Native Warm-Season Grasses Show Limited Response to Phosphorus and Potassium
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Data Collection
2.4. Root Sampling for Mycorrhizal Assessment
2.5. Root Cleaning and Staining Procedure
2.6. Statistical Analysis
3. Results and Discussion
3.1. NWSG Yield Response to Potassium and Phosphorus
3.2. Temporal Effects on DM Yield
3.3. Forage Nutritive Values
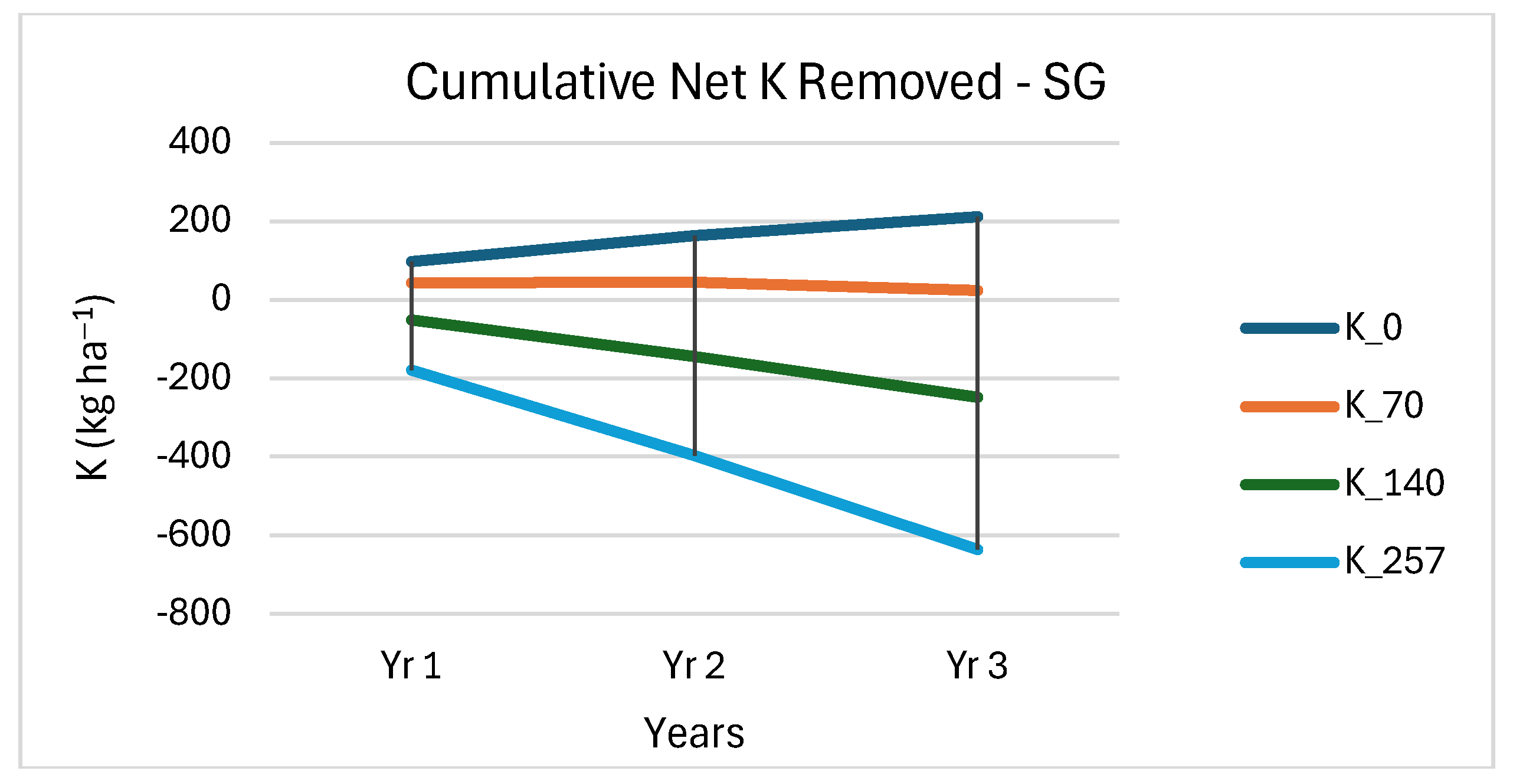
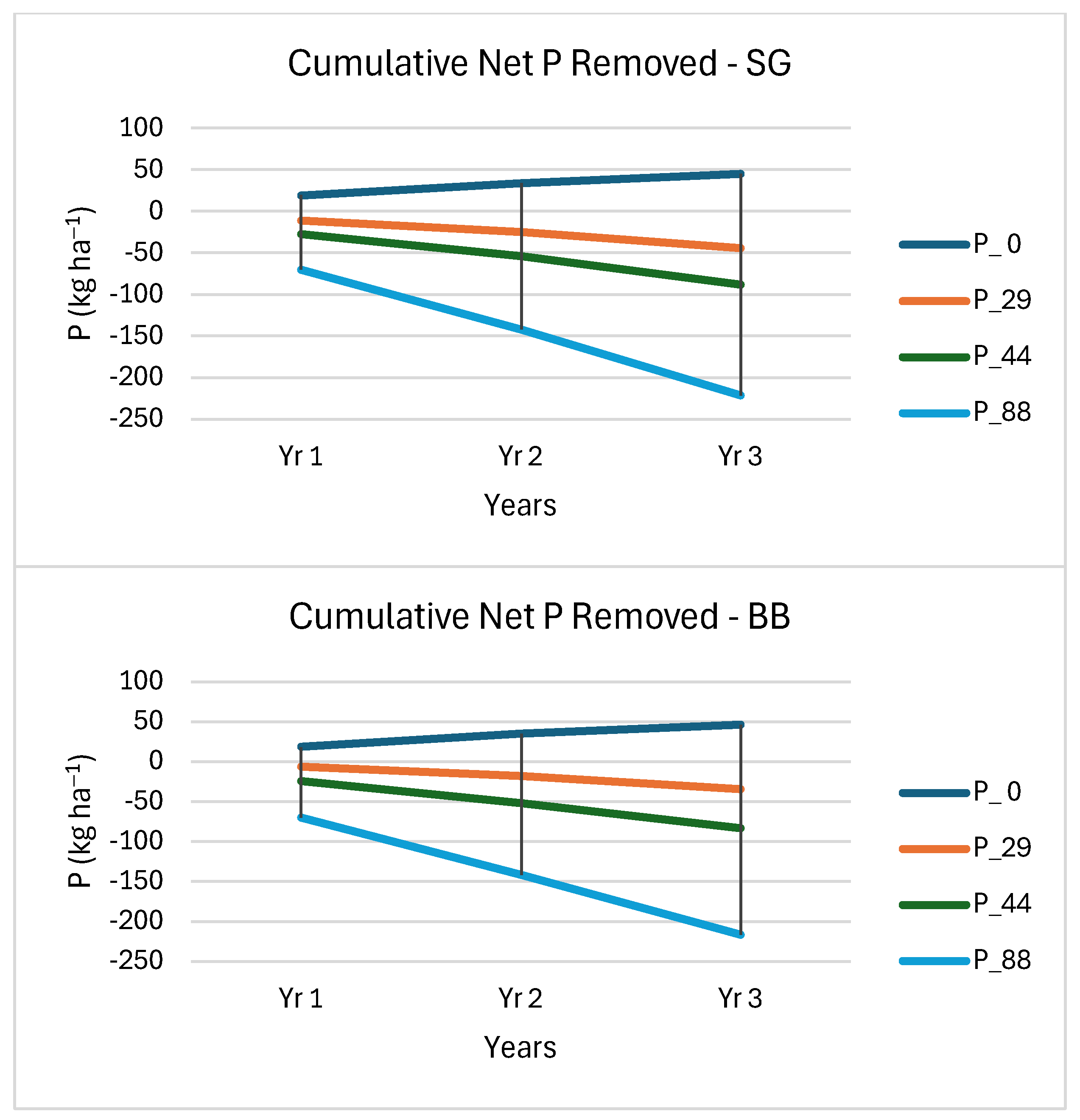
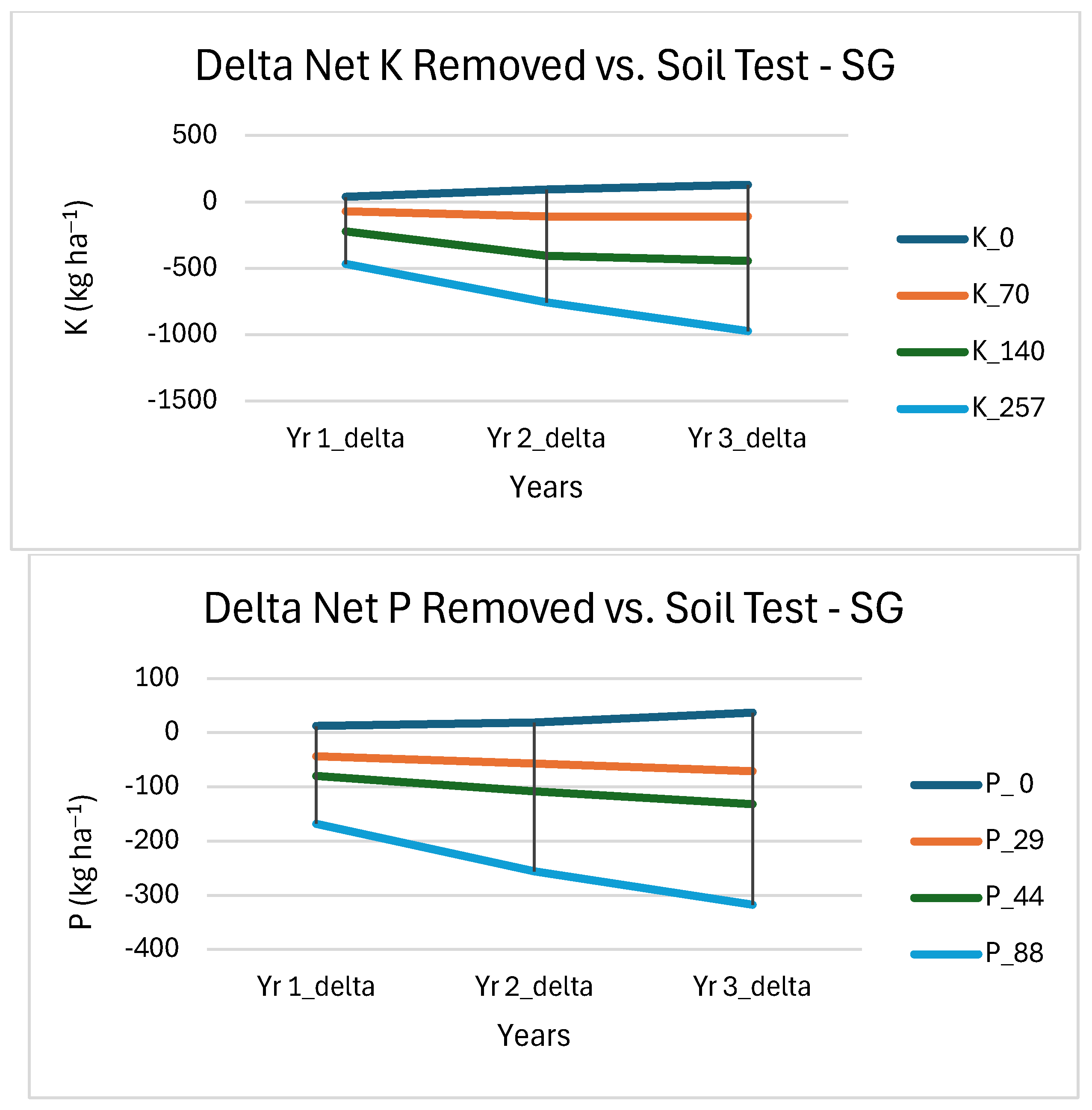
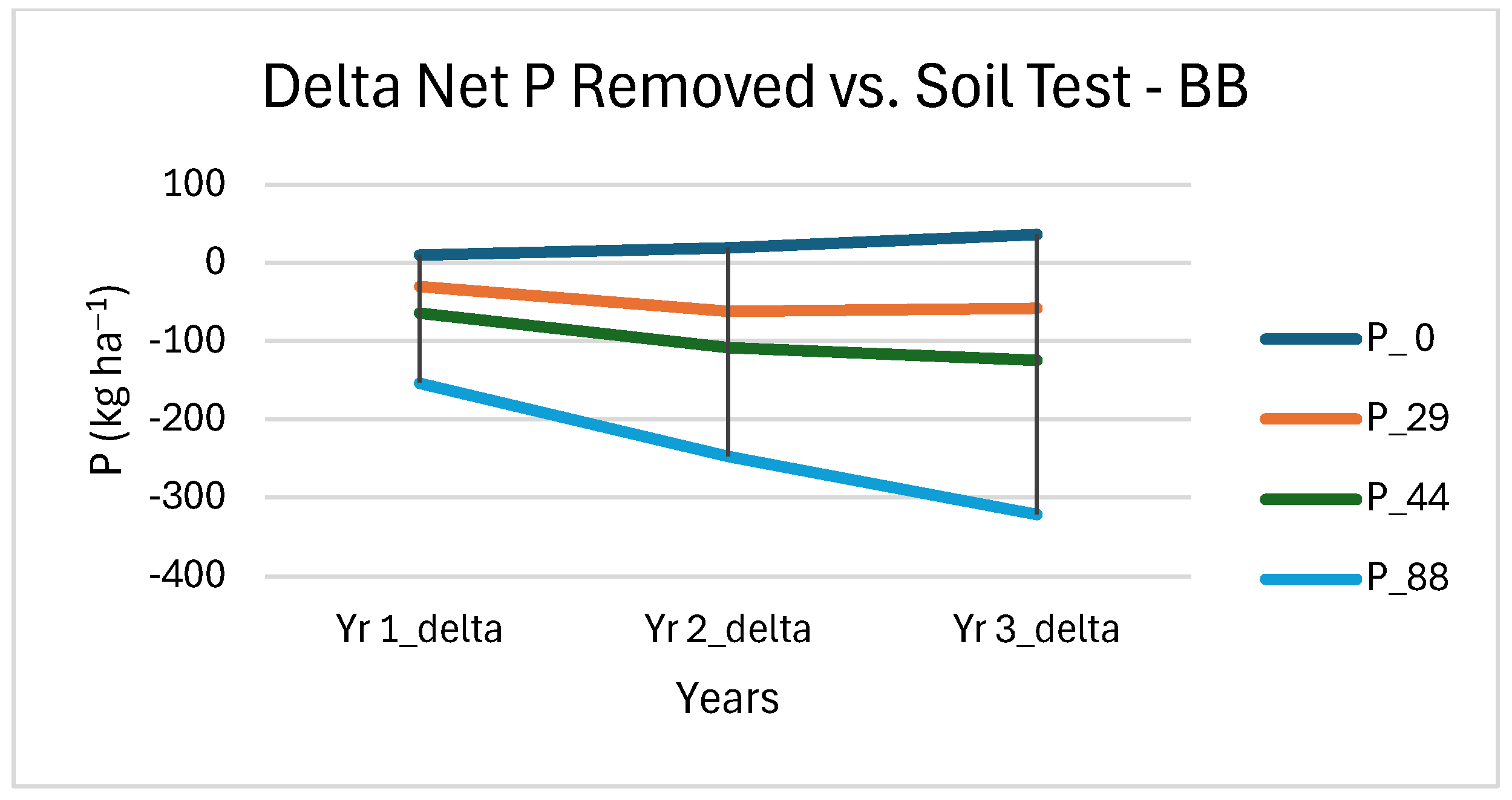
3.4. Soil Nutrient Removal
3.5. Mycorrhizal Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Backus, W.M.; Waller, J.C.; Bates, G.E.; Harper, C.A.; Saxton, A.; McIntosh, D.W.; Birckhead, J.; Keyser, P.D. Management of native warm-season grasses for beef cattle and biomass production in the Mid-South USA. J. Anim. Sci. 2017, 95, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Rushing, J.B.; Lemus, R.W.; White, J.A.; Lyles, J.C.; Thornton, M.T. Yield of native warm-season grasses in response to nitrogen and harvest frequency. Agron. J. 2020, 111, 193–199. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Schmer, M.; Owens, V.; Keyser, P.; Elbersen, W. Crop management of switchgrass. In Switchgrass: A Valuable Biomass Crop for Energy; Springer: London, UK, 2012; pp. 87–112. [Google Scholar]
- Awada, T.; Moser, L.E.; Schacht, W.H.; Reece, P.E. Stomatal variability of native warm-season grasses from the Nebraska Sandhills. Can. J. Plant Sci. 2002, 82, 349–355. [Google Scholar] [CrossRef]
- Gitz, D.C., III; Baker, J.T.; Stout, J.E.; Brauer, D.K.; Lascano, R.J.; Velten, J.P. Suitability of eastern gamagrass for in situ precipitation catchment forage production in playas. Agron. J. 2013, 105, 907–914. [Google Scholar] [CrossRef]
- Jung, G.A.; Shaffer, J.A.; Stout, W.L. Switchgrass and big bluestem responses to amendments on strongly acid soil. Agron. J. 1988, 80, 669–676. [Google Scholar] [CrossRef]
- Buttrey, E.K.; Bean, B.W. Yield, water use efficiency, and nutritive value of six warm-season perennial grasses in response to irrigation level. Forage Grazinglands 2011, 9, 1–8. [Google Scholar] [CrossRef]
- Gelley, C.H.; Ashworth, A.J.; Keyser, P.D.; Nave, R.L.; Rhinehart, J.D. Water-use efficiency of forage crops in the southeastern United States. Agronomy 2020, 10, 1377. [Google Scholar] [CrossRef]
- Bisangwa, E.; Richwine, J.D.; Keyser, P.D.; Ashworth, A.J.; Walker, F.R. Native warm-season grass response to nitrogen fertilization. Agronomy 2024, 14, 180. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Moore, P.A., Jr.; King, R.; Pote, D.H.; Douglas, J.L.; Jacobs, A.A. Switchgrass nitrogen fertility response and nutrient cycling in a hay system. Agron. J. 2020, 112, 1963–1971. [Google Scholar] [CrossRef]
- Brundrett, M. Mycorrhizas in natural ecosystems. In Advances in Ecological Research; Academic Press: Cambridge, MA, USA, 1991; Volume 21, pp. 171–313. [Google Scholar]
- Hestrin, R.; Lee, M.R.; Whitaker, B.K.; Pett-Ridge, J. The switchgrass microbiome: A review of structure, function, and taxonomic distribution. Phytobiomes J. 2021, 5, 14–28. [Google Scholar] [CrossRef]
- Stutz, J.C.; Morton, J.B. Successive pot cultures reveal high species richness of arbuscular endomycorrhizal fungi in arid ecosystems. Can. J. Bot. 1996, 74, 1883–1889. [Google Scholar] [CrossRef]
- Bever, J.D.; Schultz, P.A.; Pringle, A.; Morton, J.B. Arbuscular mycorrhizal fungi: More diverse than meets the eye, and the ecological tale of why: The high diversity of ecologically distinct species of arbuscular mycorrhizal fungi within a single community has broad implications for plant ecology. Bioscience 2001, 51, 923–931. [Google Scholar] [CrossRef]
- Cooke, J.C.; Butler, R.H.; Madole, G. Some observations on the vertical distribution of vesicular arbuscular mycorrhizae in roots of salt marsh grasses growing in saturated soils. Mycologia 1993, 85, 547–550. [Google Scholar] [CrossRef]
- Zajicek, J.M.; Hetrick, B.D.; Owensby, C.E. The influence of soil depth on mycorrhizal colonization of forbs in the tallgrass prairie. Mycologia 1986, 78, 316–320. [Google Scholar] [CrossRef]
- Thompson, J.P. Improving the mycorrhizal condition of the soil through cultural practices and effects on growth and phosphorus uptake by plants. In Phosphorus Nutrition of Grain Legumes in the Semi-Arid Tropics; ICRISAT: Andhra Pradesh, India, 1991; pp. 117–137. [Google Scholar]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Ris, E.A.; Boller, T.; Wiemken, A. Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems. N. Phytol. 2005, 165, 273–283. [Google Scholar] [CrossRef]
- Berg, W.A. Response of a mixed native warm-season grass planting to nitrogen fertilization. J. Range Manag. 1995, 48, 64–67. [Google Scholar] [CrossRef]
- Haque, M.; Biermacher, J.T.; Kering, M.; Guretzky, J.A. Managing Nitrogen and Phosphorus Nutrients for Switchgrass Produced for Bioenergy Feedstock in Phosphorus-Deficient Soil. 2012. Available online: https://digitalcommons.unl.edu (accessed on 13 January 2025).
- Brejda, J.J.; Moser, L.E.; Vogel, K.P. Evaluation of switchgrass rhizosphere microflora for enhancing seedling yield and nutrient uptake. Agron. J. 1998, 90, 753–758. [Google Scholar] [CrossRef]
- Brejda, J.J. Fertilization of native warm-season grasses. In Native Warm-Season Grasses: Research Trends and Issues; CSSA: Madison, WI, USA, 2000; Volume 30, pp. 177–200. [Google Scholar]
- Muir, J.P.; Sanderson, M.A.; Ocumpaugh, W.R.; Jones, R.M.; Reed, R.L. Biomass production of ‘Alamo’switchgrass in response to nitrogen, phosphorus, and row spacing. Agron. J. 2001, 93, 896–901. [Google Scholar] [CrossRef]
- McLaughlin, S.; Bouton, J.; Bransby, D.; Conger, B.; Ocumpaugh, W.; Parrish, D.; Taliaferro, C.; Vogel, K.; Wullschleger, S. Developing switchgrass as a bioenergy crop. Perspect. N. Crops N. Uses 1999, 56, 282–299. [Google Scholar]
- Sanderson, M.A.; Reed, R.L.; McLaughlin, S.B.; Wullschleger, S.D.; Conger, B.V.; Parrish, D.J.; Wolf, D.D.; Taliaferro, C.; Hopkins, A.A.; Tischler, C.R. Switchgrass as a sustainable bioenergy crop. Bioresour. Technol. 1996, 56, 83–93. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Keyser, P.D.; Allen, F.L.; Tyler, D.D.; Taylor, A.M.; West, C.P. Displacing inorganic nitrogen in lignocellulosic feedstock production systems. Agron. J. 2016, 108, 109–116. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Rocateli, A.C.; West, C.P.; Brye, K.R.; Popp, M.P. Switchgrass growth and effects on biomass accumulation, moisture content, and nutrient removal. Agron. J. 2017, 109, 1359–1367. [Google Scholar] [CrossRef]
- Daniel, T.C.; Sharpley, A.N.; Lemunyon, J.L. Agricultural phosphorus and eutrophication: A symposium overview. J. Environ. Qual. 1998, 27, 251–257. [Google Scholar] [CrossRef]
- Scholz, R.W.; Ulrich, A.E.; Eilittä, M.; Roy, A. Sustainable use of phosphorus: A finite resource. Sci. Total Environ. 2013, 461, 799–803. [Google Scholar] [CrossRef]
- Lavelle, P.; Dugdale, R.; Scholes, R.; Berhe, A.A.; Carpenter, E.; Codispoti, L.; Izac, A.M.; Lemoalle, J.; Luizao, F.; Treguer, P.; et al. Chapter 12: Nutrient Cycling. In Ecosystems and Human Well-Being: Current State and Trends. Findings of the Condition and Trends Working Group. Millennium Ecosystem Assessment Series; World Resources Institute: Washington, DC, USA; Island Press: Washington, DC, USA, 2005; Volume 1. [Google Scholar]
- Capece, J.C.; Campbell, K.L.; Bohlen, P.J.; Graetz, D.A.; Portier, K.M. Soil phosphorus, cattle stocking rates, and water quality in subtropical pastures in Florida, USA. Rangel. Ecol. Manag. 2007, 60, 19–30. [Google Scholar] [CrossRef]
- Silveira, M.L.; Vendramini, J.M.; Sollenberger, L.E. Phosphorus management and water quality problems in grazingland ecosystems. Int. J. Agron. 2010, 2010, 517603. [Google Scholar] [CrossRef]
- Garnache, C.; Swinton, S.M.; Herriges, J.A.; Lupi, F.; Stevenson, R.J. Solving the phosphorus pollution puzzle: Synthesis and directions for future research. Am. J. Agric. Econ. 2016, 98, 1334–1359. [Google Scholar] [CrossRef]
- Schroeder, D. Structure and weathering of potassium containing minerals. IPI Res. Top. 1978, 37, 5–25. [Google Scholar]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Moller, I.S.; White, P. Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, H., Ed.; Academic Press: London, UK, 2012; pp. 178–189. [Google Scholar]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Bennett, E.M. Human impact on erodible phosphorus and eutrophication: A global perspective. BioScience 2001, 51, 227–234. [Google Scholar]
- Conley, D.J.; Humborg, C.; Rahm, L.; Savchuk, O.P.; Wulff, F. Hypoxia in the Baltic Sea and basin-scale changes in phosphorus biogeochemistry. Environ. Sci. Technol. 2002, 36, 5315–5320. [Google Scholar] [CrossRef] [PubMed]
- American Scientist. Does peak phosphorus loom? Am. Sci. 2010, 98, 91. [Google Scholar]
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olson, C.; Sztein, A.E.; Sparks, D.L. Soil and human security in the 21st century. Science 2015, 348, 1261071. [Google Scholar] [CrossRef]
- Morris, R.J.; Fox, R.H.; Jung, G.A. Growth, P Uptake, and Quality of Warm and Cool-Season Grasses on a Low Available P Soil 1. Agron. J. 1982, 74, 125–129. [Google Scholar] [CrossRef]
- Popp, M.P.; Ashworth, A.J.; Moore, P.A., Jr.; Owens, P.R.; Douglas, J.L.; Pote, D.H.; Jacobs, A.A.; Lindsay, K.R.; Dixon, B.L. Fertilize r recommendations for switchgrass: Quantifying economic effects on quality and yield. Agron. J. 2018, 110, 1854–1861. [Google Scholar] [CrossRef]
- Miller, R.M.; Wilson, G.W.; Johnson, N.C. Arbuscular mycorrhizae and grassland ecosystems. In Biocomplexity of Plant–Fungal Interactions; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 59–84. [Google Scholar]
- Daniel, H.A.; Harper, H.J. The relation between total calcium and phosphorus in mature prairie grass and available plant food in the soil. J. Am. Soc. Agron. 1934, 36, 986–992. [Google Scholar] [CrossRef]
- Murphy, H.F. Recovery of phosphorus from prairie grasses growing on central Oklahoma soils treated with superphosphate. J. Agric. Res. 1933, 47, 911–917. [Google Scholar]
- Garland, C.D.; Bates, G.E.; Clark, C.D.; Dalton, D.A.; Gerloff, D.C.; Goddard, J.J.; Goddard, K.J.; Howard, L.; Keyser, P.D.; Newman, M.A.; et al. SP701-A-Growing and Harvesting Switchgrass for Ethanol Production in Tennessee. 2008. Available online: https://trace.tennessee.edu/utk_agexbiof/6/ (accessed on 1 January 2025).
- Popp, M.P.; Ashworth, A.J.; West, C.P. Simulating the feasibility of dual use switchgrass on cow-calf operations. Energies 2021, 14, 2422. [Google Scholar] [CrossRef]
- Silveira, M.L.; Vendramini, J.M.; da Silva, H.M.; Borges, B.M.; Ribeirinho, V.S.; Lacerda, J.J.; Azenha, M.V.; Viegas, P.R.A.; Aguiar, A.D. Potassium and phosphorus fertilization impacts on bermudagrass and limpograss herbage accumulation, nutritive value, and persistence. Crop Sci. 2017, 57, 2881–2890. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Moore, P.A., Jr.; King, R.; Pote, D.H.; Douglas, J.L.; Jacobs, A.A.; Pratt, E. Switchgrass forage yield and compositional response to phosphorus and potassium. Agrosystems Geosci. Environ. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Moyer, J.L.; Sweeney, D.W. Responses of eastern gamagrass [Tripsacum dactyloides (L.) L.] forage quality to nitrogen application and harvest system. J. Plant Nutr. 2016, 39, 17–26. [Google Scholar] [CrossRef]
- Obour, A.K.; Harmoney, K.; Holman, J.D. Nitrogen fertilizer application effects on switchgrass herbage mass, nutritive value and nutrient removal. Crop Sci. 2017, 57, 1754–1763. [Google Scholar] [CrossRef]
- Keyser, P.D.; Hancock, D.W.; Marks, L.; Dillard, L. Establishing native grass forages in the southeast. Univ. Tenn. Ext. PB 2015, 1873, 1–28. [Google Scholar]
- McIntosh, D.; Aderson-Husmoen, B.J.; Kern-Lunbery, R.; Goldblatt, P.; Lemus, R.; Griggs, T.; Bauman, L.; Boone, S.; Shewmaker, G.; Teutsch, C. Guidelines for Optimal Use of NIRSC Forage and Feed Calibrations in Membership Laboratories, 2nd ed.; The University of Tennessee Press: Knoxville, TN, USA, 2022. [Google Scholar]
- McIntosh, D.; Bates, G.; Berghazi, P.; Labbe, N.; Eckelkamp, E.; Lemus, R.; Anderson, B. The impact of moisture content on forage nutritive predictions using near infrared spectroscopy (NIRS) on prepared ground samples and how this affects producers and researchers. Abstract and presentation. In Proceedings of the Western Alfalfa and Hay Symposium, Reno, NV, USA, 19–21 November 2019. [Google Scholar]
- Murray, I.; Cowe, I. Sample preparation. In Near-Infrared Spectroscopy in Agriculture; Roberts, C.A., Workman, J.J., Reeves, J.B., Eds.; ASA: Madison, WI, USA; CSSA: Madison, WI, USA; SSSA: Madison, WI, USA, 2004; pp. 75–112. [Google Scholar]
- Koske, R.; Gemma, J. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989, 92, 486. [Google Scholar] [CrossRef]
- McGonigle, T.; Miller, M.; Evans, D.; Fairchild, G.; Swsan, J. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. N. Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- SAS Institute. The SAS System for Windows, Version 9.4; SAS Institute: Cary, NC, USA, 2013. [Google Scholar]
- Piepho, H.P. Letters in mean comparisons: What they do and don’t mean. Agron. J. 2018, 110, 431–434. [Google Scholar] [CrossRef]
- Waramit, N.; Moore, K.J.; Fales, S.L. Forage quality of native warm-season grasses in response to nitrogen fertilization and harvest date. Anim. Feed Sci. Technol. 2012, 174, 46–59. [Google Scholar] [CrossRef]
- Mcintosh, D.W.; Bates, G.E.; Keyser, P.D.; Allen, F.L.; Harper, C.A.; Waller, J.C.; Birckhead, J.L.; Backus, W.M. Forage harvest timing impact on biomass quality from native warm-season grass mixtures. Agron. J. 2016, 108, 1524–1530. [Google Scholar] [CrossRef]
- NOAA. NOWData—NOAA Online Weather Data, Knoxville Exp Sta, TN. 2020. Available online: https://www.weather.gov/wrh/Climate?wfo=mrx (accessed on 2 November 2023).
- NOAA. NOWData—NOAA Online Weather Data, Springfield Exp Sta, TN. 2020. Available online: https://www.weather.gov/wrh/Climate?wfo=mrx (accessed on 6 March 2025).
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Jungers, J.M.; Sheaffer, C.C.; Lamb, J.A. The effect of nitrogen, phosphorus, and potassium fertilizers on prairie biomass yield, ethanol yield, and nutrient harvest. BioEnergy Res. 2015, 8, 279–291. [Google Scholar] [CrossRef]
- Pant, H.K.; Mislevy, P.; Rechcigl, J.E. Effects of phosphorus and potassium on forage nutritive value and quantity: Environmental implications. Agron. J. 2004, 96, 1299–1305. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Meena, V.D.; Basak, B.B.; Meena, R.S. Potassium uptake by crops as well as microorganisms. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V., Maurya, B., Verma, J., Meena, R., Eds.; Springer: New Delhi, India, 2016; pp. 267–280. [Google Scholar]
- Mulkey, V.R.; Owens, V.N.; Lee, D.K. Management of warm-season grass mixtures for biomass production in South Dakota USA. Bioresour. Technol. 2008, 99, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Veeh, R.H.; Skogley, E.O. Small grain response to potassium fertilizers as related to soil and site characteristics. Soil Sci. Soc. Am. J. 1986, 50, 373–378. [Google Scholar] [CrossRef]
- McLean, E.O. Influence of clay content and clay composition on potassium availability. In Potassium in Soils and Crops, Proceedings of the Symposium of the Potash Research Institute of India, New Delhi, India, 16–17 November 1978; Potash Research Institute of India: Haryana, India, 1978; pp. 1–19. [Google Scholar]
- Barber, S.A. A diffusion and mass-flow concept of soil nutrient. In Soil Nutrient Availability: Chemistry and Concepts; Van Nostrand Reinhold: New York, NY, USA, 1985; p. 255. [Google Scholar]
- Wang, C.; White, P.J.; Li, C. Colonization and community structure of arbuscular mycorrhizal fungi in maize roots at different depths in the soil profile respond differently to phosphorus inputs on a long-term experimental site. Mycorrhiza 2017, 27, 369–381. [Google Scholar] [CrossRef]
- Ryan, M.H.; Small, D.R.; Ash, J.E. Phosphorus controls the level of colonisation by arbuscular mycorrhizal fungi in conventional and biodynamic irrigated dairy pastures. Aust. J. Exp. Agric. 2000, 40, 663–670. [Google Scholar] [CrossRef]
- Ryan, M.; Ash, J. Effects of phosphorus and nitrogen on growth of pasture plants and VAM fungi in SE Australian soils with contrasting fertiliser histories (conventional and biodynamic). Agric. Ecosyst. Environ. 1999, 73, 51–62. [Google Scholar] [CrossRef]
- Rubio, R.; Borie, F.; Schalchli, C.; Castillo, C.; Azcón, R. Occurrence and effect of arbuscular mycorrhizal propagules in wheat as affected by the source and amount of phosphorus fertilizer and fungal inoculation. Appl. Soil Ecol. 2003, 23, 245–255. [Google Scholar] [CrossRef]
- Hetrick, B.D.; Wilson, G.W.T.; Todd, T.C. Differential responses of C3 and C4 grasses to mycorrhizal symbiosis, phosphorus fertilization, and soil microorganisms. Can. J. Bot. 1990, 68, 461–467. [Google Scholar] [CrossRef]
- Brundrett, M. Diversity and classification of mycorrhizal associations. Biol. Rev. 2004, 79, 473–495. [Google Scholar] [CrossRef]
- Konvalinková, T.; Püschel, D.; Řezáčová, V.; Gryndlerová, H.; Jansa, J. Carbon flow from plant to arbuscular mycorrhizal fungi is reduced under phosphorus fertilization. Plant Soil 2017, 419, 319–333. [Google Scholar] [CrossRef]
- Edayilam, N.; Montgomery, D.; Ferguson, B.; Maroli, A.S.; Martinez, N.; Powell, B.A.; Tharayil, N. Phosphorus stress-induced changes in plant root exudation could potentially facilitate uranium mobilization from stable mineral forms. Environ. Sci. Technol. 2018, 52, 7652–7662. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Huertas, R.; Torres-Jerez, I.; Liu, W.; Watson, B.; Scheible, W.R.; Udvardi, M. Transcriptional, metabolic, physiological and developmental responses of switchgrass to phosphorus limitation. Plant Cell Environ. 2021, 44, 186–202. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Cruz-Ramırez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Johnson, N.C. Can fertilization of soil select less mutualistic mycorrhizae? Ecol. Appl. 1993, 3, 749–757. [Google Scholar] [CrossRef]
- Lekberg, Y.; Arnillas, C.A.; Borer, E.T.; Bullington, L.S.; Fierer, N.; Kennedy, P.G.; Leff, J.W.; Luis, A.D.; Seabloom, E.W.; Henning, J.A. Nitrogen and phosphorus fertilization consistently favor pathogenic over mutualistic fungi in grassland soils. Nat. Commun. 2021, 12, 3484. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Ferrández, T.; Morales, M.A.; Morte, A.; Alarcón, J.J. Variations in water status, gas exchange, and growth in Rosmarinus officinalis plants infected with Glomus deserticola under drought conditions. J. Plant Physiol. 2004, 161, 675–682. [Google Scholar] [CrossRef]
- Gosling, P.; Proctor, M.; Jones, J.; Bending, G.D. Distribution and diversity of Paraglomus spp. in tilled agricultural soils. Mycorrhiza 2014, 24, 1. [Google Scholar] [CrossRef]
- Huang, L.; Chen, D.; Zhang, H.; Song, Y.; Chen, H.; Tang, M. Funneliformis mosseae enhances root development and Pb phytostabilization in Robinia pseudoacacia in Pb-contaminated soil. Front. Microbiol. 2019, 10, 2591. [Google Scholar] [CrossRef]





| Phosphorus | Potassium | ||
|---|---|---|---|
| Big Bluestem | Switchgrass | Big Bluestem | Switchgrass |
| 0 | 0 | 0 | 0 |
| 29 | 29 | 70 | 70 |
| 44 | 44 | 140 | 140 |
| 88 | 88 | 257 | 257 |
| Springfield | Knoxville | |||
|---|---|---|---|---|
| Year | First Harvest | Second Harvest | First Harvest | Second Harvest |
| 2013 | 20 June † | 15 August | 11 June | 14 August |
| 2014 | 20 June | 19 November ⊥ | 3 June | 11 December |
| 2015 | 27 May | 7 December | 2 June | 30 July |
| 2016 | 7 June | 3 August | 25 May | 1 August |
| 2017 | 7 June | 28 November | 2 June | 11 December |
| 2018 | 28 June | 11 December | 15 June | 6 September |
| 2019 | 8 July | 5 December | 14 June | 19 August |
| Potassium | Phosphorus | |||
|---|---|---|---|---|
| Main Effect | F Value | p > F | F Value | p > F |
| Big Bluestem | ||||
| Rate | 1.92 | 0.159 | 0.11 | 0.953 |
| Year | 49.62 | <0.001 | 28.29 | <0.001 |
| Year × rate | 0.62 | 0.828 | 0.41 | 0.984 |
| Switchgrass | ||||
| Rate | 0.78 | 0.505 | 1.35 | 0.259 |
| Year | 40.89 | <0.001 | 20.11 | <0.001 |
| Year × rate | 0.49 | 0.959 | 0.32 | 0.996 |
| Fertilizer | Rate | Big Bluestem | Switchgrass |
|---|---|---|---|
| Potassium | 0 | 8.45 | 11.76 |
| 70 | 9.34 | 12.55 | |
| 140 | 8.33 | 12.32 | |
| 257 | - | 12.33 | |
| Phosphorus | |||
| 0 | 8.65 | 9.99 | |
| 29 | 8.86 | 9.95 | |
| 44 | 8.57 | 10.57 | |
| 88 | 8.75 | 11.03 | |
| Big Bluestem (Phosphorus) | Switchgrass (Phosphorus) | Switchgrass (Potassium) | ||||
|---|---|---|---|---|---|---|
| Effect | F Value | p > F | F Value | p > F | F Value | p > F |
| Crude Protein | ||||||
| Rate | 0.78 | 0.512 | 0.80 | 0.504 | 2.05 | 0.123 |
| Year | 191.71 | <0.001 | 56.23 | <0.001 | 65.55 | <0.001 |
| Rate × year | 1.58 | 0.138 | 1.02 | 0.453 | 0.69 | 0.752 |
| Total Digestible Nutrient | ||||||
| Rate | 0.43 | 0.731 | 0.69 | 0.563 | 0.18 | 0.912 |
| Year | 110.13 | <0.001 | 56.67 | <0.001 | 60.29 | <0.001 |
| Rate × Year | 0.42 | 0.947 | 0.69 | 0.749 | 0.53 | 0.884 |
| Total Colonization | Hyphal Colonization | Arbuscule Colonization | Vesicle Colonization | |||||
|---|---|---|---|---|---|---|---|---|
| F value | p > F | F Value | p > F | F Value | p > F | F Value | p > F | |
| Grass species | 0.03 | NS | 0.02 | NS | 1.46 | NS | 0.28 | NS |
| P rate | 4.91 | 0.02 | 2.20 | NS | 2.89 | NS | 0.26 | NS |
| Grass × P rate | 1.63 | NS | 0.46 | NS | 0.14 | NS | 2.30 | NS |
| Grass Species Main Effect | Colonization (Percentage) |
|---|---|
| Switchgrass | 61.9 |
| Big bluestem | 63.0 |
| P rate main effect | |
| 0 P | 58.9 b † |
| 29 P | 58.3 b |
| 88 P | 70.2 a |
| SG 0 P | SG 29P | SG 88 P | BB 0 P | BB 29 P | BB 88 P | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fungal Species | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2018 | 2018 | 2018 |
| Paraglomus occultum | + | + | + | + | + | + | + | + | + |
| Septoglomus deserticola | + | + | + | + | + | + | + | + | + |
| Cetrospora pellucida | + | + | + | + | + | + | + | ||
| Funneliformis mosseae | + | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisangwa, E.; Richwine, J.D.; Keyser, P.D.; Ashworth, A.J.; Butler, D.M.; Shrestha, U.; Walker, F.R. Native Warm-Season Grasses Show Limited Response to Phosphorus and Potassium. Agronomy 2025, 15, 669. https://doi.org/10.3390/agronomy15030669
Bisangwa E, Richwine JD, Keyser PD, Ashworth AJ, Butler DM, Shrestha U, Walker FR. Native Warm-Season Grasses Show Limited Response to Phosphorus and Potassium. Agronomy. 2025; 15(3):669. https://doi.org/10.3390/agronomy15030669
Chicago/Turabian StyleBisangwa, Eric, Jonathan D. Richwine, Patrick D. Keyser, Amanda J. Ashworth, David M. Butler, Utsala Shrestha, and Forbes R. Walker. 2025. "Native Warm-Season Grasses Show Limited Response to Phosphorus and Potassium" Agronomy 15, no. 3: 669. https://doi.org/10.3390/agronomy15030669
APA StyleBisangwa, E., Richwine, J. D., Keyser, P. D., Ashworth, A. J., Butler, D. M., Shrestha, U., & Walker, F. R. (2025). Native Warm-Season Grasses Show Limited Response to Phosphorus and Potassium. Agronomy, 15(3), 669. https://doi.org/10.3390/agronomy15030669






