Effects of Long-Term Input of Controlled-Release Urea on Maize Growth Monitored by UAV-RGB Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Image Preprocessing
2.3.1. UAV Image Data Information Extraction and Index Construction
2.3.2. OTSU Method
2.4. Data Analysis
2.4.1. ReliefF Feature Weight Selection
2.4.2. Variance Expansion Factor
2.4.3. Linear Regression Modeling
2.5. Model Evaluation
3. Results
3.1. OTSU Threshold Segmentation
3.2. Vegetation Indices Constructed Under Different Fertilization Conditions and Growth Periods
3.3. Correlation Analysis of Yield and Indices in Different Growth Periods
3.3.1. Weight Selection of ReliefF Features
3.3.2. Construct Collinearity Analysis Between Vegetation Indices
3.3.3. Correlation Analysis
3.4. Optimum Yield Estimation Model for Each Growth Period Using Linear Regression
4. Discussion
4.1. Optimum Vegetation Index for Growth Monitoring in Different Growth Periods
4.2. The Best Yield Estimation Model for Each Growth Period
4.3. Optimal Nutrient Management Model
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IFAD; UNICEF. The State of Food Security and Nutrition in the World 2017; FAO: Rome, Italy, 2017. [Google Scholar]
- Chen, X.; Cui, Z.; Fan, M.; Vitousek, P.; Zhao, M.; Ma, W.; Wang, Z.; Zhang, W.; Yan, X.; Yang, J. Producing more grain with lower environmental costs. Nature 2014, 514, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Batyrbek, M.; Ikram, K.; Ahmad, S.; Kamran, M.; Misbah; Khan, R.S.; Hou, F.; Han, Q. Nitrogen management improves lodging resistance and production in maize (Zea mays L.) at a high plant density. J. Integr. Agric. 2023, 22, 417–433. [Google Scholar] [CrossRef]
- Elisante, E.; Muzuka, A. Occurrence of nitrate in Tanzanian groundwater aquifers: A review. Appl. Water Sci. 2015, 7, 71–87. [Google Scholar] [CrossRef]
- Du, L.; Zhao, T.; Zhang, C.; An, Z.; Wu, Q.; Liu, B.; Li, P.; Ma, M. Investigations on nitrate pollution of soil, groundwater and vegetable from three typical farmlands in Beijing region, China. Agric. Sci. China 2011, 10, 423–430. [Google Scholar] [CrossRef]
- Ke, J.; He, R.; Hou, P.; Ding, C.; Ding, Y.; Wang, S.; Liu, Z.; Tang, S.; Ding, C.; Chen, L.; et al. Combined controlled-released nitrogen fertilizers and deep placement effects of N leaching, rice yield and N recovery in machine-transplanted rice. Agric. Ecosyst. Environ. 2018, 265, 402–412. [Google Scholar] [CrossRef]
- Xiao, Y.; Peng, F.; Zhang, Y.; Wang, J.; Zhuge, Y.; Zhang, S.; Gao, H. Effect of bag-controlled release fertilizer on nitrogen loss, greenhouse gas emissions, and nitrogen applied amount in peach production. J. Clean. Prod. 2019, 234, 258–274. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, W.; Wang, X.; Zhang, L.; Zhang, W.; Liu, D.; Chen, X. Agronomic, environmental, and ecosystem economic benefits of controlled-release nitrogen fertilizers for maize production in Southwest China. J. Clean. Prod. 2021, 312, 127611. [Google Scholar] [CrossRef]
- Ji, P.; Peng, Y.; Cui, Y.; Li, X.; Tao, P.; Zhang, Y. Effects of reducing and postponing controlled-release urea application on soil nitrogen regulation and maize grain yield. Int. J. Agric. Biol. Eng. 2022, 15, 116–123. [Google Scholar] [CrossRef]
- Nyiraneza, J.; N’Dayegamiye, A.; Chantigny, M.H.; Laverdière, M.R. Variations in corn yield and nitrogen uptake in relation to soil attributes and nitrogen availability indices. Soil Sci. Soc. Am. J. 2009, 73, 317–327. [Google Scholar] [CrossRef]
- Meng, Q.; Hou, P.; Wu, L.; Chen, X.; Cui, Z.; Zhang, F. Understanding production potentials and yield gaps in intensive maize production in China. Field Crop. Res. 2013, 143, 91–97. [Google Scholar] [CrossRef]
- Miphokasap, P.; Honda, K.; Vaiphasa, C.; Souris, M.; Nagai, M. Estimating canopy nitrogen concentration in sugarcane using field imaging spectroscopy. Remote Sens. 2012, 4, 1651–1670. [Google Scholar] [CrossRef]
- Qiu, Z.; Xiang, H.; Ma, F.; Du, C. Qualifications of rice growth indicators optimized at different growth stages using unmanned aerial vehicle digital imagery. Remote Sens. 2020, 12, 3228. [Google Scholar] [CrossRef]
- Wang, R.; Bowling, L.; Cherkauer, K. Estimation of the effects of climate variability on crop yield in the Midwest USA. Agric. For. Meteorol. 2016, 216, 141–156. [Google Scholar] [CrossRef]
- Battude, M.; Al Bitar, A.; Morin, D.; Cros, J.; Huc, M.; Marais Sicre, C.; Le Dantec, V.; Demarez, V. Estimating maize biomass and yield over large areas using high spatial and temporal resolution Sentinel-2 like remote sensing data. Remote Sens. Environ. 2016, 184, 668–681. [Google Scholar] [CrossRef]
- Berni, J.; Zarco-Tejada, P.; Suarez, L.; Fereres, E. Thermal and narrowband multispectral remote sensing for vegetation monitoring from an unmanned aerial vehicle. IEEE Trans. Geosci. Remote Sens. 2009, 47, 722–738. [Google Scholar] [CrossRef]
- Yu, N.; Li, L.; Schmitz, N.; Tian, L.F.; Greenberg, J.A.; Diers, B.W. Development of methods to improve soybean yield estimation and predict plant maturity with an unmanned aerial vehicle based platform. Remote Sens. Environ. 2016, 187, 91–101. [Google Scholar] [CrossRef]
- Jeong, S.; Ko, J.; Kim, M.; Kim, J. Construction of an unmanned aerial vehicle remote sensing system for crop monitoring. J. Appl. Remote Sens. 2016, 10, 026027. [Google Scholar] [CrossRef]
- Li, B.; Xu, X.; Zhang, L.; Han, J.; Bian, C.; Li, G.; Liu, J.; Jin, L. Above-ground biomass estimation and yield prediction in potato by using UAV-based RGB and hyperspectral imaging. ISPRS J. Photogramm. Remote Sens. 2020, 162, 161–172. [Google Scholar] [CrossRef]
- Do-Duy, T.; Nguyen, L.D.; Duong, T.Q.; Khosravirad, S.R.; Claussen, H. Joint optimisation of real-time deployment and resource allocation for UAV-aided disaster emergency communications. IEEE J. Sel. Areas Commun. 2021, 39, 3411–3424. [Google Scholar] [CrossRef]
- Huang, Y.; Thomson, S.J.; Hoffmann, W.C.; Lan, Y.; Fritz, B.K. Development and prospect of unmanned aerial vehicle technologies for agricultural production management. Int. J. Agric. Biol. Eng. 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Li, Z.; Huang, X.; Shen, T.; Zou, X. Simultaneous and nondestructive diagnostics of nitrogen/magnesium/potassium-deficient cucumber leaf based on chlorophyll density distribution features. Biosyst. Eng. 2021, 212, 458–467. [Google Scholar] [CrossRef]
- Bendig, J.; Bolten, A.; Bennertz, S.; Broscheit, J.; Eichfuss, S.; Bareth, G. Estimating biomass of barley using crop surface models (CSMs) derived from UAV-based RGB imaging. Remote Sens. 2014, 6, 10395–10412. [Google Scholar] [CrossRef]
- Fu, Z.; Yu, S.; Zhang, J.; Xi, H.; Gao, Y.; Lu, R.; Zheng, H.; Zhu, Y.; Cao, W.; Liu, X. Combining UAV multispectral imagery and ecological factors to estimate leaf nitrogen and grain protein content of wheat. Eur. J. Agron. 2022, 132, 126405. [Google Scholar] [CrossRef]
- Astaoui, G.; Dadaiss, J.E.; Sebari, I.; Benmansour, S.; Mohamed, E. Mapping wheat dry matter and nitrogen content dynamics and estimation of wheat yield using UAV multispectral imagery machine learning and a variety-based approach: Case study of morocco. AgriEngineering 2021, 3, 29–49. [Google Scholar] [CrossRef]
- Zheng, H.; Zhou, X.; Cheng, T.; Yao, X.; Tian, Y.; Cao, W.; Zhu, Y. Evaluation of a UAV-based hyperspectral frame camera for monitoring the leaf nitrogen concentration in rice. In Proceedings of the 2016 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Beijing, China, 10–15 July 2016; pp. 7350–7353. [Google Scholar]
- Ge, H.; Xiang, H.; Ma, F.; Li, Z.; Qiu, Z.; Tan, Z.; Du, C. Estimating plant nitrogen concentration of rice through fusing vegetation indices and color moments derived from UAV-RGB images. Remote Sens. 2021, 13, 1620. [Google Scholar] [CrossRef]
- Everaerts, J. The use of unmanned aerial vehicles (UAVs) for remote sensing and mapping. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2008, 37, 1187–1192. [Google Scholar]
- Louhaichi, M.; Borman, M.M.; Johnson, D.E. Spatially located platform and aerial photography for documentation of grazing impacts on wheat. Geocarto. Int. 2001, 16, 65–70. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Arkebauer, T.J.; Rundquist, D.C.; Keydan, G.; Leavitt, B. Remote estimation of leaf area index and green leaf biomass in maize canopies. Geophys. Res. Lett. 2003, 30, 1248–1251. [Google Scholar] [CrossRef]
- Bendig, J.; Yu, K.; Aasen, H.; Bolten, A.; Bennertz, S.; Broscheit, J.; Gnyp, M.L.; Bareth, G. Combining UAV-based plant height from crop surface models, visible, and near infrared vegetation indices for biomass monitoring in barley. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 79–87. [Google Scholar] [CrossRef]
- Nie, S.; Wang, C.; Dong, P.; Xi, X.; Luo, S.; Zhou, H. Estimating leaf area index of maize using airborne discrete-return LiDAR data. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2016, 9, 3259–3266. [Google Scholar] [CrossRef]
- Meyer, G.E.; Neto, J.C. Verification of color vegetation indices for automated crop imaging applications. Comput. Electron. Agric. 2008, 63, 282–293. [Google Scholar] [CrossRef]
- Tilly, N.; Aasen, H.; Bareth, G. Fusion of plant height and vegetation indices for the estimation of barley biomass. Remote Sens. 2015, 7, 11449–11480. [Google Scholar] [CrossRef]
- Ostu, N. A threshold selection method from gray-histogram. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Robnik-Sikonja, M.; Kononenko, I. Theoretical and empirical analysis of ReliefF and RReliefF. Mach. Learn. 2003, 53, 23–69. [Google Scholar] [CrossRef]
- Verrelst, J.; Schaepman, M.E.; Koetz, B.; Kneubühler, M. Angular sensitivity analysis of vegetation indices derived from CHRIS/PROBA data. Remote Sens. Environ. 2008, 112, 2341–2353. [Google Scholar] [CrossRef]
- Kaufman, Y.J.; Tanre, D. Atmospherically resistant vegetation index (ARVI) for EOS-MODIS. IEEE Trans. Geosci. Remote Sens. 1992, 30, 261–270. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, Z.; Ren, B.; Zhao, B.; Liu, P.; Zhang, J. Effects of humic acid added to controlled-release fertilizer on summer maize yield, nitrogen use efficiency and greenhouse gas emission. Agriculture 2022, 12, 448. [Google Scholar] [CrossRef]
- Li, G.; Cheng, G.; Lu, W.; Lu, D. Differences of yield and nitrogen use efficiency under different applications of slow release fertilizer in spring maize. J. Integr. Agric. 2021, 20, 554–564. [Google Scholar] [CrossRef]
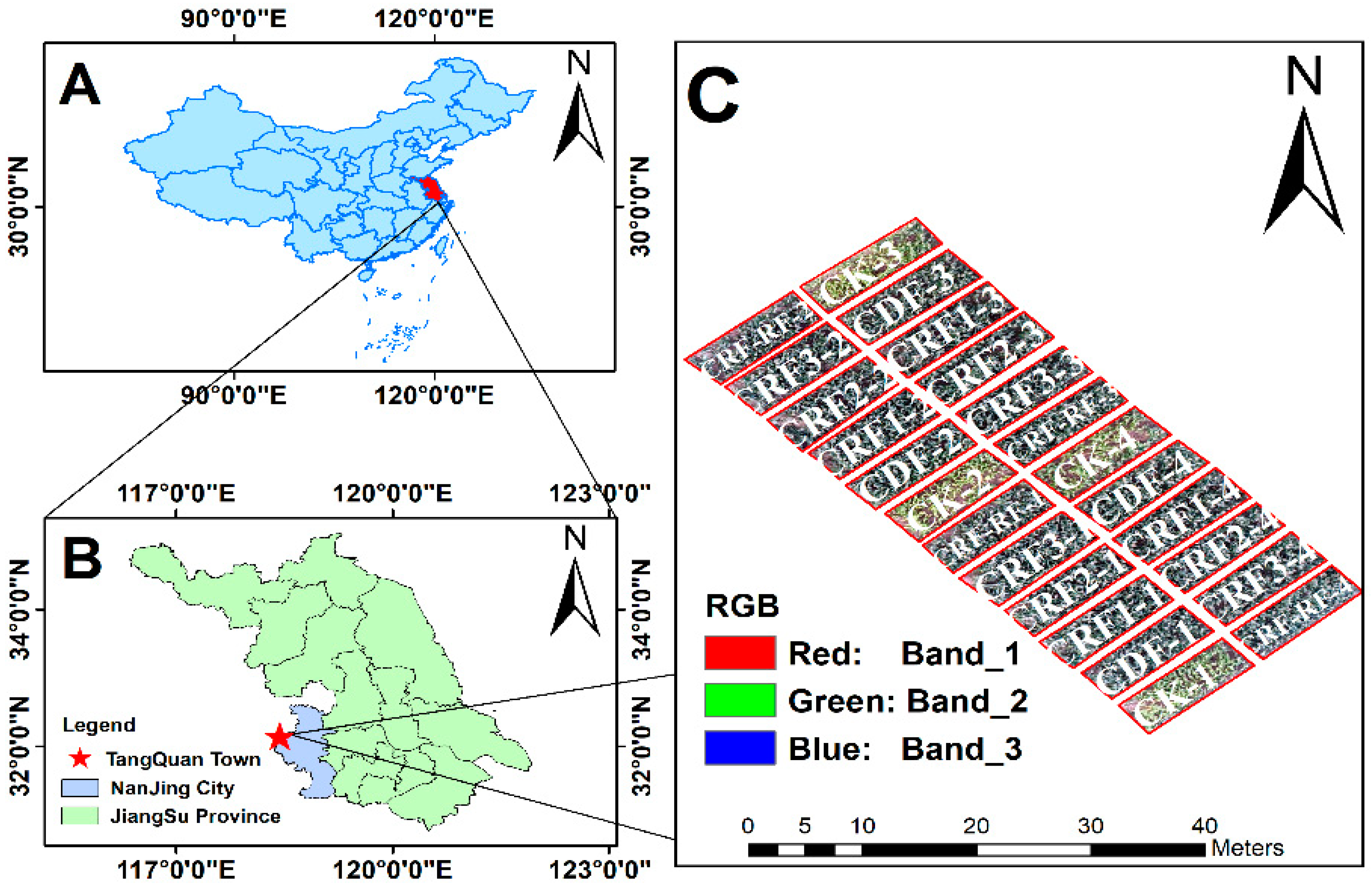



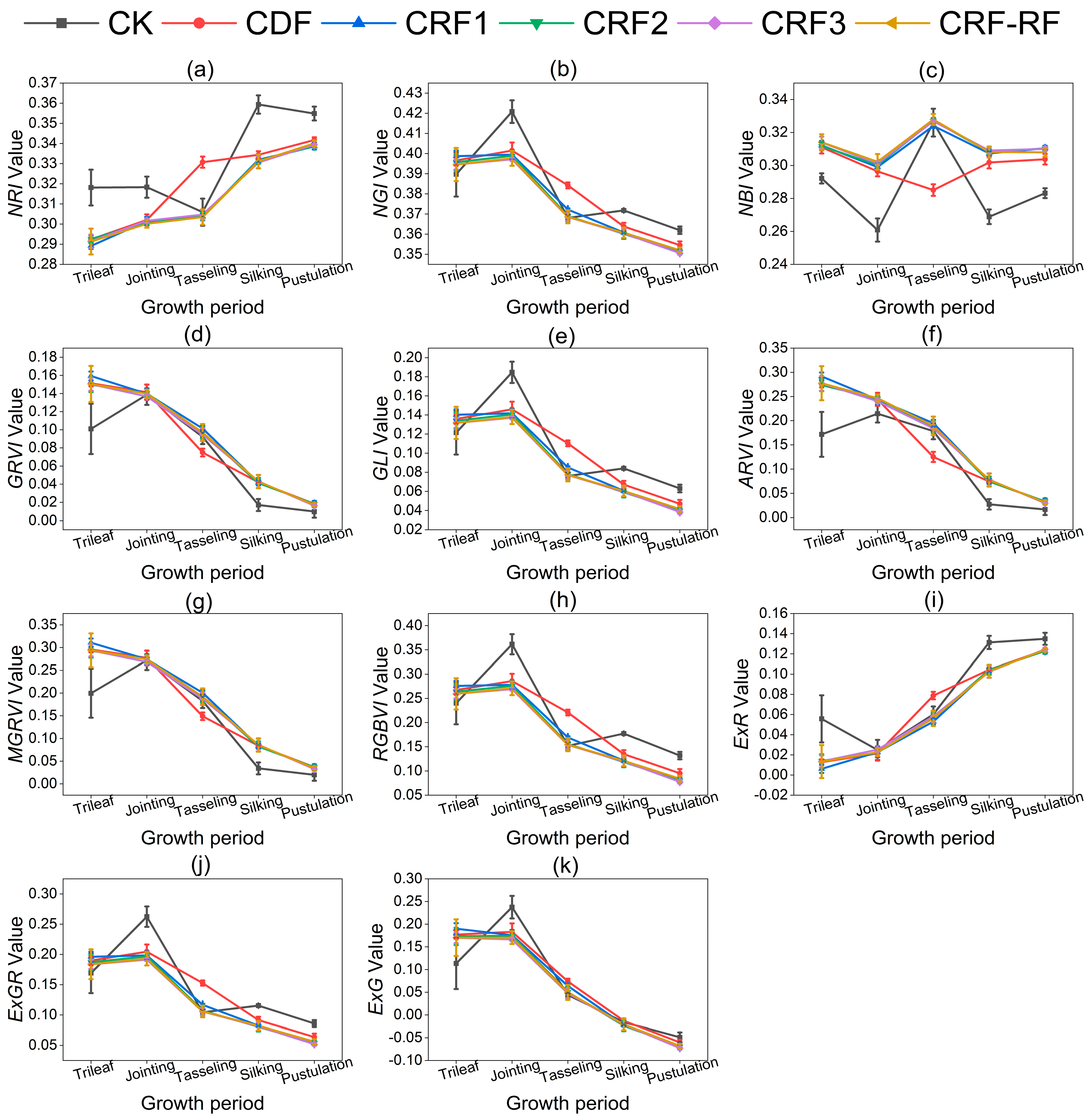
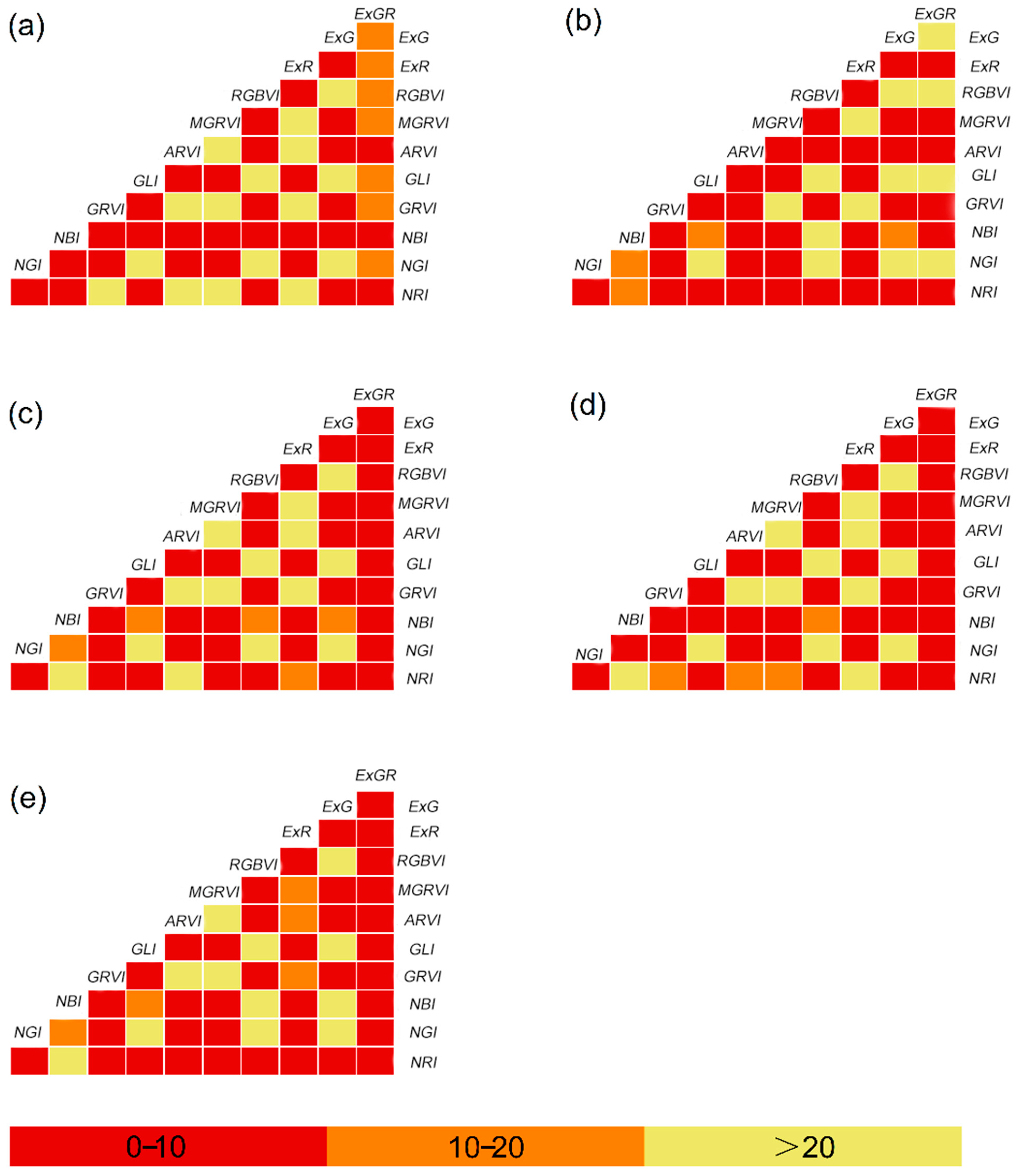
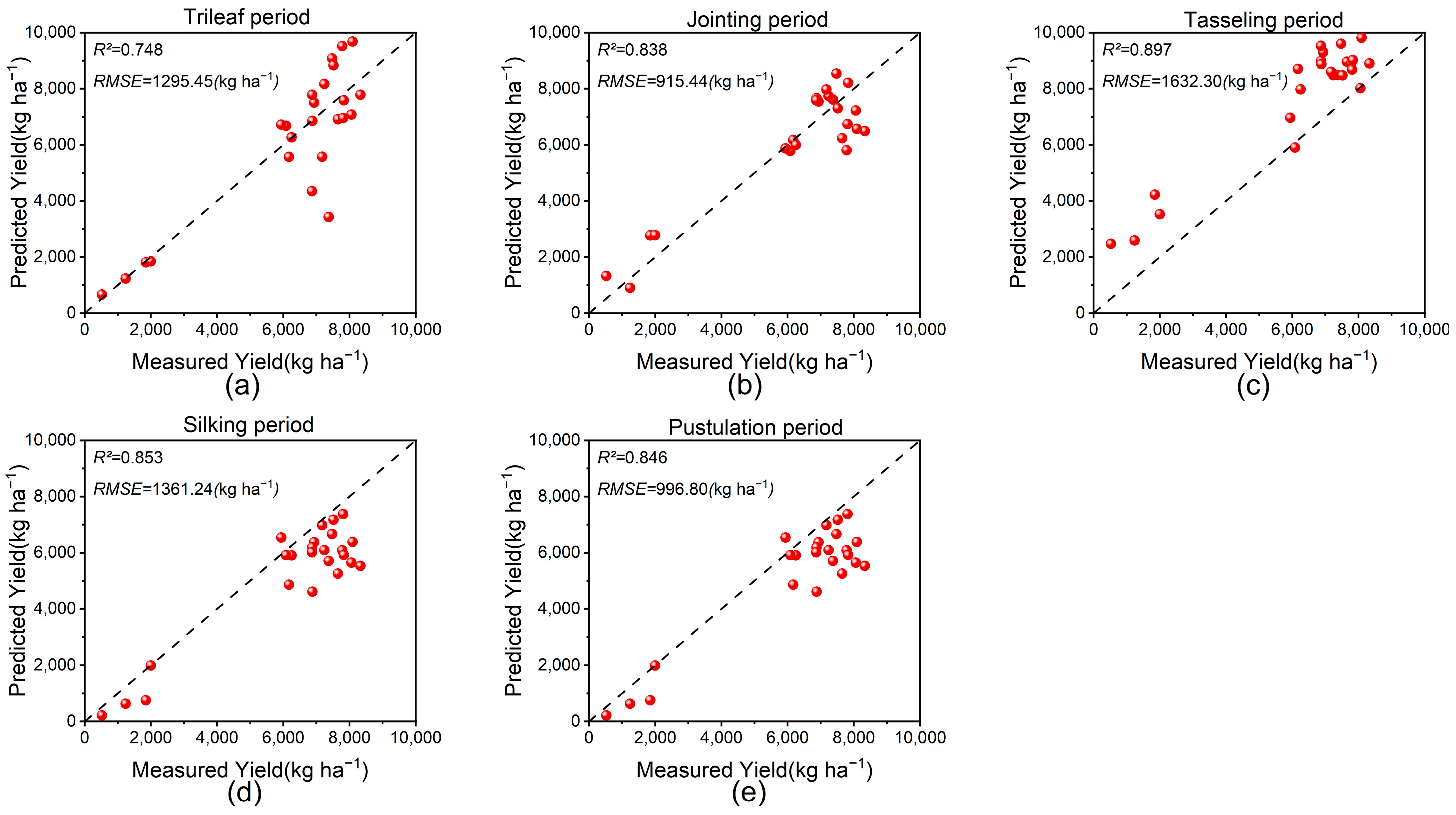
| Treatment | Fertilization Method | N (kg ha−1) | P2O5 (kg ha−1) | K2O (kg ha−1) | CRN Ratio (%) |
|---|---|---|---|---|---|
| CK | No fertilization | - | - | - | - |
| CDF | Conventional fertilizer, twice-split | 240 | 60 | 135 | 0 |
| CRF1 | Controlled-release fertilizer, one application | 240 | 60 | 135 | 30 |
| CRF2 | Controlled-release fertilizer, one application | 240 | 60 | 135 | 40 |
| CRF3 | Controlled-release fertilizer, one application | 240 | 60 | 135 | 50 |
| CRF-RF | Controlled-release fertilizer, one application, 15% N reduced | 204 | 60 | 135 | 50 |
| Date of Data Collection | Growth Period |
|---|---|
| 14 July 2019 | Trileaf period |
| 26 July 2019 | Jointing period |
| 12 August 2019 | Tasseling period |
| 27 August 2019 | Silking period |
| 8 September 2019 | Pustulation period |
| Name | Index | Formulation | References |
|---|---|---|---|
| Normalized Red light Index | NRI | / | |
| Normalized Green light Index | NGI | / | |
| Normalized Blue light Index | NBI | / | |
| Green Leaf Index | GLI | [29] | |
| Green Red Vegetation Index | GRVI | [30] | |
| Atmospherically Resistant Vegetation Index | ARVI | [31] | |
| Modified Green Red Vegetation Index | MGRVI | [32] | |
| Red–Green–Blue Vegetation Index | RGBVI | [33] | |
| Excess Red Index | ExR | [34] | |
| Excess Green Index | ExG | [34] | |
| Excess Green minus Excess Red Index | ExGR | [35] |
| Index | Trileaf Period | Jointing Period | Tasseling Period | Silking Period | Pustulation Period |
|---|---|---|---|---|---|
| NRI | 0.0003 | 0.1469 | 0.1935 | 0.2762 | 0.1832 |
| NGI | 0.1538 | 0.1936 | 0.2034 | 0.3351 | 0.2473 |
| NBI | 0.0772 | 0.0203 | 0.0891 | 0.3354 | 0.0376 |
| GRVI | 0.0004 | 0.0203 | 0.1929 | 0.2663 | 0.1834 |
| GLI | 0.0968 | 0.0363 | 0.1223 | 0.2951 | 0.0500 |
| ARVI | 0.0797 | 0.1473 | 0.0891 | 0.2723 | 0.0376 |
| MGRVI | 0.0006 | 0.1505 | 0.1871 | 0.2662 | 0.1962 |
| RGBVI | 0.0766 | 0.0220 | 0.1033 | 0.3221 | 0.0873 |
| ExR | 0.0003 | 0.1469 | 0.1935 | 0.2646 | 0.1832 |
| ExG | 0.0314 | 0.1093 | 0.0839 | 0.3351 | 0.0695 |
| ExGR | 0.2366 | 0.2366 | 0.2366 | 0.3512 | 0.2366 |
| Index | Trileaf Period | Jointing Period | Tasseling Period | Silking Period | Pustulation Period |
|---|---|---|---|---|---|
| NRI | −0.842 ** | −0.858 ** | −0.890 ** | −0.920 ** | −0.918 ** |
| NGI | 0.328 | −0.922 ** | −0.909 ** | −0.879 ** | −0.905 ** |
| NBI | 0.850 ** | 0.943 ** | 0.931 ** | 0.944 ** | 0.940 ** |
| GRVI | 0.736 ** | −0.065 | 0.690 ** | 0.798 ** | 0.584 ** |
| GLI | 0.331 | −0.922 ** | −0.907 ** | −0.878 ** | −0.904 ** |
| ARVI | 0.786 ** | 0.607 ** | 0.794 ** | 0.819 ** | 0.643 ** |
| MGRVI | 0.736 ** | −0.063 | 0.691 ** | 0.798 ** | 0.584 ** |
| RGBVI | 0.330 | −0.926 ** | −0.908 ** | −0.904 ** | −0.915 ** |
| ExR | −0.744 ** | −0.015 | −0.773 ** | −0.852 ** | −0.676 ** |
| ExG | 0.328 | −0.922 ** | −0.940 ** | −0.882 ** | −0.906 ** |
| ExGR | 0.564 ** | −0.861 ** | −0.698 ** | −0.216 | −0.715 ** |
| Maize Growth Period | Equation of Regression |
|---|---|
| Trileaf period | |
| Jointing period | |
| Tasseling period | |
| Silking period | |
| Pustulation period |
| Vegetation Index | Applicable Growth Period | Specific Expressions | Relationship with the Literature | References |
|---|---|---|---|---|
| NRI NBI | All growth periods | Real-time maize growth monitoring throughout lifecycle | This study revealed the dynamic responses of plant canopy images to CRF input. | / |
| ARVI | Trileaf period Jointing period | Superior performance was demonstrated comparied with the traditional NDVI under specific atmospheric conditions. | ARVI considered the atmospheric disturbance, and this study validated its applicability under specific climatic conditions | [39] |
| RGBVI ExG ExR ExGR | Jointing period Tasseling period Silking period Pustulation period | Vegetation coverage was reflected by improved monitoring effectiveness under CRF input | These VIs expanded the description of the long-term effects of CRF input on maize regarding promoting maize growth | [33,34,35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Lin, F.; Ma, F.; Du, C. Effects of Long-Term Input of Controlled-Release Urea on Maize Growth Monitored by UAV-RGB Imaging. Agronomy 2025, 15, 716. https://doi.org/10.3390/agronomy15030716
Chen X, Lin F, Ma F, Du C. Effects of Long-Term Input of Controlled-Release Urea on Maize Growth Monitored by UAV-RGB Imaging. Agronomy. 2025; 15(3):716. https://doi.org/10.3390/agronomy15030716
Chicago/Turabian StyleChen, Xingyu, Fenfang Lin, Fei Ma, and Changwen Du. 2025. "Effects of Long-Term Input of Controlled-Release Urea on Maize Growth Monitored by UAV-RGB Imaging" Agronomy 15, no. 3: 716. https://doi.org/10.3390/agronomy15030716
APA StyleChen, X., Lin, F., Ma, F., & Du, C. (2025). Effects of Long-Term Input of Controlled-Release Urea on Maize Growth Monitored by UAV-RGB Imaging. Agronomy, 15(3), 716. https://doi.org/10.3390/agronomy15030716






