Microbiome Analysis of Rhizosphere Soil of Wild Succulent Shrubs Zygophyllum coccineum and Haloxylon salicornicum
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Soil Samples
2.2. Soil and Plant Chemical Analysis
2.3. DNA Extraction and PCR Amplification
2.4. Metagenomic Sequencing and Analysis
2.5. Data Analysis
3. Results
3.1. Nutrient Elements in Soil and Plant Samples
3.2. Data Processing and Statistics
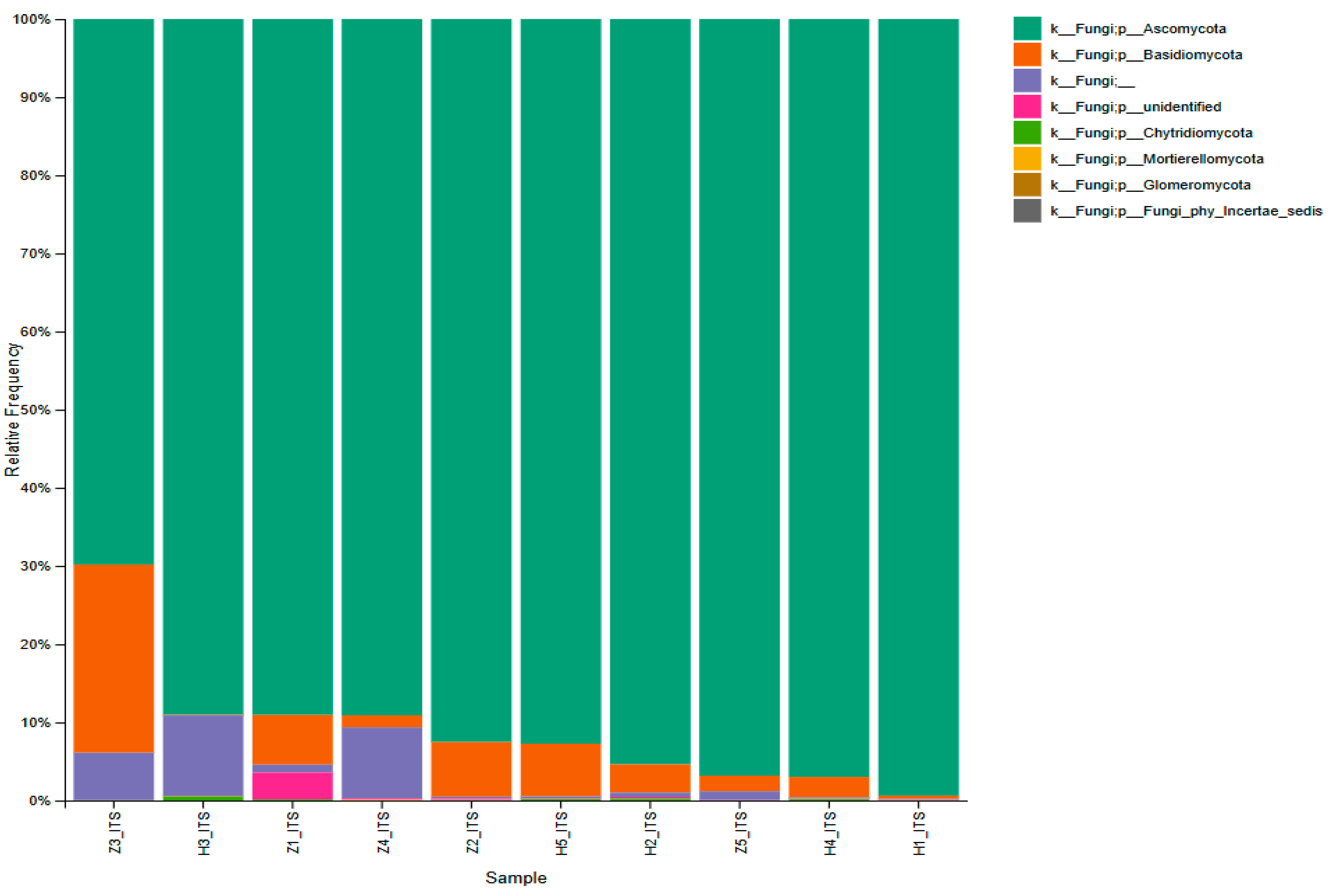
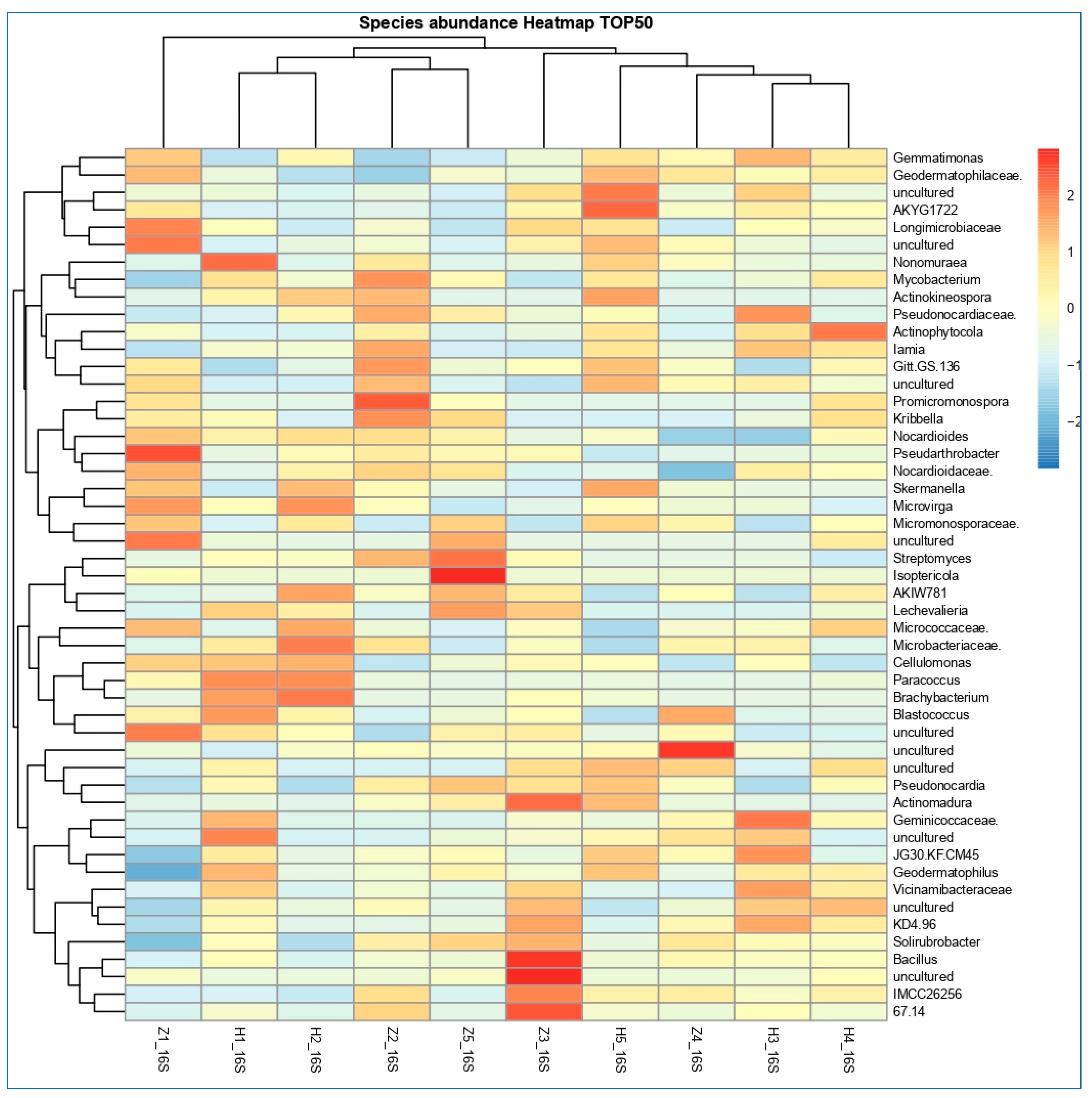
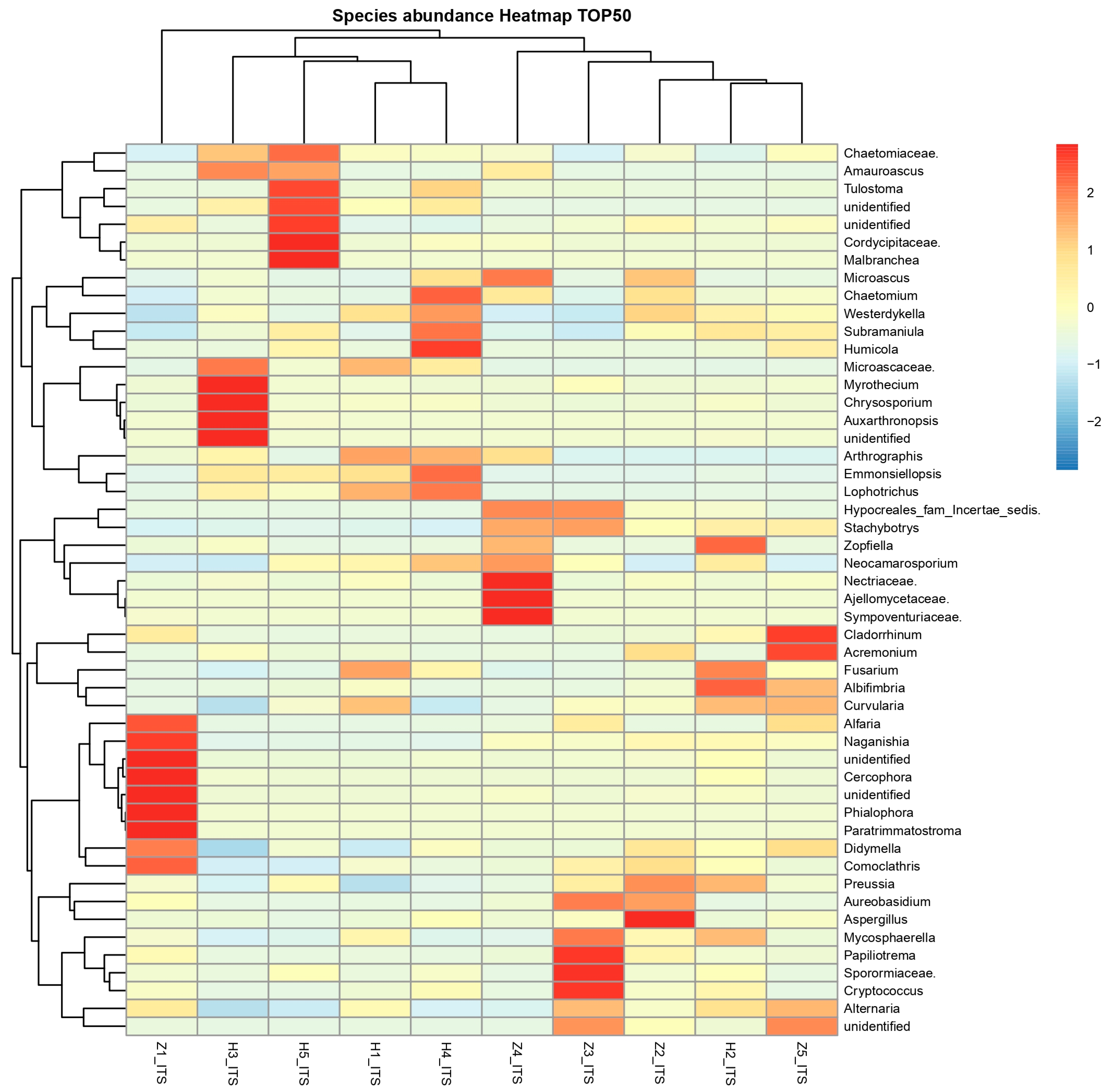
3.3. Microbial Abundance and Distribution
3.4. Diversity Analysis of the Microbial Community
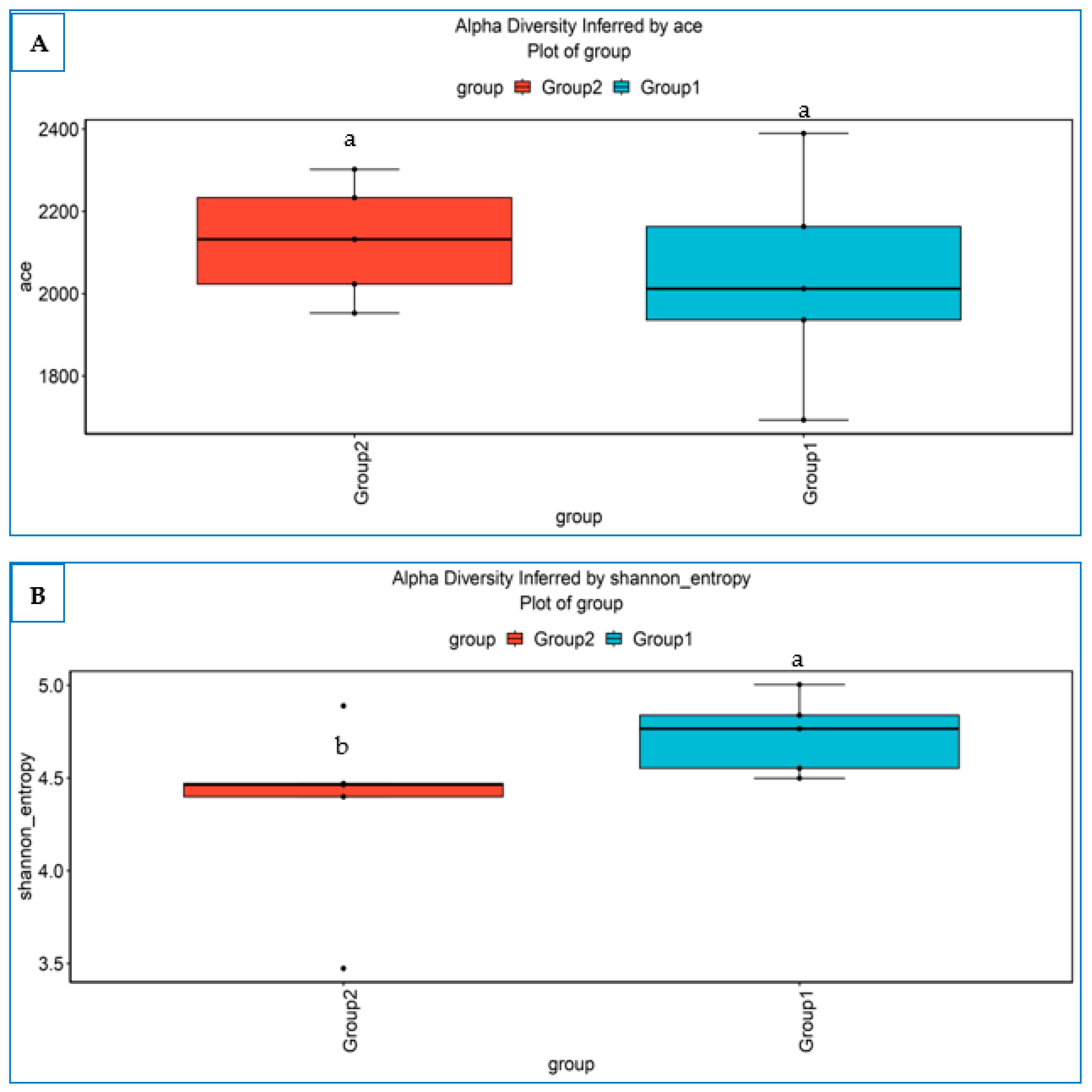
3.4.1. Alpha Diversity
3.4.2. Beta Diversity
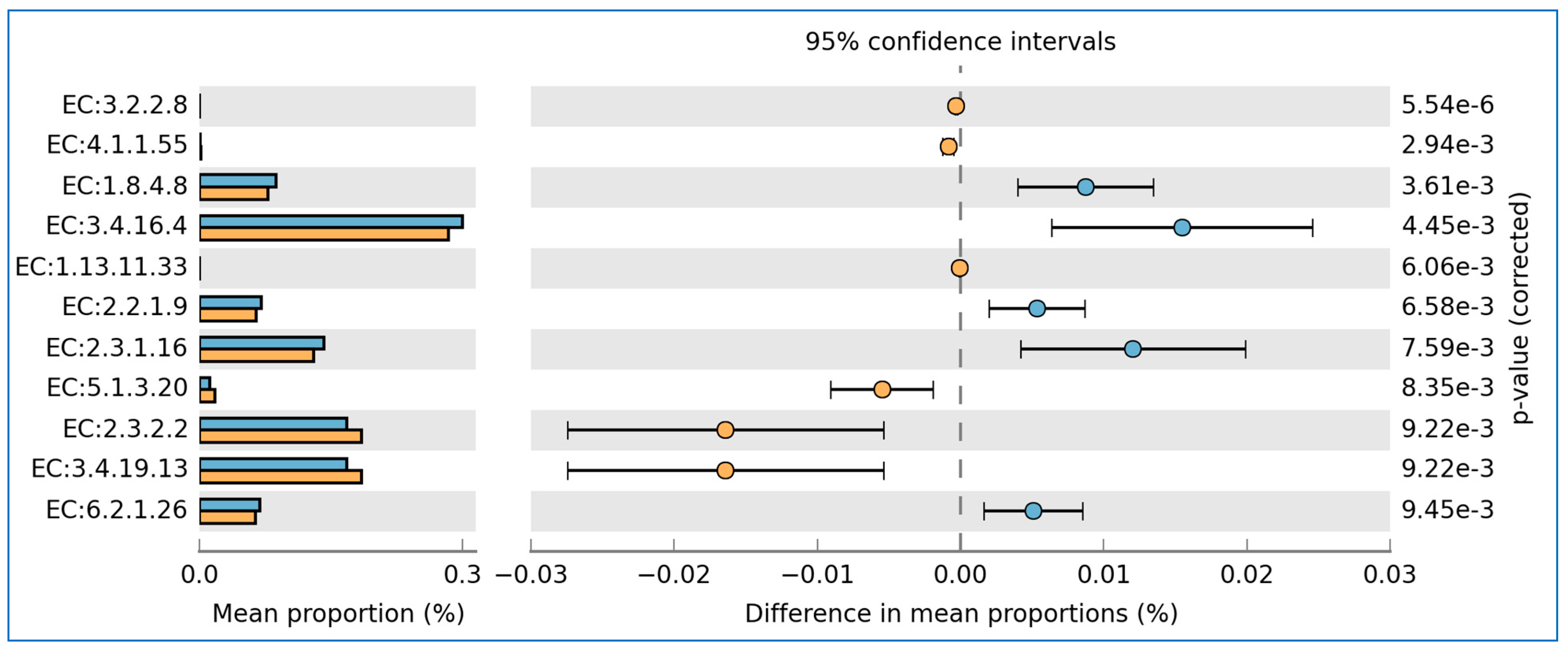
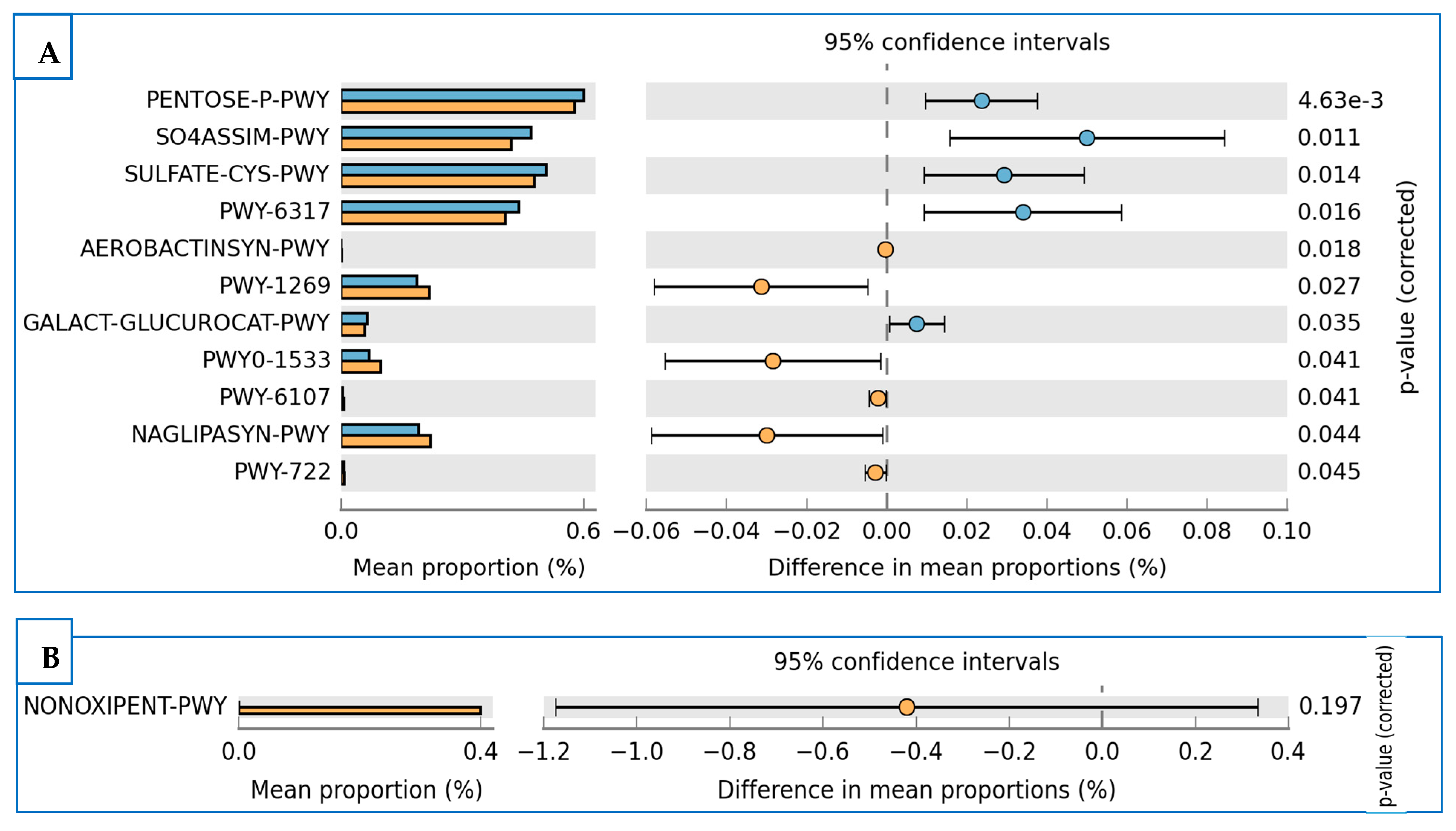
3.5. Prediction of Functional Enzymes and Pathways
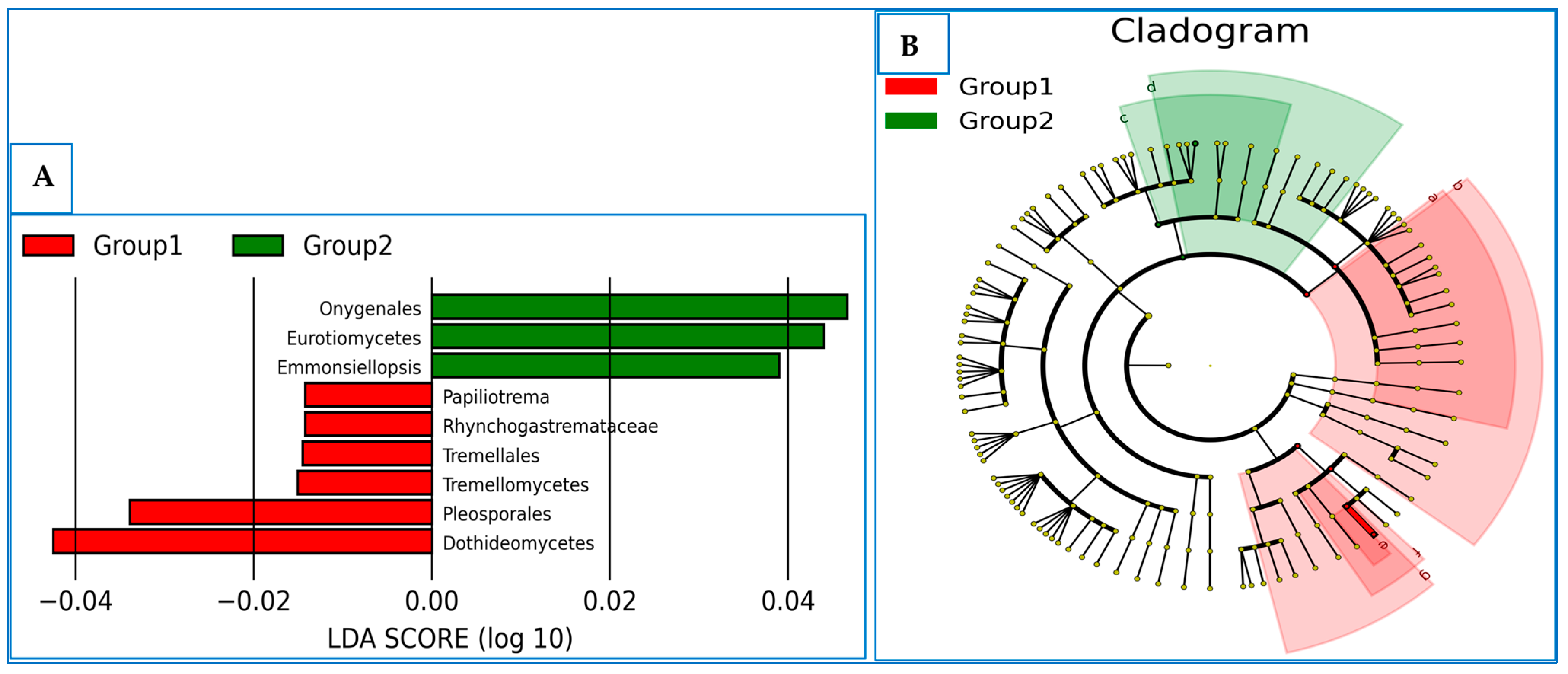
3.6. The Differentially Abundance in Microbial Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Esawi, M.A.; Elshamy, M.M.; Heikal, Y.M. Genetic Variation and Molecular Characterization of Zygophyllum coccineum L. Ecotypes of the Iron Mining Area of El-Wahat El-Bahariya in Egypt. Plant Physiol. Biochem. 2022, 190, 212–230. [Google Scholar] [CrossRef] [PubMed]
- Hofland, N.; Hatim, M.Z.; Thomas, J.; Janssen, J.A.M.; Alharti, A.S.; Alfarhan, A.H.; Alatar, A.A.; Schaminée, J.H.J.; El-Sheikh, M.A. The Vegetation around the Foothills of the Hijaz Mountains, Saudi Arabia. Rend. Lincei Sci. Fis. Nat. 2024, 35, 437–449. [Google Scholar] [CrossRef]
- El-Amier, Y.A.; El-Shora, H.M.; Hesham, M. Ecological Study on Zygophyllum coccineum L. in Coastal and Inland Desert of Egypt. J. Agric. Ecol. Res. 2016, 6, 1–17. [Google Scholar] [CrossRef]
- Yosri, M.; Elaasser, M.M.; Abdel-Aziz, M.M.; Hassan, M.M.; Alqhtani, A.H.; Al-Gabri, N.; Ali, A.B.A.; Pokoo-Aikins, A.; Amin, B.H. Determination of Therapeutic and Safety Effects of Zygophyllum coccineum Extract in Induced Inflammation in Rats. Biomed. Res. Int. 2022, 2022, 7513155. [Google Scholar] [CrossRef]
- Mohamed, E.; Kasem, A.M.M.A.; Gobouri, A.A.; Elkelish, A.; Azab, E. Influence of Maternal Habitat on Salinity Tolerance of Zygophyllum coccineum with Regard to Seed Germination and Growth Parameters. Plants 2020, 9, 1504. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.-J.; Chen, H.-Y.; Bai, W.-P.; Yang, R.-C.; Yang, P.-Z.; Chen, R.-J.; Hu, T.-M.; Wang, S.-M. Sodium-Related Adaptations to Drought: New Insights from the Xerophyte Plant Zygophyllum xanthoxylum. Front. Plant Sci. 2018, 9, 1678. [Google Scholar] [CrossRef]
- Cao, Y.-H.; Ren, W.; Gao, H.-J.; Lü, X.-P.; Zhao, Q.; Zhang, H.; Rensing, C.; Zhang, J.-L. HaASR2 from Haloxylon Ammodendron Confers Drought and Salt Tolerance in Plants. Plant Sci. 2023, 328, 111572. [Google Scholar] [CrossRef]
- Mansour, H.A.; Newairy, A.-S.A.; Yousef, M.I.; Sheweita, S.A. Biochemical Study on the Effects of Some Egyptian Herbs in Alloxan-Induced Diabetic Rats. Toxicology 2002, 170, 221–228. [Google Scholar] [CrossRef]
- El-Afify, S.M.; El-Metwaly, M.A.; Abbas, M.A.; El-Amier, Y.A. In Vitro Assessment of Antioxidant and Cytotoxic Activities of Zygophyllum coccineum L. Methanolic Extract. Egypt. J. Chem. 2024, 67, 393–401. [Google Scholar] [CrossRef]
- Gibbons, S.; Oriowo, M.A. Antihypertensive Effect of an Aqueous Extract of Zygophyllum coccineum L. in Rats. Phytother. Res. 2001, 15, 452–455. [Google Scholar] [CrossRef]
- Amro, A.N.; Abhary, M.K. Removal of Lead and Copper Ions from Water Using Powdered Zygophyllum coccineum Biomass. Int. J. Phytoremediat. 2019, 21, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, I.; Corréal, E.; Lutts, S. Cadmium Tolerance and Accumulation in the Noxious Weed Zygophyllum Fabago. Can. J. Bot. 2005, 83, 1655–1662. [Google Scholar] [CrossRef]
- Singh, J.; Rathore, V.S.; Roy, M. Notes about Haloxylon Salicornicum (Moq.) Bunge Ex Boiss., a Promising Shrub for Arid Regions. Genet. Resour. Crop Evol. 2015, 62, 451–463. [Google Scholar] [CrossRef]
- Elagamy, N.A.; Soliman, H.M.; Abbas, M.A.; El-Shaer, M.M.; El-Amier, Y.A. Chemical Composition, Antioxidant and Antimicrobial Activities of Haloxylon Salicornicum Methanolic Extract. Egypt. J. Chem. 2024, 67, 453–461. [Google Scholar] [CrossRef]
- Purohit, H.J.; Pandit, P.; Pal, R.; Warke, R.; Warke, G.M. Soil Microbiome: An Intrinsic Driver for Climate Smart Agriculture. J. Agric. Food Res. 2024, 18, 101433. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, Y.; Shi, F.; Liu, Y.; Wang, F.; Dong, S.; Li, M. Composition and Diversity of Soil Microbial Community Associated With Land Use Types in the Agro–Pastoral Area in the Upper Yellow River Basin. Front. Plant Sci. 2022, 13, 819661. [Google Scholar] [CrossRef]
- Vincze, É.-B.; Becze, A.; Laslo, É.; Mara, G. Beneficial Soil Microbiomes and Their Potential Role in Plant Growth and Soil Fertility. Agriculture 2024, 14, 152. [Google Scholar] [CrossRef]
- Chauhan, P.; Sharma, N.; Tapwal, A.; Kumar, A.; Verma, G.S.; Meena, M.; Seth, C.S.; Swapnil, P. Soil Microbiome: Diversity, Benefits and Interactions with Plants. Sustainability 2023, 15, 14643. [Google Scholar] [CrossRef]
- Pascale, A.; Proietti, S.; Pantelides, I.S.; Stringlis, I.A. Modulation of the Root Microbiome by Plant Molecules: The Basis for Targeted Disease Suppression and Plant Growth Promotion. Front. Plant Sci. 2020, 10, 1741. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root Exudation of Primary Metabolites: Mechanisms and Their Roles in Plant Responses to Environmental Stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Vimal, S.R.; Singh, J.S.; Arora, N.K.; Singh, S. Soil-Plant-Microbe Interactions in Stressed Agriculture Management: A Review. Pedosphere 2017, 27, 177–192. [Google Scholar] [CrossRef]
- Verbon, E.H.; Liberman, L.M. Beneficial Microbes Affect Endogenous Mechanisms Controlling Root Development. Trends Plant Sci. 2016, 21, 218–229. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The Rhizosphere Microbiome: Significance of Plant Beneficial, Plant Pathogenic, and Human Pathogenic Microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The Unseen Majority: Soil Microbes as Drivers of Plant Diversity and Productivity in Terrestrial Ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Inui Kishi, R.N.; Galdiano Júnior, R.F.; Val-Moraes, S.P.; Kishi, L.T. Soil Microbiome and Their Effects on Nutrient Management for Plants. In Probiotics in Agroecosystem; Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer: Singapore, 2017; pp. 117–143. ISBN 978-981-10-4059-7. [Google Scholar]
- Dai, Z.; Ahmed, W.; Yang, J.; Yao, X.; Zhang, J.; Wei, L.; Ji, G. Seed Coat Treatment by Plant-Growth-Promoting Rhizobacteria Lysobacter Antibioticus 13–6 Enhances Maize Yield and Changes Rhizosphere Bacterial Communities. Biol. Fertil. Soils 2023, 59, 317–331. [Google Scholar] [CrossRef]
- Ahmed, W.; Dai, Z.; Zhang, J.; Li, S.; Ahmed, A.; Munir, S.; Liu, Q.; Tan, Y.; Ji, G.; Zhao, Z. Plant-Microbe Interaction: Mining the Impact of Native Bacillus Amyloliquefaciens WS-10 on Tobacco Bacterial Wilt Disease and Rhizosphere Microbial Communities. Microbiol. Spectr. 2022, 10, e01471-22. [Google Scholar] [CrossRef]
- Omar, A.F.; Abdelmageed, A.H.A.; Al-Turki, A.; Abdelhameid, N.M.; Sayyed, R.Z.; Rehan, M. Exploring the Plant Growth-Promotion of Four Streptomyces Strains from Rhizosphere Soil to Enhance Cucumber Growth and Yield. Plants 2022, 11, 3316. [Google Scholar] [CrossRef]
- Li, J. Editorial: Relationships between Plant Disease and Microbiomes. Front. Plant Sci. 2023, 14, 1285682. [Google Scholar] [CrossRef]
- De Zutter, N.; Ameye, M.; Bekaert, B.; Verwaeren, J.; De Gelder, L.; Audenaert, K. Uncovering New Insights and Misconceptions on the Effectiveness of Phosphate Solubilizing Rhizobacteria in Plants: A Meta-Analysis. Front. Plant Sci. 2022, 13, 858804. [Google Scholar] [CrossRef]
- Rehan, M.; Al-Turki, A.; Abdelmageed, A.H.A.; Abdelhameid, N.M.; Omar, A.F. Performance of Plant-Growth-Promoting Rhizobacteria (PGPR) Isolated from Sandy Soil on Growth of Tomato (Solanum lycopersicum L.). Plants 2023, 12, 1588. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.A. Flora of the Kingdom of Saudi Arabia Illustrated; Ministry of Agriculture & Water, National Herbarium: Riyadh, Saudi Arabia, 1999; ISBN 9789960180106. [Google Scholar]
- Page, A.L. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Wiley: Hoboken, NJ, USA, 1982; ISBN 9780891180722. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall, Inc.: Englewood Cliffs, NJ, USA, 1958. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive Functional Profiling of Microbial Communities Using 16S RRNA Marker Gene Sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Khiddir, S.M. A Statistical Approach in the Use of Parametric Systems Applied to the FAO Framework for Land Evaluation. Ph.D. Thesis, State University Ghent, Ghent, Belgium, 1986. 141p. [Google Scholar]
- Mashaly, I.; El-Amier, Y. Phytosociological and Ecological Studies of Five Species of Genus Zygophyllum in Egypt. Mansoura J. Biol. 2015, 40, 79–96. [Google Scholar]
- Větrovský, T.; Kohout, P.; Kopecký, M.; Machac, A.; Man, M.; Bahnmann, B.D.; Brabcová, V.; Choi, J.; Meszárošová, L.; Human, Z.R. A Meta-Analysis of Global Fungal Distribution Reveals Climate-Driven Patterns. Nat. Commun. 2019, 10, 5142. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Chen, W.; Wang, J.; Du, N.; Li, Q.; Wei, G. Soil Microbiomes with Distinct Assemblies through Vertical Soil Profiles Drive the Cycling of Multiple Nutrients in Reforested Ecosystems. Microbiome 2018, 6, 146. [Google Scholar] [CrossRef]
- Dai, Z.; Guo, X.; Lin, J.; Wang, X.; He, D.; Zeng, R.; Meng, J.; Luo, J.; Delgado-Baquerizo, M.; Moreno-Jiménez, E.; et al. Metallic Micronutrients Are Associated with the Structure and Function of the Soil Microbiome. Nat. Commun. 2023, 14, 8456. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Correction to: Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 119. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kumar, V.; Dhaliwal, H.S.; Prasad, R.; Saxena, A.K. Microbiome in Crops: Diversity, Distribution, and Potential Role in Crop Improvement. In Crop Improvement Through Microbial Biotechnology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 305–332. [Google Scholar]
- Islam, W.; Noman, A.; Naveed, H.; Huang, Z.; Chen, H.Y.H. Role of Environmental Factors in Shaping the Soil Microbiome. Environ. Sci. Pollut. Res. 2020, 27, 41225–41247. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Zarandi, M.; Etesami, H.; Glick, B.R. Fostering Plant Resilience to Drought with Actinobacteria: Unveiling Perennial Allies in Drought Stress Tolerance. Plant Stress 2023, 10, 100242. [Google Scholar] [CrossRef]
- Smercina, D.N.; Evans, S.E.; Friesen, M.L.; Tiemann, L.K. To Fix or Not to Fix: Controls on Free-Living Nitrogen Fixation in the Rhizosphere. Appl. Environ. Microbiol. 2019, 85, e02546-18. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Wang, Z.; Luo, W.; Zhou, J.; Xin, X.; Guo, R.; Zhu, Q.; Wang, L.; Song, S. Examining the Diversity of Rhizosphere Soil Bacterial Communities and Screening of Growth-Promoting Bacteria from the Rhizosphere Soil of Haloxylon Ammodendron in Xinjiang. Microbiol. Res. 2024, 15, 1346–1358. [Google Scholar] [CrossRef]
- Bala, S.; Jianqing, D.; Rémy, B.; Simone, C.; Nico, E.; Kai, X.; Yanfen, W.; Helge, B.; Tesfaye, W. Functional Potential of Soil Microbial Communities and Their Subcommunities Varies with Tree Mycorrhizal Type and Tree Diversity. Microbiol. Spectr. 2023, 11, e04578-22. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and Function of the Global Topsoil Microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Fierer, N.; Wood, S.A.; Bueno de Mesquita, C.P. How Microbes Can, and Cannot, Be Used to Assess Soil Health. Soil. Biol. Biochem. 2021, 153, 108111. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The Microbial Nitrogen-Cycling Network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Rhizobacteria AK1 Remediates the Toxic Effects of Salinity Stress via Regulation of Endogenous Phytohormones and Gene Expression in Soybean. Biochem. J. 2019, 476, 2393–2409. [Google Scholar] [CrossRef]
- Kong, Z.; Glick, B.R. The Role of Plant Growth-Promoting Bacteria in Metal Phytoremediation. Adv. Microb. Physiol. 2017, 71, 97–132. [Google Scholar] [PubMed]


| Plant | N | Soil Sample Code | Location (Longitude and Latitude) |
|---|---|---|---|
| Zygophyllum coccineum (Group1) | 1 | Z1 | 26°25′58.2″ N 43°52′52.9″ E |
| 2 | Z2 | 26°07′45.6″ N 43°52′18.5″ E | |
| 3 | Z3 | 26°15′20.6″ N 43°46′58.1″ E | |
| 4 | Z4 | 26°22′48.4″ N 43°48′38.9″ E | |
| 5 | Z5 | 26°24′23.4″ N 43°51′10.6″ E | |
| Haloxylon salicornicum (Group 2) | 6 | H1 | 26°09′53.5″ N 43°50′20.8″ E |
| 7 | H2 | 26°07′45.1″ N 43°52′18.6″ E | |
| 8 | H3 | 26°11′21.2″ N 43°49′16.3″ E | |
| 9 | H4 | 26°17′14.4″ N 43°45′56.2″ E | |
| 10 | H5 | 26°22′55.1″ N 43°48′42.7″ E |
| Plant | Sample ID | pH | EC | Soluble Cations (meq/L) | Soluble Anions (meq/L) | SAR | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| dS/m | Ca | Mg | Na | K | HCO3 | Cl | SO4 | ||||
| Zygophyllum coccineum | Z1 | 8.19 | 3.7 | 10 | 5 | 18.2 | 0.5 | 2.5 | 3.1 | 28.1 | 6.6 |
| Z2 | 8.39 | 1.16 | 5 | 1.3 | 4.7 | 0.5 | 2 | 1.1 | 8.4 | 2.6 | |
| Z3 | 8.16 | 2.7 | 11.4 | 4 | 12.6 | 0.6 | 2.5 | 2.3 | 23.8 | 4.5 | |
| Z4 | 8.2 | 2.5 | 10.7 | 3 | 16 | 0.8 | 3.6 | 2.6 | 24.3 | 6.1 | |
| Z5 | 8.6 | 1.06 | 4.2 | 2.8 | 4 | 0.8 | 3.5 | 1.1 | 7.2 | 2.1 | |
| Haloxylon salicornicum | H1 | 8.26 | 2.89 | 11.2 | 4.8 | 14.7 | 0.5 | 2.6 | 2.7 | 25.9 | 5.2 |
| H2 | 8.53 | 1.76 | 4.7 | 5 | 8.4 | 1.4 | 3.8 | 1.5 | 14.2 | 3.8 | |
| H3 | 8.19 | 2.2 | 9.6 | 4.6 | 13.6 | 0.4 | 1.5 | 2 | 24.7 | 5.1 | |
| H4 | 8.27 | 2.96 | 9 | 2.8 | 16.8 | 1.3 | 2.3 | 2.5 | 25.1 | 6.9 | |
| H5 | 8.24 | 2.45 | 11.4 | 2.5 | 12 | 0.1 | 2.3 | 2.45 | 21.2 | 4.6 | |
| Zygophyllum | Avr ± SD | 8.21 ± 0.04 b | 2.79 ± 0.57 a | 10.72 ± 0.86 a | 4.18 ± 1.01 a | 14.22 ± 2.45 a | 0.42 ± 0.19 b | 2.28 ± 0.45 a | 2.51 ± 0.42 a | 24.74 ± 2.55 a | 5.21 ± 0.86 a |
| Haloxylon | 8.40 ± 0.17 a | 1.89 ± 0.83 a | 6.72 ± 2.94 b | 2.98 ± 1.32 a | 9.98 ± 6.10 a | 0.96 ± 0.38 a | 3.04 ± 0.83 a | 1.76 ± 0.74 a | 15.84 ± 8.52 a | 4.33 ± 2.11 a | |
| Plant (Group) | Location | Soil Available Nutrients (ppm) | Plant Macro Nutrients (%) | Plant Micro Nutrients (ppm) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total-N | P | K | Fe | Zn | Mn | Cu | N | P | K | Ca | Mg | Fe | Zn | Mn | Cu | ||
| Zygophyllum (Group1) | 1 | 311.22 | 4.0 | 45 | 0.96 | 1.44 | 4.53 | 0.23 | 0.70 | 0.06 | 1.43 | 2.78 | 1.08 | 162 | 62.5 | 11.9 | 67.5 |
| 2 | 154.28 | 3.2 | 57 | 0.96 | 0.59 | 2.64 | 0.28 | 0.71 | 0.05 | 1.05 | 2.38 | 1.15 | 237 | 100 | 4.75 | 72.5 | |
| 3 | 406.98 | 2.4 | 52 | 0.59 | 1.08 | 2.1 | 0.44 | 3.67 | 0.33 | 1.13 | 2.85 | 1.2 | 390 | 52.5 | 11.5 | 111 | |
| 4 | 377.72 | 2.7 | 35 | 0.17 | 0.77 | 2.33 | 0.41 | 0.48 | 0.45 | 2.13 | 3.2 | 1.24 | 725 | 55 | 155 | 141 | |
| 5 | 619.78 | 3.0 | 40 | 0.96 | 1.23 | 3.18 | 0.49 | 0.78 | 0.11 | 1.75 | 3.23 | 1.19 | 747 | 68.2 | 28 | 122 | |
| Haloxylon (Group2) | 6 | 896.42 | 2.1 | 53 | 0.99 | 2.85 | 4.98 | 2.40 | 0.49 | 0.03 | 1.08 | 1.63 | 0.86 | 420 | 48.7 | 17.2 | 77.5 |
| 7 | 103.74 | 1.1 | 82 | 0.87 | 1.08 | 4.5 | 0.34 | 7.37 | 0.17 | 1.93 | 1.97 | 1.05 | 192 | 44.2 | 64.2 | 47.5 | |
| 8 | 962.92 | 4.7 | 80 | 0.57 | 1.09 | 6.78 | 0.61 | 2.23 | 0.06 | 1.65 | 1.73 | 1.03 | 755 | 47.5 | 10.7 | 85.5 | |
| 9 | 736.82 | 4.2 | 140 | 0.45 | 1.20 | 3.84 | 0.47 | 0.63 | 0.45 | 2.75 | 1.99 | 1.01 | 555 | 107 | 27.5 | 160 | |
| 10 | 353.78 | 2.9 | 123 | 0.99 | 1.05 | 4.29 | 0.55 | 0.84 | 0.11 | 1.3 | 2.29 | 1.20 | 595 | 67.5 | 81.7 | 155 | |
| Zygophyllum Haloxylon | Average ± SD | 374.00 ± 168.62 a | 3.06 ± 0.60 a | 45.80 ± 8.87 b | 0.73 ± 0.35 a | 1.02 ± 0.35 a | 2.96 ± 0.97 b | 0.37 ± 0.11 a | 1.27 ± 1.35 a | 0.20 ± 0.18 a | 1.50 ± 0.45 a | 2.89 ± 0.35 a | 1.17 ± 0.06 a | 452.20 ± 271.90 a | 67.64 ± 19.12 a | 42.23 ± 63.62 a | 102.80 ± 31.86 a |
| 610.74 ± 368.99 a | 3.03 ± 1.49 a | 95.60 ± 35.23 a | 0.77 ± 0.25 a | 1.45 ± 0.78 a | 4.88 ± 1.14 a | 0.87 ± 0.86 a | 2.31 ± 2.91 a | 0.16 ± 0.17 a | 1.74 ± 0.65 a | 1.92 ± 0.26 b | 1.03 ± 0.12 b | 503.40 ± 211.12 a | 62.98 ± 26.24 a | 40.26 ± 31.06 a | 105.10 ± 49.92 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, A.; Rehan, M. Microbiome Analysis of Rhizosphere Soil of Wild Succulent Shrubs Zygophyllum coccineum and Haloxylon salicornicum. Agronomy 2025, 15, 717. https://doi.org/10.3390/agronomy15030717
Alharbi A, Rehan M. Microbiome Analysis of Rhizosphere Soil of Wild Succulent Shrubs Zygophyllum coccineum and Haloxylon salicornicum. Agronomy. 2025; 15(3):717. https://doi.org/10.3390/agronomy15030717
Chicago/Turabian StyleAlharbi, Abdulaziz, and Medhat Rehan. 2025. "Microbiome Analysis of Rhizosphere Soil of Wild Succulent Shrubs Zygophyllum coccineum and Haloxylon salicornicum" Agronomy 15, no. 3: 717. https://doi.org/10.3390/agronomy15030717
APA StyleAlharbi, A., & Rehan, M. (2025). Microbiome Analysis of Rhizosphere Soil of Wild Succulent Shrubs Zygophyllum coccineum and Haloxylon salicornicum. Agronomy, 15(3), 717. https://doi.org/10.3390/agronomy15030717







