A Rotational Cultivation System for Indoor-Grown Lettuce: Feasibility in Terms of Yields, Resource Efficiency, Quality, and Postharvest Storage Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Germination
2.2. Plant Growth
- A control cultivation system, horizontal (Figure 1A). Lettuce grown in this cultivation system is referred to as CT lettuce. Cultivation frames (140 × 210 cm) were installed in a growth chamber and equipped with a drip irrigation system and white light LED lamps (LO250 Floodlight, LO250-PSHW06040, ‘Vegeled’, Ghent, Belgium). Lettuce was cultivated in pots (2113 cm3) filled with potting soil (VER4, ‘Florentaise’, Saint-Mars-du-Désert, France), with a density of 30 plants m−2.
- A Gigrow® rotational cultivation system from Futura Gaïa Technologies, Rodilhan, France (Figure 1B). Lettuce grown in this cultivation system is referred to as ROT lettuce. The lettuce was transplanted in five-hole stainless steel trays (72 × 15 × 4.5; l × w × h, in cm) filled with potting soil (VER4, ‘Florentaise’, Saint-Mars-du-Désert, France), with a density of 30 plants m−2. Plants were rotating according to a horizontal rotation axis at a speed of 50 min per revolution, such a speed providing a centrifugal force of 1.33 × 10−5 Newton, considered neglectable. Light was provided using ceramic metal-halide lamps with a 4200 K color temperature (630 W double ended, ‘Lumatek Ltd.’, Saint Julian’s, Malta).
2.3. Measurement and Calculation Methods of Agronomic Parameters
2.4. Glucose, Fructose, and Sucrose Contents by HPAEC
2.5. Photosynthetic and Chlorophyll Fluorescence Parameters
2.6. Untargeted Liquid Chromatography Analysis
2.6.1. Sample Extraction
2.6.2. Untargeted UPLC-ESI-QTOF-MSE Profiling
2.6.3. Data Processing and Data Analysis
2.7. Postharvest Processing and Measurements
2.8. Statistical Analyses
3. Results
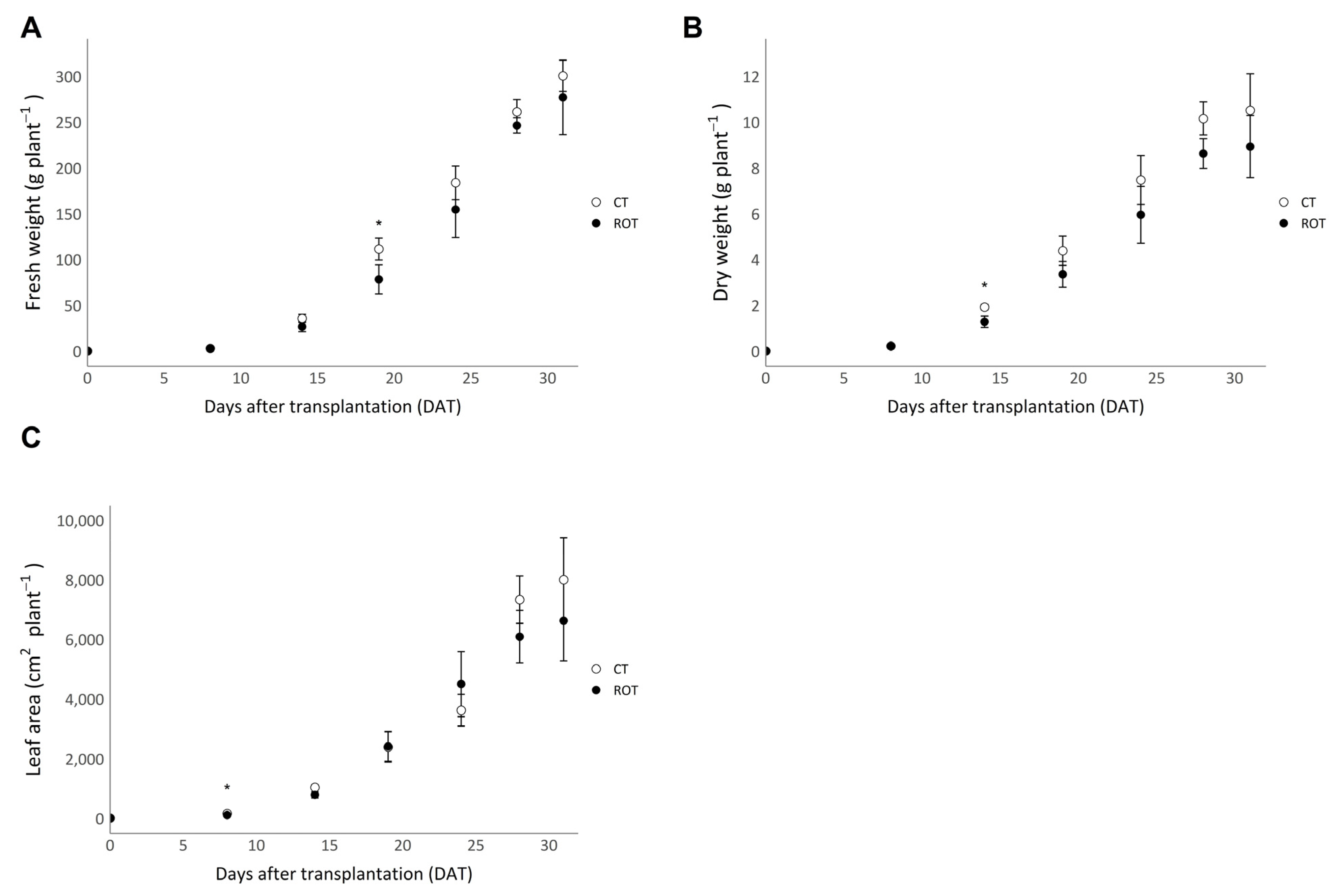
3.1. Yields, Efficiencies, and Agronomic Parameters
3.2. Glucose, Fructose, Sucrose, and Total Soluble Sugar (TSS) Contents
3.3. Photosynthetic Parameters
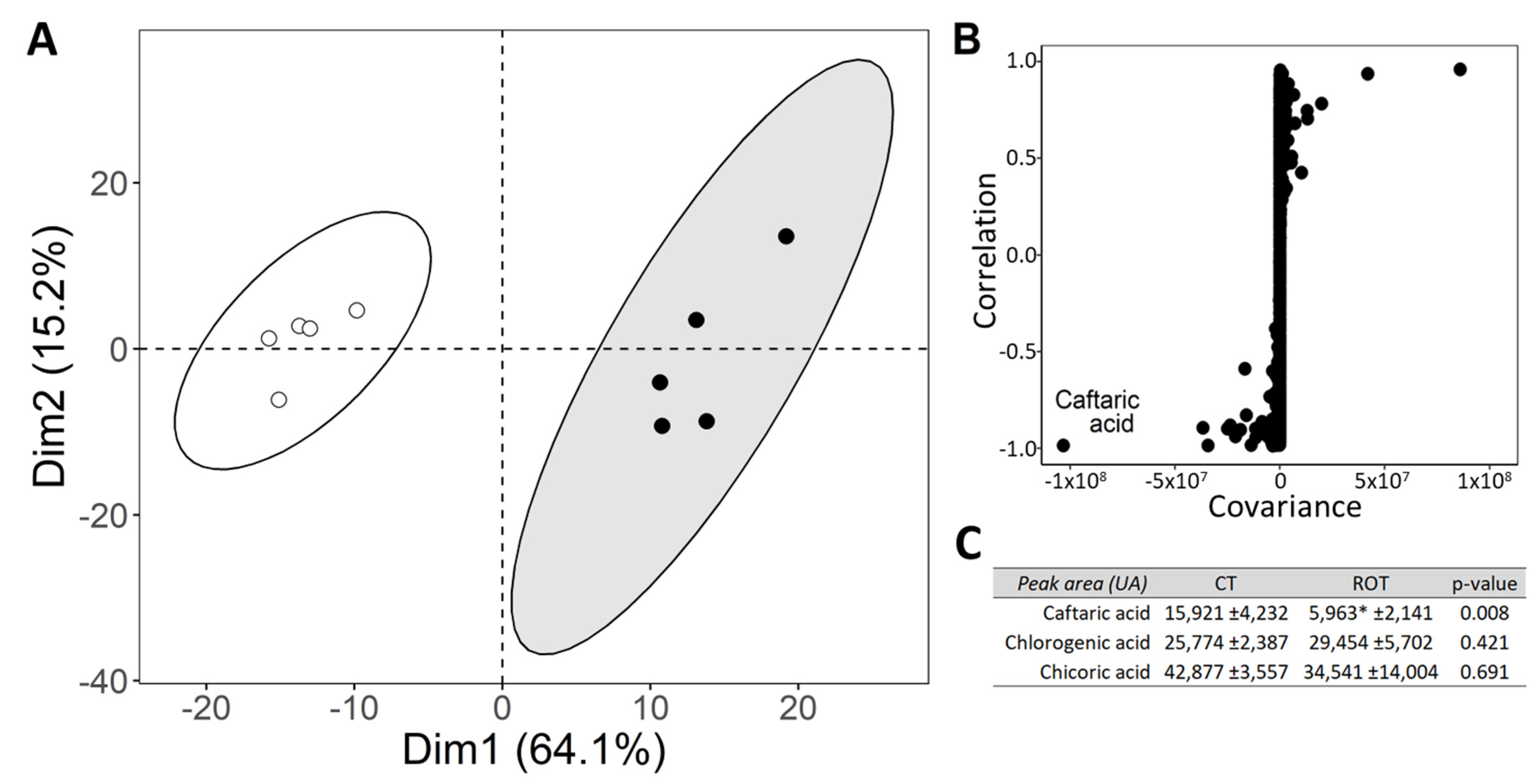
3.4. Untargeted Liquid Chromatography Profiling and Main Polyphenols Content at Harvest
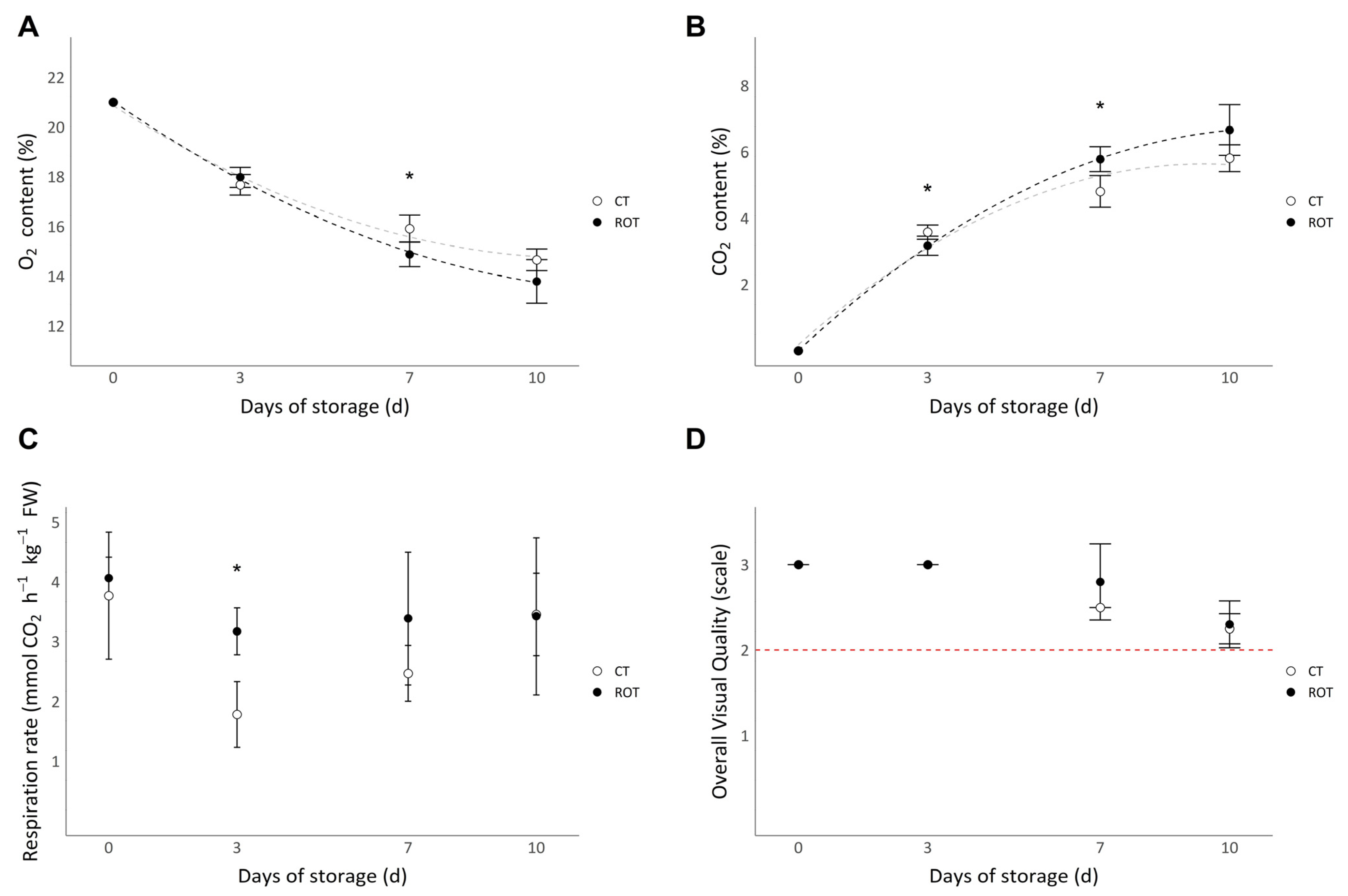
3.5. Postharvest Analyses
4. Discussion
4.1. The Yields in Control or the Rotational Cultivation Systems Were Similar
4.2. The Observed Decrease in Net Photosynthesis Can Be Attributed to the Perturbation of Plant Gravity Perception
4.3. Energy and Land Surface Use Efficiencies Were Higher in the Rotational Cultivation System but Still Need to Be Maximized
4.4. The Rotational Cultivation System Induced Changes in the Plant’s Quality Without Altering the Postharvest Storage Capacity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LUE | Light use efficiency |
| WUE | Water use efficiency |
| EUE | Energy use efficiency |
| LSUE | Land surface use efficiency |
References
- Gu, D.; Andreev, K.; Dupre, M.E. Major Trends in Population Growth Around the World. China CDC Wkly. 2021, 3, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Mempel, H.; Jüttner, I.; Wittmann, S. The Potentials of Indoor Farming for Plant Production. Automatisierungstechnik 2021, 69, 287–296. [Google Scholar] [CrossRef]
- Graamans, L.; Baeza, E.; van den Dobbelsteen, A.; Tsafaras, I.; Stanghellini, C. Plant Factories versus Greenhouses: Comparison of Resource Use Efficiency. Agric. Syst. 2018, 160, 31–43. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G. Chapter 4—Plant Factory as a Resource-Efficient Closed Plant Production System. In Plant Factory; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 69–90. ISBN 978-0-12-801775-3. [Google Scholar]
- Righini, I.; Stanghellini, C.; Hemming, S.; Graamans, L.; Marcelis, L.F.M. Resources for Plant-Based Food: Estimating Resource Use to Meet the Requirements of Urban and Peri-Urban Diets. Food Energy Secur. 2023, 12, e462. [Google Scholar] [CrossRef]
- Barbosa, G.L.; Gadelha, F.D.A.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.M.; Halden, R.U. Comparison of Land, Water, and Energy Requirements of Lettuce Grown Using Hydroponic vs. Conventional Agricultural Methods. Int. J. Environ. Res. Public. Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef]
- Tasgal, P. The Economics of Local Vertical & Greenhouse Farming Are Getting Competitive. Available online: https://agfundernews.com/the-economics-of-local-vertical-and-greenhouse-farming-are-getting-competitive (accessed on 13 January 2025).
- Avgoustaki, D.D.; Xydis, G. How Energy Innovation in Indoor Vertical Farming Can Improve Food Security, Sustainability, and Food Safety? In Advances in Food Security and Sustainability; Elsevier: Amsterdam, The Netherlands, 2020; Volume 5, pp. 1–51. ISBN 978-0-12-820711-6. [Google Scholar]
- Kozai, T. Resource Use Efficiency of Closed Plant Production System with Artificial Light: Concept, Estimation and Application to Plant Factory. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2013, 89, 447–461. [Google Scholar] [CrossRef]
- Avgoustaki, D.; Xydis, G. Plant Factories in the Water-Food-Energy Nexus Era: A Systematic Bibliographical Review. Food Secur. 2020, 12, 253–268. [Google Scholar] [CrossRef]
- Harbick, K.; Albright, L.D. Comparison of Energy Consumption: Greenhouses and Plant Factories. Acta Hortic. 2016, 1134, 285–292. [Google Scholar] [CrossRef]
- Kelly, N.; Choe, D.; Meng, Q.; Runkle, E.S. Promotion of Lettuce Growth under an Increasing Daily Light Integral Depends on the Combination of the Photosynthetic Photon Flux Density and Photoperiod. Sci. Hortic. 2020, 272, 109565. [Google Scholar] [CrossRef]
- Palmer, S.; van Iersel, M.W. Increasing Growth of Lettuce and Mizuna under Sole-Source LED Lighting Using Longer Photoperiods with the Same Daily Light Integral. Agronomy 2020, 10, 1659. [Google Scholar] [CrossRef]
- Pennisi, G.; Pistillo, A.; Orsini, F.; Cellini, A.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Crepaldi, A.; Gianquinto, G.; Marcelis, L.F.M. Optimal Light Intensity for Sustainable Water and Energy Use in Indoor Cultivation of Lettuce and Basil under Red and Blue LEDs. Sci. Hortic. 2020, 272, 109508. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of Environment Lighting on the Growth, Photosynthesis, and Quality of Hydroponic Lettuce in a Plant Factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Lee, M.-J.; Son, K.-H.; Oh, M.-M. Increase in Biomass and Bioactive Compounds in Lettuce under Various Ratios of Red to Far-Red LED Light Supplemented with Blue LED Light. Hortic. Environ. Biotechnol. 2016, 57, 139–147. [Google Scholar] [CrossRef]
- Liu, J.; van Iersel, M.W. Far-Red Light Effects on Lettuce Growth and Morphology in Indoor Production Are Cultivar Specific. Plants 2022, 11, 2714. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Landolfo, M.; Pistillo, A.; Crepaldi, A.; Nicola, S.; Fernández, J.A.; Marcelis, L.F.M.; Gianquinto, G. Optimal Photoperiod for Indoor Cultivation of Leafy Vegetables and Herbs. Eur. J. Hortic. Sci. 2020, 85, 329–338. [Google Scholar] [CrossRef]
- Pinho, P.; Jokinen, K.; Halonen, L. The Influence of the LED Light Spectrum on the Growth and Nutrient Uptake of Hydroponically Grown Lettuce. Light. Res. Technol. 2017, 49, 866–881. [Google Scholar] [CrossRef]
- Agati, G.; Franchetti, B.; Rispoli, F.; Venturini, P. Thermo-Fluid Dynamic Analysis of the Air Flow inside an Indoor Vertical Farming System. Appl. Therm. Eng. 2024, 236, 121553. [Google Scholar] [CrossRef]
- Despommier, D. The Vertical Farm: Controlled Environment Agriculture Carried out in Tall Buildings Would Create Greater Food Safety and Security for Large Urban Populations. J. Für Verbraucherschutz Und Leb. 2011, 6, 233–236. [Google Scholar] [CrossRef]
- Lee, J.G.; Choi, C.S.; Jang, Y.A.; Jang, S.W.; Lee, S.G.; Um, Y.C. Effects of Air Temperature and Air Flow Rate Control on the Tipburn Occurrence of Leaf Lettuce in a Closed-Type Plant Factory System. Hortic. Environ. Biotechnol. 2013, 54, 303–310. [Google Scholar] [CrossRef]
- Naranjani, B.; Najafianashrafi, Z.; Pascual, C.; Agulto, I.; Chuang, P.-Y.A. Computational Analysis of the Environment in an Indoor Vertical Farming System. Int. J. Heat Mass Transf. 2022, 186, 122460. [Google Scholar] [CrossRef]
- Paquet, G.; Nguyen, T.T.A.; Brégard, A.; Barrada, A.; Poirier, G.; Dorais, M. The Agronomic Potential of a New Vertical Growing Farming System Using Biofertilizers and Peat-Based Wood Fibre Growing Medium: Lettuce as a Case Study. Acta Hortic. 2022, 1355, 455–462. [Google Scholar] [CrossRef]
- Yang, X.; Gil, M.I.; Yang, Q.; Tomás-Barberán, F.A. Bioactive Compounds in Lettuce: Highlighting the Benefits to Human Health and Impacts of Preharvest and Postharvest Practices. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4–45. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Orsini, F.; Pennisi, G.; Zulfiqar, F.; Gianquinto, G. Sustainable Use of Resources in Plant Factories with Artificial Lighting (PFALs). Eur. J. Hortic. Sci. 2020, 85, 297–309. [Google Scholar] [CrossRef]
- Rusch, A.; Suchail, S.; Valantin-Morison, M.; Sarthou, J.-P.; Roger-Estrade, J. Nutritional State of the Pollen Beetle Parasitoid Tersilochus Heterocerus Foraging in the Field. BioControl 2013, 58, 17. [Google Scholar] [CrossRef]
- Black, C.; Haughey, S.A.; Chevallier, O.P.; Galvin-King, P.; Elliott, C.T. A Comprehensive Strategy to Detect the Fraudulent Adulteration of Herbs: The Oregano Approach. Food Chem. 2016, 210, 551–557. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. In Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Gargaro, M.; Murphy, R.J.; Harris, Z.M. Let-Us Investigate; A Meta-Analysis of Influencing Factors on Lettuce Crop Yields within Controlled-Environment Agriculture Systems. Plants 2023, 12, 2623. [Google Scholar] [CrossRef]
- Matysiak, B.; Ropelewska, E.; Wrzodak, A.; Kowalski, A.; Kaniszewski, S. Yield and Quality of Romaine Lettuce at Different Daily Light Integral in an Indoor Controlled Environment. Agronomy 2022, 12, 1026. [Google Scholar] [CrossRef]
- Moore, R.; Evans, M.L. How Roots Perceive and Respond to Gravity. Am. J. Bot. 1986, 73, 574–587. [Google Scholar] [CrossRef]
- Masson, P.H.; Tasaka, M.; Morita, M.T.; Guan, C.; Chen, R.; Boonsirichai, K. Arabidopsis thaliana: A Model for the Study of Root and Shoot Gravitropism. Arab. Book 2002, 1, e0043. [Google Scholar] [CrossRef]
- Mano, E.; Horiguchi, G.; Tsukaya, H. Gravitropism in Leaves of Arabidopsis thaliana (L.) Heynh. Plant Cell Physiol. 2006, 47, 217–223. [Google Scholar] [CrossRef]
- Gago, J.; Daloso, D.D.M.; Figueroa, C.M.; Flexas, J.; Fernie, A.R.; Nikoloski, Z. Relationships of Leaf Net Photosynthesis, Stomatal Conductance, and Mesophyll Conductance to Primary Metabolism: A Multispecies Meta-Analysis Approach. Plant Physiol. 2016, 171, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Gorton, H.L.; Williams, W.E.; Binns, M.E.; Gemmell, C.N.; Leheny, E.A.; Shepherd, A.C. Circadian Stomatal Rhythms in Epidermal Peels from Vicia Faba. Plant Physiol. 1989, 90, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, T.L.; Field, C.B. Oscillations in Carbon Assimilation and Stomatal Conductance under Constant Conditions. Plant Physiol. 1991, 96, 6. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.C.; Brown, C.S.; Levine, H.G.; Krikorian, A.D. Growth and Photosynthetic Responses of Wheat Plants Grown in Space. Plant Physiol. 1996, 110, 801–806. [Google Scholar] [CrossRef]
- Kirkham, M.B. Horizontal Root Growth: Water Uptake and Stomatal Resistance under Microgravity. Vadose Zone J. 2008, 7, 1125–1131. [Google Scholar] [CrossRef]
- Chater, C.C.C.; Oliver, J.; Casson, S.; Gray, J.E. Putting the Brakes on: Abscisic Acid as a Central Environmental Regulator of Stomatal Development. New Phytol. 2014, 202, 376–391. [Google Scholar] [CrossRef]
- Han, W.; Rong, H.; Zhang, H.; Wang, M.-H. Abscisic Acid Is a Negative Regulator of Root Gravitropism in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2009, 378, 695–700. [Google Scholar] [CrossRef]
- Walton, D.C. Biochemistry and Physiology of Abscisic Acid. Annu. Rev. Plant Physiol. 1980, 31, 453–489. [Google Scholar] [CrossRef]
- Jiao, S.; Hilaire, E.; Paulsen, A.Q.; Guikema, J.A. Brassica Rapa Plants Adapted to Microgravity with Reduced Photosystem I and Its Photochemical Activity. Physiol. Plant. 2004, 122, 281–290. [Google Scholar] [CrossRef]
- Stutte, G.W.; Monje, O.; Goins, G.D.; Tripathy, B.C. Microgravity Effects on Thylakoid, Single Leaf, and Whole Canopy Photosynthesis of Dwarf Wheat. Planta 2005, 223, 46–56. [Google Scholar] [CrossRef]
- Yamamoto, Y. Quality Control of Photosystem II. Plant Cell Physiol. 2001, 42, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Halstead, T.W.; Dutcher, F.R. Plants in Space. Annu. Rev. Plant Physiol. 1987, 38, 317–345. [Google Scholar] [CrossRef] [PubMed]
- Stutte, G.W.; Monje, O.; Hatfield, R.D.; Paul, A.-L.; Ferl, R.J.; Simone, C.G. Microgravity Effects on Leaf Morphology, Cell Structure, Carbon Metabolism and mRNA Expression of Dwarf Wheat. Planta 2006, 224, 1038–1049. [Google Scholar] [PubMed]
- Jin, W.; Formiga Lopez, D.; Heuvelink, E.; Marcelis, L.F.M. Light Use Efficiency of Lettuce Cultivation in Vertical Farms Compared with Greenhouse and Field. Food Energy Secur. 2022, 12, e391. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource Use Efficiency of Indoor Lettuce (Lactuca sativa L.) Cultivation as Affected by Red:Blue Ratio Provided by LED Lighting. Sci. Rep. 2019, 9, 14127. [Google Scholar] [CrossRef]
- Eaton, M.; Shelford, T.; Cole, M.; Mattson, N. Modeling Resource Consumption and Carbon Emissions Associated with Lettuce Production in Plant Factories. J. Clean. Prod. 2023, 384, 135569. [Google Scholar] [CrossRef]
- Avgoustaki, D.D.; Xydis, G. Indoor Vertical Farming in the Urban Nexus Context: Business Growth and Resource Savings. Sustainability 2020, 12, 1965. [Google Scholar] [CrossRef]
- Xu, D.; Ahmed, H.A.; Tong, Y.; Yang, Q.; van Willigenburg, L.G. Optimal Control as a Tool to Investigate the Profitability of a Chinese Plant Factory–Lettuce Production System. Biosyst. Eng. 2021, 208, 319–332. [Google Scholar] [CrossRef]
- Zhuang, Y.; Lu, N.; Shimamura, S.; Maruyama, A.; Kikuchi, M.; Takagaki, M. Economies of Scale in Constructing Plant Factories with Artificial Lighting and the Economic Viability of Crop Production. Front. Plant Sci. 2022, 13, 992194. [Google Scholar] [CrossRef]
- Kartika, H.; Li, Q.X.; Wall, M.M.; Nakamoto, S.T.; Iwaoka, W.T. Major Phenolic Acids and Total Antioxidant Activity in Mamaki Leaves, Pipturus Albidus. J. Food Sci. 2007, 72, S696–S701. [Google Scholar] [CrossRef] [PubMed]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic Acids, Flavonoids and Total Antioxidant Capacity of Selected Leafy Vegetables. J. Funct. Foods 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Carotti, L.; Potente, G.; Pennisi, G.; Ruiz, K.B.; Biondi, S.; Crepaldi, A.; Orsini, F.; Gianquinto, G.; Antognoni, F. Pulsed LED Light: Exploring the Balance between Energy Use and Nutraceutical Properties in Indoor-Grown Lettuce. Agronomy 2021, 11, 1106. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Galardi, C.; Sani, G.; Cimato, A.; Heimler, D. Polyphenols in Greenhouse and Open-Air-Grown Lettuce. Food Chem. 2002, 79, 337. [Google Scholar]
- Yang, M.; Wu, C.; Zhang, T.; Shi, L.; Li, J.; Liang, H.; Lv, X.; Jing, F.; Qin, L.; Zhao, T.; et al. Chicoric Acid: Natural Occurrence, Chemical Synthesis, Biosynthesis, and Their Bioactive Effects. Front. Chem. 2022, 10, 888673. [Google Scholar] [CrossRef]
- Popova, A.F. Comparative Characteristic of Mitochondria Ultrastructural Organization in Chlorella Cells under Altered Gravity Conditions. Adv. Space Res. 2003, 31, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Sytnik, K.M.; Popova, A.F.; Nechitailo, G.S.; Mashinsky, A.L. Peculiarities of the Submicroscopic Organization of Chlorella Cells Cultivated on a Solid Medium in Microgravity. Adv. Space Res. 1992, 12, 103–107. [Google Scholar] [CrossRef]
- Vasilenko, A.; Popova, A.F. Energetic Metabolism Response in Algae and Higher Plant Species from Simulation Experiments with the Clinostat. Adv. Space Res. Off. J. Comm. Space Res. COSPAR 1996, 17, 103–106. [Google Scholar] [CrossRef]



| Glucose (mg g−1 of DW) | Fructose (mg g−1 of DW) | Sucrose (mg g−1 of DW) | Total Soluble Sugars (mg g−1 of DW) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CT | ROT | CT | ROT | CT | ROT | CT | ROT | |||||||||||||
| DAT | Mean | sd | Mean | sd | p-Value | Mean | sd | Mean | sd | p-Value | Mean | sd | Mean | sd | p-Value | Mean | sd | Mean | sd | p-Value |
| 18 | 108 | ±19.8 | 81 * | ±6.4 | 0.0079 | 161 | ±23.2 | 101 * | ±10.2 | 0.0079 | 114 | ±21.3 | 99 | ±10.1 | 0.1508 | 383 | ±23.2 | 281 * | ±15.7 | 0.0079 |
| 31 | 129 | ±14.8 | 95 | ±19.2 | 0.0635 | 130 | ±16.5 | 101 | ±19.7 | 0.1111 | 13 | ±2 | 19 | ±8.5 | 0.2857 | 273 | ±33.2 | 214 | ±32.8 | 0.0635 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dresch, C.; Vidal, V.; Suchail, S.; Chevallier, O.; Sallanon, H.; Truffault, V.; Charles, F. A Rotational Cultivation System for Indoor-Grown Lettuce: Feasibility in Terms of Yields, Resource Efficiency, Quality, and Postharvest Storage Capacity. Agronomy 2025, 15, 744. https://doi.org/10.3390/agronomy15030744
Dresch C, Vidal V, Suchail S, Chevallier O, Sallanon H, Truffault V, Charles F. A Rotational Cultivation System for Indoor-Grown Lettuce: Feasibility in Terms of Yields, Resource Efficiency, Quality, and Postharvest Storage Capacity. Agronomy. 2025; 15(3):744. https://doi.org/10.3390/agronomy15030744
Chicago/Turabian StyleDresch, Cédric, Véronique Vidal, Séverine Suchail, Olivier Chevallier, Huguette Sallanon, Vincent Truffault, and Florence Charles. 2025. "A Rotational Cultivation System for Indoor-Grown Lettuce: Feasibility in Terms of Yields, Resource Efficiency, Quality, and Postharvest Storage Capacity" Agronomy 15, no. 3: 744. https://doi.org/10.3390/agronomy15030744
APA StyleDresch, C., Vidal, V., Suchail, S., Chevallier, O., Sallanon, H., Truffault, V., & Charles, F. (2025). A Rotational Cultivation System for Indoor-Grown Lettuce: Feasibility in Terms of Yields, Resource Efficiency, Quality, and Postharvest Storage Capacity. Agronomy, 15(3), 744. https://doi.org/10.3390/agronomy15030744







