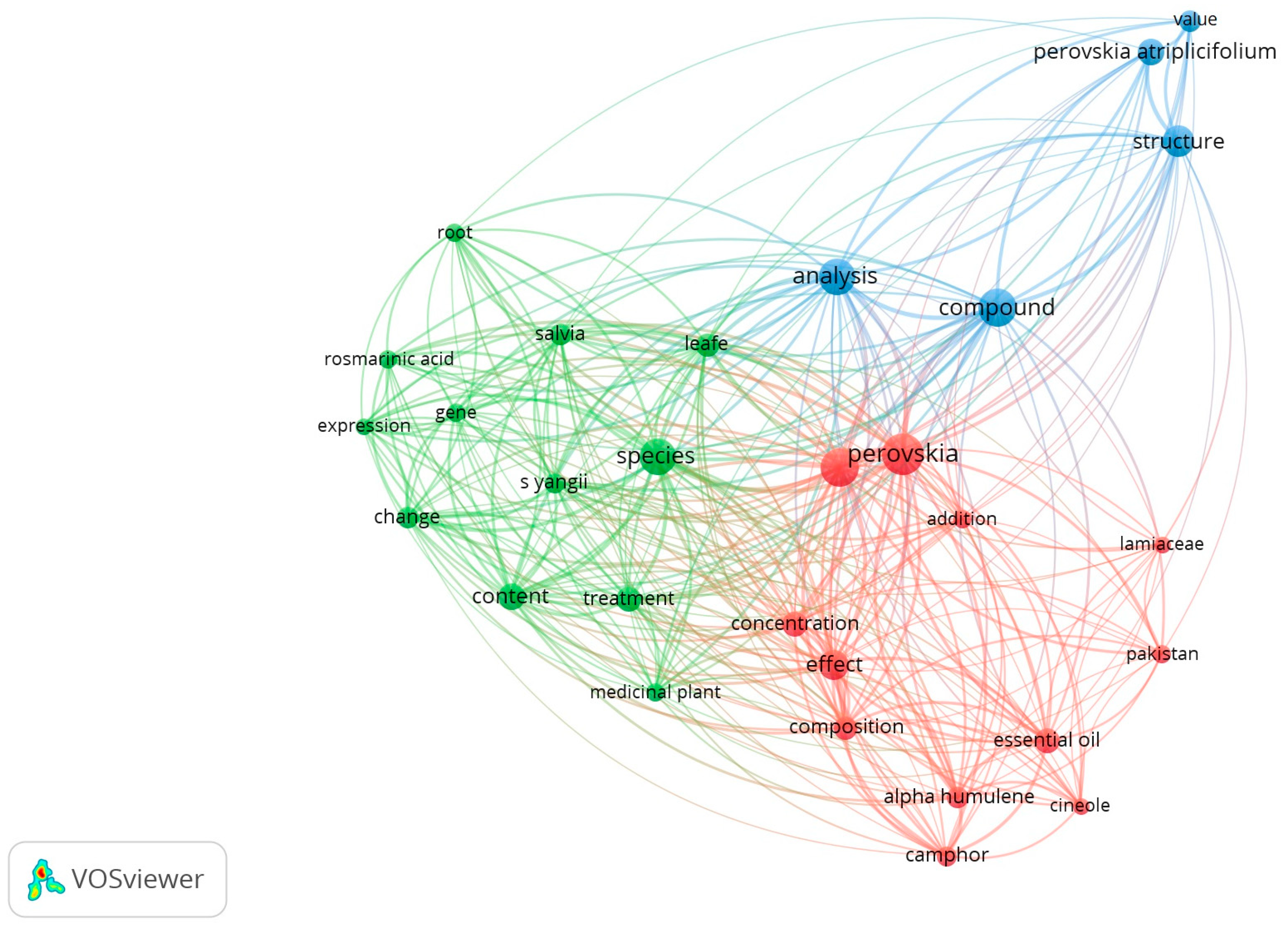
3.1. Bibliometric Analysis Regarding the Evolution of Knowledge About the Species S. yangii
3.2. Taxonomy
3.3. Morphological Description
3.4. Culture
3.5. Anatomical and Micromorphological Aspects
3.6. Biotic Interactions
3.7. Chemical Compounds from Salvia yangii
3.7.1. Terpenes
- Diterpenoids
- Triterpenoids.
- Monoterpenes, sesquiterpenes, and their derivates from essential oils
- 1,8-Cineole-dominant chemotype: This chemotype is the most widespread, observed in several samples, with 1,8-cineole present in high concentrations (27.5% in one sample) [78]. Other common components include limonene, α-pinene, α-humulene, and Δ3-carene.
- Insecticidal effect of essential oil
- b.
- Antibacterial effect of the essential oil
- c.
- Antifungal effect of the essential oil
3.7.2. Phenolic Compounds
3.7.3. Other Chemical Compounds
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moshari-Nasirkandi, A.; Alirezalu, A.; Alipour, H.; Amato, J. Screening of 20 Species from Lamiaceae Family Based on Phytochemical Analysis, Antioxidant Activity and HPLC Profiling. Sci. Rep. 2023, 13, 16987. [Google Scholar] [CrossRef]
- Venkatesh, Y.N.; Neethu, T.; Ashajyothi, M.; Kumar, V.; Hiremath, C. Pollinator Activity and Their Role on Seed Set of Medicinal and Aromatic Lamiaceae Plants. J. Apic. Res. 2024, 63, 1136–1144. [Google Scholar] [CrossRef]
- Ślusarczyk, S.; Senol Deniz, F.S.; Abel, R.; Pecio, Ł.; Pérez-Sánchez, H.; Cerón-Carrasco, J.P.; den-Haan, H.; Banerjee, P.; Preissner, R.; Krzyżak, E.; et al. Norditerpenoids with Selective Anti-Cholinesterase Activity from the Roots of Perovskia atriplicifolia Benth. IJMS 2020, 21, 4475. [Google Scholar] [CrossRef]
- Mohammadhosseini, M.; Venditti, A.; Akbarzadeh, A. The Genus Perovskia Kar.: Ethnobotany, Chemotaxonomy and Phytochemistry: A Review. Toxin Rev. 2021, 40, 484–505. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, 2024, Kew. Published on the Internet. Available online: http://www.plantsoftheworldonline.org/ (accessed on 18 January 2025).
- Erdemgil, F.Z.; Ilhan, S.; Korkmaz, F.; Kaplan, C.; Mercangöz, A.; Arfan, M.; Ahmad, S. Chemical Composition and Biological Activity of the Essential Oil of Perovskia atriplicifolia From Pakistan. Pharm. Biol. 2007, 45, 324–331. [Google Scholar]
- Jiang, Z.-Y.; Yu, Y.-J.; Huang, C.-G.; Huang, X.-Z.; Hu, Q.-F.; Yang, G.-Y.; Wang, H.-B.; Zhang, X.-Y.; Li, G.-P. Icetexane Diterpenoids from Perovskia atriplicifolia. Planta Med. 2015, 81, 241–246. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Sefidkon, F.; Hosseini, S.G. Supercritical Carbon Dioxide Extraction of Essential Oils from Perovskia atriplicifolia Benth. J. Agric. Food Chem. 2003, 51, 5414–5419. [Google Scholar] [CrossRef]
- Oreizi, E.; Asghari, G. Comparison the Essential Oil Composition of Perovskia abrotonoides Karel. & Perovskia atriplicifolia Benth. in Iran. In First National Conference on Medicinal Plants, Traditional Medicine and Organic Agriculture; Shahid Mofateh University: Hamedan, Iran, 2015. [Google Scholar]
- Miroliaei, M.; Aminjafari, A.; Ślusarczyk, S.; Nawrot-Hadzik, I.; Rahimmalek, M.; Matkowski, A. Inhibition of Glycation-Induced Cytotoxicity, Protein Glycation, and Activity of Proteolytic Enzymes by Extract from Perovskia atriplicifolia Roots. Phcog. Mag. 2017, 13, 676. [Google Scholar] [CrossRef]
- Boz, I.; Pădurariu, C.; Zamfirache, M.; Burzo, I.; Dunca, S.; Ştefan, M.; Ivănescu, L.; Olteanu, Z.; Badea, M.L.; Gostin, I.; et al. Chemical composition and antimicrobial activities of volatile oils in some Lamiaceae species. J. Biodivers. Ecol. Sci. 2011, 1, 21–27. [Google Scholar]
- Djenbaev, B.M.; Jalilova, A.A.; Abdijapar, S.; Shamshiev, A.B.; Jolboldiev, B. Radiating assessment in biosphere territories of the Issyk-Kul, Advanced monitoring techniques of hazardous wastes. In Proceedings of the 4th CCMS/NATO Workshop on Management of Industrial Toxic Wastes and Substances Research, Ioannina, Greece, 26–27 August 2006; pp. 11–18. [Google Scholar]
- Zamfirache, M.M.; Burzo, I.; Gostin, I.; Stefan, M.; Padurariu, C.; Olteanu, Z.; Badea, L.M.; Lamban, C.; Truta, E.; Ivanescu, L.; et al. Micromorphological traits and biochemical pattern of Perovskia atriplicifolia Benth., plant with phytoremediative potential. Carpathian J. Earth Environ. Sci. 2011, 6, 261–268. [Google Scholar]
- Sardashti, A. Variation in the Essential Oil Composition of Perovskia atriplicifolia Plant at Different Growth Stages in Taftan Aera. Plant Biotechnol. Persa 2021, 3, 26–33. [Google Scholar]
- Kozłowska, W.; Matkowski, A.; Zielińska, S. Light Intensity and Temperature Effect on Salvia yangii (B. T. Drew) Metabolic Profile In Vitro. Front. Plant Sci. 2022, 13, 888509. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, M.; Qing, X.; Li, G.; Tian, C. Bibliometric Analysis of Global Remote Sensing Research during 2010–2015. IJGI 2017, 6, 332. [Google Scholar] [CrossRef]
- Drew, B.T.; González-Gallegos, J.G.; Xiang, C.-L.; Kriebel, R.; Drummond, C.P.; Walked, J.B.; Sytsma, K.J. Salvia United: The Greatest Good for the Greatest Number. Taxon 2017, 66, 133–145. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-Tool for Comprehensive Science Mapping Analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, M.P. A Journey of Social Sustainability in Organization during MDG & SDG Period: A Bibliometric Analysis. Socio-Econ. Plan. Sci. 2023, 88, 101668. [Google Scholar] [CrossRef]
- Popescu, I.E.; Gostin, I.N.; Blidar, C.F. An Overview of the Mechanisms of Action and Administration Technologies of the Essential Oils Used as Green Insecticides. AgriEngineering 2024, 6, 1195–1217. [Google Scholar] [CrossRef]
- Walker, J.B.; Sytsma, K.J.; Treutlein, J.; Wink, M. Salvia (Lamiaceae) Is Not Monophyletic: Implications for the Systematics, Radiation, and Ecological Specializations of Salvia and Tribe Mentheae. Am. J. Bot. 2004, 91, 1115–1125. [Google Scholar] [CrossRef]
- Hu, G.-X.; Takano, A.; Drew, B.T.; Liu, E.-D.; Soltis, D.E.; Soltis, P.S.; Peng, H.; Xiang, C.-L. Phylogeny and Staminal Evolution of Salvia (Lamiaceae, Nepetoideae) in East Asia. Ann. Bot. 2018, 122, 649–668. [Google Scholar] [CrossRef]
- Will, M.; Claßen-Bockhoff, R. Time to Split Salvia s.l. (Lamiaceae)–New Insights from Old World Salvia Phylogeny. Mol. Phylogenetics Evol. 2017, 109, 33–58. [Google Scholar] [CrossRef]
- Will, M.; Schmalz, N.; Classen-Bockhoff, R. Towards a New Classification of Salvia s.l.: (Re)Establishing the Genus Pleudia Raf. Turk. J. Bot. 2015, 39, 693–707. [Google Scholar] [CrossRef]
- Frodin, D.G. History and Concepts of Big Plant Genera. Taxon 2004, 53, 753–776. [Google Scholar] [CrossRef]
- Colţun, M.; Gille, E.; Necula, R.D.; Grigoraş, V. Biological and chemical study of volatile oil for Perovskia atriplicifolia Benth species. Rev. Bot. 2013, 7, 74–83. [Google Scholar]
- Li, H.W.; Hedge, I.C. Perovskia atriplicifolia. In Flora of China; Wu, Z., Raven, P.H., Eds.; Verbenaceae Through Solanaceaea; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 1994; Volume 17, ISBN 978-0-915279-24-1. [Google Scholar]
- Colţun, M.; Gille, E.; Necula, R.D.; Grigoraş, V. Studiul complex al uleiului volatil din specia Perovschia atriplicifolia Benth. Stud. Univ. Mold. 2017, 101, 51–56. [Google Scholar]
- Zamfirache, M.M.; Burzo, I.; Gostin, I.; Olteanu, Z.; Stefan, M.; Badea, M.L.; Padurariu, C.; Gales, R.C.; Adumitresei, L.; Lamban, C.; et al. Glandular trichomes and essential oil constituents of Perovskia atriplicifolia Benth. J. Exp. Mol. Biol. 2009, 10, 73–80. [Google Scholar]
- Ahmad, I.; Waheed, A.; Tahir, N.B.; Rais, A.K. Anti-Inflammatory Constituents from Perovskia atriplicifolia. Pharm. Biol. 2015, 53, 1628–1631. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-K. Confirmation of Distyly in Perovskia and Floral Dimorphism of P. abrotanoides (Salviinae: Lamiaceae). Flora 2021, 283, 151905. [Google Scholar] [CrossRef]
- Dabiri, M.; Sefidkon, F. Analysis of the Essential Oil from Aerial Parts of Perovskia atriplicifolia Benth. at Different Stages of Plant Growth. Flavour. Fragr. J. 2001, 16, 435–438. [Google Scholar] [CrossRef]
- Colţun, M. Aspects for domestication of Perovskia atriplicifolia Benth species. In Proceedings of the Simpozionul “Conservation of Plant Diversity”, Chișinău, Moldova, 16–19 May 2012; pp. 295–297. [Google Scholar]
- Afshari, M.; Rahimmalek, M.; Sabzalian, M.R.; Bielecka, M.; Matkowski, A.; Talebi, M. Changes in Physiological, Phytochemical Traits and Gene Expression of Two Perovskia Species in Response to Water Deficit. Sci. Hortic. 2022, 293, 110747. [Google Scholar] [CrossRef]
- Mirzaei, S.; Banijamali, S.M. Investigating Drought Stress Tolerance in Endemic Perovskia for Using in the Urban Landscape. Iran. J. Hortic. Sci. Technol. 2021, 22, 325–348. [Google Scholar]
- Bredikhina, Y.; Turovtseva, N.; Podorozhniy, S.; Pyurko, O.; Lohvina-Byk, T. The use of medicinal plants in landscape design in the steppe zone of Ukraine. In Proceedings of the International Multidisciplinary Scientific GeoConference: SGEM, Albena, Bulgaria, 26 June–5 July 2021; Volume 21, pp. 321–327. [Google Scholar]
- Khodadadi, F.; Ahmadi, F.S.; Talebi, M.; Moshtaghi, N.; Matkowski, A.; Szumny, A.; Rahimmalek, M. Essential Oil Composition, Physiological and Morphological Variation in Salvia Abrotanoides and S. yangii under Drought Stress and Chitosan Treatments. Ind. Crops Prod. 2022, 187, 115429. [Google Scholar] [CrossRef]
- Ali, M.S.; Saleem, M.; Erian, A.W. A New Acylated Steroid Glucoside from Perovskia Atriplicifolia. Fitoterapia 2001, 72, 712–714. [Google Scholar] [CrossRef]
- Devecchi, M. The use of Labiatae of ornamental interest in the design of parks and gardens. Acta Hortic. 2006, 723, 51–58. [Google Scholar] [CrossRef]
- Scoggins, H.L. Determination of Optimum Fertilizer Concentration and Corresponding Substrate Electrical Conductivity for Ten Taxa of Herbaceous Perennials. Hort. Sci. 2005, 40, 1504–1506. [Google Scholar] [CrossRef]
- Dumitraşcu, M. Vegetative propagation of Perovskia atriplicifolia. Acta Hortic. 2008, 766, 215–218. [Google Scholar] [CrossRef]
- Mirzaei, S. Investigating Propagation of Different Endemic Species of Perovskia spp. JMPB 2023, 1, 29–35. [Google Scholar] [CrossRef]
- Oreizi, E.; Rahiminejad, M.; Asghari, G.R. Taxonomic study in Perovskia Karel. by checking the morphologic: Anatomic and phytochemical aspects in Iran. Cumhur. Sci. J. 2015, 36, 1717–1725. [Google Scholar]
- Perveen, A. Micromorphological studies of the family Labiatae from Pakistan with special reference to trichomes and its use as a taxonomic evidence, Int. J. Biol. Biotech. 2006, 3, 285–300. [Google Scholar]
- Nikolakaki, A.; Christodoulakis, N.S. Secretory structures and cytochemical investigation of the leaf of Phlomis fruticosa, a sonally dimorphic subshrub. Secreting activity of the leaf–originating calluses. Flora 2007, 202, 429–436. [Google Scholar] [CrossRef]
- Gostin, I.N. Glandular and Non-Glandular Trichomes from Phlomis herba-venti subsp. pungens Leaves: Light, Confocal, and Scanning Electron Microscopy and Histochemistry of the Secretory Products. Plants 2023, 12, 2423. [Google Scholar] [CrossRef]
- Gostin, I.N. Perovskia atriplicifolia leaf Anatomy. Alexandru Ioan Cuza University, Iasi, Romania. 2009; Unpublished work. [Google Scholar]
- Westerhold, C.M. Horticulture for Pollinator Conservation. Master’s Thesis, Graduate College at the University of Nebraska, Lincoln, NE, USA, 2017. [Google Scholar]
- Giovannetti, M.; Malabusini, S.; Giuliani, C.; Lupi, D. The attraction of bees: A preference expressed by Anthidium spp. to Salvia yangii. In Proceedings of the 27th Conference of the Italian National Congress of Entomology, Palermo, Italy, 12–16 June 2023. [Google Scholar]
- Coplen, S. Cold Stratification of Salvia azurea var. grandiflora Benth.(Lamiaceae) seeds to Break Dormancy. Okla. Nativ. Plant Rec. 2021, 21, 53–61. [Google Scholar]
- Graham, K.K.; Eaton, K.; Obrien, I.; Starks, P.T. Anthidium manicatum, an invasive bee, excludes a native bumble bee, Bombus impatiens, from floral resources. Biol. Invasions 2018, 21, 1089–1099. [Google Scholar] [CrossRef]
- Bailey, K. Evaluating Herbaceous Perennials for Landscape Performance and Pollinator Behavior in North-Central Texas. Master’s Thesis, Texas A&M University-Commerce, Commerce, TX, USA, December 2022. [Google Scholar]
- Loza-Tavera, H. Monoterpenes in Essential Oils. In Chemicals via Higher Plant Bioengineering; Shahidi, F., Kolodziejczyk, P., Whitaker, J.R., Munguia, A.L., Fuller, G., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1999; Volume 464, pp. 49–62. [Google Scholar] [CrossRef]
- Friedman, A.L.L. Rosemary beetle Chrysolina americana: A new invasive leaf beetle (Coleoptera: Chrysomelidae: Chrysomelinae) in Israel. Isr. J. Entomol. 2016, 46, 87–91. [Google Scholar]
- Bhatla, S.C. Secondary Metabolites. In Plant Physiology, Development and Metabolism; Springer Nature: Singapore, 2018; pp. 1099–1166. ISBN 9789811320224. [Google Scholar]
- Powder-George, Y.L.; Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Terpenoids. In Pharmacognosy; Elsevier: London, UK, 2024; pp. 253–294. ISBN 978-0-443-18657-8. [Google Scholar]
- Naeini, A.A.; Ziegelmeier, A.A.; Chain, W.J. Recent Developments with Icetexane Natural Products. Chem. Biodivers. 2022, 19, e202200793. [Google Scholar] [CrossRef]
- Xu, G.; Hou, A.-J.; Zheng, Y.-T.; Zhao, Y.; Li, X.-L.; Peng, L.-Y.; Zhao, Q.-S. Przewalskin B, a Novel Diterpenoid with an Unprecedented Skeleton from Salvia Przewalskii Maxim. Org. Lett. 2007, 9, 291–293. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Huang, C.-G.; Xiong, H.-B.; Tian, K.; Liu, W.-X.; Hu, Q.-F.; Wang, H.-B.; Yang, G.-Y.; Huang, X.-Z. Perovskatone A: A Novel C23 Terpenoid from Perovskia Atriplicifolia. Tetrahedron Lett. 2013, 54, 3886–3888. [Google Scholar] [CrossRef]
- Barbosa, C.S.; Ahmad, A.; Maluf, S.E.C.; Moura, I.M.R.; Souza, G.E.; Guerra, G.A.H.; Barros, R.R.M.; Gazarini, M.L.; Aguiar, A.C.C.; Burtoloso, A.C.B.; et al. Synthesis, Structure–Activity Relationships, and Parasitological Profiling of Brussonol Derivatives as New Plasmodium falciparum Inhibitors. Pharmaceuticals 2022, 15, 814. [Google Scholar] [CrossRef]
- Forzato, C.; Nitti, P. New Diterpenes with Potential Antitumoral Activity Isolated from Plants in the Years 2017–2022. Plants 2022, 11, 2240. [Google Scholar] [CrossRef]
- Bielecka, M.; Pencakowski, B.; Stafiniak, M.; Jakubowski, K.; Rahimmalek, M.; Gharibi, S.; Matkowski, A.; Ślusarczyk, S. Metabolomics and DNA-Based Authentication of Two Traditional Asian Medicinal and Aromatic Species of Salvia Subg. Perovskia. Cells 2021, 10, 112. [Google Scholar] [CrossRef]
- Salamatin, A.A.; Khaliullina, A.S.; Khaziev, R.S.H. Extraction of Aromatic Abietane Diterpenoids from Salvia officinalis Leaves by Petroleum Ether: Data Resolution Analysis. Ind. Crops Prod. 2020, 143, 111909. [Google Scholar] [CrossRef]
- López-Cabeza, R.; Rodríguez-Sabina, S.; Reyes, C.P.; Expósito, D.G.; Giménez, C.; Jiménez, I.A.; Cabrera, R.; Bazzocchi, I.L. Bio-guided Isolation of Aromatic Abietane Diterpenoids from Salvia canariensis as Biopesticides in the Control of Phytopathogenic Fungi. Pest. Manag. Sci. 2024, 80, 2199–2207. [Google Scholar] [CrossRef]
- Bielecka, M.; Stafiniak, M.; Pencakowski, B.; Ślusarczyk, S.; Jastrzębski, J.P.; Paukszto, Ł.; Łaczmański, Ł.; Gharibi, S.; Matkowski, A. Comparative Transcriptomics of Two Salvia Subg. Perovskia Species Contribute towards Molecular Background of Abietane-Type Diterpenoid Biosynthesis. Sci. Rep. 2024, 14, 3046. [Google Scholar] [CrossRef]
- Liu, W.-X.; Zhao, J.-W.; Zuo, A.-X.; Yang, Z.; Gao, L.; Zhou, M.; Jiang, Z.-Y. Two Novel Terpenoids from the Cultured Perovskia atriplicifolia. Fitoterapia 2018, 130, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.-D.; Cao, Y.-F.; Che, Y.-Y.; Li, J.; Shang, Z.-P.; Zhao, W.-J.; Qiao, Y.-J.; Zhang, J.-Y. Danshen: A phytochemical and pharmacological overview. Chin. J. Nat. Med. 2019, 17, 59–80. [Google Scholar]
- Perveen, S.; AL-Taweel, A.M.; Fawzy, G.A.; Ibrahim, T.A.; Malik, A.; Khan, A. Cholinesterase Inhibitory Triterpenes from Perovskia atriplicifolia. Asian J. Chem. 2014, 26, 6163–6166. [Google Scholar] [CrossRef]
- Scott, L.J.; Goa, K.L. Galantamine: A Review of Its Use in Alzheimer’s Disease. Drugs 2000, 60, 1095–1122. [Google Scholar] [CrossRef]
- Perveen, S.; Malik, A.; Bakhsh Tareen, R. Structural Determination of Atricins A and B, New Triterpenes from Perovskia atriplicifolia, by 1D and 2D NMR Spectroscopy. Magn. Reson. Chem. 2009, 47, 266–269. [Google Scholar] [CrossRef]
- Luchnikova, N.A.; Grishko, V.V.; Ivshina, I.B. Biotransformation of Oleanane and Ursane Triterpenic Acids. Molecules 2020, 25, 5526. [Google Scholar] [CrossRef]
- Shanaida, M.; Pryshlyak, A.; Golembiovska, O. Determination of Triterpenoids in Some Lamiaceae Species. Rese. Jour. Pharm. Technol. 2018, 11, 3113. [Google Scholar] [CrossRef]
- Hamed, A.N.; Attia, E.; Desoukey, S.Y. A review on various classes of secondary metabolites and biological activities of Lamiaceae (Labiatae) (2002–2018). J. Adv. Biomed. Pharm. Sci. 2021, 4, 16–31. [Google Scholar] [CrossRef]
- Shevchuk, O.M.; Feskov, S.A. Chemotypic Diversity of Essential Oil of Species and Cultivars of the Genus Perovskia Kar. in the Conditions of the South Coast of Crimea//Zemledelie. 2020. No. 7. pp. 16–24. Available online: https://jurzemledelie.ru/arkhiv-nomerov/7-2020/1719-khemotipicheskoe-raznoobrazie-efirnogo-masla-vidov-i-kultivarov-roda-perovskia-kar-v-usloviyakh-yuzhnogo-berega-kryma (accessed on 25 January 2025). [CrossRef]
- Ramos Da Silva, L.R.; Ferreira, O.O.; Cruz, J.N.; De Jesus Pereira Franco, C.; Oliveira Dos Anjos, T.; Cascaes, M.M.; Almeida Da Costa, W.; Helena De Aguiar Andrade, E.; Santana De Oliveira, M. Lamiaceae Essential Oils, Phytochemical Profile, Antioxidant, and Biological Activities. Evid.-Based Complement. Altern. Med. 2021, 2021, 6748052. [Google Scholar] [CrossRef]
- Mucciarelli, M.; Maffei, M.E.; Sacco, T. Oli essenziali e tricomi ghiandolari di Perovskia atriplicifolia Benth. e P. scrophulariaefolia Bunge. Riv. Ital. EPPOS 1993, 9, 3–7. [Google Scholar]
- Sefldkon, F.; Ahmadl, L.; Mirza, M. Volatile Components of Perovskia atriplicifolia Benth. J. Essent. Oil Res. 1997, 9, 101–103. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Ahmad, V.U.; Tareen, R.B. Constituents of the Essential Oil of Perovskia atriplicifolia Benth. Flavour. Fragr. J. 1999, 14, 38–40. [Google Scholar] [CrossRef]
- Burzo, I.; Dobrescu, A.; Bădulescu, L. The composition of volatile oils extracted from Perovskia atriplicifolia Benth flowers and leaves. Lucr. Științifice Ser. B Hortic. 2010, 54, 629–632. [Google Scholar]
- Logvinenko, L.A.; Khlypenko, L.A.; Marko, N.V. Aromatic plant of Lamiaceae family for use in phytotherapy. Farm. Farmakol. 2016, 4, 34–47. [Google Scholar] [CrossRef]
- Faqeryar, N.; Azuma, J.; Mori, J. Variation in the volatile oils of P. atriplicifolia Benth. and its two cultivars. In Proceedings of the 48th International Symposium on Essential Oils (ISEO2017), Pécs, Hungary, 10–13 September 2017; p. 77. [Google Scholar]
- Sadeghi, Z.; Farimani, M.M.; Khorrami, F.; Abdollahi, V. Insecticidal and Oviposition Deterrent Effects of Pure and Combined Essential Oils of Salvia Subg. Perovskia Species against Phthorimaea operculella. S. Afr. J. Bot. 2022, 151, 126–132. [Google Scholar] [CrossRef]
- Ghaffari, Z.; Rahimmalek, M.; Sabzalian, M.R.; Arzani, A.; Kiani, R.; Gharibi, S.; Wróblewska, K.; Szumny, A. Chemical Composition, Physiological and Morphological Variations in Salvia Subg. Perovskia Populations in Response to Different Salinity Levels. IJMS 2024, 25, 12566. [Google Scholar] [CrossRef]
- Zuzarte, M.; Sousa, C.; Alves-Silva, J.; Salgueiro, L. Plant Monoterpenes and Essential Oils as Potential Anti-Ageing Agents: Insights from Preclinical Data. Biomedicines 2024, 12, 365. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Hobbie, F.; Keerthi, A.; Oun, A.; Kortholt, A.; Boddeke, E.; Dolga, A. Linalool Attenuates Oxidative Stress and Mitochondrial Dysfunction Mediated by Glutamate and NMDA Toxicity. Biomed. Pharmacother. 2019, 118, 109295. [Google Scholar] [CrossRef]
- Sarmento-Neto, J.; Do Nascimento, L.; Felipe, C.; De Sousa, D. Analgesic Potential of Essential Oils. Molecules 2015, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Askarova, O.K.; Bobakulov, K.M.; Botirov, E.K.; Zhuraev, M.U.; Yarylkaganova, A.M.; Sasmakov, S.A.; Azimova, S.S. Constituent Composition and Antimicrobial Activity of Essential Oil from Perovskia botschantzevii. Chem. Nat. Compd. 2024, 60, 174–176. [Google Scholar]
- Haris, A.; Azeem, M.; Abbas, M.G.; Mumtaz, M.; Mozūratis, R.; Binyameen, M. Prolonged Repellent Activity of Plant Essential Oils against Dengue Vector, Aedes aegypti. Molecules 2023, 28, 1351. [Google Scholar] [CrossRef]
- Ahmadi, M.; Abd-alla, A.M.M.; Moharramipour, S. Combination of Gamma Radiation and Essential Oils from Medicinal Plants in Managing Tribolium castaneum Contamination of Stored Products. Appl. Radiat. Isot. 2013, 78, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.I.; Anwar, F.; Nigam, P.S.; Sarker, S.D.; Moore, J.E.; Rao, J.R.; Mazumdar, A. Antibacterial Activity of Some Lamiaceae Essential Oils Using Resazurin as an Indicator of Cell Growth. LWT-Food Sci. Technol. 2011, 44, 1199–1206. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial Activity and Mechanism of Cinnamon Essential Oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Duda-Madej, A.; Viscardi, S.; Grabarczyk, M.; Topola, E.; Kozłowska, J.; Mączka, W.; Wińska, K. Is Camphor the Future in Supporting Therapy for Skin Infections? Pharmaceuticals 2024, 17, 715. [Google Scholar] [CrossRef]
- Afshari, M.; Rahimmalek, M.; Miroliaei, M.; Sabzalian, M.R.; Sadeghi, M.; Matkowski, A.; Szumny, A. Attenuation of Protein Glycation by Phenolic Compounds of Salvia Subg. Perovskia: Insights from Experimental and Computational Studies. Ind. Crops Prod. 2024, 208, 117859. [Google Scholar] [CrossRef]
- Stafiniak, M.; Ślusarczyk, S.; Pencakowski, B.; Matkowski, A.; Rahimmalek, M.; Bielecka, M. Seasonal Variations of Rosmarinic Acid and Its Glucoside and Expression of Genes Related to Their Biosynthesis in Two Medicinal and Aromatic Species of Salvia Subg. Perovskia. Biol. 2021, 10, 458. [Google Scholar] [CrossRef]
- Perveen, S.; Khan, S.B.; Malik, A.; Tareen, R.B.; Nawaz, S.A.; Choudhary, M.I. Phenolic Constituents from Perovskia atriplicifolia. Nat. Prod. Res. 2006, 20, 347–353. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Shukla, S.; Paek, W.K.; Lim, J.; Kumar, P.; Kumar, P.; Na, M. Efficacy of (+)-Lariciresinol to Control Bacterial Growth of Staphylococcus aureus and Escherichia coli O157:H7. Front. Microbiol. 2017, 8, 804. [Google Scholar] [CrossRef]
- Tupe, R.S.; Kemse, N.G.; Khaire, A.A.; Shaikh, S.A. Attenuation of Glycation-Induced Multiple Protein Modifications by Indian Antidiabetic Plant Extracts. Pharm. Biol. 2017, 55, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S.; Manayi, A.; Gohari, A.R.; Abdollahi, M. The story of beta-sitosterol-a review. Eur. J. Med. Plants 2014, 4, 590–609. [Google Scholar]
- Ashraf, R.; Bhatti, H.N. Stigmasterol. In A Centum of Valuable Plant Bioactives; Elsevier: Amsterdam, The Netherlands, 2021; pp. 213–232. ISBN 978-0-12-822923-1. [Google Scholar]

| Plant Material/Developmental Stage | Essential Oil Yield (w/w)% | The Main Components of the Essential Oil | Collection Site | Components Numbers/ Accounting % of the Oil | Reference |
|---|---|---|---|---|---|
| Aerial part/complete flowering | - | P-thujone (45.9%), sabinene (26.6%), α-pinene (12.1%), 1,8-cineole (10.5%) | [76] | ||
| Aerial part/complete flowering | 2.3 | 1,8-cineole + limonene (40.13%), α-pinene (17.87%), Δ3-carene (9.13%), β-pinene (6.59%), camphene (6.17%), camphor (5.36%) | National Botanical Garden, Teheran, Iran | 19/92.3 | [77] |
| Leaves and flowers | 1.32 | 1,8-cineole (27.5%), Δ3-carene (22.3%), b-caryophyllene (10.8%), α-pinene (5.9%) α-humulene (5.7%) | Quetta, Pakistan | 19/96.4 | [78] |
| Leaves/complete flowering | 1.47 | β-caryophyllene (12.51%), 1,8-cineole (11.7%), limonene (9.66%), α-humulene (9.36%), camphor (7.58%) | Botanical Garden of the Research Institute of Forests and Rangelands Tehran, Iran | 46/98 | [32] |
| Stems/complete flowering | 2.1 | 1,8-cineole (15.64%), β-caryophyllene (11.92%), camphor (11.63%), α-humulene (9.55%), α-terpinyl acetate (6.28%), bornyl acetate (6.05%) | |||
| Flowers/complete flowering | 1.45 | 1,8-cineole (19.52%), α-pinene (13.97%), camphor (8.6%), limonene (4.76%), β-caryophyllene (8.36%), α-humulene (6.39%) | |||
| Aerial part/before flowering (stem 20–30 cm) | 1.69 | β-caryophyllene (15.87%), α-humulene (14.36%), limonene (11.37%), Δ3- carene (9.77%), T-cadinol (5.35%) | 48/98 | ||
| Aerial part/before flowering (stem 40–50 cm) | 1.69 | β-caryophyllene (15.52%), α-humulene (13.46%), limonene (11.36%), Δ3-carene (10.75%), camphor (8.19%), 1,8-cineole (7.98%) | |||
| Aerial part/before flowering (stem 40–60 cm) | 1.57 | β-phellandrene (13.55%), camphor (13.49%), Δ3-carene (13.15%), β-caryophyllene (11.68%), 1,8-cineole (10.27%), α-humulene (9.7%), limonene (8.95%) | |||
| Aerial part/beginning of flowering (stem 60–75 cm) | 1.6 | 1,8-cineole (19.52%), α-pinene (13.97%), camphor (8.6%), limonene (8.6%), β-caryophyllene (8.36%), α-humulene (6.39%) | |||
| Aerial part/complete flowering (stem 90–100 cm) | 1.06 | 1,8-cineole (15.74%), β-caryophyllene (12.3%), camphor (10.16%), α-humulene (9.47%), limonene (7.55%), α-pinene (6.31%), Δ3-carene (5.71%) | |||
| Aerial part/complete flowering (stem 100–110 cm) | 1.64 | 1,8-cineole (20.74%), camphor (14.52%), limonene (8.58%), β-caryophyllene (7.9%), α-pinene (7.77%), α-humulene (6.28%), Δ3-carene (6.04%) | |||
| Leaves and flowers/flowering stage | 1,8-cineole+ limonene (29%), camphor (14.8%), β-caryophyllene (8.7%), Δ3-carene (5.4%), α-pinene (7.3%), α-humulene (6.7%) | National Garden in Tehran, Iran | 28/96.9 | [8] | |
| Aerial part/flowering stage | 3.2 | camphor (28.91%), limonene (16.72%), α-globulol (10.2%), trans-caryophyllene (9.3%), α-humulene (9.25%) | Tira, Pakistan | 18/96.6 | [6] |
| Aerial part/flowering stage | - | limonene (18%), γ-terpinene (16%), β-caryophyllene (13%), α- caryophyllene (12%), cymene (11%) | SC Miroslava, Iasi, Romania | 27/96.02 | [29] |
| Flowers/flowering stage | - | 1,8-cineole (15.83%), tau-cadinol (14.67%), α-pinene (10.88%), camphor (8.91%), β-caryophyllene (7.99%), α-caryophyllene (6.96%), 3-carene (4.54%) | Bacău, Romania | 52/97.79 | [79] |
| Leaves/flowering stage | - | 1,8-cineole (21.36%), camphor (14.31%), tau-cadinol (9.63%), β-caryophyllene (5.88%), α- caryophyllene (5.42%), 3-carene (5.30%) | 66/92.22 | ||
| Aerial part/flowering stage | - | 1,8-cineole (24.62%), o-cymene (17.87%), borneol (15.37%), γ-terpinene (6.39%), bornyl acetate (4.83%) camphene (4.66%) | Iasi, Romania | 32/94 | [13] |
| Stems/flowering stage | - | 1,8-cineole (18.44%), o-cymene (18.23%), bornyl acetate (14.65%), borneol (9.61%), cariophyllene oxide (6.02%) | 34/97.16 | ||
| Leaves/flowering stage | - | borneol (20.41%), 1,8-cineole (19.7%), o-cymene (13.91%), bornil acetate (5.2%), cariophyllene oxide (4.78%), β-caryophyllene (4.66%) | 33/98.08 | ||
| Flowers/flowering stage | - | 1,8-cineole (16.3%), o-cymene(11.81%), α-pinene (7.33%), borneol (9.71%), bornil acetate (7.46%), γ-terpinene (14.37%), | 47/96.05 | ||
| Aerial part/flowering stage | - | limonene (17.75%), cymene (17.93), borneole (14.67%), cis-β-ocimene (8.37%), γ-terpinene (7.15%), camfene (4.5%) | Iasi, Romania | 28/99.3 | [11] |
| Aerial part/flowering stage | - | limonene (21.47%), 1,8-cineole (16.19%), α-pinene (8.17%), β-caryophyllene (6.20%), bornyl acetate (6.06%) | Botanical Garden of Chişinău, Republic of Moldova | 28/95.5 | [26] |
| Flowers/complete flowering | - | 1,8-cineole (18.65%), Δ3-carene (11.23%), α-pinene (9.98%), viridiflorene (8.45%), α-humulene (7.55%), | Sistan– Baluchestan region, Iran | 24/99.9 | [9] |
| Aerial part/complete flowering | 0.9 | camphor (27.2%), 1.8-cineol (14.3%), linalool (5.5%), borneol (4.8%), carvacrol (5%) | Nikitsky Botanical Garden, Crimea | 30 | [80] |
| Aerial part/complete flowering | - | α-myrcene (16.57%), 1,8-cineole (10.69%), borneol (8.52%), β-caryophyllene (8.30%), α-caryophyllene (7.42%) | Afganistan | - | [81] |
| Aerial part/complete flowering cultivar ‘Little Spire’ | - | 1,8-cineole (17.79%), camphor (14.28%), D-limonene (10.93%), Δ3-carene (6.76%), α-pinene (6.64%) | |||
| Aerial part/complete flowering cultivar ‘Blue Spire’ | - | 1,8-cineole (15.72%), β-caryophyllene (10.32%), α-caryophyllene (9.35%), borneol (9.32%), camphor (7.3%), δ-3-carene (7.10%) | |||
| Aerial part/complete flowering | 0.54 | 1,8-cineole and limonene (40.13%), α-pinene (17.87%), δ-3-carene (9.13%), β-pinene, (6.59%), camphene (6.17%), camphor (5.36%) | National Botanical Garden Chişinău, Republic of Moldova | 39/95.01 | [28] |
| Aerial part/complete flowering (sample 1) | 0.38 | camphor (21.19%), 1,8-cineole (20.1%), α-pinene (9.54%), endoborneol (6.56%), bornyl acetate (5.7%) | Crimea, Russia | 38/99.09 | [74] |
| Aerial part/complete flowering (sample 2) | 0.53 | endoborneol (29.28%), 1,8-cineole (16.17%), α-pinene (6.28%), bornyl acetate (6.16%), β-caryophyllene (5.04%) | 42/98.69 | ||
| Leaves/before flowering | 1.28 | geranyl acetate (24.17%), δ-3-carene (6%) 1,8-cineole (5.7%), α-humulene (4.96%), linalool (4.66%), α-pinene (3.8%) | Sistan, Baluchestan, Iran | 52/92 | [14] |
| Leaves/flowering | 1.15 | δ-3-carene (9.88%), 1,8-cineole (9.32%), linalyle acetate (8.15%), geranyl acetate (7.79%), linalool (6.07%), α-humulene (4.21%) | 62/86.31 | ||
| Leaves/complete flowering | 1.03 | geranyl acetate (33.26%), δ linalyle acetate (8.15%), linalool (7.49%), 1,8-cineole (5.5%), geraniol (5.07%), transcaryophyllene (4.79%), Δ3-carene (4.09%) | 43/66.46 | ||
| Aerial part/complete flowering | 0.61 | camphor (13.3%), bornyl acetate (11.9%), δ 3-carene (10.8%), 1,8-cineole (10.2%) | Baluchestan, Iran | 28/97.4 | [37] |
| Aerial part/complete flowering | 1.4 | 1,8-cineole (12.55%), linalyl acetate (11.48%), Δ3-carene (9.03%), linalool (6.15%), bornyl acetate (3. 51%) | Taftan mountain, Baluchestan, Iran | 33/99.41 | [82] |
| Aerial part dry/complete flowering | 0.43 | 1,8-cineole (24.2%), camphor (8.6%), endoborneol (7.3%), bornyl acetate (6.2%), caryophyllene (4.4%) | 57/94.0 | ||
| Aerial part/complete flowering | 0.9 | 1,8-cineole (22.02%), borneol (9.19%) bornyl acetate (10.39%), ∆3-carene (9.09%), camphor (6.08%), β-caryophyllene (5.46%) | Khash– Sistan and Baluchestan, Iran | 24/94.27 | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gostin, I.N.; Popescu, I.E. Russian Sage Revealed: Exploring Biology, Cultivation, and Chemical Dimensions of Salvia yangii. Agronomy 2025, 15, 868. https://doi.org/10.3390/agronomy15040868
Gostin IN, Popescu IE. Russian Sage Revealed: Exploring Biology, Cultivation, and Chemical Dimensions of Salvia yangii. Agronomy. 2025; 15(4):868. https://doi.org/10.3390/agronomy15040868
Chicago/Turabian StyleGostin, Irina Neta, and Irinel Eugen Popescu. 2025. "Russian Sage Revealed: Exploring Biology, Cultivation, and Chemical Dimensions of Salvia yangii" Agronomy 15, no. 4: 868. https://doi.org/10.3390/agronomy15040868
APA StyleGostin, I. N., & Popescu, I. E. (2025). Russian Sage Revealed: Exploring Biology, Cultivation, and Chemical Dimensions of Salvia yangii. Agronomy, 15(4), 868. https://doi.org/10.3390/agronomy15040868







