The Impact of Cover Crop Biomass Introduction on the Dynamics of Nutrient Changes and Crop Productivity in Sandy-Clay Soils
Abstract
1. Introduction
2. Materials and Methods
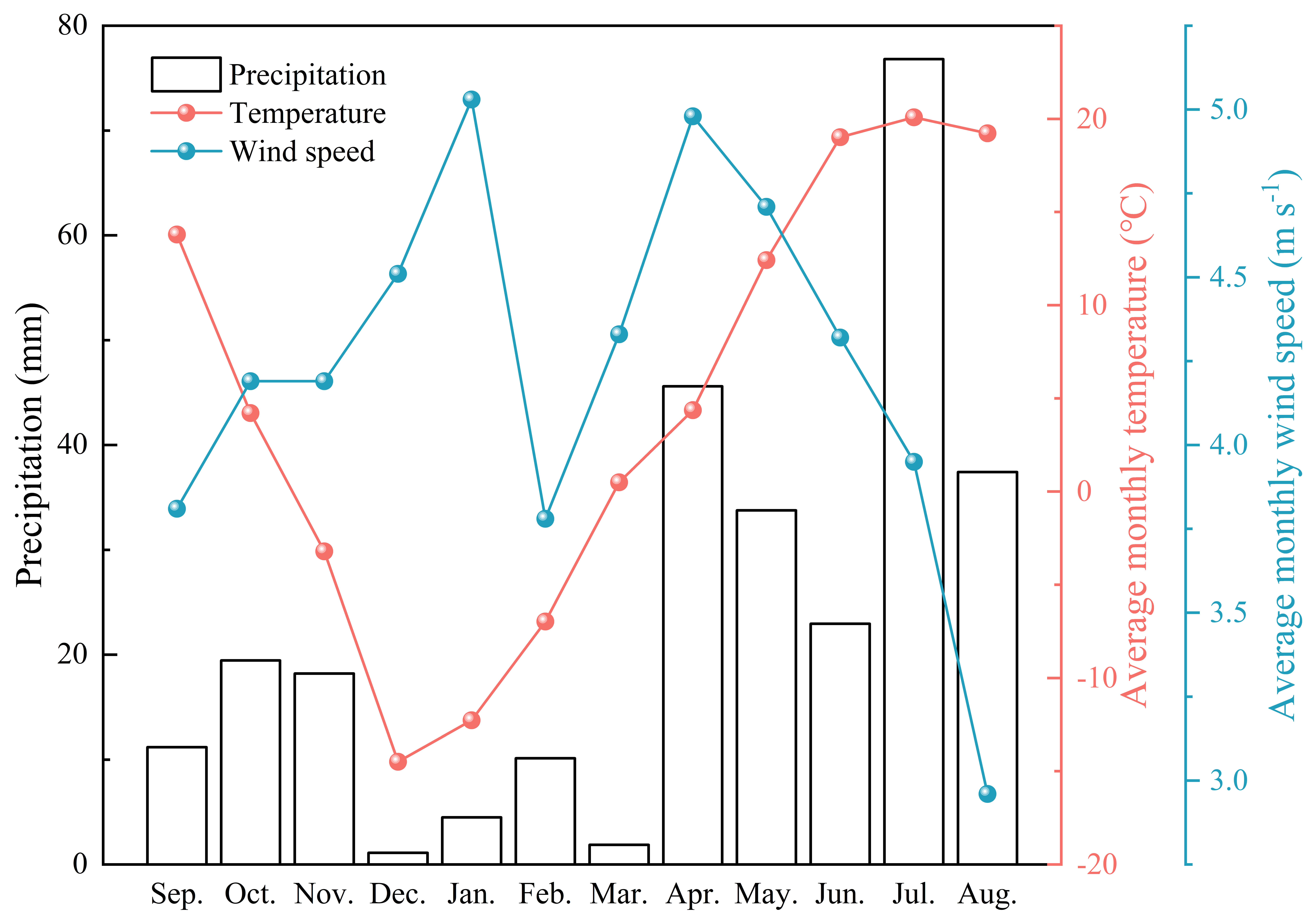
2.1. Climatic and Edaphic Conditions of Experimental Site
2.2. Experimental Site
2.3. Sampling and Measurements
2.3.1. Sampling Protocol and Analytical Determination of Plant Specimens
2.3.2. Sampling Protocol and Analytical Determination of Soil Specimens
2.3.3. Quantification of Potato Tuber Yield
2.4. Statistical Analysis
3. Results
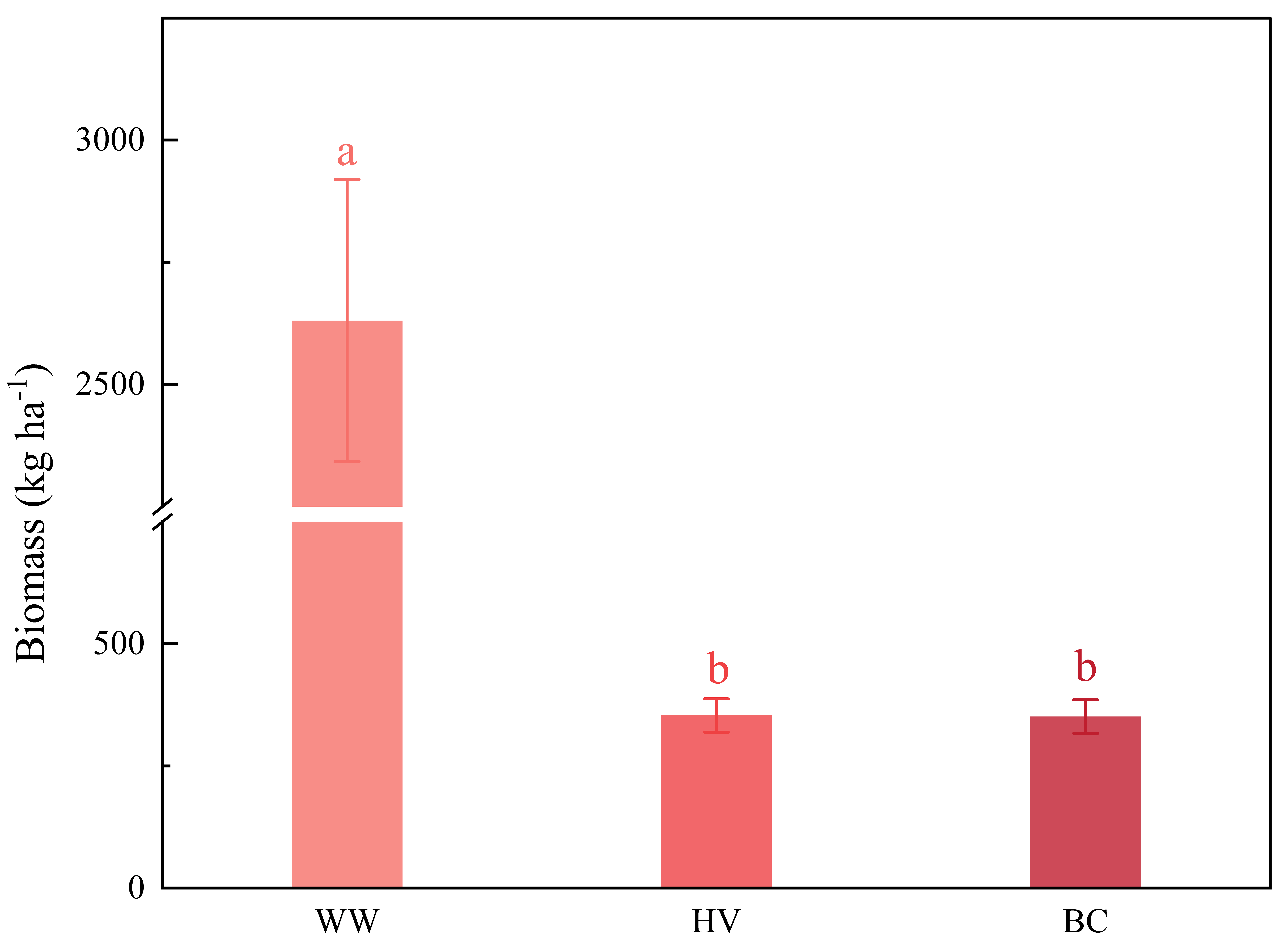
3.1. Cover Crop Biomass and Nutrient Accumulation
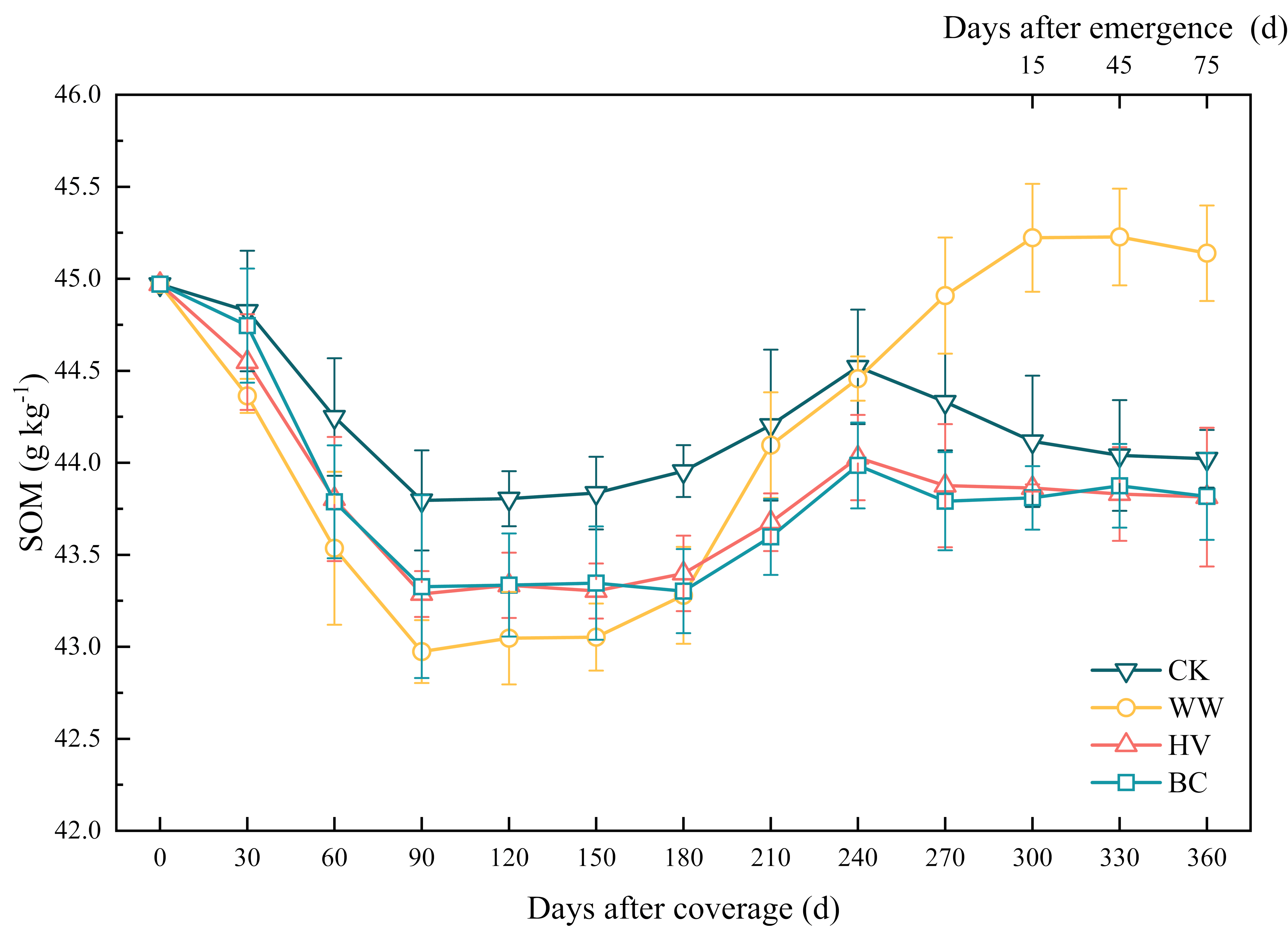
3.2. Effect of Cover Crops on SOM
3.3. Effect of Cover Crops on -N
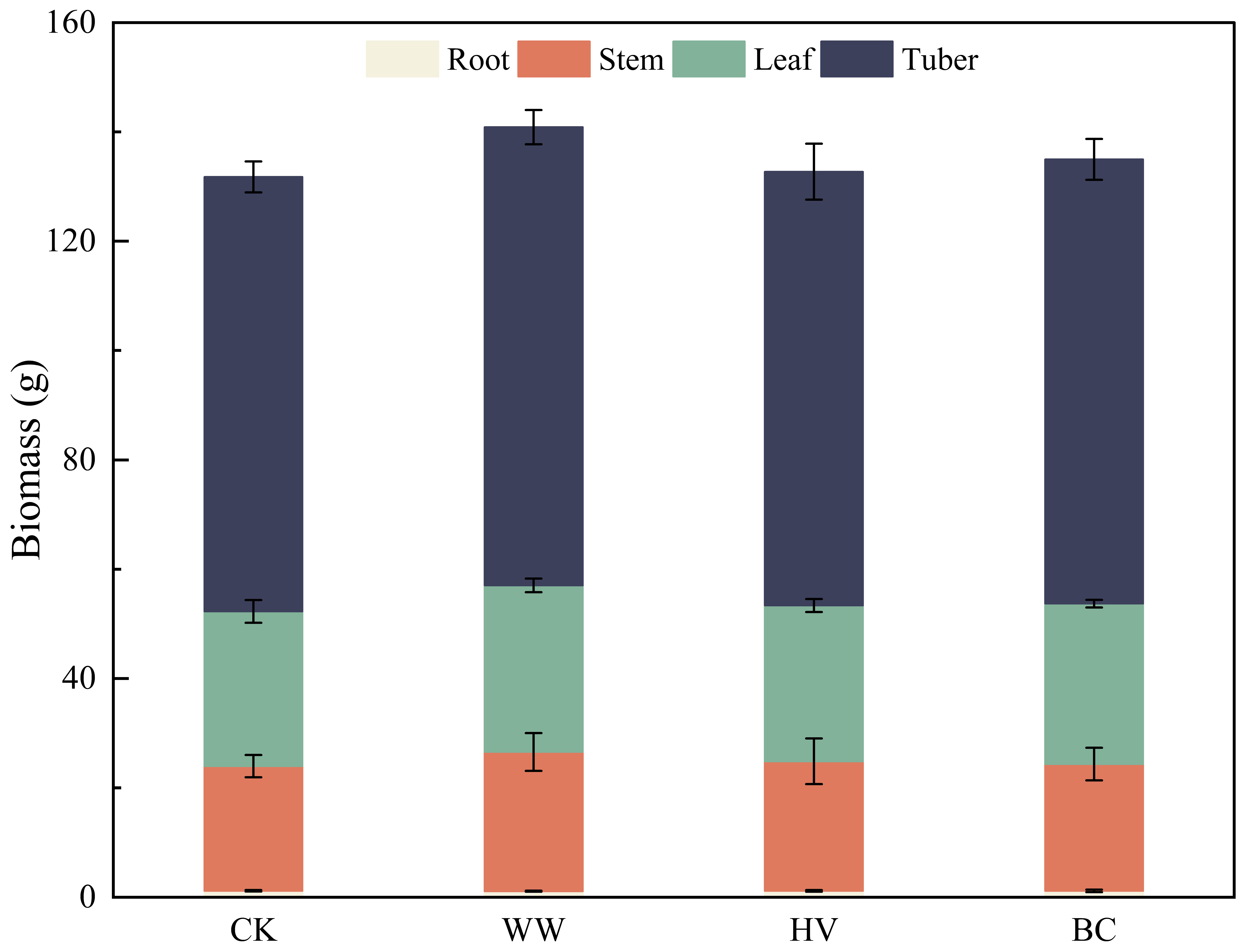
3.4. Impact of Cover Crop Incorporation on Subsequent Potato Crop Performance
4. Discussion
4.1. Factors Influencing SOM and SOM Increment Under Cover Cropping Systems
4.2. Factors Influencing -N and -N Increment Under Cover Cropping Systems
4.3. Decomposition Rate and Duration of Cover Crop Residues
4.4. Potato Biomass and Nitrogen Utilization Dynamics
4.5. Potential Future Research Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SOM | Soil organic matter |
| SOC | Soil organic carbon |
| -N | Nitrate-nitrogen |
| NUE | Nitrogen use efficiency |
| PFPN | Partial factor productivity of nitrogen |
| TC | Total carbon |
| TN | Total nitrogen |
References
- Mausel, P.W. Soil quality in Illinois—An example of a soils geography resource analysis. Prof. Geogr. 1971, 23, 127–136. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; De Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar]
- Huang, J.; Hartemink, A.E. Soil and environmental issues in sandy soils. Earth-Sci. Rev. 2020, 208, 103295. [Google Scholar]
- Chen, Z.; Ma, S.S. Study on Capability to Resist Wind Erosion of Faemland Strip Intercropping with Wheat and Potato in the Cross Region between Farmland and Grassland in North Area of Yinshan Mountain. J. Henan Agric. Univ. 2006, 40, 370–374. [Google Scholar]
- Tian, H.; Lu, C.; Yang, J.; Banger, K.; Huntzinger, D.N.; Schwalm, C.R.; Michalak, A.M.; Cook, R.; Ciais, P.; Hayes, D.; et al. Global patterns and controls of soil organic carbon dynamics as simulated by multiple terrestrial biosphere models: Current status and future directions. Glob. Biogeochem. Cycles 2015, 29, 775–792. [Google Scholar]
- Doran, J.W.; Timothy, B.P. Defining and assessing soil quality. Defin. Soil Qual. A Sustain. Environ. 1994, 35, 1–21. [Google Scholar]
- Karlen, D.L.; Eash, N.S.; Unger, P.W. Soil and crop management effects on soil quality indicators. Am. J. Altern. Agric. 1992, 7, 48–55. [Google Scholar]
- Maurya, S.; Abraham, J.S.; Somasundaram, S.; Toteja, R.; Gupta, R.; Makhija, S. Indicators for assessment of soil quality: A mini-review. Environ. Monit. Assess. 2020, 192, 604. [Google Scholar]
- Khadka, D.; Lamichhane, S.; Bhurer, K.P.; Chaudhary, J.N.; Ali, M.F.; Lakhe, L. Soil fertility assessment and mapping of regional agricultural research station, Parwanipur, Bara, Nepal. J. Nepal Agric. Res. Counc. 2018, 4, 33–47. [Google Scholar]
- Merckx, R.; Diels, J.; Vanlauwe, B.; Sanginga, N.; Denef, K.; Oorts, K. Soil organic matter and soil fertility. Sustain. Soil Fertil. West Afr. 2001, 58, 69–89. [Google Scholar]
- Gerke, J. The central role of soil organic matter in soil fertility and carbon storage. Soil Syst. 2022, 6, 33. [Google Scholar] [CrossRef]
- Assefa, S.; Tadesse, S. The principal role of organic fertilizer on soil properties and agricultural productivity—A review. Agric. Res. Technol. Open Access J. 2019, 22, 556192. [Google Scholar]
- Saini, P.K.; Yadav, R.K.; Yadav, G.C. Green manures in agriculture: A review. Bhartiya Krishi Anusandhan Patrika 2019, 34, 1–10. [Google Scholar]
- Tian, Y.; Wang, Q.; Gao, W.; Luo, Y.; Wu, L.; Rui, Y.; Huang, Y.; Xiao, Q.; Li, X.; Zhang, W. Organic amendments facilitate soil carbon sequestration via organic carbon accumulation and mitigation of inorganic carbon loss. Land Degrad. Dev. 2022, 33, 1423–1433. [Google Scholar]
- Clark, A.; Buric, D.; Sarrantonio, M.; Sears, E.; Chowdhury, A.; Brannen, C. Managing Cover Crops Profitably, 3rd ed.; Diane Publishing: Collingdale, PA, USA, 2008; pp. 17–24. [Google Scholar]
- Liang, Z.; Rasmussen, J.; Poeplau, C.; Elsgaard, L. Priming effects decrease with the quantity of cover crop residues–Potential implications for soil carbon sequestration. Soil Biol. Biochem. 2023, 184, 109110. [Google Scholar]
- Poeplau, C.; Don, A. Carbon sequestration in agricultural soils via cultivation of cover crops—A meta-analysis. Agric. Ecosyst. Environ. 2015, 200, 33–41. [Google Scholar]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar]
- Wang, Y.; Ying, H.; Yin, Y.; Zheng, H.; Cui, Z. Estimating soil nitrate leaching of nitrogen fertilizer from global meta-analysis. Sci. Total Environ. 2019, 657, 96–102. [Google Scholar]
- Tian, L.; Zhu, B.; Akiyama, H. Seasonal variations in indirect N2O emissions from an agricultural headwater ditch. Biol. Fertil. Soils 2017, 53, 651–662. [Google Scholar]
- Whittaker, J.; Nyiraneza, J.; Zebarth, B.J.; Jiang, Y.; Burton, D.L. The effects of forage grasses and legumes on subsequent potato yield, nitrogen cycling, and soil properties. Field Crops Res. 2023, 290, 108747. [Google Scholar]
- Zhang, Z.; Wang, J.; Huang, W.; Chen, J.; Wu, F.; Jia, Y.; Han, Y.; Wang, G.; Feng, L.; Li, X.; et al. Cover crops and N fertilization affect soil ammonia volatilization and N2O emission by regulating the soil labile carbon and nitrogen fractions. Agric. Ecosyst. Environ. 2022, 340, 108188. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; McElroy, M.S.; Chapagain, T.; Papadopoulos, Y.A.; Raizada, M.N. Belowground nitrogen transfer from legumes to non-legumes under managed herbaceous cropping systems. A review. Agron. Sustain. Dev. 2016, 36, 58. [Google Scholar]
- Wagger, M.G.; Cabrera, M.L.; Ranells, N.N. Nitrogen and carbon cycling in relation to cover crop residue quality. J. Soil Water Conserv. 1998, 53, 214–218. [Google Scholar]
- Drinkwater, L.E.; Snapp, S. Nutrients in agroecosystems: Rethinking the management paradigm. Adv. Agron. 2007, 92, 163–186. [Google Scholar]
- Salmerón, M.; Isla, R.; Cavero, J. Effect of winter cover crop species and planting methods on maize yield and N availability under irrigated Mediterranean conditions. Field Crops Res. 2011, 123, 89–99. [Google Scholar]
- Abdalla, M.; Hastings, A.; Cheng, K.; Yue, Q.; Chadwick, D.; Espenberg, M.; Truu, J.; Rees, R.M.; Smith, P. A critical review of the impacts of cover crops on nitrogen leaching, net greenhouse gas balance and crop productivity. Glob. Change Biol. 2019, 25, 2530–2543. [Google Scholar]
- Salazar, O.; Balboa, L.; Peralta, K.; Rossi, M.; Casanova, M.; Tapia, Y.; Singh, R.; Quemada, M. Effect of cover crops on leaching of dissolved organic nitrogen and carbon in a maize-cover crop rotation in Mediterranean Central Chile. Agric. Water Manag. 2019, 212, 399–406. [Google Scholar]
- Meisinger, J.J.; Ricigliano, K.A. Nitrate leaching from winter cereal cover crops using undisturbed soil-column lysimeters. J. Environ. Qual. 2017, 46, 576–584. [Google Scholar]
- Thapa, R.; Mirsky, S.B.; Tully, K.L. Cover crops reduce nitrate leaching in agroecosystems: A global meta-analysis. J. Environ. Qual. 2018, 47, 1400–1411. [Google Scholar]
- Qin, Y.H.; Wu, Y.H.; Wang, L.; Dan, Y.B.; Chen, H.; Hao, X.S.; Wang, D.; Yang, J. Decomposition and nutrient release characteristics of four winter green manures in Hanzhong. Pratacultural Sci. 2024, 41, 2306–2315. [Google Scholar]
- Garba, I.I.; Bell, L.W.; Williams, A. Cover crop legacy impacts on soil water and nitrogen dynamics, and on subsequent crop yields in drylands: A meta-analysis. Agron. Sustain. Dev. 2022, 42, 34. [Google Scholar]
- Toler, H.D.; Augé, R.M.; Benelli, V.; Allen, F.L.; Ashworth, A.J. Global meta-analysis of cotton yield and weed suppression from cover crops. Crop Sci. 2019, 59, 1248–1261. [Google Scholar]
- Aiken, R.M.; O’Brien, D.M.; Olson, B.L.; Murray, L. Replacing fallow with continuous cropping reduces crop water productivity of semiarid wheat. Agron. J. 2013, 105, 199–207. [Google Scholar]
- Turgut, I.; Bilgili, U.; Duman, A.; Acikgoz, E. Effect of green manuring on the yield of sweet corn. Agron. Sustain. Dev. 2005, 25, 433–438. [Google Scholar]
- Yang, L.; Bai, J.; Liu, J.; Zeng, N.; Cao, W. Green manuring effect on changes of soil nitrogen fractions, maize growth, and nutrient uptake. Agronomy 2018, 8, 261. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, G.; Chang, D.; Liang, H.; Nie, J.; Liao, Y.; Lu, Y.; Xu, C.; Liu, J.; Wu, J.; et al. Southern China can produce more high-quality rice with less N by green manuring. Resour. Conserv. Recycl. 2023, 196, 107025. [Google Scholar]
- Blanco-Canqui, H.; Ruis, S.J. Cover crop impacts on soil physical properties: A review. Soil Sci. Soc. Am. J. 2020, 84, 1527–1576. [Google Scholar]
- Qin, Y.L.; Yu, J.; Chen, Y.; Jia, L.G.; Suyala, Q.Q.G.; Fan, M.S. Situation of Fertilization and Fertilizer Use Efficiency on Irrigated Potato in InnerMongolia. China Veg. 2019, 11, 75–79. [Google Scholar]
- T/SDAS 302-2021; Determination of Total Carbon in Plant. Shandong Standardization Association: Jinan, China, 2021.
- ISO 20483:2013; Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method. ISO/TC 34/SC 4: Beijing, China, 2013.
- ISO 10694:1995; Soil Quality—Determination of Organic and Total Carbon After Dry Combustion (Elementary Analysis). ISO/TC 190/SC 3: Berlin, Germany, 1995.
- Lu, R.K.; Zhu, H.Z.; He, P.A.; Chen, C.Z.; Chen, H.M.; Zhou, J.M.; Su, D.C.; Xu, J.M.; Qin, H.Y.; Bao, S.D.; et al. Methods of Soil and Agricultural Chemistry Analysis, 1st ed.; China Agricultural Science and Technology Press: Beijing, China, 1999; pp. 107–110. [Google Scholar]
- GB/T 42487-2023; Soil Quality—Determination of Nitrate, Nitrite and Ammonium in Soils—Extraction with Potassium Chloride Solution and Determination Using Automated Method with Segmented Flow Analysis. China-Soil Quality: Nanjing, China, 2023.
- Lal, R. Soil carbon management and climate change. Carbon Manag. 2013, 4, 439–462. [Google Scholar]
- Nieder, R.; Benbi, D.K. Carbon and Nitrogen in the Terrestrial Environment, 1st ed.; Springer Science & Business Media: Berlin, Germany, 2008; pp. 161–218. [Google Scholar]
- Zhang, N.; Chen, X.; Wang, J.; Dong, H.; Han, X.; Lu, X.; Yan, J.; Zou, W. Anthropogenic soil management performs an important role in increasing soil organic carbon content in northeastern China: A meta-analysis. Agric. Ecosyst. Environ. 2023, 350, 108481. [Google Scholar] [CrossRef]
- Coucheney, E.; Kätterer, T.; Meurer, K.H.; Jarvis, N. Improving the sustainability of arable cropping systems by modifying root traits: A modelling study for winter wheat. Eur. J. Soil Sci. 2024, 75, e13524. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Castellano, M.J.; Vogel, C.; Wiesmeier, M.; Mueller, C.W. Unlocking complex soil systems as carbon sinks: Multi-pool management as the key. Nat. Commun. 2023, 14, 2967. [Google Scholar] [CrossRef]
- Tao, F.; Huang, Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L.; et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Yue, S.; Chen, X.; Cui, Z.; Ye, Y.; Ma, W.; Tong, Y.; Zhang, F. Understanding dry matter and nitrogen accumulation with time-course for high-yielding wheat production in China. PLoS ONE 2013, 8, e68783. [Google Scholar] [CrossRef] [PubMed]
- Kebede, E. Contribution, utilization, and improvement of legumes-driven biological nitrogen fixation in agricultural systems. Front. Sustain. Food Syst. 2021, 5, 767998. [Google Scholar] [CrossRef]
- Kumar, U.; Thomsen, I.K.; Eriksen, J.; Vogeler, I.; Mäenpää, M.; Hansen, E.M. Delaying sowing of cover crops decreases the ability to reduce nitrate leaching. Agric. Ecosyst. Environ. 2023, 355, 108598. [Google Scholar] [CrossRef]
- Fernandez Pulido, C.R.; Rasmussen, J.; Eriksen, J.; Abalos, D. Cover crops for nitrogen loss reductions: Functional groups, species identity and traits. Plant Soil 2023, 507, 127–140. [Google Scholar] [CrossRef]

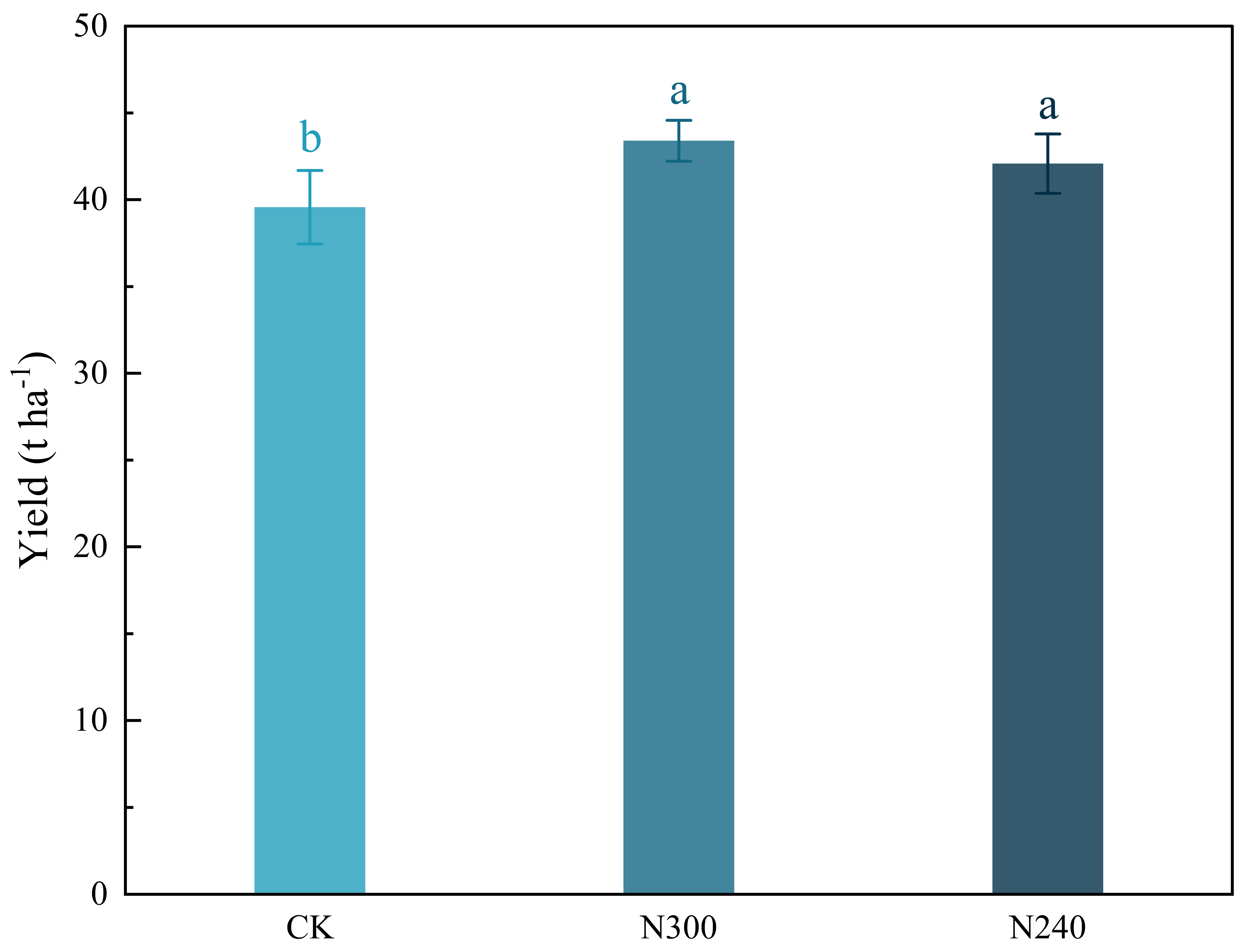
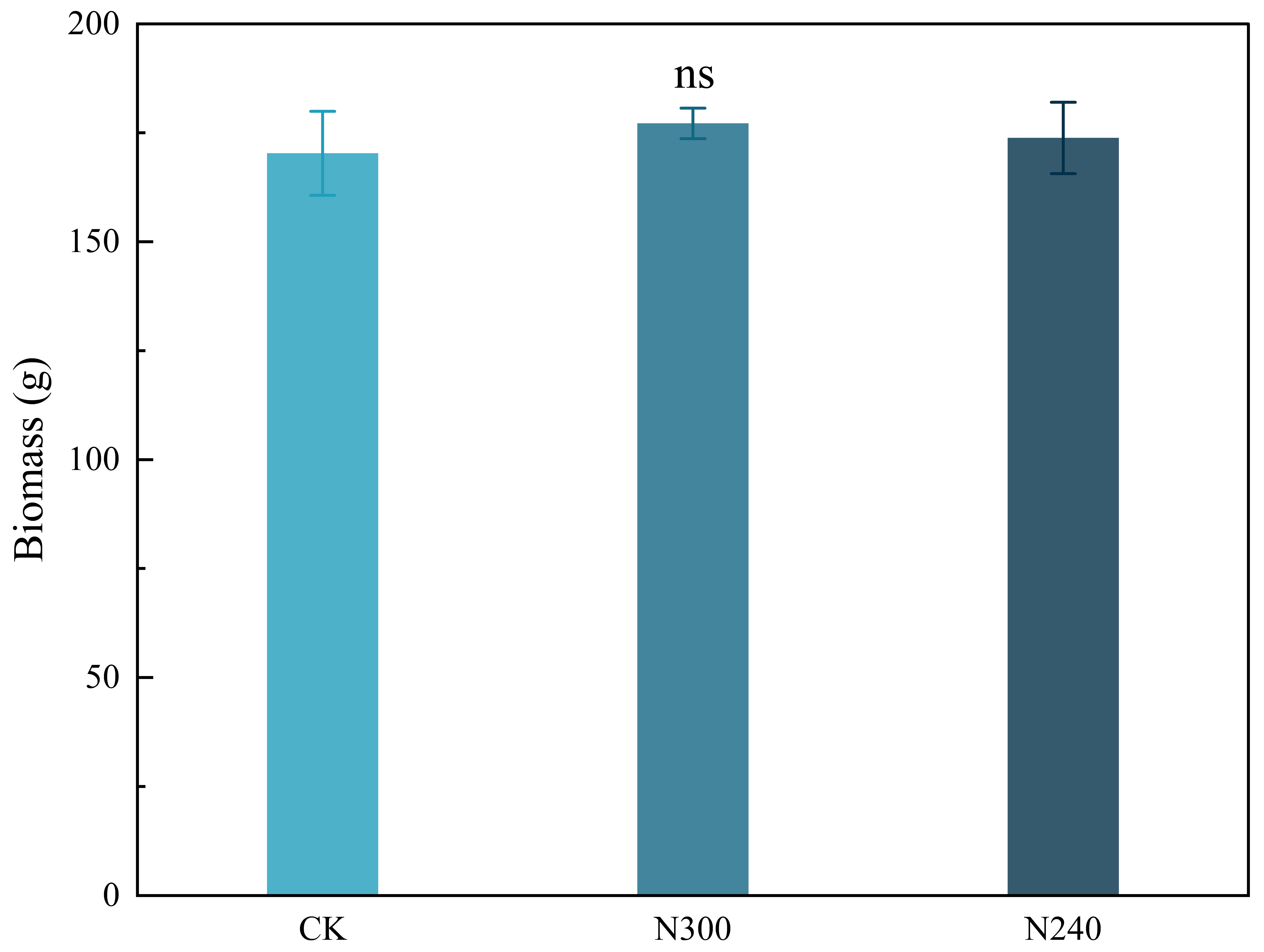
| Soil Horizon (cm) | -N (mg kg−1) | -N (mg kg−1) | Available Phosphorus (mg kg−1) | Available Potassium (mg kg−1) | Soil Organic Matter (g kg−1) | Volume Weight (g cm−3) | pH |
|---|---|---|---|---|---|---|---|
| 0–20 | 36.71 ± 3.41 | 1.62 ± 0.30 | 44.55 ± 4.15 | 170 ± 13.62 | 44.97 ± 3.68 | 1.37 ± 0.18 | 7.8 ± 1.57 |
| 20–40 | 22.01 ± 2.92 | 1.35 ± 0.20 | 18.74 ± 1.22 | 153 ± 10.71 | 40.58 ± 2.52 | 1.40 ± 0.25 | 7.7 ± 1.42 |
| Main Factor | Soil Horizon (cm) | -N (mg kg−1) | Available Phosphorus (mg kg−1) | Available Potassium (mg kg−1) | Soil Organic Matter (g kg−1) | Volume Weight (g cm−3) | pH |
|---|---|---|---|---|---|---|---|
| Bare | 0–20 | 11.63 ± 1.47 | 20.97 ± 1.57 | 74.53 ± 5.03 | 40.31 ± 0.12 | 1.37 ± 0.19 | 7.8 ± 1.23 |
| 20–40 | 23.58 ± 3.23 | 20.13 ± 0.73 | 75.20 ± 0.80 | 38.72 ± 0.14 | 1.39 ± 0.18 | 7.9 ± 1.21 | |
| Cover | 0–20 | 18.18 ± 2.14 | 17.28 ± 1.35 | 83.16 ± 4.41 | 41.64 ± 0.05 | 1.34 ± 0.11 | 7.7 ± 1.18 |
| 20–40 | 18.77 ± 2.82 | 17.19 ± 1.45 | 86.00 ± 8.61 | 39.31 ± 0.05 | 1.37 ± 0.17 | 7.8 ± 1.20 |
| Treatment | Fertilizer | Subsequent Crop | N Rate (kg N ha−1) | P Rate (kg P2O5 ha−1) | K Rate (kg K2O ha−1) |
|---|---|---|---|---|---|
| CK | None | Potato | 300 | 180 | 300 |
| WW | None | Potato | 300 | 180 | 300 |
| HV | None | Potato | 300 | 180 | 300 |
| BC | None | Potato | 300 | 180 | 300 |
| Treatment | Cover Factor | N Rate (Basal) (kg N ha−1) | N Rate (DAE 25 d) (kg N ha−1) | N Rate (DAE 40 d) (kg N ha−1) |
|---|---|---|---|---|
| CK | Bare | 90 | 120 | 90 |
| CK0 | 0 | 0 | 0 | |
| N300 | Cover | 90 | 120 | 90 |
| N240 | 30 | 120 | 90 | |
| N0 | 0 | 0 | 0 |
| TC (%) | Caccumulation (kg ha−1) | TN (%) | Naccumulation (kg ha−1) | |
|---|---|---|---|---|
| WW | 61.39 ± 1.83 a | 1612.43 ± 150.83 a | 3.36 ± 0.02 b | 88.44 ± 9.44 a |
| HV | 60.69 ± 1.59 a | 214.42 ± 23.41 b | 3.87 ± 0.18 b | 13.61 ± 1.91 b |
| BC | 60.36 ± 1.82 a | 212.27 ± 26.85 b | 2.35 ± 0.15 b | 8.34 ± 1.28 b |
| CK | N300 | N240 | |
|---|---|---|---|
| NUE (%) | 42.62 ± 1.05 b | 46.19 ± 5.74 b | 53.45 ± 4.88 a |
| PFPN (kg kg−1) | 131.88 ± 7.06 c | 144.64 ± 3.92 b | 175.32 ± 7.14 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Shi, X.; Kong, S.; Jia, L.; Qin, Y.; Yu, J.; Liu, K.; Fan, M. The Impact of Cover Crop Biomass Introduction on the Dynamics of Nutrient Changes and Crop Productivity in Sandy-Clay Soils. Agronomy 2025, 15, 856. https://doi.org/10.3390/agronomy15040856
Li C, Shi X, Kong S, Jia L, Qin Y, Yu J, Liu K, Fan M. The Impact of Cover Crop Biomass Introduction on the Dynamics of Nutrient Changes and Crop Productivity in Sandy-Clay Soils. Agronomy. 2025; 15(4):856. https://doi.org/10.3390/agronomy15040856
Chicago/Turabian StyleLi, Chenyi, Xiaohua Shi, Shuo Kong, Liguo Jia, Yonglin Qin, Jing Yu, Kun Liu, and Mingshou Fan. 2025. "The Impact of Cover Crop Biomass Introduction on the Dynamics of Nutrient Changes and Crop Productivity in Sandy-Clay Soils" Agronomy 15, no. 4: 856. https://doi.org/10.3390/agronomy15040856
APA StyleLi, C., Shi, X., Kong, S., Jia, L., Qin, Y., Yu, J., Liu, K., & Fan, M. (2025). The Impact of Cover Crop Biomass Introduction on the Dynamics of Nutrient Changes and Crop Productivity in Sandy-Clay Soils. Agronomy, 15(4), 856. https://doi.org/10.3390/agronomy15040856







