Abstract
Agroforestry contributes to slowing deforestation, favoring ecosystem regeneration and improving land use sustainability. This study evaluated the impact of silvopastoral systems on soil recovery and their capacity to sequester and stabilize carbon (C) and nitrogen (N) in degraded soils of a native Nothofagus obliqua forest in Ranchillo Alto (37°04′52″ S, 71°39′14″ W), Ñuble Region, Chile. Three open (Op), semi-open (SOp), and semi-closed (SC) silvopastoral systems were analyzed and compared with a control (Ctr) without silvopastoral management across four soil depths (0–10, 10–20, 20–30, 30–60 cm). Physical, chemical, and biological analyses were performed, along with soil physical organic matter (SOM) fractionation. The highest C levels were found in the 0–10 cm depth (13.9, 11.8, 11.5, and 8.5% for Op > SC > SOp and Ctr, respectively). Despite its higher degradation, Op presented the highest levels of C, N, and non-oxidizable C (Cnox), possibly due to pyrogenic carbon from old potato burns. Furthermore, the same trend was observed for mineral associated organic matter (MAOM) fraction and C stocks in all silvopastoral systems compared to the control. These results underline the potential of silvopastoral practices to improve soil quality and increase long-term carbon sequestration, contributing to sustainable soil restoration strategies.
1. Introduction
In Chile, decades of overuse of natural resources have led to 37.8% of the national territory showing soil degradation, in moderate to severe conditions, concentrated mainly in the central part of the country [1,2]. This degradation is mainly caused by the felling of more than 44% of native forests, affecting more than 8 million hectares (Mha). The genus Nothofagus has been one of the most impacted, decreasing its original area by more than 70%. This reduction is mainly due to anthropogenic activities, including the expansion of urban areas, croplands, and forest plantations, although most of the surface has been cleared for the growth of pastures for livestock use, reaching up to 3 Mha [3].
Given this scenario of increasing soil and environmental degradation, various sustainable practices have been promoted in recent years to mitigate the impact of human activities. Among them, silvopastoral systems (SPSs) have played a fundamental role. According to several studies [4,5,6], the integration of trees in production systems reduces erosion, improves soil fertility and quality, protects biodiversity, and contributes to the mitigation of climate change through carbon (C) sequestration, being able to store between 1.8 and 6.1 Mg of soil organic carbon (SOC) per year [5,7].
Recent studies have highlighted the importance of including native forests and agroforestry systems in conservation and climate change mitigation policies [6,8]. Since 2019, the Intergovernmental Panel on Climate Change (IPCC) [9] has underlined the crucial role of these systems in C capture and their potential to reduce greenhouse gas emissions, conserve biodiversity, and protect soil [10,11]. FAO [12] as well called for collaboration between the agricultural and forestry sectors to expand agroforestry practices, defining agroforestry as a key climate solution due to its capacity to store large amounts of soil organic matter (SOM), which helps the provision of multiple ecosystem services [13]. According to Voltr et al. [14], in agroecosystems with a marked presence of woody species, high amounts of SOM are produced, which translate into annual net increases in ecosystem C, of which around 60% of the C is in the form of SOC.
SOM can be defined as the complex mixture of different compounds from plants and soil microorganisms [15]. The physical separation of SOM by particle size operationally into particulate organic matter (POM) and matrix-associated organic matter (MAOM) minerals is an approach that helps to understand the distribution of SOM and responses to environmental change [16,17,18,19,20]. It is proposed that POM and MAOM have different formation pathways and mean residence times in soil. It is postulated that POM (labile fraction of C) comes from the fragmentation or depolymerization of plant litter, while MAOM (stable fraction of C) comes from the transformations or modifications carried out by soil microorganisms and/or their exoenzymes, which grant it different degrees of physicochemical protection [21].
Understanding the mechanisms of SOM formation and stabilization, as well as its sensitivity to disturbances and environmental changes, is fundamental for predicting its future dynamics [22]. Representing SOM pools based on the processes that regulate their formation, and stabilization is crucial for developing sustainable management strategies. In this context, SPSs stand out for their ability to restore degraded soils by facilitating the incorporation and recycling of nutrients through SOM contributions. However, knowledge about the implementation of these systems in degraded native forests, particularly at different soil depths, and their capacity to stabilize SOC in the long term remains limited. Therefore, the objective of this study was to evaluate the effect of agroforestry on the recovery of degraded soils in a native roble (Nothofagus obliqua) forest and its ability to capture and stabilize soil C and nitrogen (N).
2. Materials and Methods
2.1. Description of the Study Area
The study was conducted in Ranchillo Alto public property located in the foothills of the Ñuble region, Chile (37°04′52″ S, 71°39′14″ W; 1200–2000 m a.s.l.), covering approximately 635 ha (Figure 1). This property corresponds to a native forest that has undergone prolonged degradation mainly due to indiscriminate logging, overgrazing, and extraction of saw-quality timber and firewood (browsing, depredation). The foothills of the Ñuble region have a humid temperate Mediterranean climate with a marked winter season, and annual temperatures of 13.5 °C, but can exceed 25 °C in summer. The annual rainfall may exceed 3.000 mm in a normal year. Snowfall occurs mostly between May and September, reaching when there are low temperatures and frequent frosts. The soils are Andisols, “Santa Barbara” series (medial, amorphic, mesic Typic Haploxerands), and locally known as “trumaos” [23].
Figure 1.
Location of the native forest study site (Ranchillo Alto) in south-central Chile.
2.2. Conditioning of Experimental Treatments
In order to restore the ecosystem value of the forest, experimental treatments of silvopastoral systems (SPSs) were implemented in the northern and southern sectors of the property in 2016. This study was carried out in the period 2023–2024 and focused on three SPSs located in the southern sector, which cover approximately 10 ha. These SPSs represent different levels of open canopy, defined according to the degree of previous disturbance of the forest. The sites were classified as open (Op) +++, semi-open (SOp) ++, and semi-closed (SC) +. In addition, a control of degraded native forest without silvopastoral intervention (Ctr) was included (Table 1).

Table 1.
General information for each tree covers conditions in the silvopastoral systems located in the southern sector of Ranchillo Alto.
The SPSs located in the southern sector already had the three canopy conditions defined for this study previously established in 2016 [24], so, in 2023 we validated mean percentages of solar radiation at ground level as well as leaf area indices and determined that no modifications in tree density were necessary as these levels had not changed over time. To quantify and compare the percentages of cover for each treatment and their evolution over time, the results were corroborated with a second series of hemispheric photos (15–20 random points in each plot) taken with a Solariscope SOL 300B (Behling, Germany) [25].
The woody component in the three silvopastoral systems (SPSs) is represented by roble (Nothofagus obliqua). In the study area, current land use is primarily based on controlled grazing practices. In 2016, during its establishment, a mixture of 12 species of grasses and legumes was sown, with the objective of improving the quality of feed for livestock, while maintaining the vegetation cover and favoring the regeneration of the ecosystem, since the previous degradation had strongly reduced the availability of grazing. High-quality feed contributes to improved availability and animal nutrition. Additionally, it is more palatable food, considering that livestock tends to consume selectively. The herbaceous component included oats (Avena sativa L.), vetch (Fabaceae purpurea L.), clover (Trifolium incarnatum L., T. subterraneum L. and T. vesiculosum L.), Lolium multiflorum var. Westerwoldicum, Phalaris aquatica L., Lolium perenne L., and Dactylis glomerata L., in addition to the regrowth of radal (Lomatia hirsuta).
Currently, due to the conditions of the forest in the foothills, as well as the low temperatures and the presence of snow in winter, more resistant species have prevailed, notably quilla (Chusquea quila), radal, and Festuca arundinacea L. The latter is present in all three silvopastoral systems, especially in Op and SOp, and to a lesser extent in SC, which is related to the degree of solar radiation available, as it requires sun for optimal development.
Likewise, in the Op system there is a marked presence of radal, a pioneer species indicative of forest degradation, which rapidly colonizes open areas after a disturbance. Its abundance in Op, the most degraded site, is associated with the clearing carried out for potato (Solanum tuberosum L.) cultivation more than 50 years ago, where stubble burning was also practiced. These activities have caused changes in soil properties and carbon dynamics.
It is important to highlight that the silvopastoral systems were established within a native forest with the aim of restoring soil quality and the ecosystem value of the forest. As part of this strategy, livestock stocking rates have been carefully managed and kept low, preventing further disturbances since the initial sowing.
From a statistical perspective, efforts were made to minimize variability in cultural practices among the sites, ensuring that the main source of variation was canopy openness, defined according to the previous level of forest degradation. Other practices, such as species sowing, were carried out uniformly across all sites to prevent them from becoming a confounding factor in the analysis. The experimental control allows us to attribute the observed differences in the results mainly to the initial degradation conditions and the effects of the silvopastoral treatment, enabling a more accurate assessment of the impact of these practices in relation to the study’s objectives.
2.3. Soil Sampling
A completely randomized design with three replicates (plots) randomly distributed at each tree cover level treatment was used for soil sampling.
Soil samples were collected from each plot, consisting of five random sub-samples taken at different depths: 0–10 cm, 10–20 cm, 20–30 cm, and 30–60 cm, following the recommendations of Kögel-Knabner [23]. In addition, a sample of the shallow depth (0–30 cm) was taken in the field for chemical and physical analyses, which were not the main focus of this study and where we expected less variability in the first three depths. The other depths (0–10, 10–20, and 20–30) were included to evaluate differences that might be found in the first 30 cm of soil, particularly in biological analyses and labile and stable carbon forms.
The samples were placed in a thermal box with ice to be transported from the forest to the laboratory and stored at 4 °C. All samples were sieved through a 2 mm stainless steel sieve and then divided into 2 parts, one of which was air-dried for subsequent physical and chemical analyses, and the other of which was conditioned to 60% water-filled pore space (WFPS), ideal for biological analyses. Chemical and physical analyses were carried out in the Soil Laboratories for corresponding analyses and for biological ones in the Spectroscopy Laboratory (Vis-IR) and Sustainable Soil Management department of the Faculty of Agronomy of the University of Concepción.
2.4. Soil Analysis
2.4.1. Evaluation of Physical Parameters
For physical analyses, bulk density (BD) was determined using the cylinder method. A soil sample was collected in the field at the corresponding depth using a metal cylinder of known volume. The sample was then carefully extracted, avoiding compaction, and transported to the laboratory. In the laboratory, the sample was oven-dried at 105 °C for 24 h to remove all moisture. The dry weight of the soil was then measured, and BD was calculated as the ratio of dry soil mass to cylinder volume. Particle density (PD) was assessed by the pycnometer method and texture was analyzed by the Bouyoucos hydrometer method, all following the methodology described by Sandoval et al. [24].
2.4.2. Evaluation of Chemical Parameters
Chemical analyses of the soil, including pH(WATER), organic matter (OM), N(AVAILABLE), P, K, effective cation exchange capacity (ECEC), exchangeable Al, Ca, Mg, K, Na (AlEXCH, Ca2+EXCH, Mg2+EXCH, K+EXCH, Na+EXCH), NH4+, NO3−; Al, K, Ca, and Mg saturation (AlSAT, K+SAT, Ca2+SAT and Mg2+SAT) were performed according to the protocols of Sadzawka et al. [25].
2.4.3. Evaluation of Biological Parameters
Total enzymatic activity in soil was assessed by fluorescein diacetate (FDA) hydrolysis, a method that estimated the enzymatic activity of hydrolytic enzymes involved in OM degradation. For analysis, 1.0 g of moist soil was weighed into screw-capped test tubes (triplicates plus a blank). A total of 9.9 mL of sodium phosphate buffer was added to the samples and 10 mL to the blank, followed by 0.1 mL of fluorescein diacetate (FDA) only to the samples. After vortexing, the tubes were incubated at 25 °C for 1 h. After incubation, they were cooled in an ice bath. For colorimetry, 10 mL of acetone was added to each tube, vortexed, and filtered. The absorbance of the filtrate was measured at 490 nm against a reagent blank prepared with acetone and distilled water [26].
The activity of β-glucosidase, a key component of carbohydrate degradation in soil, was assessed using the method [27], based on the release of p-nitrophenol after incubating the soil with p-nitrophenyl-β-D-glucopyranoside (25 mM) in MUB-HCl (pH 6) at 37 °C for 1 h. It was then cooled on ice, centrifuged at 6000 rpm for 5 min, and after the addition of 0.5 M CaCl2 and THAM-NaOH buffer (pH 12), the absorbance at 400 nm was measured. Both the absorbance of β-glucosidase and FDA enzymatic activity were measured using a UV-visible spectrophotometer (AA3, BRAN + LUEBBE, Norderstedt, Germany).
For soil respiration, 20 g of moist soil conditioned at 60% WFPS per treatment (in triplicate) was weighed into a Falcon tube. These vials were sealed with specialized caps that allow gas (CO2) extraction using a precision syringe, which was then injected into a gas analyzer (see below) to perform microbial respiration analysis. The vials with soils were kept in an incubation chamber at 22 °C for 14 days. This requires that the soil has been conditioned at 60% WPFS. Basal soil CO2 emission was calculated at 3, 5, 7, 10, and 14 days of incubation. To perform the gas extraction process, a homogenization of the Falcon’s headspace was previously performed by extracting 1 mL of gas and injecting it again three times. The 1 mL gas sample was then injected into a unidirectional dual-wavelength non-dispersive infrared (NDIR) gas analyzer (LI-820, Li-COR Bioscience, Lincoln, NE, USA), using a modification as described by Craine et al. [28].
2.4.4. Soil Physical Fractionation
The physical fractionation of the soil was conducted following the method described by Lavallee et al. [21] to enhance understanding of soil C fraction dynamics. Briefly, soils were sieved to 2 mm, and 5 g of oven-dried soil at 105 °C was shaken with 15 mL of 0.5% sodium hexametaphosphate solution and five 1 mm glass beads for 18 h to disperse the soil. The dispersed soil was then rinsed through a 53 µm sieve, with the fraction passing through (<53 µm) collected as MAOM, while the remaining material was classified as POM.
2.4.5. Total Carbon, Oxidizable Carbon, Non-Oxidizable Carbon, and Carbon Stocks
Total carbon (TC) and total nitrogen were determined in each of the soil samples and their fractions using the dry combustion method based on the Dumas principle [29]. The different samples and their fractions were subjected to chemical oxidation with sodium dichromate dihydrate to determine the proportion of oxidizable carbon (Cox), following the Walkley–Black method [1,25]. Since there is no inorganic C in this volcanic soil, TC = total organic carbon (TOC). Non-oxidizable carbon (Cnox) was determined by the difference between TC and Cox (Cnox = TOC − Cox).
The carbon stock (C stock) for each soil layer was calculated by multiplying the OC concentration by the BD and the thickness of the sampled soil layer (cm). The formula used was as follows:
2.5. Statistical Analysis
To evaluate the effect of Op, SOp, SC, and Ctr canopy cover on the different chemical, physical, and biological analyses at the different depths (0–10; 10–20; 20–30; and 30–60 cm), a two-way ANOVA was performed, and for significant differences, Tukey post hoc analyses were performed, using the R program (RStudio 2024). A principal component analysis (PCA) was performed to identify patterns in the variability of the variates studied and to reduce the dimensionality of the data. This analysis allowed us to observe the distribution of the samples based on the treatments and soil depths, and to evaluate the relationship between the different soil variables studied. The PCA was performed using the R program (RStudio 2024), employing the prcomp function with previously centered and scaled data.
3. Results and Discussion
Our physical analyses of the soil revealed that BD and PD, as well as the texture, remained within the typical range for volcanic soils under the different site conditions (Table 2) [1].

Table 2.
Physical properties of soil under different open canopy levels and depth.
Individually, the BD showed representative values for Andisols ranging between 0.60 and 0.76 g/cm3. The density variation depended more on the soil depth than on the canopy opening, being more significant in the surface horizon (p ≤ 0.05). These observations coincide with the results of Gomez et al. [30], who reported similar findings in Andisol soils from forests under silvopastoral management in Argentine Patagonia.
In addition, the PD, like the BD, presented variations in terms of soil depth, giving significant differences (p ≤ 0.05). The PD ranged from 2.06 to 2.21 g cm−3 with a mean value of 2.1 g cm−3, which is within representative ranges of volcanic soils rich in OM, according to Ortiz et al. [31] and Nissen et al. [32], who estimated ranges of 1.9 to 2.1 (0 to 15 cm) and 1.9 to 2.0 (0 to 20 cm) for PD in forest soils (0–15 cm).
The soil texture, like the rest of the physical variables, did not show differences in terms of canopy opening levels, but did show significant differences between soil depths for sand and silt (p ≤ 0.05). The results found for texture coincide with those reported by Gomez et al. [30].
Chemical analyses of the soil generally showed results representative of Andisol soils (Table 3). The observed pH values are slightly acidic, fluctuating between 5.41 and 5.66, a typical characteristic of Andisol soils. The pH did not show significant differences (p > 0.05), although there was a trend in both depths, with the Op condition having the lowest values, which could be attributed to a higher acidity favored by a high OM content [33].

Table 3.
Chemical properties of soil under different open canopy levels and depth.
OM showed a decrease with depth in all treatments, an expected pattern given that OM is mostly concentrated in the surface horizon due to the accumulation of leaf litter and roots. In the surface horizon (0–30 cm), the Op system presents the highest OM value (22.07%), followed by SC, SOp, and Ctr respectively, reflecting the direct contribution of OM in the silvopastoral treatments compared to the control. The OM values are relatively higher than those reported in other studies [30]; this is mainly due to the location of the forest in the foothills, where low temperatures throughout the year slow down the decomposition processes of soil microorganisms [34].
Available N decreased with depth in all treatments, which is consistent with that presented in OM. In silvopastoral systems, nitrogen levels were considerably higher than in the Ctr, especially in the surface horizon (p ≤ 0.05). These results suggest that SPSs favor microbial activity and nitrogen mineralization, which increases the availability of this nutrient for plants. This is consistent with previous studies, indicating that this type of practice can improve nitrogen retention and availability in soils, mainly due to the incorporation of organic waste from animals in these systems, which provides an additional source of nitrogen [35]. On the other hand, legumes planted in 2016 in SPSs failed to persist due to forest conditions. However, despite their visible disappearance, the initial establishment of these plants may have influenced soil fertility, contributing to higher long-term nitrogen availability in these systems. In contrast, the Ctr, which did not receive legume seeding, lacks this additional source of nitrogen, which could explain the observed differences in nutrient dynamics between the sites [36].
Phosphorus (P) presented low values in all treatments, which is a common characteristic of Andisols due to the high phosphate adsorption capacity of the short-range-order (SRO) minerals present in volcanic soils in Chile. These minerals are important for retaining nutrients in the soil, limiting the availability of the nutrient for plants [37]. In addition, there was a tendency for Op and SOp systems to have the lowest P values, which could be related to the prominent presence of radal in these two conditions. Radal, as a species of the Proteaceae family, has a remarkable ability to compete and adapt to phosphorus-poor soils, which allows it to be particularly efficient in the absorption of this nutrient. This efficiency, however, can reduce the availability of P for other plant species, thus contributing to a reduction in the concentration of this nutrient in the soil [38].
ECEC values were highest at the 0–30 cm depth (p ≤ 0.05). At this depth, ECEC showed a tendency to decrease with increasing canopy disturbance (+). This capacity is directly related to mineralogical composition, particularly the higher clay content, and furthermore, to the values observed for K, Ca, and Mg, as the ECEC favors the accumulation of amorphous minerals, which contribute significantly to the cation retention capacity [39]. The chemical properties of the soil, specifically the cations K, Ca, and Mg, have remained constant over time [31], without significant changes. This suggests that the ecosystem is recycling nutrients efficiently. Other chemical properties, such as the exchangeable cations NH4+EXCH, K+EXCH, Na+EXCH, as well as the anion NO3− and the saturation percentages of AlSAT, K+SAT, Ca2+SAT, and Mg2+SAT, are presented in Table A1.
FDA activity is a measure of overall enzymatic activity in soil, used as an indicator of total microbial activity. Figure 2 shows the results of microbial biomass activity as a function of open canopy and soil depth. FDA hydrolysis analyses reveal significant differences both between different soil depths (p ≤ 0.05) and between different canopy openness conditions (p ≤ 0.05).

Figure 2.
Microbial biomass activity determined by FDA hydrolysis as a function of open canopy (Ctr: control; Op: open; SOp: semi-open; SC: semi-closed) and soil depth (0–10; 10–20; 20–30; and 30–60 cm). Uppercase letters indicate significant differences between open canopy conditions, while lowercase letters indicate significant differences between soil depths. Differences were considered significant according to Tukey’s test (p < 0.05). The box-and-whisker plot shows the distribution of the data in the box and the whiskers show the deviation. The line shows the mean of the data.
It is observed that FDA activity decreases as soil depth increases, regardless of the type of management. This is related to the fact that soil microorganisms, which are responsible for the hydrolysis of FDA, are more concentrated in the surface layers of the soil, where there is greater availability of OM and nutrients. On the other hand, significant differences are observed between the plots with silvopasture compared to the control, which may suggest that including SPSs helps soils recover their microbial functional capacity after having been degraded.
The results obtained for the FDA in this study are consistent with those of previous research carried out at the site by Ortiz et al. [8]. However, they are higher than those reported by Reyes et al. [40], who evaluated the biological activities of the soil in a relict forest in south-central Chile within a mixed forest community. Their results are very similar to those of the Ctr, while SPSs present higher values. This indicates that these systems promote greater biological activity in the soil, possibly due to the combination of plant species and management practices that favor the availability of OM and microbial diversity.
Like FDA activity, soil respiration showed significant differences between conditions and soil depths (p ≤ 0.05) (Figure 3). The accumulated CO2 fluxes, measured in a closed system for 14 days, were higher at shallow depths (0–10 cm) compared to the other depths for the four treatments. The SOp and SC conditions were those that showed the greatest increase in CO2 emissions. This relationship could be influenced by variations in litter contributions, since a higher tree density leads to a greater incorporation of OM into the soil and a greater metabolic activity of microorganisms. In addition, closed systems limit the incidence of sunlight, which helps to preserve the humidity and temperature conditions in the litter, favoring the subsequent proliferation of fungi and bacteria.

Figure 3.
Cumulative soil respiration fluxes over a 14-day period. Each line represents a time series of CO2 measurements for different open canopy levels: (a) depth 0–10 cm; (b) depth 10–20 cm; (c) depth 20–30 cm; and (d) 30–60 cm. Analysis of variance (ANOVA) was used to determine significant differences. Uppercase letters indicate significant differences between open canopy levels (in each figure), while lowercase letters indicate significant differences between soil depths (between figures). Differences were considered significant at p < 0.05.
β-Glucosidase, unlike FDA activity and respiration, did not show significant differences between conditions (p > 0.05) (Figure 4). However, it was observed that Op > SC > Sop > Ctr showed a similar trend to the rest of the biological analyses. This is because this indicator is less sensitive to changes in soil conditions, since its activity is more related to specific processes of the C cycle, while FDA and respiration reflect a broader range of microbial metabolic activities and responses to environmental variations [40]. Regarding depth, it did show significant differences, finding greater β-Glucosidase activity in the surface horizons. Since β-Glucosidase is an important indicator of the decomposition of SOM, especially of polysaccharides such as cellulose, this result could have been conditioned by the fact that in the superficial horizons (0–10 cm) there is the greatest availability of C-rich substrates, such as those from the decomposition of leaf litter, and as one goes deeper into the profile (30–60 cm), the contributions of fresh OM and oxygen conditions decrease, which limits enzymatic activity.

Figure 4.
β-Glucosidase activity as a function of open canopy (Ctr: control; Op: open; SOp: semi-open; SC: semi-closed) and soil depth (0–10; 10–20; 20–30; and 30–60 cm). Uppercase letters indicate significant differences between open canopy levels, while lowercase letters indicate significant differences between soil depths. Differences were considered significant according to Tukey’s test (p < 0.05). The box-and-whisker plot shows the distribution of the data in the box and the whiskers show the deviation. The line shows the mean of the data.
The OC content in the soil showed significant differences between the evaluated depths (p ≤ 0.05) (Figure 5a). The highest concentrations were found in the surface horizons (0–10 cm). This pattern is consistent with the dynamics of C in soils, since the surface horizons receive greater contributions of leaf litter and other organic residues that, when decomposed, favor the accumulation of C and as the depth of the profile increases, the percentage of C decreases due to the lower amount of fresh OM and the more limited microbial activity [41].
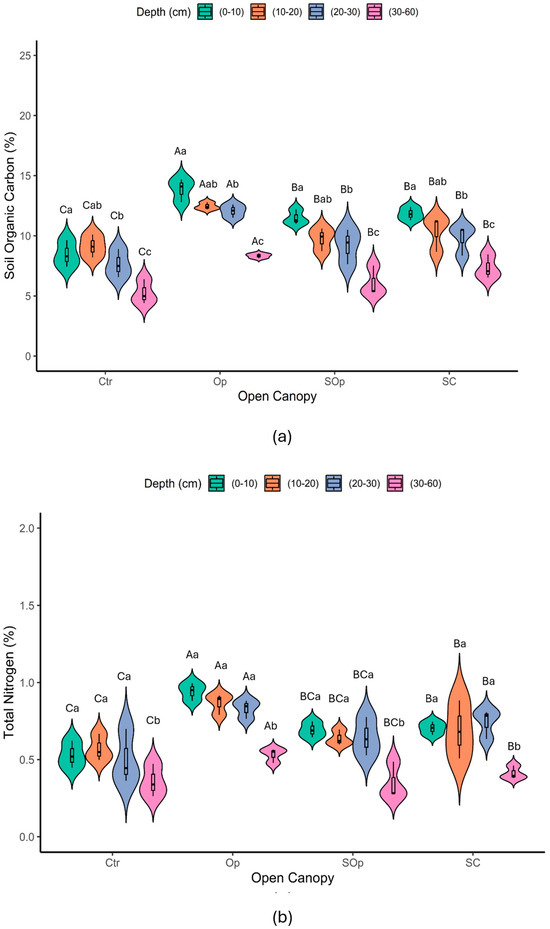
Figure 5.
Violin plot of variables (a) organic carbon (%) and (b) total nitrogen as a function of the factors open canopy (Ctr: control; Op: open; SOp: semi-open; SC: semi-closed) and soil depth (0–10; 10–20; 20–30; and 30–60 cm). Uppercase letters indicate significant differences between open canopy levels, while lowercase letters indicate significant differences between soil depths. Differences were considered significant according to Tukey’s test (p < 0.05).
On the other hand, significant differences were observed between the different open canopy levels (p ≤ 0.05). The Op canopy showed the highest concentrations of C, despite having the least tree cover and being the most degraded area. This finding coincides with previous studies [31] and could be associated with the history of agricultural burning in this area, mainly of potato cultivation, which generates pyrogenic carbon (PyC) [24]. This form of C is evidenced by the presence of charcoal fragments and an intense black color in the soil samples. PyC is highly resistant to oxidation due to its polyaromatic structure, being a persistent fraction of the TC, making it an important long-term C reservoir [42].
Although the study sites are close to the Op zone, the distribution of treatments does not suggest a uniform pattern of PyC influence across the other treatments. Although SOp is close to Op, it does not show similar values, indicating that the transport of pyrogenic carbon is not a dominant factor. Therefore, we maintain that only the Op condition presents PyC, which supports our results. Another important point is the presence of black charcoal in the Op samples, a material previously identified in earlier studies by Ortiz et al. However, this material has not been visually observed in the samples from the other treatments, reinforcing the idea that the influence of pyrogenic carbon is concentrated exclusively in Op.
Furthermore, silvopastoral management conditions showed a higher percentage C compared to the non-silvopastoral treatment (Op > SC > SOp > Ctr). This suggests that SPSs promote soil recovery, increasing C accumulation, possibly through the increase in the amount of available OM contributed by animals to the system. In general, the estimated C concentrations were higher than those observed in other soil conservation systems; an example of this is the study by Poblete-Grant et al. [43], which reports OC levels in Andisol soils under grasslands in southern Chile with prolonged application of poultry manure, which were lower than those found in this study. Our results are, however, similar to those reported by Gomez et al. [30], who evaluated these parameters under similar agroforestry conditions in a temperate native forest in Argentina, working with the tree species Nothofagus antartica. This species, like N. obliqua, is a deciduous tree that contributes a continuous level of leaf litter to the soil throughout the year, which is related to high C level inputs.
The total N% showed significant differences between the open canopy conditions evaluated and soil depths (p ≤ 0.05) (Figure 5b). The total N% of (0–10 cm) varied between 0.45 and 1.0 (±0.15) where Op > SC > Sop > Ctr. The values obtained in this study are higher than those reported by Crovo et al. [44] for native forest Andisols. However, the inorganic forms of nitrogen available to plants, NO3− and NH4+, were lower than the minimum requirements necessary for plant growth (Table A1. Average NO3− values ranged from 7.77 to 14.7 mg/kg (Ctr > SC > Op > SOp), while NH4+ values ranged from 3.01 to 12.83 mg/kg (SOp > SC > Op > Ctr), respectively. Previous studies have shown a similar trend or behavior at the same site in previous years [31,45], although an increase in NH4+ levels as a form of bioavailable nitrogen would have been expected due to the incorporation of animal feces, or also an increase in the potential N mineralization pool due to the leguminous species incorporated in 2016.
In terms of C sequestration, the results indicate that the Op treatment is the most effective, as it presents the highest SOC stocks at all depths (Figure 6). The highest Op levels with respect to the rest of the treatments were observed in the surface horizons 0–10 cm and deep horizons 30–60 cm, where values of 84.2 Mg C ha−1 and 189.6 Mg C ha−1 were reached, respectively. On the other hand, the SOp and SC treatments also showed high values at all depths analyzed, although not as high as Op; this could be due to differences in management practices associated with agricultural burning, which influence the dynamics of C in the soil. The Ctr presented the lowest C stock values with respect to the rest of the treatments, especially at the depth of 30–60 cm, where it barely reached values close to 90 Mg C ha−1. This suggests that soil in unmanaged degraded native forests has a lower capacity to retain C, and that the implementation of practices such as SPSs are effective in promoting long-term C storage.
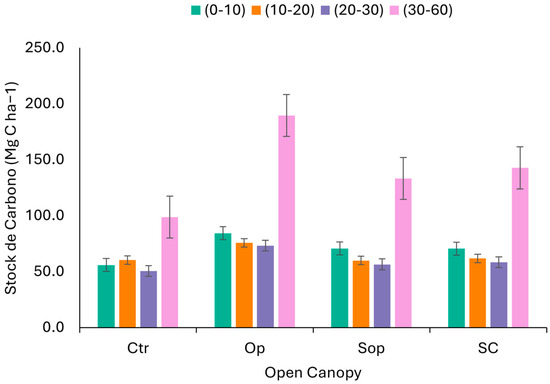
Figure 6.
SOC stocks as a function of open canopy (Ctr: control; Op: open; SOp: semi-open; SC: semi-closed) and soil depth (0–10; 10–20; 20–30; and 30–60 cm). The error bars represent the standard deviation SD.
The results revealed that the C:N ratio (C/N) of the unfractionated samples did not show significant differences in terms of open canopy (p > 0.05) but did differ in terms of soil depth (p ≤ 0.05) (Figure 7a). The C/N ratio of the unfractionated samples varied between 12.66 and 19.25 with an average of 15.6 ±1, which is within the distribution of C/N averages (9.9 to 25.8) found for soils around the world [46], although it is below the C/N ratio reported by Katsumi [47], who worked with Andisols (n = 14) under different land uses and found an average C/N ratio of 26.3. These results for the site could be highly related to the quality of the substrate, since the tree species present at the site is deciduous and its litter has a relatively lower lignin content (lignin: N of 21.79) and, consequently, lower C:N ratios [48].
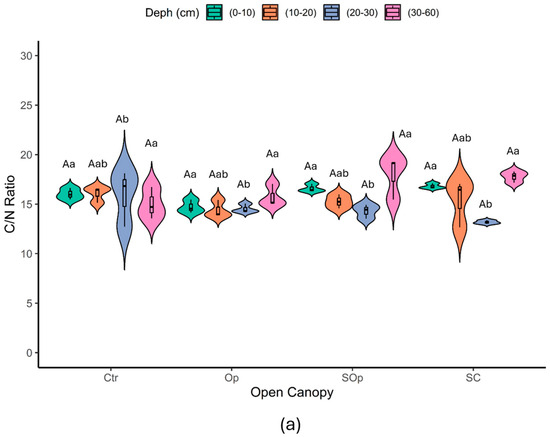
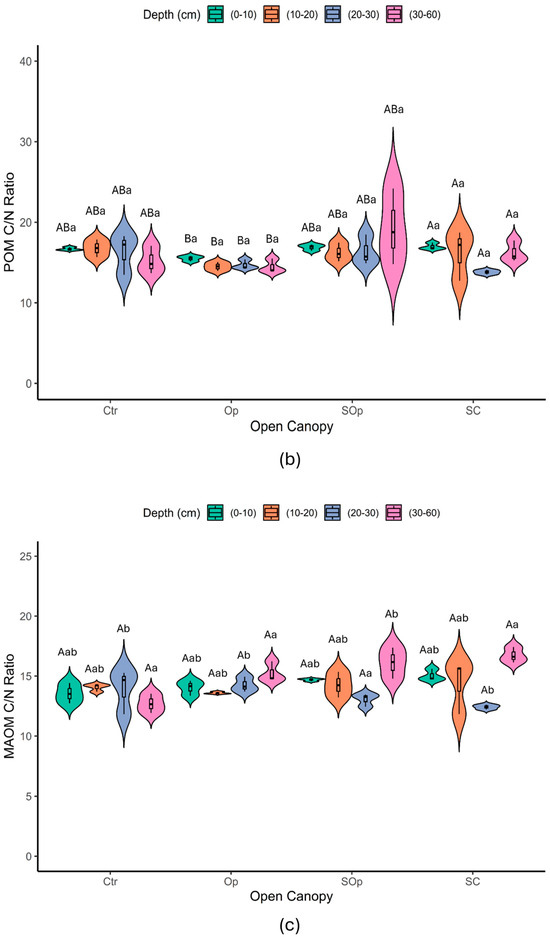
Figure 7.
Violin plot of the variables: (a) C/N ratio, (b) POM C/N ratio, and (c) MAOM C/N ratio as a function of open canopy (Ctr: control; Op: open; SOp: semi-open; SC: semi-closed) and soil depth (0–10; 10–20; 20–30 and 30–60 cm). Uppercase letters indicate significant differences between open canopy levels, while lowercase letters indicate significant differences between soil depths. Differences were considered significant according to Tukey’s test (p < 0.05).
The C/N ratio of the POM fraction showed significant differences depending on the open canopy conditions (p ≤ 0.05) (Figure 7b). On the other hand, the C/N ratio of the MAOM fraction showed significant differences both in depth and in open canopy (p ≤ 0.05) (Figure 7c). In the particulate fraction, the highest C/N ratios were observed at depths of 30–60 cm, particularly in SC, while in the surface horizons (0–10 cm), the values were more uniform between treatments. On the other hand, in the mineral fraction, C/N values were more homogeneous throughout the depths, although the surface horizons showed lower C/N ratios, with Ctr being the treatment with the lowest values.
Overall, the C/N ratio of MAOM (11–17, mean = 14.24 ± 1) was very similar to that of POM (12–24, mean = 16.02 ± 2), although more contrasting values between both fractions would have been expected (Figure 7c). As reported by Lavallee et al. [21], POM generally presents C/N ratios between 10 and 40, while MAOM has narrower C/N ratios, between 8 and 13. This would be expected, given that the C/N ratios of POM and MAOM largely reflect the characteristics of the source material. POM is associated with the C/N of plant litter, while MAOM corresponds to the C/N of microbial necromass and metabolites. Plant litter shows high variability in its C/N ratio (e.g., from 40 to 120 in a subset of NEON sites; [49]), while soil microorganisms have a significantly lower and restricted C/N ratio (between 3 and 15; [50]). This analysis would be based on the hypothesis that MAOM is dominated by microbially derived OM; however, POM and MAOM fractions are also known to contain mixtures of faster and slower cycling C pools with likely contributions from both plant and microbial detritus [51,52,53]. A study by Yu et al. [54] suggests that plant-derived OM may also contribute substantially to MAOM, especially in humid forests (with MAOM C/N > 15), which could explain the similarity observed between fractions in this study.
Cnox concentrations in unfractionated samples varied significantly with respect to opening condition and depth (p ≤ 0.05) (Figure 8a). Cnox decreases with depth in all treatments. The highest values are found in the Op and SC treatments in the surface horizons despite being the most different treatments in terms of degradation condition, but this is not the case at the depth of 30–60 cm where the differences between all treatments tend to be less marked.

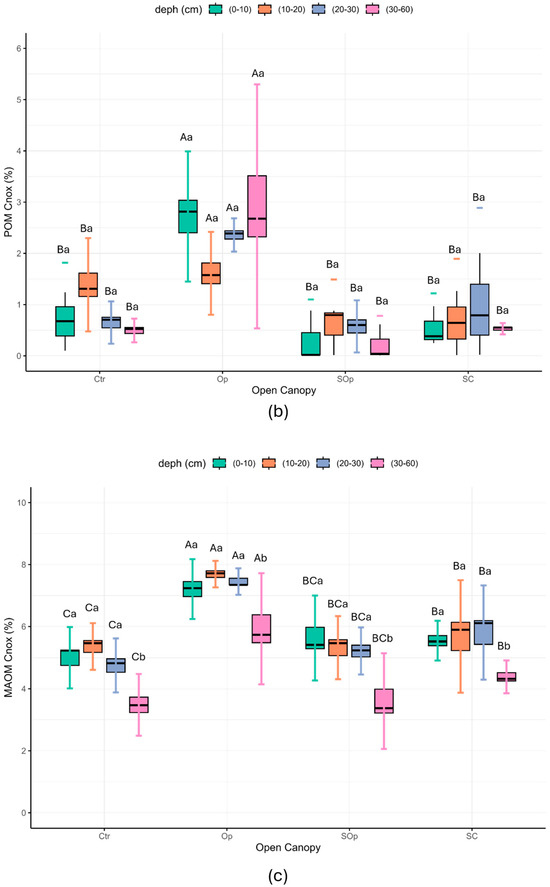
Figure 8.
(a) Non-oxidizable carbon (Cnox %), (b) non-oxidizable carbon in particulate fraction (POM Cnox %), (c) non-oxidizable carbon in mineral fraction (MAOM Cnox %) as a function of open canopy (Ctr: control; Op: open; SOp: semi-open; SC: semi-closed) and soil depth (0–10; 10–20; 20–30 and 30–60 cm). Uppercase letters indicate significant differences between open canopy levels, while lowercase letters indicate significant differences between soil depths. Differences were considered significant according to Tukey’s test (p < 0.05). The box-and-whisker plot shows the distribution of the data in the box and the whiskers show the deviation. The line shows the mean of the data. The concentration of POM Cnox and MAOM Cnox showed significant differences depending on the open canopy conditions (p ≤ 0.05). In addition, the MAOM Cnox fraction also showed significant differences depending on the depth (p ≤ 0.05) (Figure 8b,c). In general, the values of POM Cnox and MAOM Cnox tend to be higher in the surface horizons (0–10 cm) and progressively decrease with depth, following a pattern similar to that observed in the unfractionated samples.
As reported by Sierra et al. [55], it is expected that as the soil profile goes deeper, the fraction of Cnox will increase proportionally, reflecting the accumulation of more recalcitrant and stable forms of OM in the deeper layers. This phenomenon is due to the presence of recalcitrant compounds such as lignin, tannins [56], cutins, and suberins [57], which are more abundant in the roots [58]. On the other hand, it is expected that the concentrations of Cox will be higher in both fractions (POM and MAOM) in the surface horizons, due to the contribution of OC from leaf litter.
In this study, both Cox and Cnox showed the highest concentrations in the surface horizon (0–10 cm) (Figure A1a). Yu et al. [54], in a study that included a large transect of soil types, found that forest soils tend to vary the chemical and isotopic properties of the OM fractions of the soil and that this occurs mainly in the surface horizons. These authors found that in the surface horizons of forest soils there is a high abundance of aliphatic compounds (aliphatic C–H/C=O) compared to carbonyl groups (C=O), which are associated with more oxidized compounds. These observations reinforce the relationship between C dynamics in the different OM fractions and the fact that the C stabilization processes in our study are not only occurring at depth.
In terms of open canopy, the Op treatment showed the highest concentrations of both fractions. However, MAOM Cnox concentrations are more homogeneous throughout the depths compared to the POM fraction, which presents greater variability. This behavior could be related to the different dynamics of C stabilization in each fraction, where MAOM is more associated with stabilized microbial SOM, while POM reflects a more direct influence of litter and other materials in initial decomposition processes. In addition, Cnox is determined as a more stable fraction of C and is expected to include highly recalcitrant forms of C, including PyC or black carbon, and/or organic compounds strongly associated with the clay fraction. This is why the Op condition, despite being the most degraded, presents the highest values and this is associated with the historical anthropogenic practices of burning stubble.
PCA explained 80.4% of the total variation in the data with the first two components (Figure 9). The results revealed clear clusters for biological and C and N analyses in the samples and their fractions indicating that agroforestry practices have a significant impact on SOC properties and dynamics.
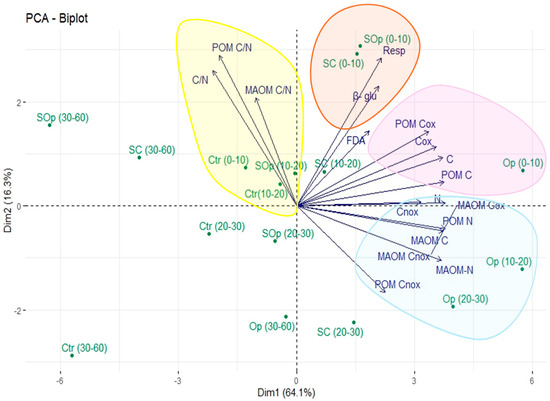
Figure 9.
Principal component analysis (PCA) of soil chemical and biological indicators under different open canopy levels (Ctr, Op, SOp, and SC) and soil depths (0–10, 10–20, 20–30, and 30–60 cm). Arrows represent the original variables projected into the space of the first two principal components (Dim1 and Dim2), which explain 80.4% of the total variance, respectively. Colored ellipses group observations according to their similarities, providing information on the relationship between variables under different open canopy levels and depths. Arrows indicate the contribution of each response variable to the variability explained by the principal components, highlighting the differences in parameters under each treatment.
At shallow depths of 0–10 cm and 10–20 cm for the SOp and SC systems, they are grouped together, showing an association with the variables of enzymatic activity and soil respiration. This indicates that SPSs at shallow depths present a high biological activity, which is consistent with their greater exposure to the input of OM and conditions that favor microbial activity. The Op system and the depths of 20–30 cm and 30–60 cm tend to group together, showing an association with the content of Cox and Cnox in different fractions. This suggests that in this system, although it is more degraded, the accumulation of stable C could be promoted by historical management practices (such as burning), which generate PyC resistant to degradation. The Ctr is not grouped with the variables of labile C content and enzymatic activity. This suggests a lower level of C and lower biological activity, which could be determined since this system does not present silvopastoralism.
This study provides relevant information on the impact of silvopastoral systems on carbon and nitrogen dynamics in degraded soils. Although previous studies exist at this site, long-term research (more than 20 years) is required to evaluate the persistence of these effects, which could be addressed in future studies. In addition, environmental factors such as climatic variability, soil type, and tree component can significantly influence the response of these systems, which underscores the need for studies under diverse ecological conditions, especially those that include the native forest component, which are still limited. Also, the inclusion of additional indicators, such as microbial biodiversity, would allow a more complete evaluation of the impact of silvopastoral systems. Analysis of the soil microbial community, which includes the diversity of bacteria, fungi, and other microorganisms, would provide a detailed profile that would help identify the composition and relative abundance of different microbial groups and the contribution and pathways of carbon formation in soils. In addition, molecular compositional techniques would be useful to understand the pathways of formation and the contribution of plant material in the generation of soil organic matter. Furthermore, the analysis of Al and Fe pedogenic oxides, stable aggregates (≥63 µm) [59], and/or Al-organomineral complexes [60] will also contribute to a better understanding of C stabilization in C-rich soils in temperate climates.
4. Conclusions
This study highlights the critical role agroforestry systems based on native forests can play in restoring degraded soils. The increased levels of stable C and nitrogen observed in SPS treatments, compared to the Ctr, emphasize the potential of these practices to enhance long-term C sequestration and improve soil quality in degraded landscapes. The distinct dynamics of C across various OM fractions, coupled with the observation that C stabilization in this study is not confined to deeper soil layers, may be characteristic of humid forest ecosystems. PyC contributed to the higher concentration found in the Op treatments. However, our findings do not support the fact that its transport to the other treatments is a dominant factor. Future research should incorporate more advanced methodologies, such as fractionation techniques that separate SOM by particle size and density, and target more specific analyses to identify PyC. These approaches would enable a more nuanced understanding of the mechanisms and pathways driving SOM formation and stabilization, thereby advancing our knowledge of its dynamics across diverse ecological and management contexts.
Author Contributions
Conceptualization, E.Z., F.D. and C.R.; methodology, C.R., E.Z., F.D., L.P., S.T. and J.P.F.; validation, C.R., E.Z., F.D., L.P., S.T. and J.P.F.; formal analysis, C.R. and E.Z.; investigation, C.R., E.Z., J.I.-Á. and J.O.; resources, E.Z. and F.D.; data curation, C.R., E.Z., S.T., F.D. and J.O.; writing—original draft preparation, C.R. and E.Z.; writing—review and editing, C.R., E.Z., F.D., J.I.-Á., L.P., S.T. and J.P.F.; visualization, C.R., E.Z., J.I.-Á. and J.O.; supervision, C.R. and E.Z.; project administration, E.Z., F.D. and C.R.; funding acquisition, E.Z. and C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANID (Agencia Nacional de Investigación y Desarrollo)/Programa/Beca Doctorado Nacional 2022-21221453 to the Doctoral Program in Agronomy Sciences, Faculty of Agronomy 2021300163 and to the Department of Soil Sciences and Natural Resources, Universidad de Concepción.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to express our gratitude to the staff of the Ranchillo Alto Research Center at the University of Concepción, as well as to the Department of Soils and Natural Resources, particularly to the Spectroscopy Laboratory (Vis-IR) and Sustainable Soil Management department. We extend special thanks to Katherine Rebolledo for her valuable collaboration. We also thank the Department of Forestry, Faculty of Forestry Sciences, and the Doctoral Program in Agronomy Sciences at the University of Concepción. Additionally, we are grateful to the National Agency for Research and Development (ANID) for the funding provided through the National Doctoral Scholarship 2022-21221453 awarded to R.C. This research is part of R.C.’s doctoral thesis, and we thank the Universidad de Concepción for approving its publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Abbreviation | Description |
| Mha | million hectares |
| SPSs | silvopastoral systems |
| C | carbon |
| SOC | soil organic carbon |
| IPCC | Intergovernmental Panel on Climate Change |
| FAO | Food and Agriculture Organization of the United Nations |
| SOM | soil organic matter |
| POM | particulate organic matter |
| MAOM | matrix-associated organic matter minerals |
| N | nitrogen |
| Op | open |
| SOp | semi-open |
| SC | semi-closed |
| Ctr | control |
| WFPS | water-filled pore space |
| BD | bulk density |
| PD | particle density |
| OM | organic matter |
| P | phosphorous |
| K+ | potassium |
| ECEC | effective cation exchange capacity |
| AlEXCH | exchangeable aluminum |
| Ca2+EXCH | exchangeable calcium |
| Mg2+EXCH | exchangeable magnesium |
| NH4+ | ammonium |
| NO3− | nitrate |
| K+EXCH | exchangeable potassium |
| Na+EXCH | exchangeable sodium |
| AlSAT | aluminum saturation |
| K+SAT | potassium saturation |
| Ca2+SAT | calcium saturation |
| Mg2+SAT | magnesium saturation |
| FDA | fluorescein diacetate |
| CO2 | carbon dioxide |
| TC | total carbon |
| Cox | oxidizable carbon |
| TOC | total organic carbon |
| Cnox | non-oxidizable carbon |
| C stock | carbon stock |
| PyC | pyrogenic carbon |
| C/N | C:N ratio |
Appendix A

Table A1.
Chemical properties of soil under different open canopy levels and depths. Supplementary results of Table 3.
Table A1.
Chemical properties of soil under different open canopy levels and depths. Supplementary results of Table 3.
| Condition/ Depths | NH4+ | NO3− | K+EXCH | Na+EXCH | AlSAT | K+SAT | Ca2+SAT | Mg2+SAT |
|---|---|---|---|---|---|---|---|---|
| Ctr (0–30) | 3.06 ± 0.07 | 14.7 ± 4.88 | 0.28 ± 1.34 | 0.07 ± 0.00 | 3.85 ± 2.19 | 8.64 ± 2.77 | 70.79 ± 8.49 | 13.75 ± 2.04 |
| Ctr (30–60) | 2.50 ± 0.00 | 5.90 ± 3.18 | 0.10 ± 0.59 | 0.07 ± 0.00 | 2.20 ± 0.43 | 8.72 ± 3.78 | 64.84 ± 1.32 | 17.24 ± 2.29 |
| OP (0–30) | 9.53 ± 0.05 | 11.60 ± 0.05 | 0.23 ± 0.05 | 0.04 ± 0.05 | 9.40 ± 0.05 | 12.54 ± 0.05 | 63.41 ± 0.05 | 12.85 ± 0.05 |
| OP (30–60) | 8.63 ± 0.04 | 5.80 ± 0.04 | 0.12 ± 0.04 | 0.04 ± 0.04 | 8.69 ± 0.04 | 13.60 ± 0.04 | 54.12 ± 0.04 | 17.81 ± 0.04 |
| SOp (0–30) | 12.83 ± 1.87 | 7.77 ± 1.87 | 0.16 ± 0.05 | 0.03 ± 0.00 | 5.14 ± 3.49 | 8.51 ± 2.64 | 71.12 ± 10.20 | 12.89 ± 2.77 |
| SOp (30–60) | 6.47 ± 1.83 | 4.37 ± 1.83 | 0.12 ± 0.06 | 0.07 ± 0.00 | 1.46 ± 0.53 | 9.88 ± 1.77 | 65.83 ± 6.86 | 15.82 ± 4.46 |
| SC (0–30) | 12.23 ± 3.03 | 14.7 ± 3.03 | 0.23 ± 0.05 | 0.04 ± 0.00 | 6.98 ± 7.49 | 7.87 ± 2.51 | 71.37 ± 11.92 | 12.30 ± 1.63 |
| SC (30–60) | 7.23 ± 0.37 | 10.07 ± 0.37 | 0.21 ± 0.10 | 0.07 ± 0.03 | 8.08 ± 8.28 | 11.28 ± 1.88 | 61.77 ± 14.10 | 14.54 ± 2.89 |
n: 24 and p < 0.05. Different capital letters mean significant differences between conditions, while lowercase letters indicate significant differences between depths. EXCH: exchangeable; SAT: saturation.
Appendix B

Figure A1.
(a) oxidizable carbon (%); (b) oxidizable carbon in the particulate fraction (POM Cox %); and (c) oxidizable carbon in the mineral fraction (MAOM Cox %) as a function of the factors of canopy openness (Ctr: control; Op: open; SOp: semi-open; SC: semi-closed) and soil depth (0–10; 10–20; 20–30; and 30–60 cm). Uppercase letters indicate significant differences between canopy conditions, while lowercase letters indicate significant differences between soil depths. Differences were considered significant according to Tukey’s test (p < 0.05).
References
- Casanova, M.; Salazar, O.; Seguel, O.; Luzio, W. Human-Induced Soil Degradation in Chile. In The Soils of Chile; World Soils Book Series; Springer: Dordrecht, The Netherlands, 2013; pp. 121–158. ISBN 978-94-007-5948-0. [Google Scholar]
- Flores, J.P.; Martínez, E.; Espinosa, M.; Avendaño, P.; Ahumada, I.; Henríquez, G.; Torres, P. Determinación de La Erosión Actual y Potencial de Los Suelos de Chile: Región Del Bío Bío. Síntesis de Resultados.(Pub. Ciren N° 148). 2010. Available online: https://bibliotecadigital.ciren.cl/items/114f1067-d6bb-4e0f-aa24-b259ad15e901 (accessed on 27 March 2025).
- Lara, A.; Solari, M.E.; Prieto, M.D.R.; Peña, M.P. Reconstrucción de La Cobertura de La Vegetación y Uso Del Suelo Hacia 1550 y Sus Cambios a 2007 En La Ecorregión de Los Bosques Valdivianos Lluviosos de Chile (35°–43° 30′ S). Bosque Valdivia 2012, 33, 3–4. [Google Scholar] [CrossRef]
- Nair, P.K.R.; Kumar, B.M.; Nair, V.D. Carbon Sequestration and Climate Change Mitigation. In An Introduction to Agroforestry; Springer International Publishing: Cham, Switzerland, 2021; pp. 487–537. ISBN 978-3-030-75357-3. [Google Scholar]
- Udawatta, R.P.; Gantzer, C.J.; Jose, S. Agroforestry Practices and Soil Ecosystem Services. In Soil Health and Intensification of Agroecosytems; Elsevier: Amsterdam, The Netherlands, 2017; pp. 305–333. ISBN 978-0-12-805317-1. [Google Scholar]
- Yasin, G.; Nawaz, M.F.; Sinha, D.; Qadir, I.; Altaf, M.; Ashraf, M.N.; Soufan, W.; Mammadov, A.; Zulfiqar, U.; Rahman, S.U. Agroforestry Status, Services, and Its Role in Climate Change Mitigation through Carbon Sequestration under Semi-Arid Conditions. Trees For. People 2024, 17, 100640. [Google Scholar] [CrossRef]
- Ramachandran Nair, P.K.; Nair, V.D.; Mohan Kumar, B.; Showalter, J.M. Carbon Sequestration in Agroforestry Systems. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2010; Volume 108, pp. 237–307. ISBN 978-0-12-381031-1. [Google Scholar]
- Ortiz, J.; Dube, F.; Neira, P.; Hernández Valera, R.R.; De Souza Campos, P.M.; Panichini, M.; Pérez-San Martín, A.; Stolpe, N.B.; Zagal, E.; Curaqueo, G. Comparative Study between Silvopastoral and Agroforest Systems on Soil Quality in a Disturbed Native Forest of South-Central Chile. Agronomy 2023, 13, 2683. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Cai, Z.; Aguilar, F.X. Economic Valuation of Agroforestry Ecosystem Services. In Agroforestry and Ecosystem Services; Udawatta, R.P., Jose, S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 477–494. ISBN 978-3-030-80059-8. [Google Scholar]
- Kay, S.; Graves, A.; Palma, J.H.N.; Moreno, G.; Roces-Díaz, J.V.; Aviron, S.; Chouvardas, D.; Crous-Duran, J.; Ferreiro-Domínguez, N.; García De Jalón, S.; et al. Agroforestry Is Paying off—Economic Evaluation of Ecosystem Services in European Landscapes with and without Agroforestry Systems. Ecosyst. Serv. 2019, 36, 100896. [Google Scholar] [CrossRef]
- FAO. Scaling up Agroecology to Achieve the Sustainable Development Goals. In Proceedings of the 2nd FAO International Symposium on Agroecology, Rome, Italy, 3–5 April 2018; FAO: Rome, Italy, 2019. [Google Scholar]
- Smith, P.; Cotrufo, M.F.; Rumpel, C.; Paustian, K.; Kuikman, P.J.; Elliott, J.A.; McDowell, R.; Griffiths, R.I.; Asakawa, S.; Bustamante, M.; et al. Biogeochemical Cycles and Biodiversity as Key Drivers of Ecosystem Services Provided by Soils. Soil 2015, 1, 665–685. [Google Scholar] [CrossRef]
- Voltr, V.; Menšík, L.; Hlisnikovský, L.; Hruška, M.; Pokorný, E.; Pospíšilová, L. The Soil Organic Matter in Connection with Soil Properties and Soil Inputs. Agronomy 2021, 11, 779. [Google Scholar] [CrossRef]
- Hayes, M.H.B.; Swift, R.S. An Appreciation of the Contribution of Frank Stevenson to the Advancement of Studies of Soil Organic Matter and Humic Substances. J. Soils Sediments 2018, 18, 1212–1231. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Particulate Soil Organic-Matter Changes across a Grassland Cultivation Sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil Carbon Storage Informed by Particulate and Mineral-Associated Organic Matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Follett, R.F.; Stewart, C.E.; Pruessner, E.G.; Kimble Retired, J.M. Great Plains Climate and Land-Use Effects on Soil Organic Carbon. Soil Sci. Soc. Am. J. 2015, 79, 261–271. [Google Scholar] [CrossRef]
- Heckman, K.; Hicks Pries, C.E.; Lawrence, C.R.; Rasmussen, C.; Crow, S.E.; Hoyt, A.M.; Von Fromm, S.F.; Shi, Z.; Stoner, S.; McGrath, C.; et al. Beyond Bulk: Density Fractions Explain Heterogeneity in Global Soil Carbon Abundance and Persistence. Glob. Change Biol. 2022, 28, 1178–1196. [Google Scholar] [CrossRef]
- Witzgall, K.; Vidal, A.; Schubert, D.I.; Höschen, C.; Schweizer, S.A.; Buegger, F.; Pouteau, V.; Chenu, C.; Mueller, C.W. Particulate Organic Matter as a Functional Soil Component for Persistent Soil Organic Carbon. Nat. Commun. 2021, 12, 4115. [Google Scholar] [CrossRef] [PubMed]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing Soil Organic Matter into Particulate and Mineral-associated Forms to Address Global Change in the 21st Century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Lavallee, J.M. Soil Organic Matter Formation, Persistence, and Functioning: A Synthesis of Current Understanding to Inform Its Conservation and Regeneration. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2022; Volume 172, pp. 1–66. ISBN 978-0-323-98953-4. [Google Scholar]
- Rumpel, C.; Kögel-Knabner, I. Deep Soil Organic Matter—A Key but Poorly Understood Component of Terrestrial C Cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Sandoval, E.; Dorner, F.; Seguel, S.; Cuevas, B.; Rivera, S. Métodos de Análisis Físicos del Suelo. 2012. Available online: https://hdl.handle.net/20.500.14001/59208 (accessed on 27 March 2025).
- Sadzawka, R.; Carrasco, R.; Adriana, M.; Grez, Z.; Mora, G.; de la Luz, M.; Flores, P.; Neaman, A. Métodos de Análisis Recomendados para los Suelos de Chile. Revisión 2006; SIDALC: Turrialba, Costa Rica, 2006. [Google Scholar]
- Alef, K. Nitrogen Mineralization in Soils. In Methods Appl. Soil Microbiol. Biochem.; Academic Press: London, UK, 1995; pp. 234–245. [Google Scholar]
- Tabatabai, M.A. Soil Enzymes. In SSSA Book Series; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; Soil Science Society of America: Madison, WI, USA, 2018; pp. 775–833. ISBN 978-0-89118-865-0. [Google Scholar]
- Craine, J.M.; Fierer, N.; McLauchlan, K.K. Widespread Coupling between the Rate and Temperature Sensitivity of Organic Matter Decay. Nat. Geosci. 2010, 3, 854–857. [Google Scholar] [CrossRef]
- Wright, A.F.; Bailey, J.S. Organic Carbon, Total Carbon, and Total Nitrogen Determinations in Soils of Variable Calcium Carbonate Contents Using a Leco CN-2000 Dry Combustion Analyzer. Commun. Soil Sci. Plant Anal. 2001, 32, 3243–3258. [Google Scholar] [CrossRef]
- Gomez, F.; Von Müller, A.; Tarabini, M.; La Manna, L. Resilient Andisols under Silvopastoral Systems. Geoderma 2022, 418, 115843. [Google Scholar] [CrossRef]
- Ortiz, J.; Dube, F.; Neira, P.; Panichini, M.; Stolpe, N.B.; Zagal, E.; Martínez-Hernández, P.A. Soil Quality Changes within a (Nothofagus Obliqua) Forest Under Silvopastoral Management in the Andes Mountain Range, South Central Chile. Sustainability 2020, 12, 6815. [Google Scholar] [CrossRef]
- Nissen, J.; Quiroz, C.; Seguel, O.; Mac Donald, R.; Sch, A.E. Variacion del potencial matrico durante el movimiento de agua en andisoles. Agro Sur 2005, 33, 36–47. [Google Scholar] [CrossRef]
- Vásquez, H.V.; Valqui, L.; Bobadilla, L.G.; Arbizu, C.I.; Alegre, J.C.; Maicelo, J.L. Influence of Arboreal Components on the Physical-Chemical Characteristics of the Soil under Four Silvopastoral Systems in Northeastern Peru. Heliyon 2021, 7, e07725. [Google Scholar] [CrossRef]
- Doetterl, S.; Stevens, A.; Six, J.; Merckx, R.; Van Oost, K.; Casanova Pinto, M.; Casanova-Katny, A.; Muñoz, C.; Boudin, M.; Zagal Venegas, E.; et al. Soil Carbon Storage Controlled by Interactions between Geochemistry and Climate. Nat. Geosci. 2015, 8, 780–783. [Google Scholar] [CrossRef]
- Renwick, L.L.R.; Celedón, A.; Nájera, F.; Fuentes Espoz, J.-P.; Celedón, D.; Arellano, C.; Salazar, O. Integrated Crop-Livestock Farms Have Higher Topsoil Nitrogen and Carbon than Crop-Only Farms in Chilean Mediterranean Climate Volcanic Soils. Agric. Syst. 2025, 222, 104172. [Google Scholar] [CrossRef]
- Banerjee, A.; Jhariya, M.K.; Yadav, D.K.; Raj, A. (Eds.) Environmental and Sustainable Development Through Forestry and Other Resources; Apple Academic Press: New York, NY, USA, 2020; ISBN 978-0-429-27602-6. [Google Scholar]
- Borie, F.; Aguilera, P.; Castillo, C.; Valentine, A.; Seguel, A.; Barea, J.M.; Cornejo, P. Revisiting the Nature of Phosphorus Pools in Chilean Volcanic Soils as a Basis for Arbuscular Mycorrhizal Management in Plant P Acquisition. J. Soil Sci. Plant Nutr. 2019, 19, 390–401. [Google Scholar] [CrossRef]
- Lambers, H.; Finnegan, P.M.; Jost, R.; Plaxton, W.C.; Shane, M.W.; Stitt, M. Phosphorus Nutrition in Proteaceae and Beyond. Nat. Plants 2015, 1, 15109. [Google Scholar] [CrossRef]
- Poirier, V.; Basile-Doelsch, I.; Balesdent, J.; Borschneck, D.; Whalen, J.K.; Angers, D.A. Organo-Mineral Interactions Are More Important for Organic Matter Retention in Subsoil Than Topsoil. Soil Syst. 2020, 4, 4. [Google Scholar] [CrossRef]
- Reyes, F.; Lillo, A.; Ojeda, N.; Reyes, M.; Alvear, M. Efecto de La Exposición y La Toposecuencia Sobre Actividades Biológicas Del Suelo En Bosque Relicto Del Centro-Sur de Chile. Bosque Valdivia 2011, 32, 255–265. [Google Scholar] [CrossRef]
- Masebo, N.; Birhane, E.; Takele, S.; Lucena, J.J.; Araceli, P.-S.; Yunta, F.; Belay, Z.; Anjulo, A. Microbial Biomass Carbon Distribution under Agroforestry Practices and Soil Depth Variations in Southern Ethiopia. Agrofor. Syst. 2025, 99, 56. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Conant, R.T.; Haddix, M.L.; Follett, R.F.; Bird, M.I.; Paul, E.A. Selective Preservation of Pyrogenic Carbon across Soil Organic Matter Fractions and Its Influence on Calculations of Carbon Mean Residence Times. Geoderma 2019, 354, 113866. [Google Scholar] [CrossRef]
- Poblete-Grant, P.; Suazo-Hernández, J.; Condron, L.; Rumpel, C.; Demanet, R.; Malone, S.L.; Mora, M.D.L.L. Soil Available P, Soil Organic Carbon and Aggregation as Affected by Long-Term Poultry Manure Application to Andisols under Pastures in Southern Chile. Geoderma Reg. 2020, 21, e00271. [Google Scholar] [CrossRef]
- Crovo, O.; Costa-Reidel, C.D.; Rodríguez, R.; Aburto, F. Livestock grazing reduces soil quality and threatens recovery of a degraded andean araucaria forest. 2021. Authorea 2021, preprints. [Google Scholar] [CrossRef]
- Alfaro, M.; Dube, F.; Zagal, E. Soil Quality Indicators in an Andisol under Different Tree Covers in Disturbed Nothofagus Forests. Chil. J. Agric. Res. 2018, 78, 106–116. [Google Scholar] [CrossRef]
- Batjes, N.H. Total Carbon and Nitrogen in the Soils of the World. Eur. J. Soil Sci. 2014, 65, 10–21. [Google Scholar] [CrossRef]
- Katsumi, T. Soil Excavation and Reclamation in Civil Engineering: Environmental Aspects. Soil Sci. Plant Nutr. 2015, 61, 22–29. [Google Scholar] [CrossRef]
- Decker, K.L.M.; Boerner, R.E.J. Mass Loss and Nutrient Release from Decomposing Evergreen and Deciduous Nothofagus Litters from the Chilean Andes. Austral Ecol. 2006, 31, 1005–1015. [Google Scholar] [CrossRef]
- Hall, S.J.; Ye, C.; Weintraub, S.R.; Hockaday, W.C. Molecular Trade-Offs in Soil Organic Carbon Composition at Continental Scale. Nat. Geosci. 2020, 13, 687–692. [Google Scholar] [CrossRef]
- Manral, V.; Bargali, K.; Bargali, S.S.; Karki, H.; Chaturvedi, R.K. Seasonal Dynamics of Soil Microbial Biomass C, N and P along an Altitudinal Gradient in Central Himalaya, India. Sustainability 2023, 15, 1651. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Nierop, K.G.J.; Simpson, M.J. Plant- or Microbial-Derived? A Review on the Molecular Composition of Stabilized Soil Organic Matter. Soil Biol. Biochem. 2021, 156, 108189. [Google Scholar] [CrossRef]
- Hall, S.J.; Silver, W.L. Reducing Conditions, Reactive Metals, and Their Interactions Can Explain Spatial Patterns of Surface Soil Carbon in a Humid Tropical Forest. Biogeochemistry 2015, 125, 149–165. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A.; Six, J.; Kaiser, M.; Benbi, D.; Chenu, C.; Cotrufo, M.F.; Derrien, D.; Gioacchini, P.; Grand, S.; et al. Isolating Organic Carbon Fractions with Varying Turnover Rates in Temperate Agricultural Soils—A Comprehensive Method Comparison. Soil Biol. Biochem. 2018, 125, 10–26. [Google Scholar] [CrossRef]
- Yu, W.; Huang, W.; Weintraub-Leff, S.R.; Hall, S.J. Where and Why Do Particulate Organic Matter (POM) and Mineral-Associated Organic Matter (MAOM) Differ among Diverse Soils? Soil Biol. Biochem. 2022, 172, 108756. [Google Scholar] [CrossRef]
- Sierra, M.; Martínez, F.J.; Verde, R.; Martín, F.J.; Macías, F. Soil-Carbon Sequestration and Soil-Carbon Fractions, Comparison between Poplar Plantations and Corn Crops in South-Eastern Spain. Soil Tillage Res. 2013, 130, 1–6. [Google Scholar] [CrossRef]
- Kraus, T.E.C.; Dahlgren, R.A.; Zasoski, R.J. Tannins in Nutrient Dynamics of Forest Ecosystems—A Review. Plant Soil 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Tegelaar, E.W.; De Leeuw, J.W.; Holloway, P.J. Some Mechanisms of Flash Pyrolysis of Naturally Occurring Higher Plant Polyesters. J. Anal. Appl. Pyrolysis 1989, 15, 289–295. [Google Scholar] [CrossRef]
- Goering, H.K.; Soest, P.J.V. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications); U.S. Agricultural Research Service: Washington, DC, USA, 1970.
- Wasner, D.; Abramoff, R.; Griepentrog, M.; Venegas, E.Z.; Boeckx, P.; Doetterl, S. The role of climate, mineralogy and stable aggregates for soil organic carbon dynamics along a geoclimatic gradient. Glob. Biogeochem. Cycles 2024, 38, e2023GB007934. [Google Scholar] [CrossRef]
- Matus, F.; Salazar, O.; Aburto, F.; Zamorano, D.; Nájera, F.; Jovanović, R.; Guerra, C.; Reyes-Rojas, L.; Seguel, O.; Pfeiffer, M.; et al. Perspective of soil carbon sequestration in Chilean volcanic soils. npj Mater. Sustain. 2024, 2, 32. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).