Foliar Application of K-Silicate and L-Cysteine Enhances Production, Quality, and Antioxidant Activities of Cape Gooseberry Fruits Under Drought Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Genetic Material, and Treatments
2.2. Plant Productivity and Fruit Nutritional Traits
2.3. POX, CAT, SOD, APX, PPO, and PAL Activities
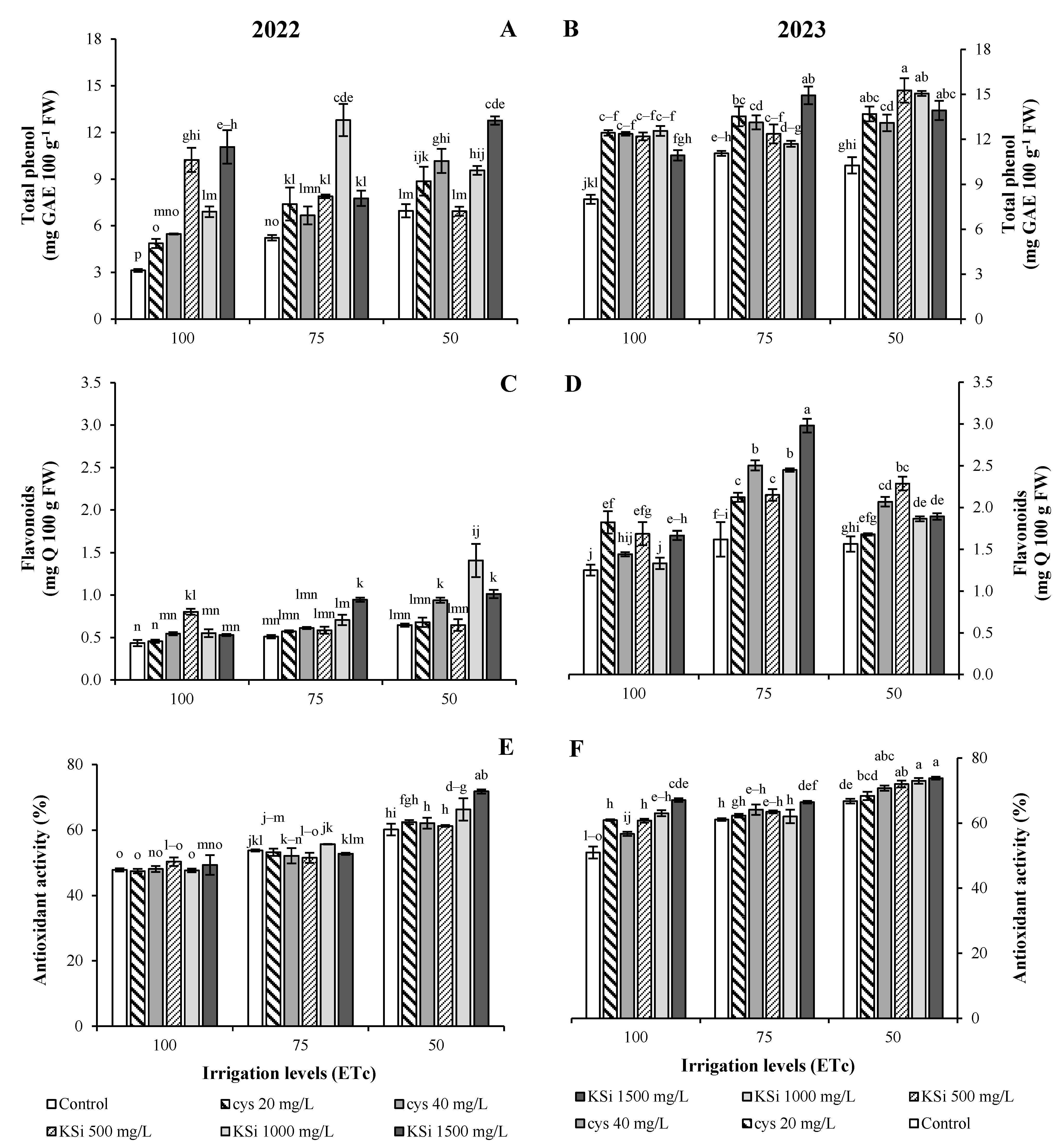
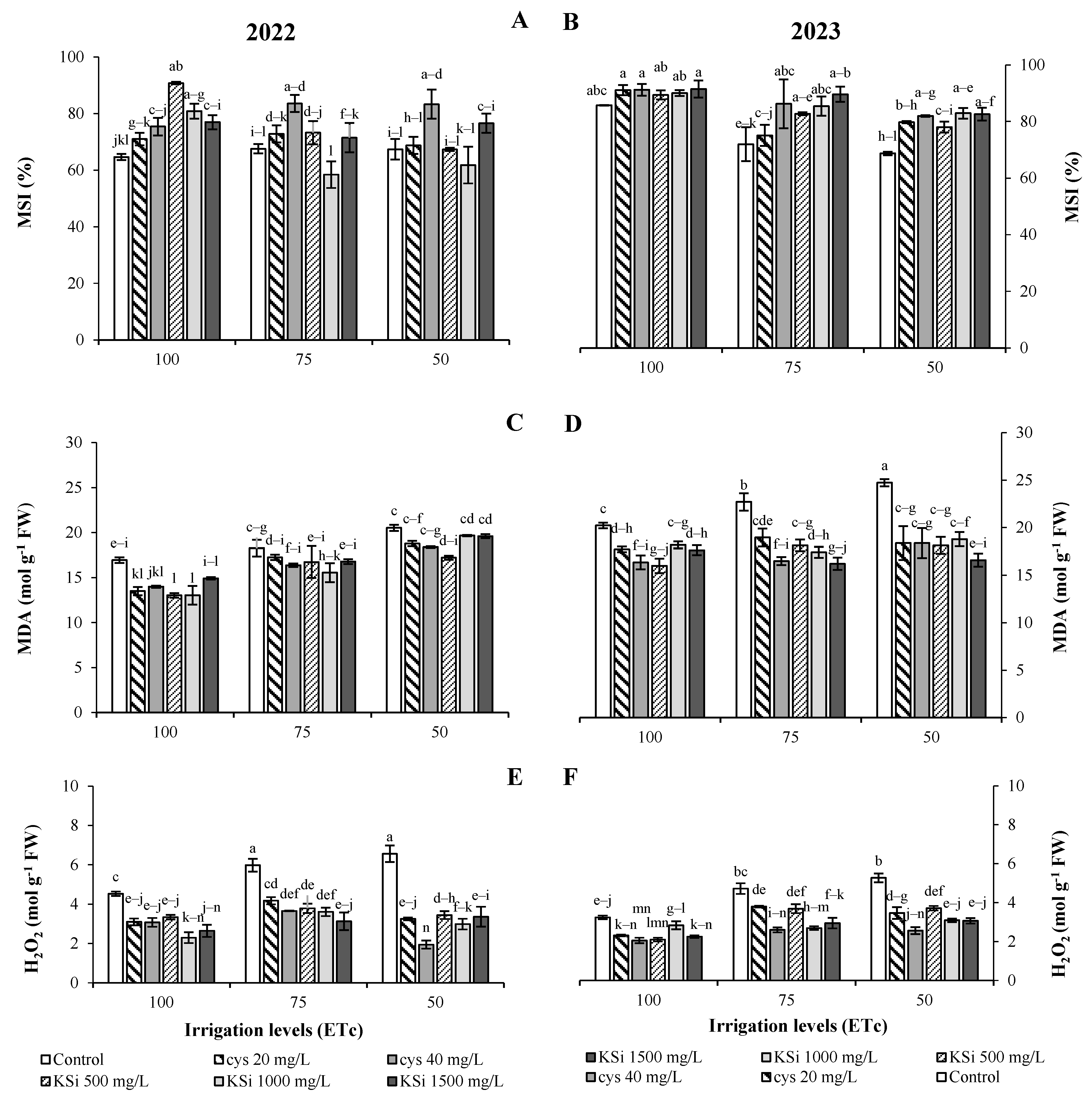
2.4. Fruit Functional Components, Antioxidant Capacity, and the Membrane Stability Index
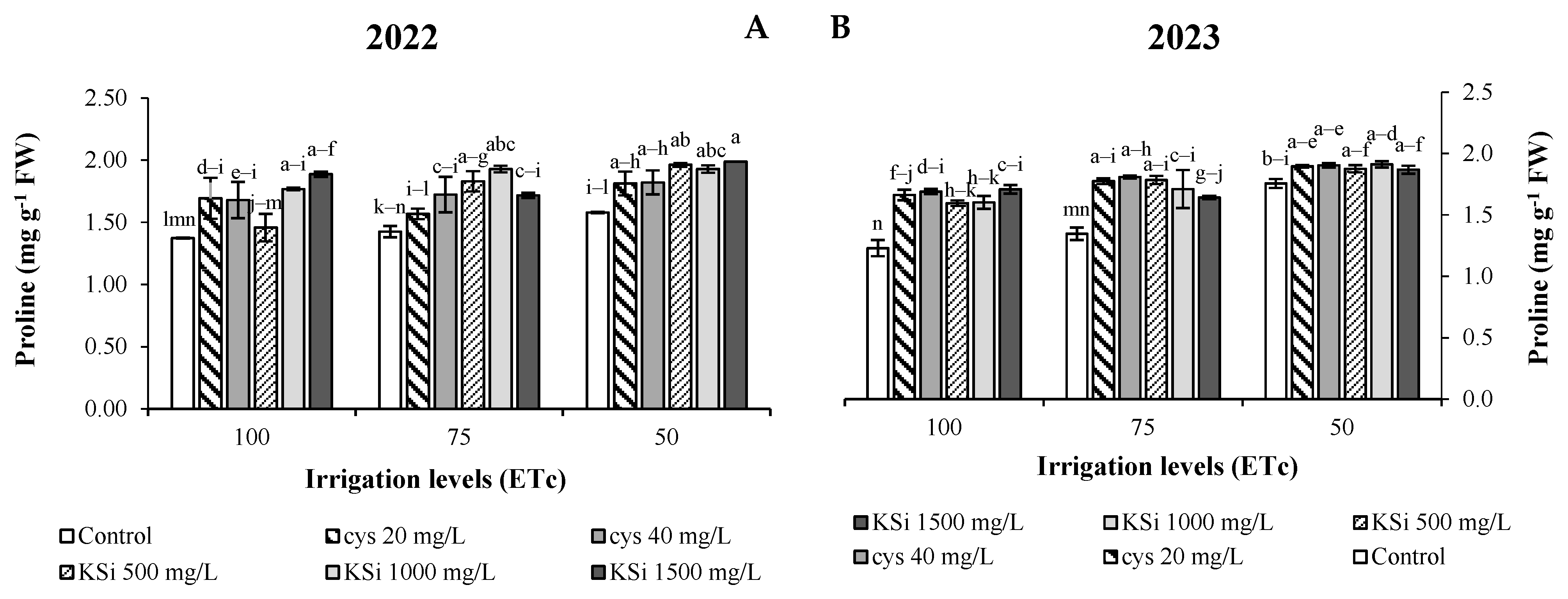
2.5. Malondialdehyde, H2O2, and Proline
2.6. Statistics
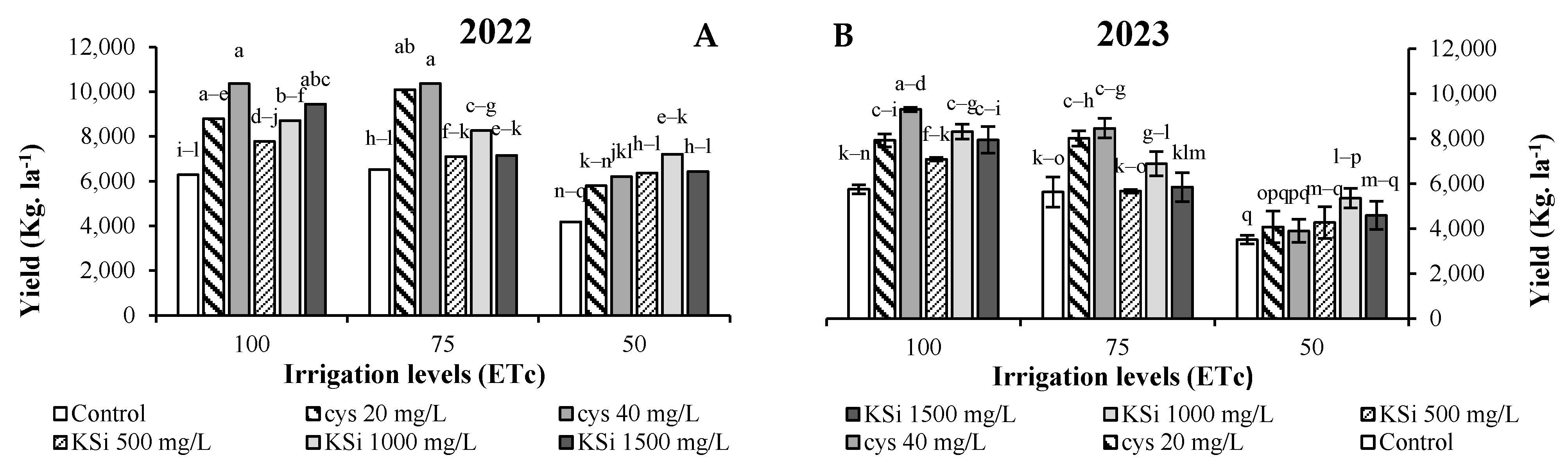
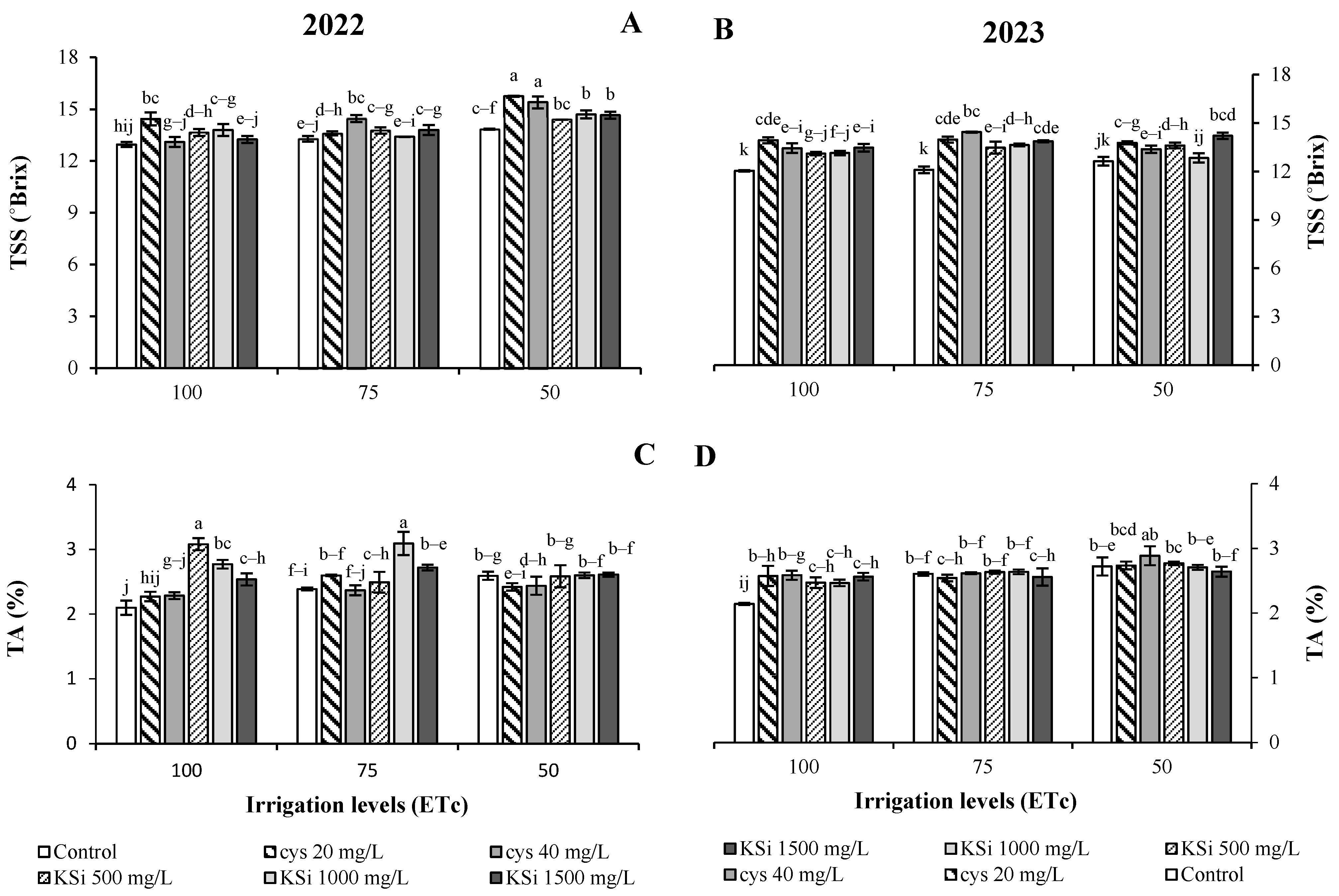
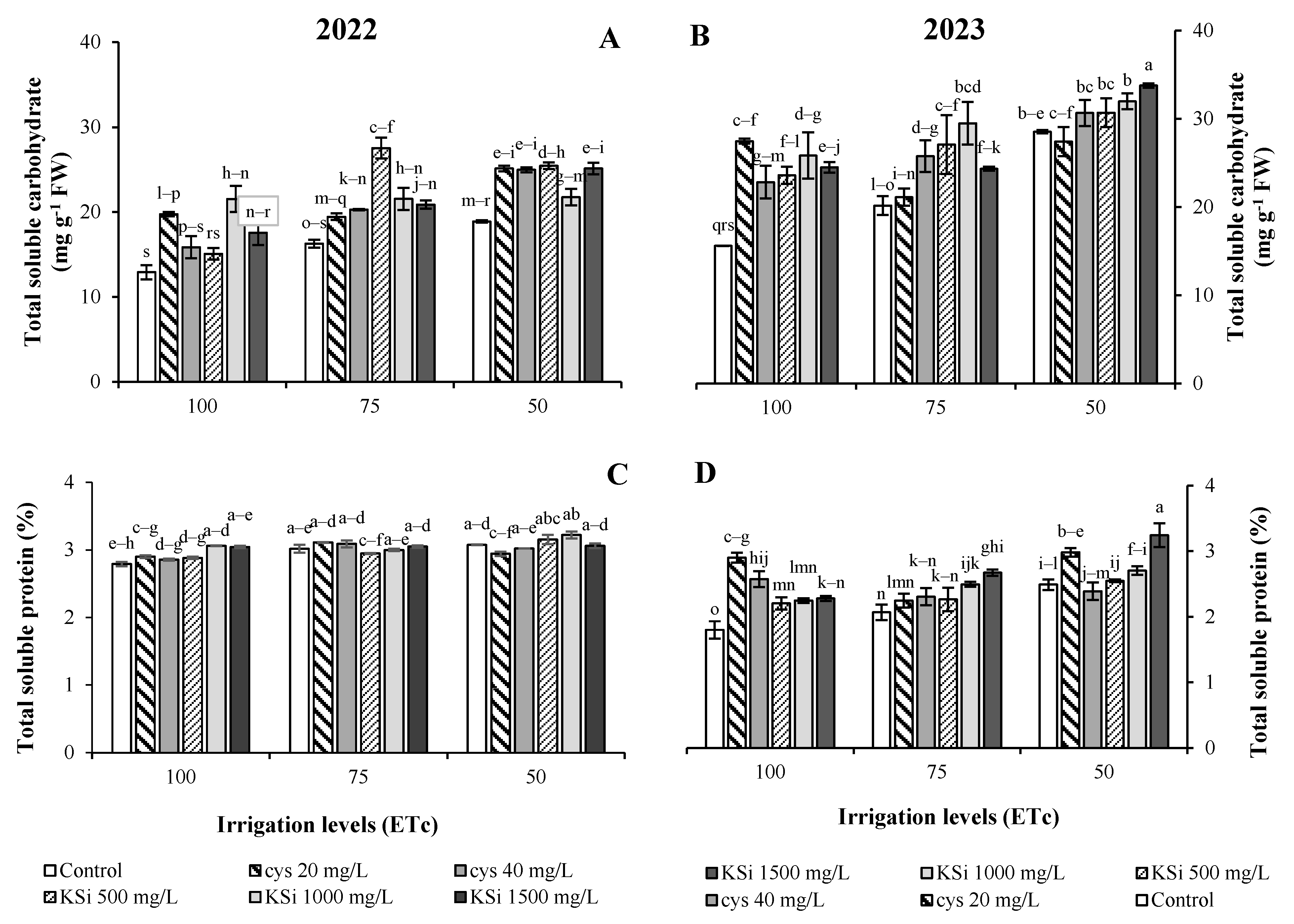
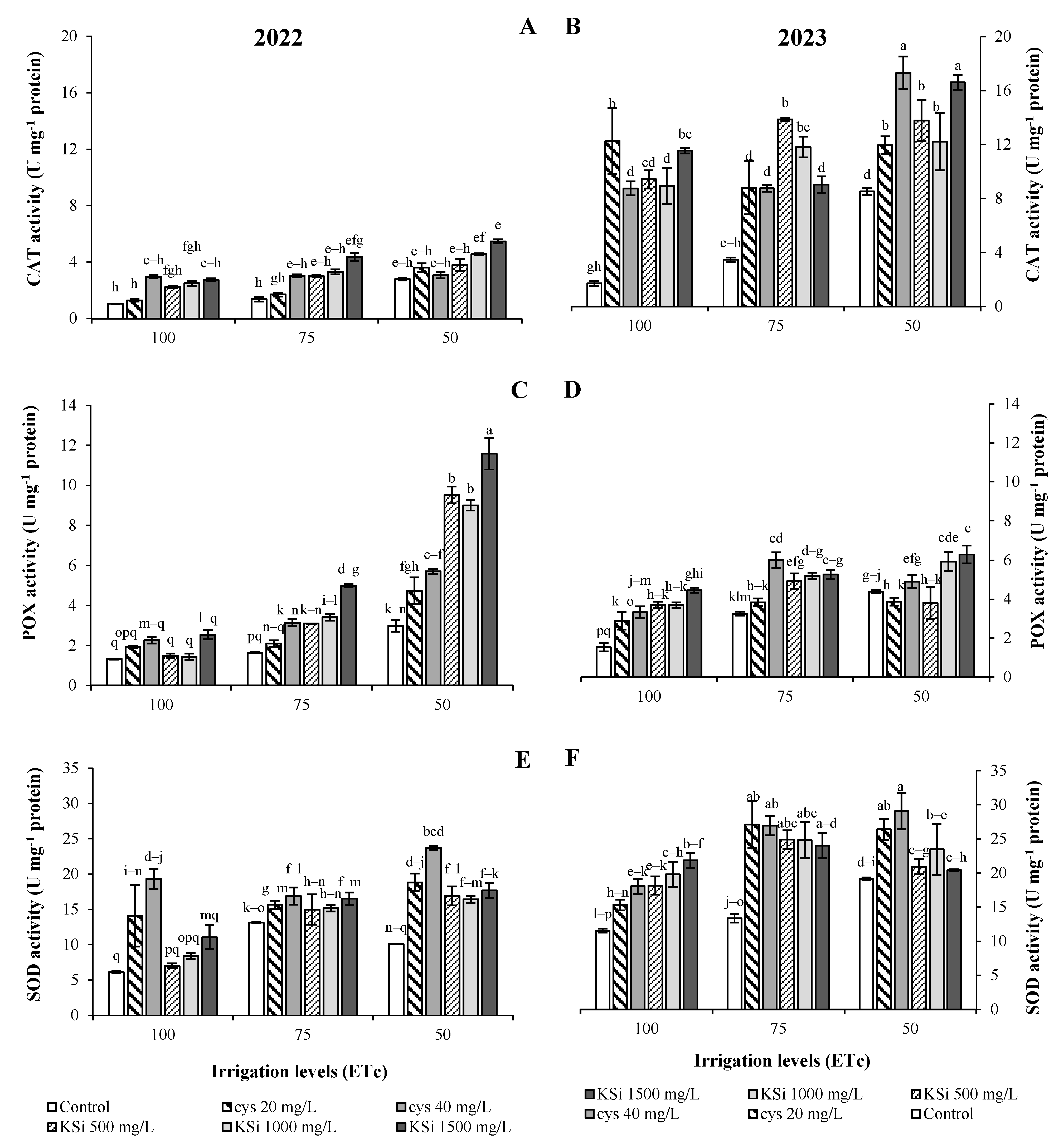
3. Results
Plant Productivity and Fruit Nutritional Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Puente, L.; Vega-Gálvez, A.; Fuentes, I.; Stucken, K.; Rodríguez, A.; Pastén, A. Effects of drying methods on the characterization of fatty acids, bioactive compounds and antioxidant capacity in a thin layer of physalis (Physalis peruviana L.) pulp. J. Food Technol. 2021, 58, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Melgarejo, L. The ecophysiology of cape gooseberry (Physalis peruviana L.)—An Andean fruit crop: A review. Rev. Colomb. Cienc. Hortícolas 2020, 14, 76–89. [Google Scholar] [CrossRef]
- Reyes, S.M.R.; Hoyos, G.R.; Júnior, D.D.C.F.; Cecílio Filho, A.B.; Fonseca, L.P.M. Physiological response of Physalis peruviana L. seedlings inoculated with Funneliformis mosseae under drought stress. Rev. Ciênc. Agrár. 2019, 42, 175–183. [Google Scholar] [CrossRef]
- Rodrigues, J.; Inzé, D.; Nelissen, H.; Saibo, N.J. Source—Sink regulation in crops under water deficit. Trends Plant Sci. 2019, 24, 652–663. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef]
- Farooq, A.; Bukhari, S.A.; Akram, N.A.; Ashraf, M.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Exogenously applied ascorbic acid-mediated changes in osmoprotection and oxidative defense system enhanced water stress tolerance in different cultivars of safflower (Carthamus tinctorious L.). Plants 2020, 9, 104. [Google Scholar] [CrossRef]
- Leite, R.D.S.; Navarro, S.H.; Nascimento, M.N.D.; Potosme, N.M.R.; Silva, A.L.D.; Santos, R.D.J. Proline and sodium nitroprusside increase the tolerance of Physalis peruviana L. plants to water deficit through chemical priming. Ciênc. Agrotec. 2022, 46, e004622. [Google Scholar] [CrossRef]
- Terra, T.G.R.; Leal, T.C.A.D.B.; Rangel, P.H.N.; Oliveira, A.B.D. Drought tolerance traits in genotypes from an upland rice core collection. Pesqui. Agropecu. Bras. 2015, 50, 788–796. [Google Scholar] [CrossRef]
- Chen, D.; Cao, B.; Wang, S.; Liu, P.; Deng, X.; Yin, L.; Zhang, S. Silicon moderated the K deficiency by improving the plant-water status in sorghum. Sci. Rep. 2016, 6, 22882. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, Z.; Wang, L.; Li, M.; Lang, D.; Zhang, X. Silicon alleviates salt and drought stress of Glycyrrhiza uralensis seedling by altering antioxidant metabolism and osmotic adjustment. J. Plant Res. 2017, 130, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.A.; Arruda, E.M.; Souza Junior, J.P.D.; de Mello Prado, R.; Santos, A.C.A.D.; Aragao, A.S.; Pedreira, N.G.; da Costa, C.F. Nutrition and production of Helianthus annuus in a function of application of leaf silicon. J. Plant Nutr. 2019, 42, 137–144. [Google Scholar] [CrossRef]
- Malhotra, C.; Kapoor, R.T. Silicon: A sustainable tool in abiotic stress tolerance in plants. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Springer: Cham, Switzerland, 2019; pp. 333–356. [Google Scholar] [CrossRef]
- Ouzounidou, G.; Giannakoula, A.; Ilias, I.; Zamanidis, P. Alleviation of drought and salinity stresses on growth, physiology, biochemistry and quality of two Cucumis sativus L. cultivars by Si application. Rev. Bras. Bot. 2016, 39, 531–539. [Google Scholar] [CrossRef]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawany, M.; Elhawat, N.; Al-Otaibi, A. Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 2019, 139, 1–10. [Google Scholar] [CrossRef]
- Cao, B.L.; Ma, Q.; Xu, K. Silicon restrains drought-induced ROS accumulation by promoting energy dissipation in leaves of tomato. Protoplasma 2020, 257, 537–547. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Zahedi, B.; Roosta, H.R. The Effect of Silicon and LED Light on Increasing the Resistance to Salinity-Alkalinity Stress in Physalis angulata L. J. Soil Plant Interact.-Isfahan Univ. Technol. 2022, 13, 45–60. [Google Scholar]
- Portu, J.; López, R.; Santamaría, P.; Garde-Cerdán, T. Elicitation with methyl jasmonate supported by precursor feeding with phenylalanine: Effect on Garnacha grape phenolic content. Food Chem. 2017, 237, 416–422. [Google Scholar] [CrossRef]
- Talukder, M.R.; Asaduzzaman, M.; Tanaka, H.; Asao, T. Light-emitting diodes and exogenous amino acids application improve growth and yield of strawberry plants cultivated in recycled hydroponics. Sci. Hortic. 2018, 239, 93–103. [Google Scholar] [CrossRef]
- Genisel, M.; Erdal, S.; Kizilkaya, M. The mitigating effect of cysteine on growth inhibition in salt-stressed barley seeds is related to its own reducing capacity rather than its effects on antioxidant system. Plant Growth Regul. 2015, 75, 187–197. [Google Scholar] [CrossRef]
- Haag, A.F.; Kerscher, B.; Dall’Angelo, S.; Sani, M.; Longhi, R.; Baloban, M.; Wilson, H.M.; Mergaert, P.; Zanda, M.; Ferguson, G.P. Role of cysteine residues and disulfide bonds in the activity of a legume root nodule-specific, cysteine-rich peptide. J. Biol. Chem. 2012, 287, 10791–10798. [Google Scholar] [CrossRef]
- Wang, J.; Wei, L.; Yan, L.; Zheng, H.; Liu, C.; Zheng, L. Effects of postharvest cysteine treatment on sensory quality and contents of bioactive compounds in goji fruit. Food Chem. 2022, 366, 130546. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, A.S.; Malik, A.U. Postharvest L-cysteine application delayed pericarp browning, suppressed lipid peroxidation and maintained antioxidative activities of litchi fruit. Postharvest Biol. Tec. 2016, 121, 135–142. [Google Scholar] [CrossRef]
- Preczenhak, A.P.; Orsi, B.; Lima, G.P.P.; Tezotto-Uliana, J.V.; Minatel, I.O.; Kluge, R.A. Cysteine enhances the content of betalains and polyphenols in fresh-cut red beet. Food Chem. 2019, 286, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Elkelish, A.; El-Mogy, M.M.; Niedbała, G.; Piekutowska, M.; Atia, M.A.; Hamada, M.M.; Shahin, M.; Mukherjee, S.; El-Yazied, A.A.; Shebl, M.; et al. Roles of exogenous α-lipoic acid and cysteine in mitigation of drought stress and restoration of grain quality in wheat. Plants 2021, 10, 2318. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration Guidelines for computing crop water requirements-FAO Irrigation and drainage paper 56. Fao Rome 1998, 300, D05109. [Google Scholar]
- Terada, M.; Watanabe, Y.; Kunitomo, M.; Hayashi, E. Differential rapid analysis of ascorbic acid and ascorbic acid 2-sulfate by dinitrophenylhydrazine method. Anal. Biochem. 1978, 84, 604–608. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, D.J.; Rao, J. Antioxidant systems of ripening avocado (Persea americana Mill.) fruit following treatment at the preclimacteric stage with aqueous 1-methylcyclopropene. Postharvest Biol. Tec. 2013, 76, 58–64. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar] [CrossRef]
- Nguyen, T.B.T.; Ketsa, S.; Van Doorn, W.G. Relationship between browning and the activities of polyphenoloxidase and phenylalanine ammonia lyase in banana peel during low temperature storage. Postharvest Biol. Tec. 2003, 30, 187–193. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Dehghan, G.; Khoshkam, Z. Tin (II)-quercetin complex: Synthesis, spectral characterisation and antioxidant activity. Food Chem. 2012, 131, 422–426. [Google Scholar] [CrossRef]
- Chen, J.Y.; He, L.H.; Jiang, Y.M.; Wang, Y.; Joyce, D.C.; Ji, Z.L.; Lu, W.J. Role of phenylalanine ammonia-lyase in heat pretreatment-induced chilling tolerance in banana fruit. Physiol. Plant. 2008, 132, 318–328. [Google Scholar] [CrossRef]
- Yi, C.; Jiang, Y.; Shi, J.; Qu, H.; Duan, X.; Yang, B.; Prasad, N.K.; Liu, T. Effect of adenosine triphosphate on changes of fatty acids in harvested litchi fruit infected by Peronophythora litchii. Postharvest Biol. Tec. 2009, 54, 159–164. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.A.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Gaafar, R.M.; Seyam, M.M.; El-Shanshory, A.R. Expression patterns of drought-related miRNAs in Chickpea (Cicer arietinum L.) under drought stress. Egypt. J. Bot. 2022, 62, 227–240. [Google Scholar] [CrossRef]
- Atilgan, A.; Rolbiecki, R.; Saltuk, B.; Jagosz, B.; Arslan, F.; Erdal, I.; Aktas, H. Deficit Irrigation Stabilizes Fruit Yield and Alters Leaf Macro and Micronutrient Concentration in Tomato Cultivation in Greenhouses: A Case Study in Turkey. J. Agron. 2022, 12, 2950. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Zhang, L. AMF inoculation and phosphorus supplementation alleviates drought induced growth and photosynthetic decline in Nicotiana tabacum by up-regulating antioxidant metabolism and osmolyte accumulation. Environ. Exp. Bot. 2020, 176, 104088. [Google Scholar] [CrossRef]
- García-Garizábal, I.; Causapé, J.; Abrahao, R. Application of the irrigation land environmental evaluation tool for flood irrigation management and evaluation of water use. Catena 2011, 87, 260–267. [Google Scholar] [CrossRef]
- Ahire, M.L.; Mundada, P.S.; Nikam, T.D.; Bapat, V.A.; Penna, S. Multifaceted roles of silicon in mitigating environmental stresses in plants. Plant Physiol. Biochem. 2021, 169, 291–310. [Google Scholar] [CrossRef]
- Sarojnee, D.Y.; Navindra, B.; Chandrabose, S. Effect of naturally occurring amino acid stimulants on the growth and yield of hot peppers. J. Anim. Plant Sci. 2009, 5, 414–424. [Google Scholar]
- Zhang, H.; Xiong, Y.; Huang, G.; Xu, X.; Huang, Q. Effects of water stress on processing tomatoes yield, quality and water use efficiency with plastic mulched drip irrigation in sandy soil of the Hetao Irrigation District. Agric. Water Manag. 2017, 179, 205–214. [Google Scholar] [CrossRef]
- Shao, G.C.; Deng, S.; Liu, N.; Wang, M.H.; She, D.L. Fruit quality and yield of tomato as influenced by rain shelters and deficit irrigation. J. Agric. Sci. Technol. 2018, 17, 691–704. [Google Scholar]
- Leite, R.D.S.; Nascimento, M.D.; Tanan, T.T.; Ramos, C.D.S.; Gonçalves Neto, L.P.; Guimarães, D.S. Physiological responses of Physalis angulata plants to water deficit. J. Agric. Sci. 2018, 10, 287–297. [Google Scholar] [CrossRef]
- Daneshpazhoh, P.; Ghasemi, A.R.; Nori Emamzadeie, M.R.; Barzegar, R. The effect of partial root-zone drying and zeolite on water use efficiency and physiological characteristics of sweet pepper. Water and Soil 2018, 32, 675–690. [Google Scholar] [CrossRef]
- Giné-Bordonaba, J.; Terry, L.A. Effect of deficit irrigation and methyl jasmonate application on the composition of strawberry (Fragaria × ananassa) fruit and leaves. Sci. Hortic. 2016, 199, 63–70. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Intrigliolo, D.S. Grape composition under abiotic constrains: Water stress and salinity. Front. Plant Sci. 2017, 8, 851. [Google Scholar] [CrossRef] [PubMed]
- Giné-Bordonaba, J.; Terry, L.A. Manipulating the taste-related composition of strawberry fruits (Fragaria × ananassa) from different cultivars using deficit irrigation. Food Chem. 2010, 122, 1020–1026. [Google Scholar] [CrossRef]
- Barzegar, Z.; Ghasemnezhad, M.; Olfati, J.; Khaledian, M.R.; Khalighi, A. The influence of silica upon quantitative, qualitative, and biochemical traits of tomato under water stress. Acta Sci. Pol. Hortorum Cultus. 2022, 21, 123–138. [Google Scholar] [CrossRef]
- Li, T.; Wu, Q.; Zhou, Y.; Yun, Z.; Duan, X.; Jiang, Y. L-Cysteine hydrochloride delays senescence of harvested longan fruit in relation to modification of redox status. Postharvest Biol. Tec. 2018, 143, 35–42. [Google Scholar] [CrossRef]
- Abdelhamid, M.T.; Sadak, M.S.; Schmidhalter, U.R.S.; El-Saady, A.K.M. Interactive effects of salinity stress and nicotinamide on physiological and biochemical parameters of faba bean plant. Acta biol. Colomb. 2013, 18, 499–509. [Google Scholar]
- Shalaby, O.A.E.S.; Konopiński, M.; Ramadan, M.E.S. Effect of chelated iron and silicon on the yield and quality of tomato plants grown under semi-arid conditions. Acta Sci. Pol. Hortorum Cultus. 2017, 16, 29–40. [Google Scholar] [CrossRef]
- Fatemi, H.; Esmaiel Pour, B.; Rizwan, M. Foliar application of silicon nanoparticles affected the growth, vitamin C, flavonoid, and antioxidant enzyme activities of coriander (Coriandrum sativum L.) plants grown in lead (Pb)-spiked soil. Environ. Sci. Pollut. Res. Int. 2021, 28, 1417–1425. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci. Rep. 2020, 10, 17672. [Google Scholar] [CrossRef]
- Mimmo, T.; Tiziani, R.; Valentinuzzi, F.; Lucini, L.; Nicoletto, C.; Sambo, P.; Scampicchio, M.; Pii, Y.; Cesco, S. Selenium biofortification in Fragaria × ananassa: Implications on strawberry fruits quality, content of bioactive health beneficial compounds and metabolomic profile. Front. Plant Sci. 2017, 8, 1887. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Karimi, M.; Teixeira da Silva, J.A. The use of nanotechnology to increase quality and yield of fruit crops. J. Sci. Food Agric. 2020, 100, 25–31. [Google Scholar] [CrossRef]
- Pakzad, R.; Fatehi, F.; Kalantar, M.; Maleki, M. Evaluating the antioxidant enzymes activities, lipid peroxidation and proteomic profile changing in UCB-1 pistachio rootstock leaf under drought stress. Sci. Hortic. 2019, 256, 108617. [Google Scholar] [CrossRef]
- Khoyerdi, F.F.; Shamshiri, M.H.; Estaji, A. Changes in some physiological and osmotic parameters of several pistachio genotypes under drought stress. Sci. Hortic. 2016, 198, 44–51. [Google Scholar] [CrossRef]
- Tang, J.; Camberato, J.J.; Yu, X.; Luo, N.; Bian, S.; Jiang, Y. Growth response, carbohydrate and ion accumulation of diverse perennial ryegrass accessions to increasing salinity. Sci. Hortic. 2013, 154, 73–81. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, X. Dark septate endophyte improves the drought-stress resistance of Ormosia hosiei seedlings by altering leaf morphology and photosynthetic characteristics. Plant Ecol. 2021, 222, 761–771. [Google Scholar] [CrossRef]
- Wang, X.; Wu, G.; Li, D.; Song, X. Moderate Nitrogen Deposition Alleviates Drought Stress of Bretschneidera sinensis. Forests 2023, 14, 137. [Google Scholar] [CrossRef]
- Li, P.; Song, A.; Li, Z.; Fan, F.; Liang, Y. Silicon ameliorates manganese toxicity by regulating manganese transport and antioxidant reactions in rice (Oryza sativa L.). Plant Soil. 2012, 354, 407–419. [Google Scholar] [CrossRef]
- Amin, M.; Ahmad, R.; Ali, A.; Hussain, I.; Mahmood, R.; Aslam, M.; Lee, D.J. Influence of silicon fertilization on maize performance under limited water supply. Silicon 2018, 10, 177–183. [Google Scholar] [CrossRef]
- Manivannan, A.; Ahn, Y.K. Silicon regulates potential genes involved in major physiological processes in plants to combat stress. Front. Plant Sci. 2017, 8, 1346. [Google Scholar] [CrossRef]
- El-Sayed, S.F.; Hassan, H.A.; Ali, A.M.; Gibrael, A.A. Effect of foliar-applied potassium silicate on growth, yield, fruit quality and antioxidant activity of tomato plants grown under deficit irrigation. Int. J. Health Sci. 2022, 6 (Suppl. 6), 10012–10032. [Google Scholar] [CrossRef]
- Nazaralian, S.; Majd, A.; Irian, S.; Najafi, F.; Ghahremaninejad, F.; Landberg, T.; Greger, M. Comparison of silicon nanoparticles and silicate treatments in fenugreek. Plant Physiol. Biochem. 2017, 115, 25–33. [Google Scholar] [CrossRef]
- Conti, V.; Mareri, L.; Faleri, C.; Nepi, M.; Romi, M.; Cai, G.; Cantini, C. Drought stress affects the response of italian local tomato (Solanum lycopersicum L.) varieties in a genotype-dependent manner. Plants 2019, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Tamasi, G.; Pardini, A.; Bonechi, C.; Donati, A.; Pessina, F.; Marcolongo, P.; Gamberucci, A.; Leone, G.; Consumi, M.; Magnani, A.; et al. Characterization of nutraceutical components in tomato pulp, skin and locular gel. Eur. Food Res. Technol. 2019, 245, 907–918. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, Q.; Tian, Y.; Meng, F. Physiological and proteomic analyses of the drought stress response in Amygdalus mira (Koehne) Yü et Lu roots. BMC Plant Biol. 2017, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Jian, W.; Zhang, J.; Zhou, Y.; Cao, J. Suppressive effect of silicon nutrient on Phomopsis stem blight development in asparagus. Hortic. Sci. 2008, 43, 811–817. [Google Scholar] [CrossRef]
- Bokor, B.; Vaculík, M.; Slováková, Ľ.; Masarovič, D.; Lux, A. Silicon does not always mitigate zinc toxicity in maize. Acta Physiol. Plant 2014, 36, 733–743. [Google Scholar] [CrossRef]
- Rady, M.M.; Belal, H.E.; Gadallah, F.M.; Semida, W.M. Selenium application in two methods promotes drought tolerance in Solanum lycopersicum plant by inducing the antioxidant defense system. Sci. Hortic. 2020, 266, 109290. [Google Scholar] [CrossRef]
- Ming, D.F.; Pei, Z.F.; Naeem, M.S.; Gong, H.J.; Zhou, W.J. Silicon alleviates PEG-induced water-deficit stress in upland rice seedlings by enhancing osmotic adjustment. J. Agron. Crop Sci. 2012, 198, 14–26. [Google Scholar] [CrossRef]
- Zeng, F.R.; Zhao, F.S.; Qiu, B.Y.; Ouyang, Y.N.; Wu, F.B.; Zhang, G.P. Alleviation of chromium toxicity by silicon addition in rice plants. Agr. Sci. China. 2011, 10, 1188–1196. [Google Scholar] [CrossRef]
- Pei, Z.F.; Ming, D.F.; Liu, D.; Wan, G.L.; Geng, X.X.; Gong, H.J.; Zhou, W.J. Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J. Plant Growth Regul. 2010, 29, 106–115. [Google Scholar] [CrossRef]
- Cao, B.L.; Ma, Q.; Zhao, Q.; Wang, L.; Xu, K. Effects of silicon on absorbed light allocation, antioxidant enzymes and ultrastructure of chloroplasts in tomato leaves under simulated drought stress. Sci. Hortic. 2015, 194, 53–62. [Google Scholar] [CrossRef]
- Gohari, G.; Molaei, S.; Kheiry, A.; Ghafouri, M.; Razavi, F.; Lorenzo, J.M.; Juárez-Maldonado, A. Exogenous application of proline and L-cysteine alleviates internal browning and maintains eating quality of cold stored flat ‘maleki’peach fruits. Horticulturae 2021, 7, 469. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Nawaz, A.; Shahid, M. Postharvest application of antibrowning chemicals modulates oxidative stress and delays pericarp browning of controlled atmosphere stored litchi fruit. J. Food Biochem. 2019, 43, 12746. [Google Scholar] [CrossRef] [PubMed]
- Sogvar, O.B.; Razavi, F.; Rabiei, V.; Gohari, G. Postharvest application of L-cysteine to prevent enzymatic browning of “Stanley” plum fruit during cold storage. J. Food Process. 2020, 44, 14788. [Google Scholar] [CrossRef]
- Özcan, M.M.; Erel, Ö.; Herken, E.E. Antioxidant activity, phenolic content, and peroxide value of essential oil and extracts of some medicinal and aromatic plants used as condiments and herbal teas in Turkey. J. Med. Food. 2009, 12, 198–202. [Google Scholar] [CrossRef]
- Amaral, J.S.; Valentão, P.; Andrade, P.B.; Martins, R.C.; Seabra, R.M. Do cultivar, geographical location and crop season influence phenolic profile of walnut leaves? Molecules 2008, 13, 1321–1332. [Google Scholar] [CrossRef]
- Shohael, A.M.; Ali, M.B.; Yu, K.W.; Hahn, E.J.; Paek, K.Y. Effect of temperature on secondary metabolites production and antioxidant enzyme activities in Eleutherococcus senticosus somatic embryos. Plant Cell Tissue Organ Cult. 2006, 85, 219–228. [Google Scholar] [CrossRef]
- Khani, A.; Barzegar, T.; Nikbakht, J.; Ghahremani, Z. Effect of foliar spray of calcium lactate on the growth, yield and biochemical attribute of lettuce (Lactuca sativa L.) under water deficit stress. Adv. Hortic. Sci. 2020, 34, 11–24. [Google Scholar]
- Weidner, S.; Karolak, M.; Karamac, M.; Kosinska, A.; Amarowicz, R. Phenolic compounds and properties of antioxidants in grapevine roots [Vitis vinifera L.] under drought stress followed by recovery. Acta Soc. Bot. Pol. 2009, 78, 97–103. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alam, P.; Alyemeni, M.N.; Wijaya, L.; Ali, S.; Ashraf, M. Silicon (Si) supplementation alleviates NaCl toxicity in mung bean [Vigna radiata (L.) Wilczek] through the modifications of physio-biochemical attributes and key antioxidant enzymes. J. Plant Growth Regul. 2019, 38, 70–82. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Wahed, S.H.A.; Y Al-Eidani, T.; Jasim, A.H. Effect of antioxidant reagents and silicon spraying on phenol, flavonoid and anthocyanin in oat varieties. Indian J. Ecol. 2019, 46, 175–179. [Google Scholar]
- Gong, H.; Chen, K. The regulatory role of silicon on water relations, photosynthetic gas exchange, and carboxylation activities of wheat leaves in field drought conditions. Acta Physiol. Plant. 2012, 34, 1589–1594. [Google Scholar] [CrossRef]
- Asgari, F.; Majd, A.; Jonoubi, P.; Najafi, F. Effects of silicon nanoparticles on molecular, chemical, structural and ultrastructural characteristics of oat (Avena sativa L.). Plant Physiol. Biochem. 2018, 127, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Çelik, Ö.; Ayan, A.; Atak, Ç. Enzymatic and non-enzymatic comparison of two different industrial tomato (Solanum lycopersicum) varieties against drought stress. Bot. Stud. 2017, 58, 32. [Google Scholar] [CrossRef]
- Mamnabi, S.; Nasrollahzadeh, S.; Ghassemi-Golezani, K.; Raei, Y. Improving yield-related physiological characteristics of spring rapeseed by integrated fertilizer management under water deficit conditions. Saudi J. Biol. Sci. 2020, 27, 797–804. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Rahman, A.; Alam, M.M.; Mahmud, J.A.; Suzuki, T.; Fujita, M. Polyamines confer salt tolerance in mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Front. Plant Sci. 2016, 7, 1104. [Google Scholar] [CrossRef]
- Raziq, A.; Wang, Y.; Mohi Ud Din, A.; Sun, J.; Shu, S.; Guo, S. A comprehensive evaluation of salt tolerance in tomato (Var. ailsa craig): Responses of physiological and transcriptional changes in RBOH’s and ABA biosynthesis and signalling genes. Int. J. Mol. Sci. 2022, 23, 1603. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qin, C.; Maodong, Q.; Dong, X.X.; Ahmad, P.; Abd_Allah, E.F.; Zhang, L. Spermine application alleviates salinity induced growth and photosynthetic inhibition in Solanum lycopersicum by modulating osmolyte and secondary metabolite accumulation and differentially regulating antioxidant metabolism. Plant Physiol. Biochem. 2019, 144, 1–13. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Pessarakli, M. Effect of silicon on photosynthetic gas exchange, photosynthetic pigments, cell membrane stability and relative water content of different wheat cultivars under drought stress conditions. J. Plant Nutr. 2016, 39, 1001–1015. [Google Scholar] [CrossRef]
- Dehghanipoodeh, S.; Ghobadi, C.; Baninasab, B.; Gheysari, M.; Shiranibidabadi, S. Effect of silicon on growth and development of strawberry under water deficit conditions. Hort Plant. J. 2018, 4, 226–232. [Google Scholar] [CrossRef]
- Ashraf, M.; Afzal, M.; Ahmad, R.; Maqsood, M.A.; Shahzad, S.M.; Aziz, A.; Akhtar, N. Silicon management for mitigating abiotic stress effects in plants. Plant Stress 2010, 4, 104–114. [Google Scholar]
- Kim, Y.H.; Khan, A.L.; Waqas, M.; Lee, I.J. Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: A review. Front. Plant Sci. 2017, 8, 510. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Arias, D.; García-Machado, F.J.; Morales-Sierra, S.; Luis, J.C.; Suarez, E.; Hernández, M.; Valdés, F.; Borges, A.A. Lettuce plants treated with L-pyroglutamic acid increase yield under water deficit stress. Environ. Exp. Bot. 2019, 158, 215–222. [Google Scholar] [CrossRef]
- Barzegar, T.; Moradi, P.; Nikbakht, J.; Ghahremani, Z. Physiological response of Okra cv. Kano to foliar application of putrescine and humic acid under water deficit stress. Int. J. Hortic. Sci. Technol. 2016, 3, 187–197. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alamri, S.A.; Alam, P.; Ashraf, M.; Ahmad, P. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE 2018, 13, 0202175. [Google Scholar] [CrossRef]
- Avestan, S.; Ghasemnezhad, M.; Esfahani, M.; Byrt, C.S. Application of nano-silicon dioxide improves salt stress tolerance in strawberry plants. J. Agron. 2019, 9, 246. [Google Scholar] [CrossRef]
- AlKahtani, M.D.; Hafez, Y.M.; Attia, K.; Rashwan, E.; Husnain, L.A.; AlGwaiz, H.I.; Abdelaal, K.A. Evaluation of silicon and proline application on the oxidative machinery in drought-stressed sugar beet. Antioxidants 2021, 10, 398. [Google Scholar] [CrossRef]
- Robatjazi, R.; Roshandel, P.; Hooshmand, S.D. Benefits of silicon nutrition on growth, physiological and phytochemical attributes of basil upon salinity stress. Int. J. Hortic. Sci. Technol. 2020, 7, 37–50. [Google Scholar] [CrossRef]
- Pang, Z.; Tayyab, M.; Islam, W.; Tarin, M.W.K.; Sarfaraz, R.; Naveed, H.; Zaman, S.; Zhang, B.; Yuan, Z.; Zhang, H. Silicon mediated improvement in tolerance of economically important crops under drought stress. Appl. Ecol. Environ. Res. 2019, 17, 6151–6170. [Google Scholar] [CrossRef]
- Asgharipour, M.R.; Mosapour, H. A foliar application silicon enhances drought tolerance in fennel. J. Anim. Plant Sci. 2016, 26, 1056–1062. [Google Scholar]


| Soil Texture | Organic Matter (%) | pH | EC (dS m−1) | N (%) | Ca (g kg−1) | Na (g kg−1) | K (g kg−1) |
|---|---|---|---|---|---|---|---|
| Loam clay | 0.94 | 7.4 | 1.49 | 0.07 | 0.12 | 0.13 | 0.20 |
| Meteorological Parameter | May | June | July | August | September | October | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |
| Maximum temperature (°C) | 30.2 | 29.2 | 33.7 | 33.1 | 34.9 | 34.8 | 32.2 | 31.8 | 27.7 | 23.8 | 15.6 | 19.0 |
| Minimum temperature (°C) | 12.2 | 12.0 | 15.6 | 14.9 | 16.5 | 15.1 | 12.2 | 12.3 | 9.0 | 8.6 | 2.9 | 5.2 |
| Average temperature (°C) | 21.2 | 20.6 | 24.7 | 24.0 | 25.7 | 25.0 | 22.2 | 22.1 | 18.4 | 16.2 | 9.3 | 12.1 |
| Average relative humidity (%) | 31.4 | 44.1 | 30.2 | 34 | 34.7 | 28.8 | 28.9 | 36.3 | 36.2 | 42.6 | 65.2 | 49.1 |
| Total rainfall (mm) | 0.1 | 40.2 | 0.0 | 8.2 | 0.0 | 2.2 | 0.0 | 0.9 | 0.5 | 4.2 | 21.1 | 2.3 |
| Sum sunshine (hr) | 10.5 | 9.7 | 11.9 | 12.3 | 11.3 | 10.8 | 10.3 | 8.3 | 9.4 | 6.6 | 6.1 | 6.7 |
| Attributes | TSS | TA | Vit C | TSC | TSP | CAT | POX | SOD | APX | PPO | PAL | Phenol | Flavonoid | AA | MSI | MDA | H2O2 | Proline | Yield |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TSS | 1 | ||||||||||||||||||
| TA | 0.027 ns | 1 | |||||||||||||||||
| Vit C | −0.088 ns | 0.007 ns | 1 | ||||||||||||||||
| TSC | 0.181 ns | 0.283 ** | −0.181 ns | 1 | |||||||||||||||
| TSP | 0.493 ** | 0.114 ns | −0.035 ns | −0.077 ns | 1 | ||||||||||||||
| CAT | −0.070 ns | 0.294 ** | −0.038 ns | 0.734 ** | −0.307 ** | 1 | |||||||||||||
| POX | 0.420 ** | 0.161 ns | −0.267 ** | 0.480 ** | 0.160 ns | 0.274 ** | 1 | ||||||||||||
| SOD | 0.198 * | 0.108 ns | −0.069n s | 0.599 ** | −0.310 ** | 0.671 ** | 0.398 ** | 1 | |||||||||||
| APX | 0.364 ** | 0.440 ** | 0.036 ns | 0.416 ** | 0.298 ** | 0.255 ** | 0.438 ** | 0.354 ** | 1 | ||||||||||
| PPO | 0.179 ns | 0.244 * | −0.257 ** | 0.547 ** | −0.259 ** | 0.526 ** | 0.395 ** | 0.656 ** | 0.455 ** | 1 | |||||||||
| PAL | −0.104 ns | 0.201 * | −0.135 ns | 0.678 ** | −0.559 ** | 0.850 ** | 0.366 ** | 0.746 ** | 0.163 ns | 0.567 ** | 1 | ||||||||
| Phenol | −0.030 ns | 0.434 ** | −0.031 ns | 0.614 ** | −0.321 ** | 0.724 ** | 0.362 ** | 0.594 ** | 0.251 ** | 0.439 ** | 0.797 ** | 1 | |||||||
| Flavonoid | −0.107 ns | 0.221 * | −0.084 ns | 0.573 ** | −0.517 ** | 0.773 ** | 0.291 ** | 0.670 ** | 0.092 ns | 0.500 ** | 0.870 ** | 0.770 ** | 1 | ||||||
| AA | 0.139 ns | 0.291 ** | −0.335 ** | 0.721 ** | −0.157 ns | 0.735 ** | 0.653 ** | 0.616 ** | 0.274 ** | 0.533 ** | 0.774 ** | 0.726 ** | 0.682 ** | 1 | |||||
| MSI | −0.064 ns | −0.001 ns | 0.313 ** | 0.241 * | −0.373 ** | 0.435 ** | −0.088 ns | 0.252 ** | −0.021 ns | 0.183 ns | 0.448 ** | 0.422 ** | 0.424 ** | 0.152 ns | 1 | ||||
| MDA | −0.219 * | −0.033 ns | −0.514 ** | 0.223 * | −0.290 ** | 0.164 ns | 0.264 ** | 0.192 * | −0.138 ns | 0.240 * | 0.361 ** | 0.176 ns | 0.245 * | 0.476 ** | −0.183 ns | 1 | |||
| H2O2 | −0.197 * | 0.054 ns | −0.431 ** | −0.254 ** | 0.092 ns | −0.298 ** | −0.187 ns | −0.276 ** | −0.264 ** | −0.142 ns | −0.298 ** | −0.341 ** | −0.267 ** | −0.089 ns | −0.509 ** | 0.396 ** | 1 | ||
| Proline | 0.480 ** | 0.364 ** | −0.096 ns | 0.528 ** | 0.339 ** | 0.338 ** | 0.551 ** | 0.471 ** | 0.567 ** | 0.360 ** | 0.240 * | 0.366 ** | 0.130 ns | 0.398 ** | −0.103 ns | −0.049 ns | −0.265 ** | 1 | |
| Yield | 0.075 ns | −0.168 ns | 0.392 ** | −0.417 ** | 0.157 ns | −0.395 ** | −0.252 ** | −0.217 * | −0.045 ns | −0.420 ** | −0.395 ** | −0.267 ** | −0.342 ** | −0.566 ** | 0.093 ns | −0.542 ** | −0.324 ** | 0.063 ns | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khani, A.; Barzegar, T.; Nikbakht, J.; Sabatino, L. Foliar Application of K-Silicate and L-Cysteine Enhances Production, Quality, and Antioxidant Activities of Cape Gooseberry Fruits Under Drought Conditions. Agronomy 2025, 15, 675. https://doi.org/10.3390/agronomy15030675
Khani A, Barzegar T, Nikbakht J, Sabatino L. Foliar Application of K-Silicate and L-Cysteine Enhances Production, Quality, and Antioxidant Activities of Cape Gooseberry Fruits Under Drought Conditions. Agronomy. 2025; 15(3):675. https://doi.org/10.3390/agronomy15030675
Chicago/Turabian StyleKhani, Arezoo, Taher Barzegar, Jaefar Nikbakht, and Leo Sabatino. 2025. "Foliar Application of K-Silicate and L-Cysteine Enhances Production, Quality, and Antioxidant Activities of Cape Gooseberry Fruits Under Drought Conditions" Agronomy 15, no. 3: 675. https://doi.org/10.3390/agronomy15030675
APA StyleKhani, A., Barzegar, T., Nikbakht, J., & Sabatino, L. (2025). Foliar Application of K-Silicate and L-Cysteine Enhances Production, Quality, and Antioxidant Activities of Cape Gooseberry Fruits Under Drought Conditions. Agronomy, 15(3), 675. https://doi.org/10.3390/agronomy15030675







