A Novel Method to Investigate Environmental Risk in Wastewater Toxicity
Abstract
1. Introduction
2. Materials and Methods
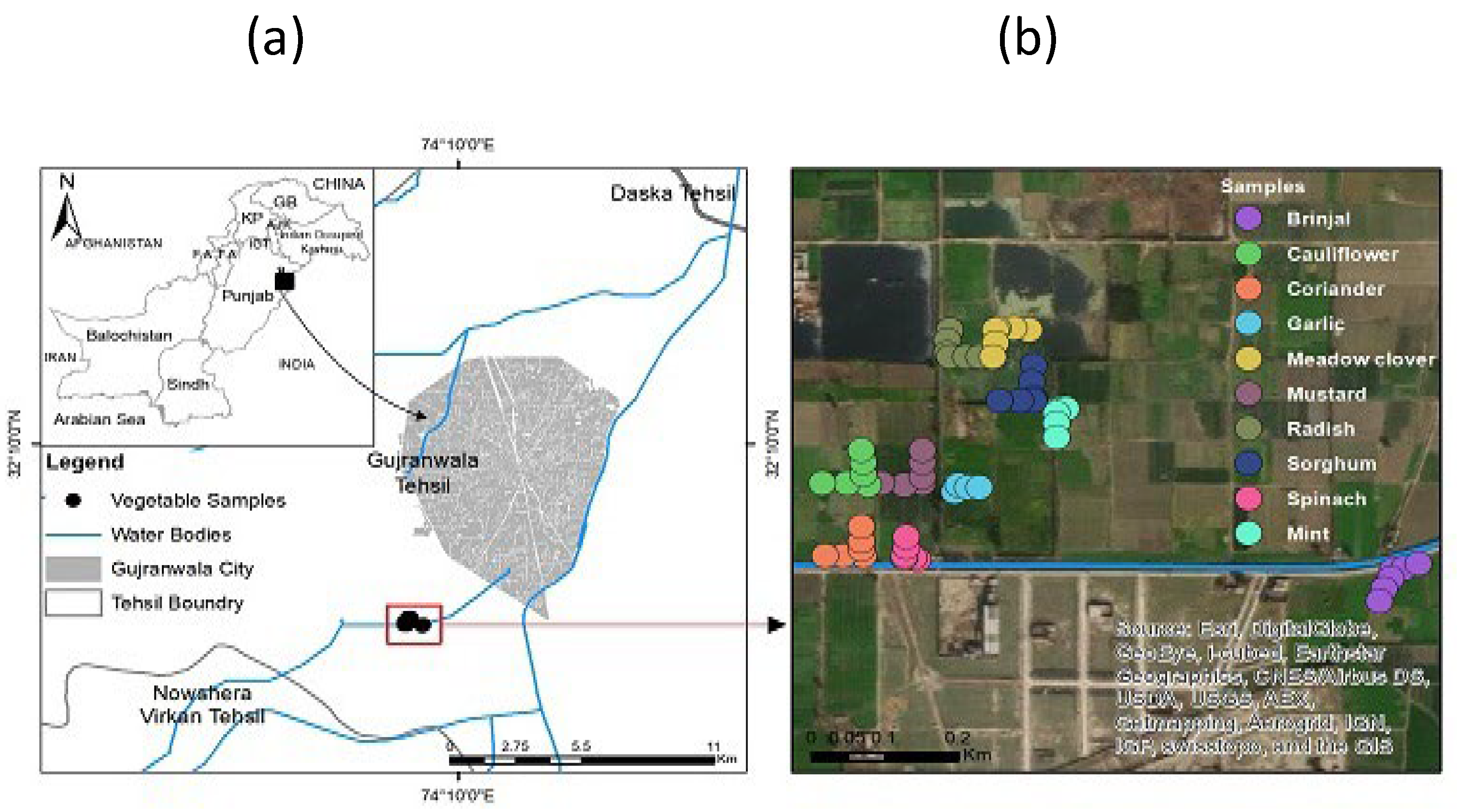
2.1. Geographical Position of the Project Site
2.2. Sampling Strategy
2.3. Physicochemical Analyses of Water, Sludge, and Soil
2.4. Spectroscopic Analysis of Samples
2.5. Transfer Factor
2.6. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical Properties of Effluents
3.2. Physico-Chemical Properties of Soil and Sludge
3.3. Toxic Metals in Samples
3.3.1. Toxic Metals in Effluents
3.3.2. Toxic Metals in Sludge
3.3.3. Toxic Metals in Soil
3.3.4. Toxic Metals in Plants
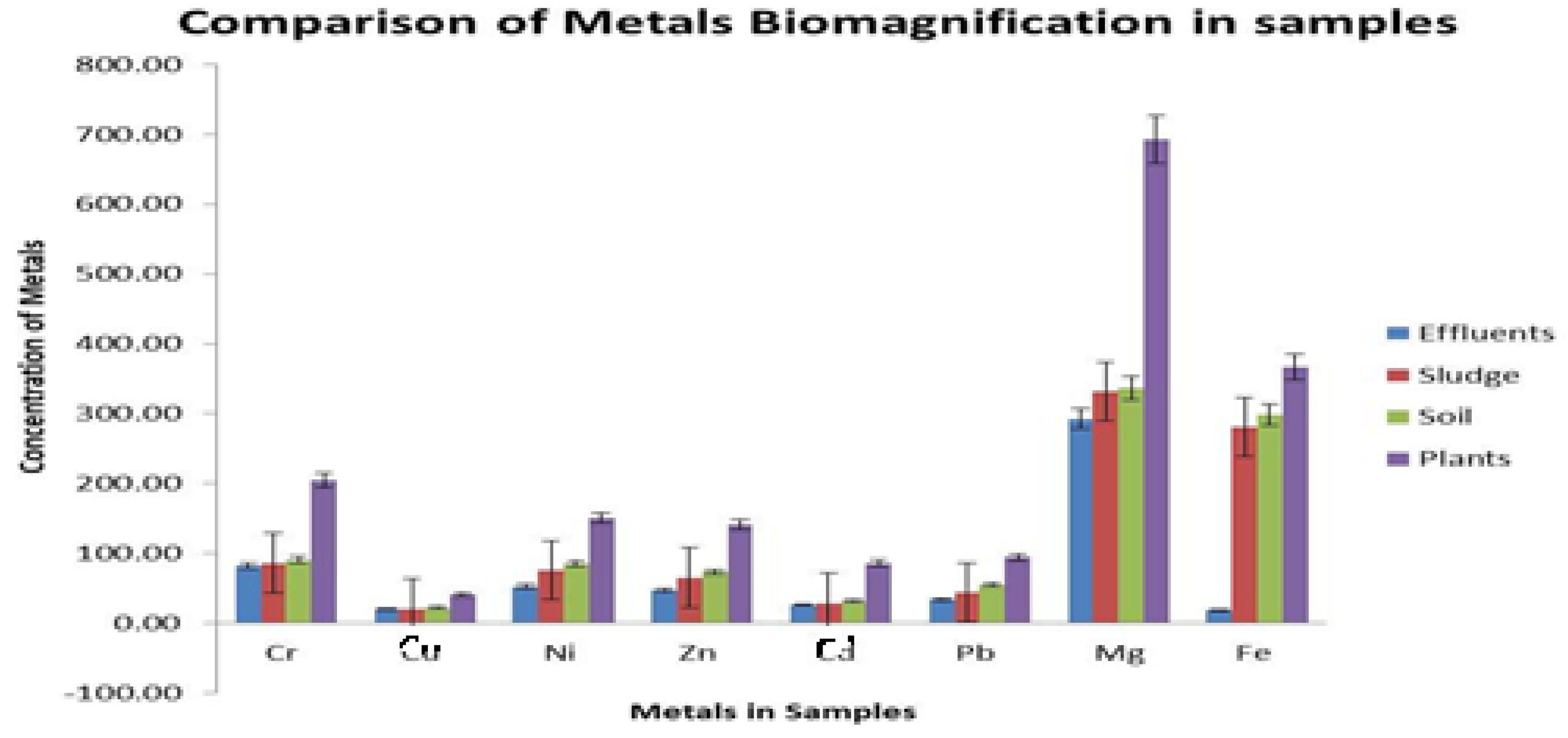
3.4. Metal Biomagnifications
3.5. Bioaccumulation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dogan, Y.; Unver, M.C.; Ugulu, I.; Calis, M.; Durkan, N. Heavy metal accumulation in the bark and leaves of Juglans regia planted in Artvin City, Turkey. Biotechnol. Biotechnol. Equip. 2014, 28, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Khan, Z.I.; Ahmad, K.; Wajid, K.; Bashir, H.; Malik, I.S.; Nadeem, M.; Ashfaq, A.; Ugulu, I. Transfer of heavy metals from different sources of fertilizers in wheat variety (Galaxy-13). Asian J. Biol. Sci. 2019, 12, 832–841. [Google Scholar] [CrossRef]
- Siddique, S.; Ahmad, K.; Khan, Z.I.; Wajid, K.; Bashir, H.; Munir, M.; Shehzadi, M. Sodium status of soil, forages, and small ruminants of Punjab, Pakistan. Pure Appl. Biol. 2019, 8, 1950–1961. [Google Scholar]
- Rasheed, M.; Ahmad, K.; Khan, Z.; Mahpara, S.; Ahmad, T.; Yang, Y.; Ugulu, I. Assessment of trace metal contents of indigenous and improved pastures and their implications for livestock in terms of seasonal variations. Rev. Chim. 2020, 71, 347–364. [Google Scholar] [CrossRef]
- Ugulu, I. Determination of heavy metal accumulation in plant samples by spectrometric techniques in Turkey. Appl. Spectrosc. Rev. 2015, 50, 113–151. [Google Scholar]
- Raza, A.; Habib, M.; Kakavand, S.N.; Zahid, Z.; Zahra, N.; Sharif, R.; Hasanuzzaman, M. Phytoremediation of cadmium: Physiological, biochemical, and molecular mechanisms. Biology 2020, 9, 177. [Google Scholar] [CrossRef]
- Hansa, A.; Devi, A.; Upadhyay, M.; Gupta, H.; Syam, K.; Asgari Lajayer, B.; Sharma, R. Toxicological implications of industrial effluents on plants: A review focusing on phytoremediation techniques. Int. J. Environ. Sci. Technol. 2024, 21, 2209–2224. [Google Scholar] [CrossRef]
- Hooda, P.S. A special issue on heavy metals in soils: Editorial foreword. Adv. Environ. Res. 2003, 8, 1–3. [Google Scholar] [CrossRef]
- Alghobar, M.A.; Suresha, S. Evaluation of metal accumulation in soil and tomatoes irrigated with sewage water from Mysore city, Karnataka, India. J. Saudi Soc. Agric. Sci. 2017, 16, 49–59. [Google Scholar] [CrossRef]
- Arora, M.; Kiran, B.; Rani, A.; Rani, S.; Kaur, B.; Mittal, M. Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem. 2008, 111, 811–815. [Google Scholar] [CrossRef]
- Gupta, N.; Khan, D.K.; Santra, S.C. Heavy metal accumulation in vegetables grown in tropical India’s long-term wastewater-irrigated agricultural land. Environ. Monit. Assess. 2012, 184, 6673–6682. [Google Scholar] [PubMed]
- Gong, L.; Wang, J.; Abbas, T. Immobilization of exchangeable Cd in soil using mixed amendment and its effect on soil microbial communities under paddy upland rotation system. Chemosphere 2021, 262, 127828. [Google Scholar] [CrossRef] [PubMed]
- Aftab, K.; Iqbal, S.; Khan, M.R.; Busquets, R.; Noreen, R.; Ahmad, N.; Ouladsmane, M. Wastewater-irrigated vegetables are a significant source of heavy metal contaminants: Toxicity and health risks. Molecules 2023, 28, 1371. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.A.; Tolosa, C.A.; Tack, F.M.G.; Verloo, M.G. Characterization of selected element concentrations and enrichment ratiosin background and anthropogenically impacted roadside areas. Arch. Environ. Contam. Toxicol. 2000, 38, 428–438. [Google Scholar] [CrossRef]
- Panseriya, H.Z.; Gosai, H.B.; Sankhwal, A.O.; Sachaniya, B.K.; Gavali, D.J.; Dave, B.P. Distribution, speciation and risk assessment of heavy metals: Geochemical exploration of Gulf of Kachchh, Gujarat, India. Environ. Earth Sci. 2020, 79, 213. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agrawal, M.; Marshall, F. Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol. Environ. Saf. 2007, 66, 258–266. [Google Scholar] [CrossRef]
- Ahmed, H.; Siddique, M.T.; Iqbal, M.; Hussain, F. Comparative study of interpolation methods for mapping soil pH in the apple orchards of Murree, Pakistan. Soil Environ. 2017, 36, 70–76. [Google Scholar] [CrossRef]
- Wang, H.; Lu, S. Spatial distribution, source identification and affecting factors of heavy metals contamination in urban-suburban soils of Lishui city, China. Environ. Earth Sci. 2011, 64, 1921–1929. [Google Scholar] [CrossRef]
- Zhang, C. Using multivariate analyses and GIS to identify pollutants and their spatial patterns in urban soils in Galway, Ireland. Environ. Pollut. 2006, 142, 501–511. [Google Scholar] [CrossRef]
- Walkley, A. A critical examination of a rapid method for determining organic carbon in soils—Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of soil, plant, and water analysis. A Man. West Asia N. Afr. Reg. 2013, 3, 65–119. [Google Scholar]
- Greenberg, A.E.; Clesseri, L.S.; Eaton, A.D. Standard Methods for Examining Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998; pp. 4–415. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis: Advanced Course; UW-Madison Libraries Parallel Press: Madison, WI, USA, 2005. [Google Scholar]
- Saeed, G. Technical Guide for Chemical Analysis of Soil Water Samples; Soil Survey of Pakistan, Lahore, Pakistan, 1980.
- Rhoades, J.D. Cation exchange capacity. Methods Soil Anal. Chem. Microbiol. Prop. 1983, 9, 149–157. [Google Scholar]
- Ball, D.F. Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soils. J. Soil Sci. 1964, 15, 84–92. [Google Scholar]
- Khan, S.; Rehman, S.; Khan, A.Z.; Khan, A.; Shah, M.T. Soil and vegetables enrichment with heavy metals from geological sources in Gilgit, northern Pakistan. Ecotoxicol. Environ. Saf. 2010, 73, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G.; Ghafoor, A.; Qadir, M.; Owens, G.; Aziz, M.; Zia, M. Disposal and use of sewage on agricultural lands in pakistan: A review. Pedosphere 2010, 20, 23–34. [Google Scholar] [CrossRef]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef]
- Kalsoom, A.; Kareem, A.; Aslam, A.; Niaz, A.; Mukhtar, N.; Sattar, A.; Ul Haq, E. Influence of wastewater irrigation on soil chemical properties and buildup of heavy metals in soil. EQA-Int. J. Environ. Qual. 2020, 37, 23–30. [Google Scholar]
- Singh, K.P.; Mohan, D.; Sinha, S.; Dalwani, R. Impact assessment of treated/untreated wastewater toxicants discharged by sewage treatment plants on health, agricultural, and environmental quality in the wastewater disposal area. Chemosphere 2004, 55, 227–255. [Google Scholar] [CrossRef]
- Chaoua, S.; Boussaa, S.; Khadra, A.; and Boumezzough, A. Efficiency of two sewage treatment systems (activated sludge and natural lagoons) for helminth egg removal in Morocco. J. Infect. Public Health 2018, 11, 197–202. [Google Scholar] [CrossRef]
- Hassan, M.A.; Tamer, T.M.; Valachová, K.; Omer, A.M.; El-Shafeey, M.; Eldin, M.S.M.; Šoltés, L. Antioxidant and antibacterial polyelectrolyte wound dressing based on chitosan/hyaluronan/phosphatidylcholine dihydroquercetin. Int. J. Biol. Macromol. 2021, 166, 18–31. [Google Scholar] [CrossRef]
- Nazir, R.; Khan, M.; Masab, M.; Rehman, H.U.; Rauf, N.U.; Shahab, S.; Ameer, N.; Sajed, M.; Ullah, M.; Rafeeq, M. Accumulation of heavy metals (Ni, Cu, Cd, Cr, Pb, Zn, Fe) in the soil, water and plants and analysis of physico-chemical parameters of soil and water collected from Tanda Dam Kohat. J. Pharm. Sci. Res. 2015, 7, 89. [Google Scholar]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Eremektar, G.; Selcuk, H.; Meric, S. Investigation of the relation between COD fractions and the toxicity in a textile finishing industry wastewater: Effect of preozonation. Desalination 2007, 211, 314–320. [Google Scholar] [CrossRef]
- Noreen, M.; Shahid, M.; Iqbal, M.; Nisar, J. Measurement of cytotoxicity and heavy metal load in drains water receiving textile effluents and drinking water near drains. Measurement 2017, 109, 88–99. [Google Scholar] [CrossRef]
- Mohsin, A.; Liu, L.; Liu, P.; Deng, W.; Ivanov, I.N.; Li, G.; Gu, G. Synthesis of millimeter-size hexagon-shaped graphene single crystals on resolidified copper. ACS Nano 2013, 7, 8924–8931. [Google Scholar] [CrossRef]
- Ma, Z.; Xu, J.; Quan, W.; Zhang, Z.; Lin, W.; Xu, X. Significant increase of surface ozone at a rural site, north of eastern China. Atmos. Chem. Phys. 2015, 16, 3969–3977. [Google Scholar] [CrossRef]
- Mehmood, A.; Mirza, M.A.; Choudhary, M.A.; Kim, K.H.; Raza, W.; Raza, N.; Sarfraz, M. Spatial distribution of heavy metals in crops in a wastewater irrigated zone and health risk assessment. Environ. Res. 2019, 168, 382–388. [Google Scholar] [CrossRef]
- Karaca, A. Effect of organic wastes on soil extractability of cadmium, copper, nickel, and zinc. Geoderma 2004, 122, 297–303. [Google Scholar] [CrossRef]
- Gupta, A.K.; Sinha, S. Chemical fractionation and heavy metal accumulation in the Sesamum indicum (L.) var plant. T55 grown on soil amended with tannery sludge: Selection of single extractants. Chemosphere 2006, 64, 161–173. [Google Scholar] [CrossRef]
- Yoo, M.S.; James, B.R. Zinc extractability as a function of pH in organic waste-amended soils. Soil Sci. 2002, 167, 246–259. [Google Scholar]
- Singh, R.; Sram, R.J.; Binkova, B.; Kalina, I.; Popov, T.A.; Georgieva, T.; Georgieva, T.; Garte, S.; Taioli, E.; Farmer, P.B. The relationship between biomarkers of oxidative DNA damage, polycyclic aromatic hydrocarbon DNA adducts, antioxidant status and genetic susceptibility following exposure to environmental air pollution in humans. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2007, 620, 83–92. [Google Scholar]
- Balkhair, K.S.; Ashraf, M.A. Field accumulation risks of heavy metals in soil and vegetable crops irrigated with sewage water in the western region of Saudi Arabia. Saudi J. Biol. Sci. 2016, 23, S32–S44. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yi, X.; Dang, Z.; Wang, Q.; Luo, H.; Tang, J. Heavy metal contamination and health risk assessment near a tailing pond in Guangdong, China. Int. J. Environ. Res. Public Health 2017, 14, 1557. [Google Scholar] [CrossRef]
- Shafiq, M.; Shaukat, T.; Nazir, A.; Bareen, F.E. Modeling of Cr contamination in the agricultural lands of three villages near the leather industry in Kasur, Pakistan, using statistical and GIS techniques. Environ. Monit. Assess. 2017, 189, 423. [Google Scholar]
- Bhuiyan, M.A.H.; Suruvi, N.I.; Dampare, S.B.; Islam, M.A.; Quraishi, S.B.; Ganyaglo, S.; Suzuki, S. Investigation of the possible sources of heavy metal contamination in lagoon and canal water in the tannery industrial area in Dhaka, Bangladesh. Environ. Monit. Assess. 2011, 175, 633–649. [Google Scholar]
- Islam, M.S.; Han, S.; Ahmed, M.K.; Masunaga, S. Assessment of trace metal contamination in water and sediment of some rivers in Bangladesh. J. Water Environ. Technol. 2014, 12, 109–121. [Google Scholar]
- Zia, M.H.; Watts, M.J.; Niaz, A.; Middleton, D.R.; Kim, A.W. Health risk assessment of potentially harmful elements and dietary minerals from vegetables irrigated with untreated wastewater, Pakistan. Environ. Heochemistry Health 2017, 39, 707–728. [Google Scholar]
- Khan, M.U.; Malik, R.N.; Muhammad, S. Human health risk from Heavy metal via food crops consumption with wastewater irrigation practices in Pakistan. Chemosphere 2013, 93, 2230–2238. [Google Scholar] [CrossRef]
- Mahmood, A.; Malik, R.N. Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore, Pakistan. Arab. J. Chem. 2014, 7, 91–99. [Google Scholar] [CrossRef]
- Ali, J.; Khan, S.; Khan, A.; Waqas, M.; Nasir, M.J. Contamination of soil with potentially toxic metals and their bioaccumulation in wheat and associated health risks. Environ. Monit. Assess. 2020, 192, 138. [Google Scholar]
- Casado-Vela, J.; Sellés, S.; Díaz-Crespo, C.; Navarro-Pedreño, J.; Mataix-Beneyto, J.; and Gómez, I. Effect of composted sewage sludge application to soil on sweet pepper crop (Capsicum annuum var. annuum) grown under two exploitation regimes. Waste Manag. 2007, 27, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Gascó, G.; Lobo, M.C. Composition of a Spanish sewage sludge and effects on treated soil and olive trees. Waste Manag. 2007, 27, 1494–1500. [Google Scholar] [CrossRef]
- Chishti, K.A.; Khan, F.A.; Shah, S.M.H.; AsifKhan, M.; Khan, J.; Shah, S.M.M.; Hussain, I. Estimation of heavy metals in the seeds of blue and white capitulum’s of silybum marianum grown in various districts of pakistan. J. Basic Appl. Sci. 2011, 7, 45–49. [Google Scholar]
- Shah, A.S.; Langrish, J.P.; Nair, H.; McAllister, D.A.; Hunter, A.L.; Donaldson, K.; Mills, N.L. Global association of air pollution and heart failure: A systematic review and meta-analysis. Lancet 2013, 382, 1039–1048. [Google Scholar]
- Nicholson, F.A.; Smith, S.R.; Alloway, B.J.; Carlton-Smith, C.; Chambers, B.J. An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci. Total Environ. 2003, 311, 205–219. [Google Scholar]
- Hunt, J.W.; Phillips, B.M.; Nicely, P.A.; de Vlaming, V.; Connor, V.; Tjeerdema, R.S. Integrated assessment of the impacts of agricultural drainwater in the Salinas River (California, USA). Environ. Pollut. 2003, 124, 523–532. [Google Scholar] [CrossRef]
- Rehman, N.H.; Lee, M.H.; Latif, M.T.; Suhartono, S.J.J.T. Forecasting of air pollution index with artificial neural network. J. Teknol. 2013, 63, 59–64. [Google Scholar]
- Sharma, N.; Taneja, S.; Sagar, V.; Bhatt, A. Forecasting air pollution load in Delhi using data analysis tools. Procedia Comput. Sci. 2018, 132, 1077–1085. [Google Scholar] [CrossRef]
- Romic, M.; Romic, D. Heavy metals distribution in agricultural topsoils in urban area. Environ. Geol. 2003, 43, 795–805. [Google Scholar]
- Kunhikrishnan, A.; Bolan, N.S.; Müller, K.; Laurenson, S.; Naidu, R.; Kim, W.I. The influence of wastewater irrigation on soil’s transformation and bioavailability of heavy metal (loid)s. Adv. Agron. 2012, 115, 215–297. [Google Scholar]
- Avci, H.; Deveci, T. Assessment of trace element concentrations in soil and plants from cropland irrigated with wastewater. Ecotoxicol. Environ. Saf. 2013, 98, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Anwar, F.; Rashid, U. Appraisal of heavy metal contents in different vegetables grown near an industrial area. Pak. J. Bot. 2008, 40, 2099–2106. [Google Scholar]
- Bashir, F.H.; Othman, M.S.; Mazlan, A.G.; Rahim, S.M.; Simon, K.D. Heavy metal concentration in fishes from the coastal waters of Kapar and Mersing, Malaysia. Turk. J. Fish. Aquat. Sci. 2013, 13, 375–382. [Google Scholar] [CrossRef]
- Elgallal, M.; Fletcher, L.; Evans, B. Assessment of potential risks associated with chemicals in wastewater used for irrigation in arid and semiarid zones: A review. Agric. Water Manag. 2016, 177, 419–431. [Google Scholar] [CrossRef]
- Cheshmazar, E.; Arfaeinia, H.; Karimyan, K.; Sharafi, H.; Hashemi, S.E. Dataset for effect comparison of irrigation by wastewater and groundwater on amount of heavy metals in soil and vegetables: Accumulation, transfer factor, and health risk assessment. Data Brief 2018, 18, 1702–1710. [Google Scholar] [CrossRef]
- Hattab, S.; Bougattass, I.; Hassine, R.; Dridi-Al-Mohandes, B. Metals and micronutrients in some edible crops and their cultivation soils in eastern-central region of Tunisia: A comparison between organic and conventional farming. Food Chem. 2019, 270, 293–298. [Google Scholar] [CrossRef]
- Lian, M.; Wang, J.; Sun, L.; Xu, Z.; Tang, J.; Yan, J.; Zeng, X. Profiles and potential health risks of heavy metals in soil and crops from the watershed of Xi River in Northeast China. Ecotoxicol. Environ. Saf. 2019, 169, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, T.; Khan, S.; Yu, X.; Amin, S.; Khan, M.A.; Sarwar, A.; Nazneen, S. Analysis of Arsenic concentration and its speciation in rice of different markets of Pakistan and its associated health risk. Environ. Technol. Innov. 2021, 21, 101252. [Google Scholar] [CrossRef]
- Haroon, U.; Khizar, M.; Liaquat, F.; Ali, M.; Akbar, M.; Tahir, K.; Munis, M.F.H. Halotolerant plant growth-promoting rhizobacteria induce salinity tolerance in wheat by enhancing the expression of SOS genes. J. Plant Growth Regul. 2021, 41, 2435–2448. [Google Scholar] [CrossRef]
- Sundström, R.; Åström, M.; österholm, P. Comparison of the metal content in acid sulfate soil runoff and industrial effluents in Finland. Environ. Sci. Technol. 2002, 36, 4269–4272. [Google Scholar] [CrossRef]
- Ali, M.H.; Al-Qahtani, K.M. Assessment of some heavy metals in vegetables, cereals, and fruits in Saudi Arabian markets. Egypt. J. Aquat. Res. 2012, 38, 31–37. [Google Scholar] [CrossRef]
- Rao, J.V.; Kavitha, P.; Reddy, N.C.; Rao, T.G. Petrosia testudinaria as a biomarker for metal contamination at Gulf of Mannar, southeast coast of India. Chemosphere 2006, 65, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Saxena, A.; Dan, A.; Swarup, D. Indian medicinal herb: A source of lead and cadmium for humans and animals. Arch. Environ. Occup. Health 2009, 64, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Ingwersen, J.; Streck, T. A regional-scale study on the crop uptake of cadmium from sandy soils: Measurement and modeling. J. Environ. Qual. 2005, 34, 1026–1035. [Google Scholar]
- Rashid, H.; Hasan, M.N.; Tanu, M.B.; Parveen, R.; Sukhan, Z.P.; Rahman, M.S.; Mahmud, Y. Heavy metal pollution and chemical profile of Khiru River, Bangladesh. Int. J. Environ. 2012, 2, 57–63. [Google Scholar]
- Bareen, F.E.; Tahira, S.A. Metal accumulation potential of wild plants in tannery effluent contaminated soil of Kasur, Pakistan: Field trials for toxic metal cleanup using Suaeda fruticose. J. Hazard. Mater. 2011, 186, 443–450. [Google Scholar] [CrossRef]
- Alloway, B.; Ayres, D.C. Chemical Principles of Environmental Pollution; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Liu, G.; Tao, L.; Liu, X.; Hou, J.; Wang, A.; Li, R. Heavy metal speciation and pollution of agricultural soils along Jishui River in non-ferrous metal mine area in Jiangxi Province, China. J. Geochem. Explor. 2013, 132, 156–163. [Google Scholar]
- Jan, F.A.; Ishaq, M.; Khan, S.; Ihsanullah, I.; Ahmad, I.; Shakirullah, M. A comparative study of human health risks via consumption of food crops grown on wastewater irrigated soil (Peshawar) and relatively clean water irrigated soil (lower Dir). J. Hazard. Mater. 2010, 179, 612–621. [Google Scholar] [CrossRef]



| Parameters | Effluents | Soil | Sludge | LSD at p = 0.05 |
|---|---|---|---|---|
| pH | 9.32 a ±0.04 | 9.72 a ±0.013 | 9.26 a ±0.04 | 1.2 |
| Temperature (°C) | 14.9 a ±0.014 | 20.2 ab ±0.04 | 22.93 b ±0.08 | 3.5 |
| NaCl (%) | 1.16 a ±0.013 | 0.07 ab ±0.03 | 0.82 b ±0.28 | 1.02 |
| TDS (ppm) | 2.26 a ±0.003 | 59.17 b ±0.065 | 52.923 b ±0.06 | 5.6 |
| EC/ECe (dScm−1) | 164.3 b ±1.01 | 95.069 a ±1.72 | 390.93 c ±0.27 | 7.9 |
| Cl− (mgL−1) | 120.72 ±1.04 | N/P | N/P | - |
| CO3−2 (mgL−1) | N/D | N/D | N/D | - |
| HCO3− (mgL−1) | 4.07 a ±0.56 | 0.065 c ±0.005 | 0.25 b ±0.004 | 2.7 |
| BOD (mgL−1) | 158.11 ±0.56 | N/P | N/P | - |
| COD (mgL−1) | 189.9 ±1.65 | N/P | N/P | - |
| TKN (mgL−1) | 5.74 a ±0.29 | 2.017 b ±0.02 | 2.53 b ±0.001 | 2.4 |
| OM (%) | 21.34 a ±0.45 | 24.17 a ±0.86 | 32.62 b ±0.06 | 3.3 |
| TOC (%) | 12.37 a ±0.2 | 14.30 ab ±0.5 | 18.9 b ±0.03 | 4.6 |
| TS (mgL−1) | 75.27 a ±0.75 | 130.7 b ±0.89 | 148.4 c ±0.22 | 5.4 |
| TSS (mgL−1) | 73.01 a ±0.02 | 71.6 a ±0.89 | 95.4 b ±0.19 | 3.9 |
| Content of Toxic Metals (mgkg−1) | Effluents | Sludge | LSD at p = 0.05 |
|---|---|---|---|
| Cr | 82.6 a ±3.37 | 85.9 a ±2.86 | 1.05 |
| Cu | 19.8 a ±1.1 | 20.77 a ±0.45 | 1.35 |
| Ni | 42.60 a ±2.7 | 75.52 b ±1.14 | 3.1 |
| Zn | 47.7 a ±1.91 | 65.13 b ±1.81 | 3.6 |
| Cd | 26.7 a ±2.27 | 28.77 a ±1.51 | 1.04 |
| Pb | 34.1 a ±1.6 | 43.7 a ±1.72 | 2.1 |
| Mg | 292.8 a ±2.04 | 331.9 a ±2.47 | 3.2 |
| Fe | 19.15 a ±1.09 | 281.07 b ±3.06 | 5.5 |
| Toxic Metals (mgkg−1) | S1B1 | S1P1 | S1Sa1 | S1G1 | S1D1 | S1L1 | S1Mo1 | S1Po1 | S1St1 | S1Sp1 | LSD at p = 0.05 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr | 37.38 a ±8.8 | 83.07 c ±15.4 | 84.96 c ±17.6 | 39.99 a ±12.3 | 31.16 a ±7.3 | 52.02 ab ±6.7 | 53.51 ab ±8.5 | 18.79 a ±5.3 | 30.28 a ±5.8 | 66.48 b ±6.4 | 2.1 |
| Cu | 8.50 a ±2.7 | 12.86 ab ±3.9 | 10.83 a ±3.1 | 12.31 ab ±3.5 | 12.87 ab ±3.7 | 14.67 b ±4.3 | 12.11 a ±3.9 | 12.74 a ±3.5 | 14.13 b ±4.5 | 12.34 a ±3.49 | 3.61 |
| Ni | 64.79 b ±6.2 | 43.45 a ±5.7 | 48.08 ab ±4.6 | 41 a ±3.9 | 60.65 b ±4.8 | 66.48 b ±4.8 | 74.60 c ±6.04 | 67.71 bc ±6.3 | 62.80 b ±5.42 | 39.74 a ±5.3 | 5.12 |
| Zn | 34.46 a ±3.2 | 41.74 a ±4.9 | 45.07 a ±6.2 | 41.63 a ±6.5 | 40.27 a ±6.8 | 49.75 b ±5.7 | 45.66 a ±6.18 | 40.41 a ±7.1 | 48.67 ab ±5.93 | 45.18 a ±6.4 | 1.3 |
| Cd | 48.02ba ±4.2 | 38.61 a ±5.2 | 34.72 a ±4.6 | 36.07 a ±5.6 | 30.72 a ±5.3 | 21.44 a ±3.1 | 17.63 a ±5.25 | 24.55 a ±4.2 | 32.27 a ±4.8 | 29.33 a ±3.8 | 3.4 |
| Pb | 24.68 ab ±4.7 | 33.14 b ±3.7 | 44.25 a ±5.1 | 30.08 b ±5.08 | 39.50 bc ±4.6 | 12.88 a ±3.7 | 32.63 b ±4.10 | 40.52 bc ±5.02 | 38.67 b ±6.2 | 67.35 c ±4.9 | 3.31 |
| Mg | 200.37 a ±11.8 | 384.7 b ±9.1 | 379.4 b ±8.7 | 369.4 b ±8.8 | 367.9 b ±8.7 | 379.2 b ±9.5 | 300.9 b ±9.6 | 290.9 ab ±11.30 | 374.4 b ±9.13 | 316 b ±7.3 | 5.1 |
| Fe | 18.51 a ±2.9 | 410.1 c ±3.5 | 17.4 a ±3.1 | 246.9 b ±3.2 | 403.8 c ±9.03 | 462.8 c ±8.5 | 20.9 a ±2.4 | 110.1 ab ±4.14 | 18.47 a ±2.9 | 434.9 c ±8.05 | 6.8 |
| Toxic Metals (mgkg−1) | P1B1 | P1P1 | P1Sa1 | P1G1 | P1D1 | P1L1 | P1Mo1 | P1Po1 | P1St1 | P1Sp1 |
|---|---|---|---|---|---|---|---|---|---|---|
| Cr | 188.0 b ±11.4 | 107.35 b ±21.1 | 166.18 b ±18.1 | 197.06 b ±10.3 | 72.53 a ±9.9 | 263.05 bc ±10.1 | 507.89 c ±14.1 | 127.78 b ±7.6 | 160.85 b ±10.1 | 147.26 b ±11.4 |
| Cu | 23.72 a ±6.3 | 32.91 a ±7.13 | 27.33 a ±8.01 | 40.01 b ±6.4 | 35.92 ab ±7.85 | 28.88 a ±7.79 | 27.36 a ±6.1 | 24.82 a ±6.9 | 32.48 ab ±7.44 | 25.24 a ±7.13 |
| Ni | 205.20 ab ±7.1 | 119 a ±7.10 | 216.78 b ±7.8 | 156.79 a ±5.01 | 147.88 a ±6.82 | 171.33 a ±7.84 | 175.11 a ±6.98 | 200.63 ab ±9.2 | 194.32 a ±9.04 | 152.49 a ±7.63 |
| Zn | 191.74 a ±2.5 | 149.31 a ±6.9 | 168.95 a ±5.07 | 179.18 a ±1.95 | 139.89 a ±1.04 | 155.97 a ±1.16 | 172.24 a ±4.01 | 119.81 a ±0.33 | 219.07 b ±1.36 | 165.72 a ±2.17 |
| Cd | 207.30 bc ±5.9 | 24.95 a ±6.16 | 74.11 ab ±4.83 | 25.09 a ±6.15 | 87.65 b ±5.67 | 87.92 b ±6.2 | 27.34 a ±5.85 | 42.01 a ±6.2 | 30.86 a ±5.3 | 315.26 c ±5.4 |
| Pb | 98.88 a ±8.8 | 86.75 a ±10.7 | 174.53 b ±10.2 | 80.24 a ±6.8 | 74.11 a ±7.1 | 85.65 a ±8.6 | 121.02 ab ±10.4 | 125.55 a ±9.58 | 53.93 a ±8.07 | 56.82 a ±7.77 |
| Mg | 867.9 cd ±11.5 | 834.9 c ±12.9 | 900.9 d ±4.14 | 847.5 c ±10.15 | 74.11 a ±7.1 | 579.2 c ±9.2 | 310.5 b ±7.6 | 798 bc ±7.1 | 845.5 cd ±7.24 | 483 b ±4.74 |
| Fe | 104.7 a ±4.8 | 143.5 a ±5.73 | 141.2 a ±7.82 | 271.2 b ±10.8 | 151.5 ab ±6.96 | 171.6 b ±6.79 | 69.3 c ±5.57 | 209.6 b ±3.32 | 231.6 b ±6.15 | 211.5 b ±7.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakoor, I.; Sultan, A.; Shaukat, K.; Alam, T.M.; Nazir, A. A Novel Method to Investigate Environmental Risk in Wastewater Toxicity. Agronomy 2025, 15, 841. https://doi.org/10.3390/agronomy15040841
Shakoor I, Sultan A, Shaukat K, Alam TM, Nazir A. A Novel Method to Investigate Environmental Risk in Wastewater Toxicity. Agronomy. 2025; 15(4):841. https://doi.org/10.3390/agronomy15040841
Chicago/Turabian StyleShakoor, Isha, Amina Sultan, Kamran Shaukat, Talha Mahboob Alam, and Aisha Nazir. 2025. "A Novel Method to Investigate Environmental Risk in Wastewater Toxicity" Agronomy 15, no. 4: 841. https://doi.org/10.3390/agronomy15040841
APA StyleShakoor, I., Sultan, A., Shaukat, K., Alam, T. M., & Nazir, A. (2025). A Novel Method to Investigate Environmental Risk in Wastewater Toxicity. Agronomy, 15(4), 841. https://doi.org/10.3390/agronomy15040841






