Impact of Agricultural Land Use on Organic Carbon Content in the Surface Layer of Fluvisols in the Vistula River Floodplains, Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Soil Sample Analyses and Laboratory Methods
- Cdec—carbon after decalcification (24 h) was achieved with 0.05 M HCl (1:10 w/v). After centrifugation, the residue was washed with distilled water until neutral.
- CHA+FA—carbon of the humic and fulvic acids after extraction (24 h) with a 0.5 M NaOH solution (with occasional mixing, followed by centrifugation).
- CFA—carbon of the fulvic acids (the resulting alkaline extract was acidified (for 24 h) with a 2 M HCl solution to pH = 2) after the precipitation of humic acids (CHA) through centrifugation.
- Chumin—carbon of humin was calculated as the difference between the content of TOC and the C content in the respective fractions.
2.3. Statistical Analyses
3. Results
3.1. Basic Parameters of Soil Samples Collected from Arable Soils and Grasslands
3.2. Properties of SOC and Humic Substances in the Surface Layer
3.3. Statistical Anaysis Results
4. Discussion
4.1. Description of Basic Parameters of Soil Samples Collected from Floodplains
4.2. Properties of SOC and Humic Substances in the Surface Layer
4.3. Recommendations for Improving Carbon Sequestration
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adler, P.R.; Grosso, S.J.D.; Parton, W.J. Life-cycle assessment of net greenhouse-gas flux for bioenergy cropping systems. Ecol. Appl. 2007, 17, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Van Oost, K.; Quine, T.; Govers, G.; De Gryze, S.; Six, J.; Harden, J.; Ritchie, J.C.; McCarty, G.W.; Heckrath, G.; Kosmas, C.; et al. The impact of agricultural soil erosion on the global carbon cycle. Science 2007, 318, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Beillouin, D.; Corbeels, M.; Demenois, J.; Berre, D.; Boyer, A.; Fallot, A.; Feder, F.; Cardinael, R. A global meta-analysis of soil organic carbon in the Anthropocene. Nat. Commun. 2023, 14, 3700. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Hedlund, K.; Jackson, L.E.; Kätterer, T.; Lugato, E.; Thomsen, I.K.; Bracht Jörgensen, H.; Söderström, B. What are the effects of agricultural management on organic carbon soil in boreo-temperate systems? Environ. Evid. 2015, 4, 23. [Google Scholar] [CrossRef]
- Ludwig, B.; Geisseler, D.; Michel, K.; Joergensen, R.G.; Schulz, E.; Merbach, I.; Raupp, J.; Rauber, R.; Hu, K.; Niu, L.; et al. Effects of fertilization and soil management on crop yields and carbon stabilization in soils. A review. Agron. Sust. Developm. 2011, 31, 361–372. [Google Scholar] [CrossRef]
- Dignac, M.F.; Derrien, D.; Barré, P.; Barot, S.; Cécillon, L.; Chenu, C.; Chevallier, T.; Freschet, G.T.; Garnier, P.; Guenet, B.; et al. Increasing soil carbon storage: Mechanisms, effects of agricultural practices and proxies. A review. Agron. Sustain. Dev. 2017, 37, 1–27. [Google Scholar] [CrossRef]
- Amelung, W.; Bossio, D.; de Vries, W.; Kögel-Knabner, I.; Lehmann, J.; Amundson, R.; Bol, R.; Collins, C.; Lal, R.; Leifeld, J.; et al. Towards a global-scale soil climate mitigation strategy. Nat. Commun. 2020, 27, 5427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Labaz, B.; Kowalska, J.B.; Kabala, C.; Kobierski, M.; Waroszewski, J.; Dudek, M.; Szopka, K.; Gruszka, D. Distribution and Pools of Soil Organic Carbon in Chernozemic Soils Impacted by Intensive Farming and Erosion in the Loess Plateau in South-East Poland. Agronomy 2024, 14, 2544. [Google Scholar] [CrossRef]
- Groß, A.; Glaser, B. Meta-analysis on how manure application changes soil organic carbon storage. Sci. Rep. 2021, 11, 5516. [Google Scholar] [CrossRef]
- Rodrigo-Comino, J.; Keshavarzi, A.; González, J. Evaluating soil quality status of fluvisols at the regional scale: A multidisciplinary approach crossing multiple variables. River Res. Appl. 2021, 39, 1367–1381. [Google Scholar] [CrossRef]
- Seitz, D.; Fischer, L.; Dechow, R.; Wiesmeier, M.; Don, A. The potential of cover crops to increase soil organic carbon storage in german croplands. Plant Soil 2022, 488, 157–173. [Google Scholar] [CrossRef]
- Morya, R.; Bhargava, A.; Lalitha, G.R.; Vamshi, M.; Verma, S.; Rastogi, M.; Raj, S. Soil Management Practices to Enhance Carbon Sequestration Rates- A Review. Int. J. Environ. Clim. 2023, 13, 3762–3776. [Google Scholar] [CrossRef]
- Rodrigues, C.I.D.; Brito, L.M.; Nunes, L.J.R. Soil Carbon Sequestration in the Context of Climate Change Mitigation: A Review. Soil Syst. 2023, 7, 64. [Google Scholar] [CrossRef]
- Tobiašová, E.; Lemanowicz, J.; Debska, B.; Kunkelová, M.; Sakác, J. The Effect of reduced and conventional tillage systems on soil aggregates and organic carbon parameters of different soil types. Agriculture 2023, 13, 818. [Google Scholar] [CrossRef]
- Baker, J.M.; Ochsner, T.E.; Venterea, R.T.; Griffis, T.J. Tillage and soil carbon sequestration—What do we really know? Agric. Ecosyst. Environ. 2007, 118, 1–5. [Google Scholar] [CrossRef]
- Yang, X.; Li, P.; Zhang, S.; Sun, B.; Xinping, C. Long-term-fertilization effects on soil organic carbon, physical properties, and wheat yield of a loess soil. J. Plant Nutr. Soil Sci. 2011, 174, 775–784. [Google Scholar] [CrossRef]
- Labaz, B.; Hartemink, A.E.; Zhang, Y.; Stevenson, A.; Kabała, C. Organic carbon in Mollisols of the world—A review. Geoderma 2024, 447, 116937. [Google Scholar] [CrossRef]
- Powlson, D.S.; Bhogal, A.; Chambers, B.J.; Coleman, K.; Macdonald, A.J.; Goulding, K.W.T.; Whitmore, A.P. The potential to increase soil carbon stocks through reduced tillage or organic material additions in England and Wales: A case study. Agric. Ecosyst. Environ. 2012, 146, 23–33. [Google Scholar] [CrossRef]
- Powlson, D.S.; Stirling, C.M.; Thierfelder, C.; White, R.P.; Jat, M.L. Does conservation agriculture deliver climate change mitigation through soil carbon sequestration in tropical agroecosystems? Agric. Ecosyst. Environ. 2016, 220, 164–174. [Google Scholar] [CrossRef]
- Lal, R. Soil management for carbon sequestration. S. Afr. J. Plant Soil. 2021, 38, 231–237. [Google Scholar] [CrossRef]
- Moinet, G.Y.K.; Hijbeek, R.; van Vuuren, D.P.; Giller, K.E. Carbon for soils, not soils for carbon. Glob. Change Biol. 2023, 29, 2384–2398. [Google Scholar] [CrossRef] [PubMed]
- Furtak, K.; Gawryjołek, K.; Marzec-Grządziel, A.; Niedźwiecki, J. The influence of human agricultural activities on the quality of selected fluvisols from the Vistula River valley, Poland–preliminary research. Agronomy 2024, 14, 480. [Google Scholar] [CrossRef]
- Lal, R. Societal value of soil carbon. J. Soils Water Conserv. 2014, 69, 186A–192A. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil Structure and Management: A Review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Grandy, A.S.; Robertson, G.P. Land-Use Intensity Effects on Soil Organic Carbon Accumulation Rates and Mechanisms. Ecosystems 2007, 10, 59–74. [Google Scholar] [CrossRef]
- Schrumpf, M.; Kaiser, K.; Guggenberger, G.; Persson, T.; Kögel-Knabner, I.; Schulze, E.-D. Storage and Stability of Organic Carbon in Soils as Related to Depth, Occlusion within Aggregates, and Attachment to Minerals. Biogeosciences 2013, 10, 1675–1691. [Google Scholar] [CrossRef]
- Giannetta, B.; Plaza, C.; Zaccone, C.; Vischetti, C.; Rovira, P. Ecosystem Type Effects on the Stabilization of Organic Matter in Soils: Combining Size Fractionation with Sequential Chemical Extractions. Geoderma 2019, 353, 423–434. [Google Scholar] [CrossRef]
- Angst, G.; Messinger, J.; Greiner, M.; Häusler, W.; Hertel, D.; Kirfel, K.; Kögel-Knabner, I.; Leuschner, C.; Rethemeyer, J.; Mueller, C.W. Soil Organic Carbon Stocks in Topsoil and Subsoil Controlled by Parent Material, Carbon Input in the Rhizosphere, and Microbial-Derived Compounds. Soil Biol. Biochem. 2018, 122, 19–30. [Google Scholar] [CrossRef]
- Gajić, B. Physical properties and organic matter of Fluvisols under forest, grassland, and 100 years of conventional tillage. Geoderma 2013, 200–201, 114–119. [Google Scholar] [CrossRef]
- Ellert, B.H.; Bethany, J.R. Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Can. J. Soil Sci. 1995, 75, 529–538. [Google Scholar] [CrossRef]
- Gifford, R.M.; Roderick, M.L. Soil carbon stocks and bulk density: Spatial or cumulative mass coordinates as a basis of expression? Glob. Change Biol. 2003, 9, 1507–1514. [Google Scholar] [CrossRef]
- Lee, J.; Hopmans, J.W.; Rolston, D.E.; Baer, S.G.; Six, J. Determining soil carbon stock changes: Simple bulk density corrections fail. Agric. Ecosyst. Environ. 2009, 134, 251–256. [Google Scholar] [CrossRef]
- Wendta, J.W.; Hauserb, S. An equivalent soil mass procedure for monitoring soil organic carbon in multiple soil layers. Eur. J. Soil Sci. 2013, 64, 58–65. [Google Scholar] [CrossRef]
- Lal, R.; Delgado, J.; Groffman, P.; Millar, N.; Dell, C.; Rotz, A. Management to mitigate and adapt to climate change. J. Soil Water Conserv. 2011, 66, 276–285. [Google Scholar] [CrossRef]
- Conant, R.T.; Cerri, C.E.P.; Osborne, B.B.; Paustian, K. Grassland management impacts on soil carbon stocks: A new synthesis. Ecol. Appl. 2016, 27, 662–668. [Google Scholar] [CrossRef]
- Oldfield, E.E.; Bradford, M.A.; Wood, S.A. Global meta-analysis of the relationship between soil organic matter and crop yield. Soil 2019, 5, 15–32. [Google Scholar] [CrossRef]
- Bossio, D.A.; Cook-Patton, S.C.; Ellis, P.W.; Fargione, J.; Sanderman, J.; Smith, P.; Wood, S.; Zomer, R.J.; von Unger, M.; Emmer, I.M.; et al. The role of soil carbon in natural climate solutions. Nat. Sustain. 2020, 3, 391–398. [Google Scholar] [CrossRef]
- Batjes, N. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Betts, R.A.; Fallon, P.D.; Goldwvijk, K.K.; Ramankutty, N. Biogeophysical effects of land use on climate: Model simulations of radiative forcing and large-scale temperature change. Agric. For. Meteorol. 2007, 142, 216–233. [Google Scholar] [CrossRef]
- Jackson, R.B.; Lajtha, K.; Crow, S.E.; Hugelius, G.; Kramer, M.G.; Piñeiro, G. The Ecology of soil carbon: Pools, vulnerabilities, and biotic and abiotic controls. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 419–445. [Google Scholar] [CrossRef]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Olsen, A.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; et al. Global Carbon Budget 2020. Earth Syst. Sci. Data 2020, 12, 3269–3340. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; Courcelles, V.d.R.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–90. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Scharlemann, J.P.; Tanner, E.V.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Li, C.; Xiao, C.; Li, M.; Xu, L.; He, N. A global synthesis of patterns in soil organic matter and temperature sensitivity along the altitudinal gradient. Front. Environ. Sci. 2022, 10, 959292. [Google Scholar] [CrossRef]
- Li, J.; He, N.; Xu, L.; Chai, H.; Liu, Y.; Wang, D.; Wang, L.; Wei, X.; Xue, J.; Wen, X.; et al. Asymmetric responses of soil heterotrophic respiration to rising and decreasing temperatures. Soil Biol. Biochem. 2017, 106, 18–27. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Zheng, S.; Chen, Z.; Cao, Y.Q.; Wen, X.F.; He, N.P. Temperature sensitivity of soil microbial respiration in soils with lower substrate availability is enhanced more by labile carbon input. Soil Biol. Biochem. 2021, 154, 108148. [Google Scholar] [CrossRef]
- Schiefer, J.; Lair, G.J.; Blum, W.E.H. Potential and limits of land and soil for sustainable intensification of European agriculture. Agric. Ecosyst. Environ. 2016, 230, 283–293. [Google Scholar] [CrossRef]
- Schiefer, J.; Lair, G.J.; Lüthgens, C.; Wild, E.M.; Steier, P.; Blum, W.E.H. The increase of soil organic carbon as proposed by the “4/1000 initiative” is strongly limited by the status of soil development—A case study along a substrate age gradient in Central Europe. Sci. Total Environ. 2018, 628–629, 840–847. [Google Scholar] [CrossRef]
- Panagos, P.; Standardi, G.; Borrelli, P.; Lugato, E.; Montanarella, L.; Bosello, F. Cost of agricultural productivity loss due to soil erosion in the European Union: From direct cost evaluation approaches to the use of macroeconomic models. Land Degrad. Dev. 2018, 29, 471–484. [Google Scholar] [CrossRef]
- Díaz, S.; Fargione, J.; Chapin, F.S., III; Tilman, D. Biodiversity loss threatens human well-being. PLoS Biol. 2006, 4, e277. [Google Scholar] [CrossRef] [PubMed]
- West, T.O.; Post, W.M. Soil organic carbon sequestration rates by tillage and crop rotation. Soil Sci. Soc. Am. J. 2002, 66, 1930–1946. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Freibauer, A.; Rounsevell, M.D.A.; Smith, P.; Verhagen, J. Carbon sequestration in the agricultural soils of Europe. Geoderma 2004, 122, 1–23. [Google Scholar] [CrossRef]
- Batjes, N.H. Reader for the soil carbon benefits module. In Proceedings of the ISRIC Spring School; Wageningen University Campus: Wageningen, The Netherlands, 2013; pp. 1–16. [Google Scholar]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Hansen, J.; Sato, M.; Kharecha, P.; Beerling, D.; Berner, R.; Masson-Delmotte, V.; Pagani, M.; Raymo, M.; Royer, D.L.; Zaches, J.C. Target atmospheric CO2: Where should humanity aim? Open Atmos. Sci. J. 2013, 2, 217–231. [Google Scholar] [CrossRef]
- Šimanský, V. Can soil properties of Fluvisols be influenced by river flow gradient? Acta Fytotechn. Zootechn. 2018, 21, 63–76. [Google Scholar] [CrossRef]
- Kobierski, M.; Kondratowicz-Maciejewska, K.; Banach-Szott, M.; Wojewódzki, P.; Peñas Castejón, J.M. Humic substances and aggregate stability in rhizospheric and non-rhizospheric soil. J. Soils Sediments. 2018, 18, 2777–2789. [Google Scholar] [CrossRef]
- Banach-Szott, M.; Kobierski, M.; Kondratowicz-Maciejewska, K. Humic substances in Fluvisols of the Lower Vistula floodplain, North Poland. Environ. Sci. Pollut. Res. 2018, 25, 23999–24002. [Google Scholar] [CrossRef]
- Kobierski, M.; Banach-Szott, M. Organic matter in riverbank sediments and Fluvisols from the flood zones of Lower Vistula River. Agronomy 2022, 12, 536. [Google Scholar] [CrossRef]
- Weber, J.; Chen, Y.; Jamroz, E.; Miano, T. Preface: Humic substances in the environment. J. Soil Sediment. 2018, 18, 2665–2667. [Google Scholar] [CrossRef]
- Tavares, R.L.; Nahas, E. Humic fractions of forest, pasture and maize crop soils resulting from microbial activity” Brazilian journal of microbiology. Braz. J. Microbiol. 2014, 45, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.H.B.; Swift, R.S. Chapter one-Vindication of humic substances as a key component of organic matter in soil and water. In Advances in Agronomy; Donald, L.S., Ed.; Academic Press: New York, NY, USA, 2020; Volume 163, pp. 1–37. [Google Scholar] [CrossRef]
- Gerke, J. The Central Role of Soil Organic Matter in Soil Fertility and Carbon Storage. Soil Syst. 2022, 6, 33. [Google Scholar] [CrossRef]
- Doni, S.; Macci, C.; Peruzzi, E.; Ceccanti, B.; Masciandaro, G. Factors controlling carbon metabolism and humification in different soil agroecosystems. Sci. World J. 2014, 2014, 416074. [Google Scholar] [CrossRef]
- Guimarães, D.V.; Gonzaga, M.I.S.; da Silva, T.O.; da Silva, T.L.; da Silva Dias, N.; Matias, M.I.S. Soil organic matter pools and carbon fractions in soil under different land uses. Soil Tillage Res. 2013, 126, 177–182. [Google Scholar] [CrossRef]
- Tan, Y.; Yang, K.; Xu, Z.; Zhang, L.; Li, H.; You, C.; Tan, B. The Contributions of Soil Fauna to the Accumulation of Humic Substances during Litter Humification in Cold Forests. Forests 2022, 13, 1235. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Kabała, C.; Charzyński, P.; Chodorowski, J.; Drewnik, M.; Glina, B.; Greinert, A.; Hulisz, P.; Jankowski, M.; Jonczak, J.; Łabaz, B.; et al. Polish Soil Classification, 6th edition–principles, classification scheme and correlations. Soil Sci. Annu. 2019, 70, 71–97. Available online: https://repozytorium.umk.pl/handle/item/6125 (accessed on 1 January 2020). [CrossRef]
- PN-ISO 11277; Soil Quality. Determination of Particle Size Distribution in Mineral Soil Material. Method by Sieving and Sedimentation. International Organization for Standardization: Geneva, Switzerland, 2005; p. 46.
- Van Reeuwijk, L.P. Procedures for Soil Analysis; International Soil Reference and Information Centre (ISRIC): Wageningen, The Netherlands, 2002. [Google Scholar]
- Schnitzer, M. Humic substances: Chemistry and reactions. In Soil Organic Matter; Schnitzer, M., Khan, S.U., Eds.; Elsevier: New York, NY, USA, 1978; pp. 1–64. [Google Scholar]
- Schnitzer, M.; Schluppli, P. Method for the sequential extraction of organic matter from soil fractions. Soil Sci. Soc. Am. J. 1989, 53, 1418–1424. [Google Scholar] [CrossRef]
- Stolbovoy, V.; Montanarella, L.; Filippi, N.; Jones, A.; Gallego, J.; Grassi, G. Soil Sampling Protocol to Certify the Changes of Organic Carbon Stock in Mineral Soil of the European Union. Version 2; EUR 21576 EN/2; Office for Official Publications of the European Communities: Luxembourg, 2007; 56p, ISBN 978-92-79-05379-5. [Google Scholar]
- Brogowski, Z.; Kwasowski, W.; Madyniak, R. Calculating particle density, bulk density, and total porosity of soil based on its texture. Soil Sci. Ann. 2014, 65, 39–149. [Google Scholar] [CrossRef]
- Tolimir, M.; Kresović, B.; Životić, L.; Dragović, S.; Dragović, R.; Sredojević, Z.; Gajić, B. The conversion of forestland into agricultural land without appropriate measures to conserve SOM leads to the degradation of physical and rheological soil properties. Sci. Rep. 2020, 10, 13668. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Liu, X.; Xiao, L.; Li, T.; Wang, D. The response of soil organic carbon to climate and soil texture in China. Front. Earth Sci. 2022, 16, 835–845. [Google Scholar] [CrossRef]
- Bormann, H.; Klaassen, K. Seasonal and land use dependent variability of soil hydraulic and soil hydrological properties of two northern German soils. Geoderma 2008, 145, 295–302. [Google Scholar] [CrossRef]
- Yue, C.; Ciais, P.; Houghton, R.A.; Nassikas, A.A. Contribution of land use to the interannual variability of the land carbon cycle. Nat. Commun. 2020, 11, 3170. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Lei, H.; Muhammad, I.; Khan, A.; Lei, M. Do changes in land use, water bodies, and grazing pastures have a detrimental influence on environmental quality? Opportunities and threats to long-term growth. J. Environ. Manag. 2023, 325, 116609. [Google Scholar] [CrossRef]
- Borek, Ł. The Use of Different Indicators to Evaluate Chernozems Fluvisols Physical Quality in the Odra River Valley: A Case Study. Polish J. Environ. Stud. 2019, 28, 4109–4116. [Google Scholar] [CrossRef]
- Panagos, P.; De Rosa, D.; Liakos, L.; Labouyrie, M.; Borrelli, P.; Ballabio, C. Soil bulk density assessment in Europe. Agric. Ecosyst. Environ. 2024, 364, 108907. [Google Scholar] [CrossRef]
- Suchara, I.; Sucharová, J.; Holá, M. Changes in selected physico-chemical properties of floodplain soils in three different land-use types after flooding. Plant Soil Environ. 2021, 67, 99–109. [Google Scholar] [CrossRef]
- Robinson, D.A.; Thomas, A.; Reinsch, S.; Lebron, I.; Feeney, C.J.; Maskell, L.C.; Wood, C.M.; Seaton, F.M.; Emmett, B.A.; Cosby, B.J. Analytical modelling of soil porosity and bulk density across the soil organic matter and land-use continuum. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Li, X.G.; Li, F.M.; Zed, R.; Zhan, Z.Y.; Singh, B. Soil physical properties and their relations to organic carbon pools as affected by land use in an alpine pastureland. Geoderma 2007, 139, 98–105. [Google Scholar] [CrossRef]
- Kodešová, R.; Jirků, V.; Kodeš, V.; Mühlhanselová, M.; Nikodem, A.; Žigová, A. Soil structure and soil hydraulic properties of haplic luvisol used as arable land and grassland. Soil Tillage Res. 2011, 111, 154–161. [Google Scholar] [CrossRef]
- Håkansson, I.; Lipiec, J. A review of the usefulness of relative bulk density values in studies of soil structure and compaction. Soil Tillage Res. 2000, 53, 71–85. [Google Scholar] [CrossRef]
- Conant, R.T.; Paustian, K.; Elliot, E.T. Grassland management and conversion into grassland: Effects on soil carbon. Ecol. Appl. 2001, 11, 343–355. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Carbon sequestration in agricultural soils via cultivation of cover crops—A meta-analysis. Agric. Ecosyst. Environ. 2015, 200, 33–41. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A.; Vesterdal, L.; Leifeld, J.; Van Wesemael, B.; Schumacher, J.; Gensior, A. Temporal dynamics of soil organic carbon after land-use change in the temperate zone–carbon response functions as a model approach. Glob. Change Biol. 2011, 17, 2415–2427. [Google Scholar] [CrossRef]
- Zhang, J.; An, T.; Chi, F.; Dan Wei, D.; Zhou, B.; Hao, X.; Jin, l.; Wang, J. Evolution over years of structural characteristics of humic acids in Black Soil as a function of various fertilization treatments. J. Soil Sediment. 2019, 19, 1959–1969. [Google Scholar] [CrossRef]
- Kawałko, D.; Jamroz, E.; Jerzykiewicz, M.; Cwielag-Piasecka, I. Characteristics of humic acids in drained floodplain soils in temperate climates: A spectroscopic study. Sustainability 2023, 15, 11417. [Google Scholar] [CrossRef]
- Debska, B.; Kotwica, K.; Banach-Szott, M.; Spychaj-Fabisiak, E.; Tobiašová, E. Soil Fertility Improvement and Carbon Sequestration through Exogenous Organic Matter and Biostimulant Application. Agriculture 2022, 12, 1478. [Google Scholar] [CrossRef]
- Kloster, N.; Avena, M. Interaction of humic acids with soil minerals: Adsorption and surface aggregation induced by Ca2. Environ. Chem. 2015, 12, 731–738. [Google Scholar] [CrossRef]
- Dudek, M.; Łabaz, B.; Bednik, M.; Medyńska-Juraszek, A. Humic Substances as Indicator of Degradation Rate of Chernozems in South-Eastern Poland. Agronomy 2022, 12, 733. [Google Scholar] [CrossRef]
- Pittarello, M.; Dal Ferro, N.; Chiarini, F.; Morari, F.; Carletti, P. Influence of tillage and crop rotations in organic and conventional farming systems on soil organic matter, bulk density and enzymatic activities in a short-term field experiment. Agronomy 2021, 11, 724. [Google Scholar] [CrossRef]
- Kizeková, M.; Kanianska, R.; Jančová, Ľ.; Čunderlík, J.; Dugátová, Z. Carbon and nitrogen stocks in agricultural soils under different natural conditions and management in Slovakia. Land 2024, 13, 179. [Google Scholar] [CrossRef]
- Jończak, J.; Parzych, A.; Sztabkowski, K. Soil-forming processes and properties of soils developed from fluvic materials in the headwater river valleys of middle Pomerania, north Poland: A case study of the Kamienna Stream. Soil Sci. Ann. 2022, 73, 156044. [Google Scholar] [CrossRef]
- Merante, P.; Dibari, C.; Ferrise, R.; Sánchez, B.; Iglesias, A.; Lesschen, J.P.; Kuikman, P.; Yeluripati, J.; Smith, P.; Bindi, M. Adopting soil organic carbon management practices in soils of varying quality: Implications and perspectives in Europe. Soil Tillage Res. 2017, 165, 95–106. [Google Scholar] [CrossRef]
- Shen, X.; Wang, L.; Yang, Q.; Xiu, W.; Li, G.; Zhao, J.; Zhang, G. Dynamics of soil organic carbon and labile carbon fractions in soil aggregates affected by different tillage managements. Sustainability 2021, 13, 1541. [Google Scholar] [CrossRef]
- Gabryszuk, M.; Barszczewski, J.; Wróbel, B. Characteristics of grasslands and their use in Poland. J. Water Land Dev. 2021, 51, 243–249. [Google Scholar] [CrossRef]
- Kitczak, T.; Podlasiński, M.; Jarnuszewski, G.; Malinowski, R. Changes within permanent grasslands used for agriculture in the West Pomeranian Voivodship. J. Water Land Dev. 2023, 59, 108–117. [Google Scholar] [CrossRef]
- Egoh, B.; Bengtsson, J.; Lindborg, R.; Bullock, J.M.; Dixon, A.P.; Rouget, M. The importance of grasslands in providing ecosystem services: Opportunities for poverty alleviation. In Routledge Handbook of Ecosystem Services; Potschin, M., Haines-Young, R., Fish, R., Turner, R.K., Eds.; Routledge: London, UK; New York, NY, USA, 2016; pp. 421–441. [Google Scholar]
- Leclère, D.; Obersteiner, M.; Barrett, M.; Butchart, S.H.M.; Chaudhary, A.; De Palma, A.; DeClerck, F.A.J.; Di Marco, M.; Doelman, J.C.; Dürauer, M.; et al. Bending the curve of terrestrial biodiversity needs an integrated strategy. Nature 2020, 585, 551–556. [Google Scholar] [CrossRef]
- Habel, J.C.; Dengler, J.; Janišová, M.; Török, P.; Wellstein, C.; Wiezik, M. European grassland ecosystems: Threatened hotspots of biodiversity. Biodivers. Conserv. 2013, 22, 2131–2138. [Google Scholar] [CrossRef]
- Bengtsson, J.J.; Bullock, M.; Egoh, B.; Everson, C.; Everson, T.; O’Connor, T.; O’Farrell, P.J.; Smith, H.G.; Lindborg, R. Grasslands—More important for ecosystem services than you might think. Ecosphere 2019, 10, e02582. [Google Scholar] [CrossRef]
- Brown, L.E.; Ramchunder, S.J.; Beadle, J.M.; Holden, J. Macroinvertebrate community assembly in pools created during peatland restoration. Sci. Total Environ. 2016, 569–570, 361–372. [Google Scholar] [CrossRef] [PubMed]
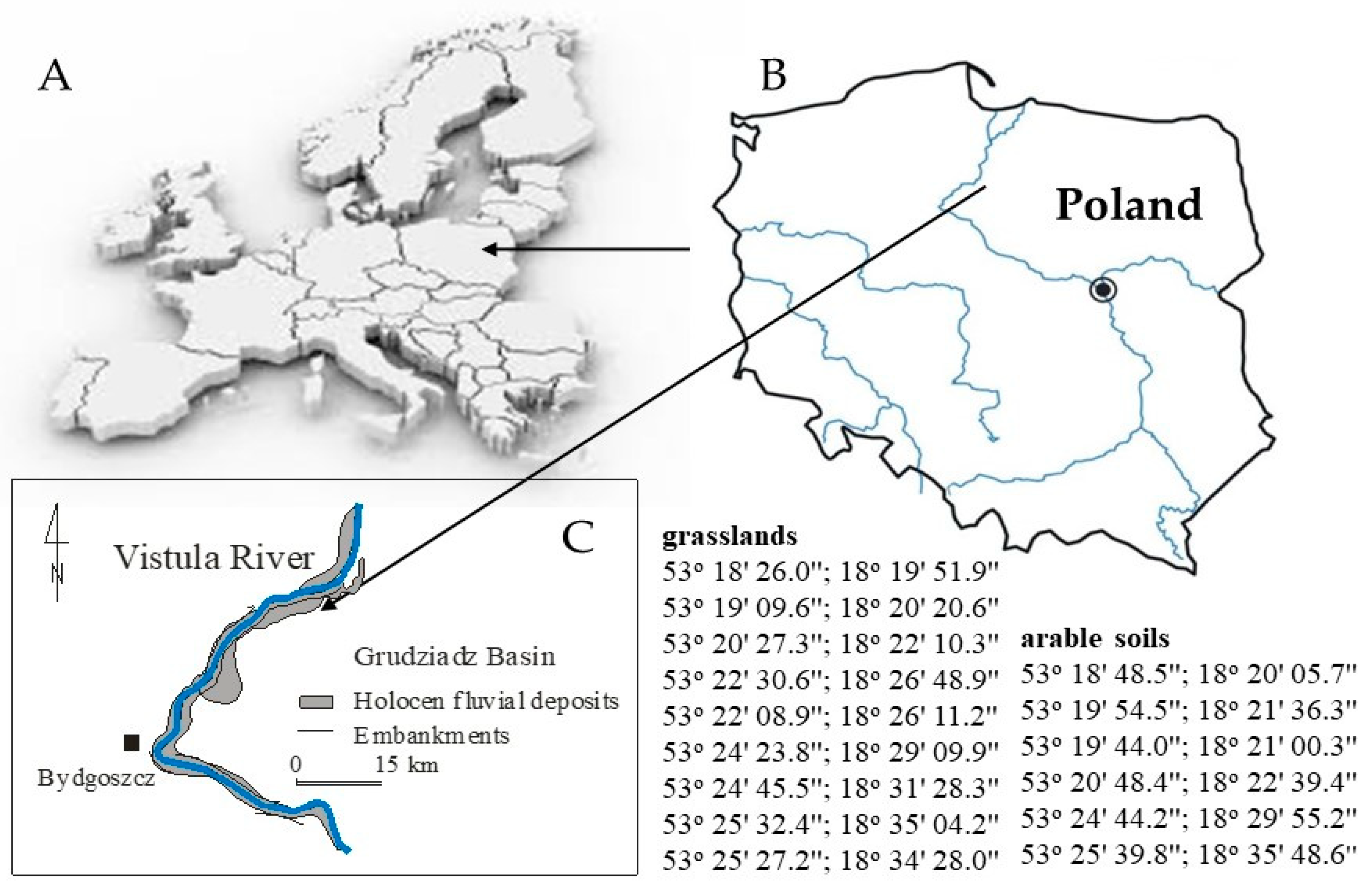


| Parameters | Arable Soils | Grasslands | Significance Level |
|---|---|---|---|
| Sand (%) 2.0–0.05 mm | 39.5 ± 14.3 | 44.8 ± 16.0 | 0.56 |
| Silt (%) 0.05–0.002 | 43.2 ± 10.5 | 38.2 ± 17.0 | 0.43 |
| Clay (%) <0.002 mm | 17.3 ± 7.4 | 17.0 ± 5.8 | 0.93 |
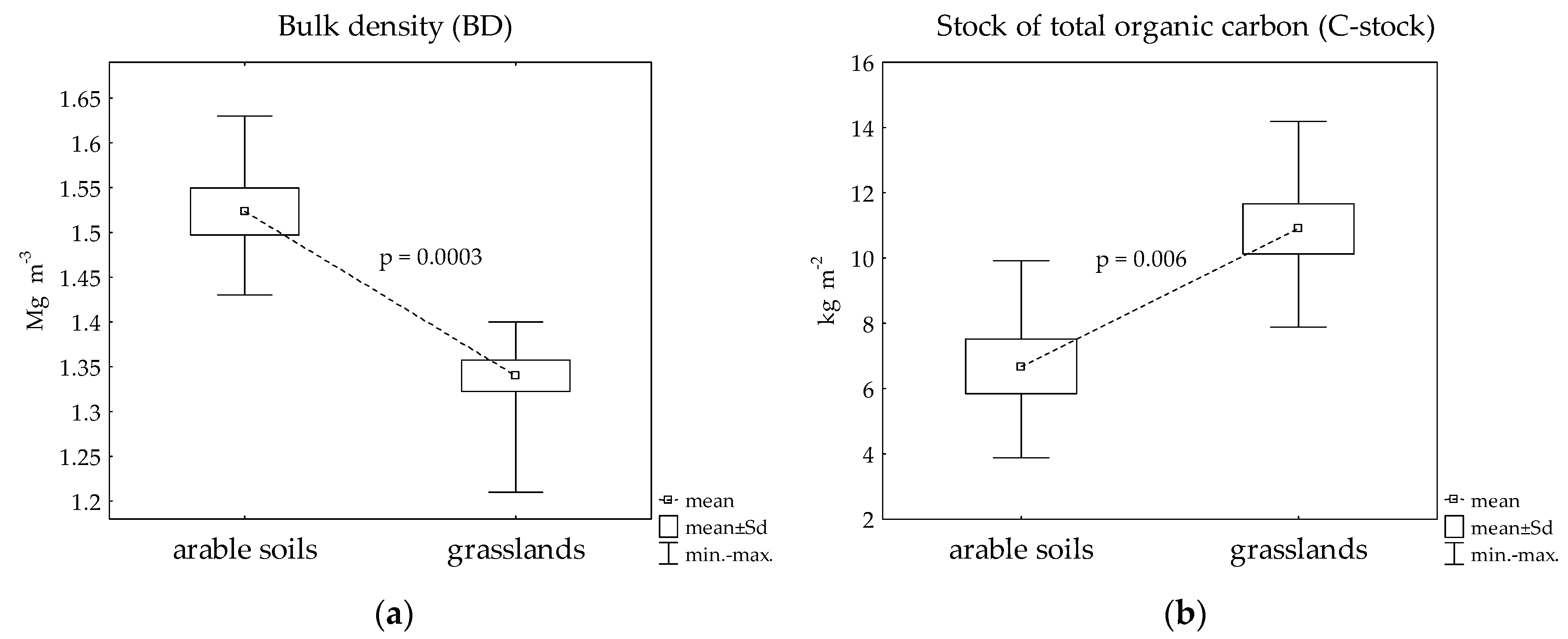
| Cumulative C density (Mg C ha−1) | 66.8 ± 20.5 | 104.1 ± 29.1 | 0.0006 * |
| Cumulative mass (kg m−2) | 445.5 ± 20.9 | 383.2 ± 18.2 | 0.0003 * |
| PD (Mg m−3) | 2.58 ± 0.035 | 2.55 ± 0.030 | 0.18 |
| TP (m3 m−3) | 0.41 ± 0.22 | 0.47 ± 0.02 | 0.0003 * |
| pH in 1M KCl | 7.23 ± 0.24 | 6.78 ± 0.35 | 0.03 * |
| CaCO3 (g kg−1) | 8.32 ± 5,7 | 6.80 ± 4.02 | 0.59 |
| EC (mS cm−1) | 0.284 ± 0.06 | 0.333 ± 0.08 | 0.28 |
| Parameters | Arable Soils | Grasslands | Significance Level |
|---|---|---|---|
| Cdec (g kg−1) | 1.15 ± 0.22 | 1.69 ± 0.35 | 0.005 * |
| CHA (g kg−1) | 4.17 ± 1.53 | 8.41 ± 2.56 | 0.003 * |
| CFA (g kg−1) | 2.66 ± 1.25 | 5.35 ± 1.82 | 0.008 * |
| Chumin (g kg−1) | 6.72 ± 1.96 | 11.81 ± 2.14 | 0.0006 * |
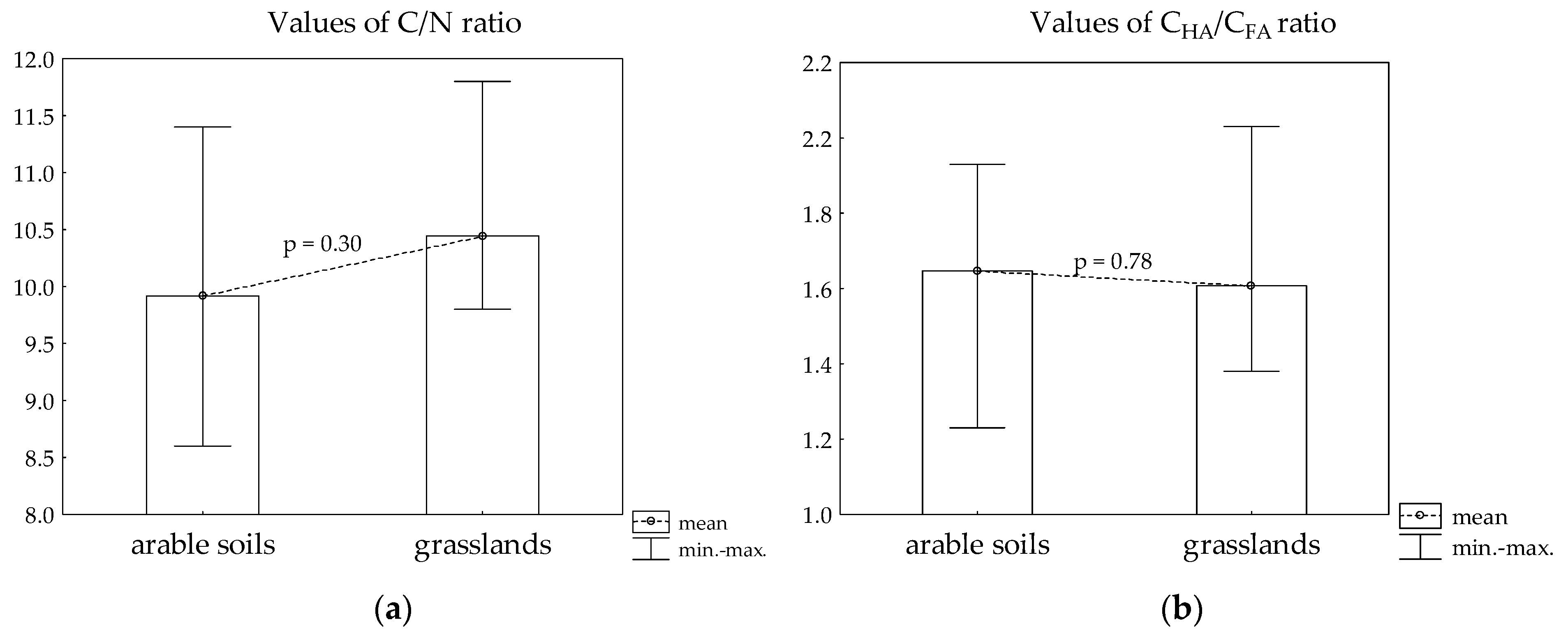
| Cdec in TOC (%) | 8.27 ± 2.06 | 6.31 ± 0.97 | 0.04 * |
| CHA in TOC (%) | 28.2 ± 2.21 | 30.5 ± 2.36 | 0.11 |
| CFA in TOC (%) | 17.5 ± 3.08 | 19.3 ± 2.84 | 0.31 |
| Chumin in TOC (%) | 46.1 ± 3.65 | 44.0 ± 3.83 | 0.34 |
| DOC (g kg−1) | 0.51 ± 0.22 | 1.15 ± 0.48 | 0.009 * |
| DON (mg kg−1) | 40.2 ± 17.0 | 68.5 ± 15.9 | 0.006 * |
| DOC in TOC (%) | 3.4 ± 0.58 | 4.09 ± 0.94 | 0.17 |
| DON in TOC (%) | 2.95 ± 0.35 | 2.77 ± 0.42 | 0.44 |
| Silt | Clay | Cdeca | CHA | CFA | CHumin | DOC | DON | BD | TP | C-stock | TOC | Nt | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sand | −0.94 * | −0.82 * | −0.08 | −0.40 | −0.45 | −0.21 | −0.27 | −0.18 | −0.12 | 0.22 | −0.41 | −0.33 | −0.38 |
| silt | 0.57 * | 0.05 | 0.32 | 0.39 | 0.14 | 0.23 | 0.08 | 0.11 | −0.19 | 0.31 | 0.27 | 0.28 | |
| clay | 0.10 | 0.42 | 0.43 | 0.26 | 0.27 | 0.31 | 0.11 | −0.20 | 0.46 | 0.35 | 0.43 | ||
| Cdeca | 0.88 * | 0.85 * | 0.92 * | 0.87 * | 0.83 * | −0.86 * | 0.81 * | 0.87 * | 0.91 * | 0.84 * | |||
| CHA | 0.97 * | 0.95 * | 0.94 * | 0.83 * | −0.80 * | 0.73 * | 0.98 * | 0.99 * | 0.98 * | ||||
| CFA | 0.91 * | 0.94 * | 0.76 * | −0.77 * | 0.69 * | 0.97 * | 0.97 * | 0.96 * | |||||
| CHumin | 0.91 * | 0.86 * | −0.87 * | 0.80 * | 0.97 * | 0.98 * | 0.95 * | ||||||
| DOC | 0.73 * | −0.79 * | 0.71 * | 0.93 * | 0.95 * | 0.92 * | |||||||
| DON | −0.78 * | 0.74 * | 0.81 * | 0.84 * | 0.79 * | ||||||||
| BD | −0.99 * | −0.78 * | −0.84 * | −0.79 * | |||||||||
| TP | 0.69 * | 0.76 * | 0.70 * | ||||||||||
| C-stock | 0.99 * | 0.99 * | |||||||||||
| TOC | 0.98 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobierski, M.; Kondratowicz-Maciejewska, K.; Labaz, B. Impact of Agricultural Land Use on Organic Carbon Content in the Surface Layer of Fluvisols in the Vistula River Floodplains, Poland. Agronomy 2025, 15, 628. https://doi.org/10.3390/agronomy15030628
Kobierski M, Kondratowicz-Maciejewska K, Labaz B. Impact of Agricultural Land Use on Organic Carbon Content in the Surface Layer of Fluvisols in the Vistula River Floodplains, Poland. Agronomy. 2025; 15(3):628. https://doi.org/10.3390/agronomy15030628
Chicago/Turabian StyleKobierski, Miroslaw, Krystyna Kondratowicz-Maciejewska, and Beata Labaz. 2025. "Impact of Agricultural Land Use on Organic Carbon Content in the Surface Layer of Fluvisols in the Vistula River Floodplains, Poland" Agronomy 15, no. 3: 628. https://doi.org/10.3390/agronomy15030628
APA StyleKobierski, M., Kondratowicz-Maciejewska, K., & Labaz, B. (2025). Impact of Agricultural Land Use on Organic Carbon Content in the Surface Layer of Fluvisols in the Vistula River Floodplains, Poland. Agronomy, 15(3), 628. https://doi.org/10.3390/agronomy15030628






