1. Introduction
Thrips (Thysanoptera) are major pests in nectarine (
Prunus persica var.
nectarina) orchards because they cause direct damage to flowers, leaves, and fruit, and also act as vectors for plant viruses such as the tomato spotted wilt virus (TSWV) [
1,
2]. Their small size, high reproductive rate, and propensity to develop insecticide resistance make them difficult to control [
3]. Reliance on synthetic insecticides has led to environmental problems, harm to non-target organisms, and evolution of resistance in thrips populations [
4,
5], thereby leading the research to more sustainable alternatives like biological control. Moreover, the continuous use of chemicals has raised concerns regarding food safety and environmental sustainability, prompting regulatory agencies and growers alike to seek integrated management approaches.
Frankliniella occidentalis (Thysanoptera: Thripidae) (Pergande, 1895), the western flower thrips, is a globally distributed and economically important pest [
6,
7,
8]. Originating in western North America, it has spread worldwide through international trade [
1,
6], including in Greece, where it was first recorded in 1988 [
9]. This polyphagous species infests more than 500 plant species across 62 families, including vegetables, fruits, and ornamentals [
10]. Damage arises not only from direct feeding and oviposition but also from indirect effects through virus transmission, particularly of TSWV [
1,
6,
10]. Feeding by larvae and adults causes necrotic lesions, chlorosis, and reduced photosynthesis, while oviposition weakens plant tissues [
10,
11,
12], leading to significant economic losses due to yield reduction and quality degradation [
12]. These multifaceted impacts underscore the critical need for effective management strategies that address both direct and indirect damage caused by thrips.
Effective management of
F. occidentalis is challenging because of its high reproductive rate, cryptic feeding habits, and widespread insecticide resistance [
1,
6,
11,
12]. Although resistance has been documented against several insecticide classes [
1,
6,
8,
10], chemical control remains the dominant strategy for thrip management. Currently, more than 20 different chemical insecticides—including organophosphates, carbamates, pyrethroids, neonicotinoids, avermectin, and spinosad—are available for various agricultural crops [
8]. However, the increasing ineffectiveness of these conventional chemical control methods, due to the development of insecticide resistance and environmental concerns, has accelerated the need for alternative sustainable pest management strategies [
10].
Integrated Pest Management (IPM) is an approach to pest control that combines various strategies to minimize the use of chemical insecticides and the environmental impact produced by agricultural practices [
1,
13,
14]. IPM is based on prevention, pest monitoring, and data-driven decision-making [
1,
11], at the same time integrating multiple control methods, such as chemical and biological techniques [
1]. Biological control plays a crucial role in IPM strategies, involving natural enemies such as entomopathogenic nematodes, entomopathogenic fungi, and predatory insects and mites [
1,
12,
14]. Recent advancements in biotechnological applications and formulation technologies have further enhanced the potential of biological control agents in modern agriculture.
Entomopathogenic nematodes (EPNs) offer a promising biological control alternative to chemical pesticides against
F. occidentalis [
15,
16,
17,
18]. These obligate insect parasites—primarily from the genera
Steinernema and
Heterorhabditis—actively search for hosts as infective juveniles (IJs) and penetrate through natural openings [
15,
17,
18]. Once inside the host, they release symbiotic bacteria (
Xenorhabdus spp. in
Steinernema and
Photorhabdus spp. in
Heterorhabditis) that cause septicemia and death within 24–72 h [
15,
16]. Several studies have demonstrated the efficacy of EPNs such as
Steinernema feltiae and
Heterorhabditis bacteriophora against
F. occidentalis [
15,
16,
17,
18]. However, EPN efficacy is influenced by biotic and abiotic factors—including nematode species, environmental conditions, application methods, and host susceptibility [
15,
16,
17,
19,
20]. One key challenge for foliar applications is that EPNs are sensitive to desiccation caused by ultraviolet (UV) radiation and low humidity [
15,
16,
18,
20]. Recent advances—such as Pickering emulsions—have improved EPN persistence and efficacy in foliar applications by enhancing moisture retention [
18]. These technological innovations not only improve the field performance of EPNs but also broaden their applicability in diverse agroecosystems facing harsh climatic conditions.
Although EPNs are generally considered safe, their impact on pollinators (especially bees) requires further investigation. Research on entomopathogenic fungi suggests minimal effects on bee mobility and foraging [
15,
21], although some studies indicate that high concentrations may negatively affect bee longevity [
15,
21,
22]. In addition, while
S. feltiae and
H. bacteriophora are typically safe for nontarget organisms, other EPN species have been linked to bee mortality [
22,
23]. Furthermore, EPN reproduction within dead bees can potentially spread infection within colonies [
23]. Given the critical role of pollinators, it is essential to mitigate potential risks by optimizing application timing and methods, selecting EPN species and concentrations that minimize nontarget effects, and using formulations that enhance nematode persistence while reducing pollinator exposure [
13,
22]. This highlights the delicate balance required in deploying biocontrol agents within an IPM framework, ensuring effective pest suppression without compromising the health of beneficial insect communities.
This study is an efficacy trial that evaluates the efficacy of EPNs against
F. occidentalis in nectarine orchards and assesses their potential nontarget effects on pollinators. In addressing the increasing insecticide resistance in
F. occidentalis and the need for sustainable solutions [
1,
6,
10], the research explores the potential of
Steinernema and
Heterorhabditis spp. [
15,
16,
17]. It also examines the challenges of EPN efficacy under field conditions, particularly for foliar applications [
15,
16,
18,
20]. Furthermore, by considering both the direct impact on pest populations and the indirect effects on crop quality, this work aims to provide a comprehensive evaluation of EPNs within an integrated pest management strategy. By investigating the interactions between EPNs and pollinators, the study aims to develop IPM strategies that maximize pest control while minimizing ecological impacts. Particularly, we hypothesize that
S. feltiae will significantly reduce
F. occidentalis populations on nectarine inflorescences and fruit damage compared to the control, while
H. bacteriophora will have a limited effect. Additionally, we expect that high exposure to certain EPN species may have negative impacts on pollinator survival. The outcomes of this research are anticipated to contribute to the refinement of biocontrol tactics and promote environmentally responsible practices in modern fruit production.
2. Materials and Methods
2.1. Thrips Field Experiment
Study Site and Plant Material
This study was conducted during the spring and summer of 2023 in a 3-hectare commercial stone fruit orchard located in Flamouria, Edessa, northern Greece (40°45′41 N, 22°01′54 E). The orchard contained 120 seven-year-old nectarine trees (Prunus persica var. nucipersica ‘Venus’) grafted onto GF 677 rootstock (Prunus persica × Prunus amygdalus). All trees in the orchard were of the same height and had undergone identical pruning practices. Consistently, over the years, the nectarine trees have been subjected to the same agricultural practices, ensuring homogeneity among them. Furthermore, soil characteristics and irrigation regimes were uniform across the orchard, minimizing confounding variables related to tree vigor and pest infestation levels.
Experimental Design and Treatments
Trees were selected based on two criteria: (1) confirmed F. occidentalis infestations, determined through pretrial crop damage assessments and population monitoring, and (2) spatial arrangement within the orchard to minimize cross-contamination between treatment groups. Overall, 32 trees were randomly assigned to two treatment groups (16 trees per group): one treated with Steinernema feltiae and the other with Heterorhabditis bacteriophora. Sixteen additional trees served as untreated controls. Border trees were excluded to minimize edge effects. For identification during spray applications, trees treated with S. feltiae were marked with red paint at the base, and those treated with H. bacteriophora were marked with white paint, while control trees remained unmarked. Randomization was performed using a computer-generated list to ensure the unbiased assignment of trees to each treatment group.
Spray Application
Two commercial EPN formulations were used: Nemasys F® (BASF S.E., Ludwigshafen, Germany) (90% S. feltiae) and Nemasys H® (BASF S.E.) (82% H. bacteriophora). Each product contained 250 million infective juveniles (IJs) per package. Initially, each product was diluted in 1 L of water and then further diluted to a final volume of 10 L. A conventional 16-L backpack sprayer (STIHL International GmbH, Waiblingen, Germany) was used for the applications of the two nematode formulations, and continuous agitation of the sprayer ensured even nematode distribution. Calibration of the sprayer was conducted prior to application to guarantee consistent flow rates and droplet sizes across treatments.
Spray applications were timed to coincide with the flowering stage of the nectarine trees. The spraying applications were applied at full bloom on 2 April 2023, early in the morning under favorable humidity conditions (above 70% relative humidity and temperature below 20 °C) to maximize nematode survival and efficacy. Weather conditions (temperature, relative humidity, and wind speed) were recorded before, during, and after application. The applications were conducted by specialized laboratory technicians, ensuring the accurate and even spraying across all treated trees and that the control trees did not receive any spray treatment.
Thrips Population Sampling
Ten inflorescences (20–30 cm in length) were randomly selected from different canopy positions on each treated tree. Sampling was conducted at four time points: (1) before the application; (2) 24 h after the application; (3) seven days after the application; and (4) fourteen days after the application. Each inflorescence was vibrated over a white paper surface to dislodge thrips, which were then transferred into vials containing 70% ethanol. A subset of the collected thrips was sent to the laboratory for species confirmation (primarily F. occidentalis) using morphological keys. Thrips numbers were recorded for each sample. In addition, sampling protocols were standardized across all trees to minimize variability introduced by the sampling methodology.
Fruit Damage Assessment
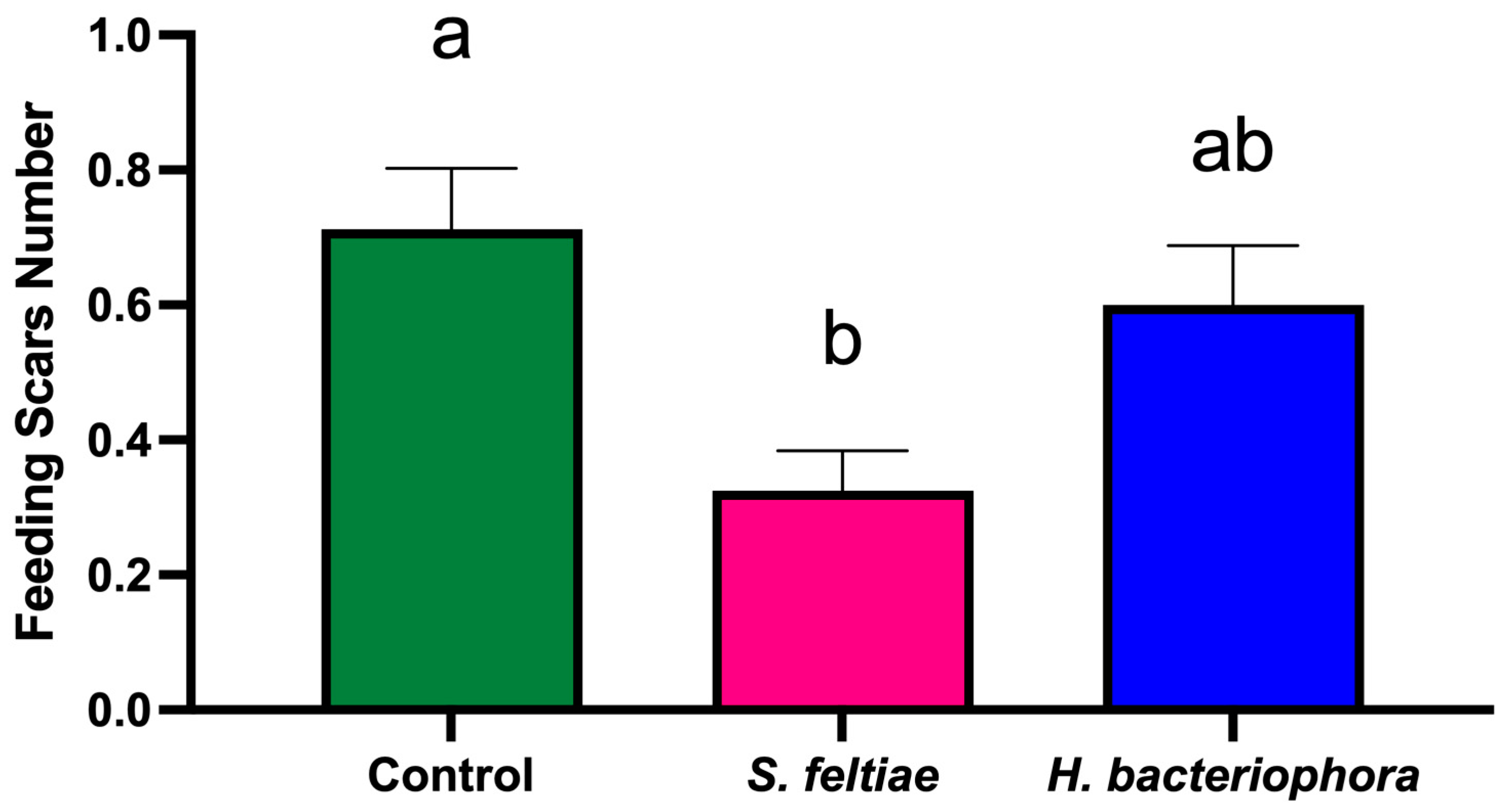
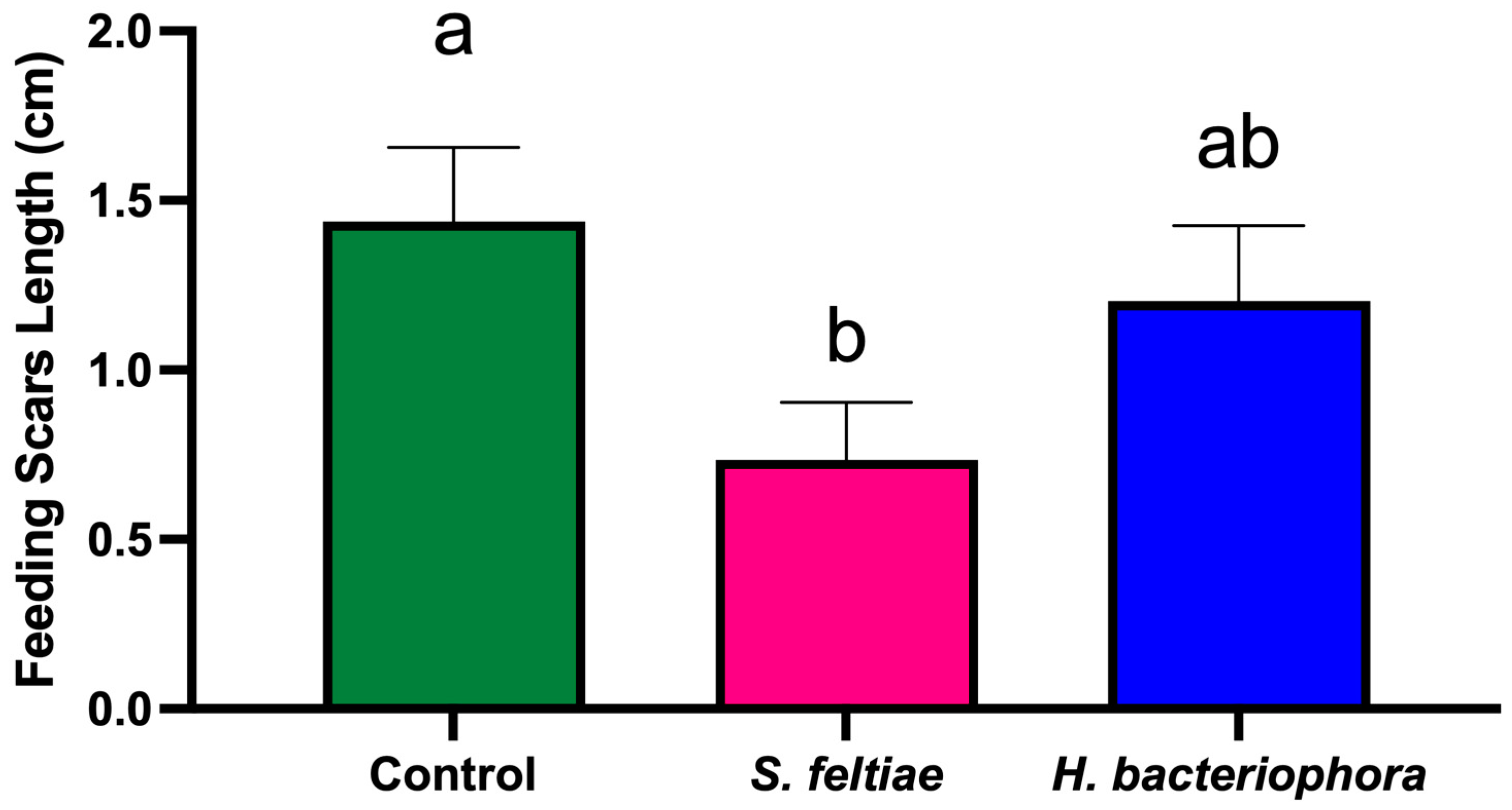
At harvest (2 August 2023), 160 fruits per treatment (10 fruits per tree) were evaluated for feeding scars. The parameters recorded for each fruit included the number of feeding scars per fruit and the length of each feeding scar (measured using calipers).
2.2. Laboratory Bioassays for Pollinator Mortality
To evaluate the potential nontarget effects of EPNs on Apis mellifera, two laboratory bioassays were conducted in 2023 and 2024, respectively, under conditions designed to simulate extreme exposure. The initial bioassay included the two EPN products used in the field experiments (Nemasys F® and Nemasys H®), as well as an untreated control. After the initial results, another bioassay was performed, in which two additional products were included: Nemasys C® (BASF S.E.) (87% Steinernema carpocapsae) and Capsanem® (Koppert B.V., Berkel en Rodenrijs, The Netherlands) (86% S. carpocapsae).
For each product, eight grams (250 million infective juveniles (IJs)) were dissolved in 1 L of water and then diluted tenfold (250 IJs/mL). Ten microliters of the final solution were applied topically to each bee using a micropipette, while control bees received 10 µL of water. A total of approximately 150 forager bees (
Apis mellifera macedonica) on average were collected from the apiary of the Agricultural University of Athens, ensuring that all bees originated from the same hive to minimize genetic variability. Bees were individually housed in ventilated Plexiglas
® containers (Polyvantis GmbH,, Weiterstadt, Germany) (10 × 10 × 10 cm
3) with ad libitum access to a syringe with 10 mL of sugar syrup (50%). The bioassay design for this efficacy trial included five replicates for each EPN product and five replicates for the control group, with 28–32 bees per replicate (yielding a total of approximately 150 honeybees in total per treatment). Bee mortality was recorded at 24, 48, and 72 h after application, and sublethal effects (e.g., reduced mobility and abnormal behavior) were also documented. A modified version of OECD Guideline 214 [
24] for acute contact toxicity testing was employed to better simulate field conditions; modifications included incorporating treated surfaces and foraging materials into the test environment. Environmental conditions were maintained at 25 ± 2 °C, 60 ± 10% relative humidity, and a 16:8 light–dark cycle. Each bioassay was independently replicated to ensure the reproducibility of the results, and control treatments were conducted concurrently to account for any background mortality.
2.3. Statistical Analyses
Data were analyzed using JMP Pro 18.0 (SAS Institute). Thrips population dynamics were analyzed using a repeated-measures analysis (treatment × time, with tree as a random effect). Fruit damage data were analyzed using one-way ANOVA. Bee mortality data were analyzed using repeated-measures analysis with a restricted maximum likelihood (REML), considering treatment and time (with cage as a random effect). Multiple comparisons were performed using Tukey’s HSD test with α = 0.05. Data were log-transformed when necessary to meet assumptions of normality and homogeneity of variance.
4. Discussion
This efficacy trial study investigated the efficacy of EPNs (
S. feltiae, S. carpocapsae, and
H. bacteriophora) for controlling
F. occidentalis in nectarine orchards while assessing their potential impact on honeybees (
A. mellifera). The significant reduction in thrips populations observed in trees treated with
S. feltiae compared to the control underscores its potential as a biocontrol agent for
F. occidentalis management—a finding that is consistent with previous research [
15,
16], where the families Steinernematidae and Heterorhabditidae have emerged as highly effective biological control agents [
14]. Moreover, it has been previously proven that
S. carpocapsae,
H. bacteriophora, and
S. feltiae are highly effective against thrips [
15,
16,
17,
20], with the latter providing the best results for controlling
F. occidentalis and
Tuta absoluta (Lepidoptera: Gelechiidae, Meyrick, 1917) [
15]. The efficacy of
S. feltiae likely stems from its active prey seeking behavior and rapid host invasion, which are enhanced by the release of symbiotic bacteria (
Xenorhabdus spp.), killing the host-insect within 48 h [
15,
25]. In contrast, the lack of significant thrips suppression by
H. bacteriophora may be due to its lower tolerance to environmental stresses (e.g., UV radiation and desiccation) [
16,
20]. Variations in environmental conditions, application methods, or strain differences might also contribute to the observed discrepancies [
17,
18]. Overall, factors such as temperature, humidity, and UV exposure appear to influence EPN efficacy [
18,
19]. Furthermore, our findings highlight that the microclimatic conditions present in commercial orchards can significantly affect the performance of biological control agents, emphasizing the need for localized field trials to determine the optimal application protocols.
The significant reduction in fruit damage, even when thrips suppression was moderate, suggests that partial pest control can substantially improve crop quality [
10,
12]. Moreover, the strategic timing of EPN applications during flowering under high-humidity conditions likely enhanced nematode persistence, supporting recommendations to optimize application conditions to counteract desiccation and UV effects [
18,
26]. This outcome not only reinforces the practical benefits of using EPNs in reducing direct pest damage but also demonstrates that even modest reductions in pest populations can lead to economically significant improvements in fruit quality and marketability.
Recent research on Africanized honeybees (
Apis mellifera L.) suggests that entomopathogenic nematodes can significantly affect pollinator longevity, depending on the species used. [
27] found that
Steinernema spp., particularly
S. feltiae and
S. rarum, led to complete mortality of worker bees within 216 h under controlled bioassay conditions, while
Heterorhabditis species demonstrated lower toxicity. These findings emphasize the importance of species selection when integrating EPNs into integrated pest management (IPM) programs, as
Steinernema species appear to present a greater risk to non-target pollinators than
Heterorhabditis species. In our study, the delayed mortality observed with
S. carpocapsae formulations further corroborates the notion that careful evaluation of EPN formulations is critical to mitigate unintended pollinator risks.
Regarding nontarget effects, laboratory bioassays revealed that although EPNs are generally considered safe,
S. carpocapsae (applied as both Nemasys C
® and Capsanem
®) and
H. bacteriophora induced delayed mortality in honeybees. This finding aligns with other studies showing that EPN toxicity can be species-specific and concentration-dependent [
21,
22]. The impact of EPNs on different
A. mellifera subspecies varies, as Africanized honeybees appear to be particularly susceptible to
Steinernema spp. exposure [
27]. Given their behavioral and physiological differences from European honeybees (
Apis mellifera ligustica), further research is needed to determine whether similar levels of susceptibility exist across other
A. mellifera subspecies. In particular, the observed mortality caused by
S. feltiae and S. rarum raises concerns about the unintended consequences of foliar EPN applications during bloom periods. These findings reinforce the necessity of optimizing application methods, such as evening sprays when pollinators are inactive, to minimize direct exposure. Additional research has linked certain EPN species to bee mortality [
22,
23], which underscores the importance of further nontarget risk assessments and the development of protective formulations. Adjusting application timing (e.g., early morning or late evening) and avoiding direct application to flowering parts of the plants could help mitigate these risks. Moreover, our results suggest that incorporating buffer zones or employing targeted application techniques may further reduce the risk of pollinator exposure in orchard settings.
In addition, in 2015, Dutka et al. [
28] conducted a study investigating the effects of commercially available formulations of EPNs on
Bombus terrestris (Hymenoptera: Apidae, Linnaeus, 1758). The results showed that exposure to formulations of
Heterorhabditis spp.,
Steinernema spp., and
Steinernema kraussei resulted in high levels of bee mortality after 72 h of exposure. Moreover, this study revealed that
S. kraussei not only caused high mortality rates but was also capable of proliferation in bumblebee bodies. This finding posed concern and encouraged further research, as this ability can potentially lead to a rapid decline in bumblebee populations and colony dynamics. These observations underscore the need for comprehensive risk assessments that evaluate both the short-term toxicity and long-term ecological impacts of EPN applications on diverse pollinator species.
At the same time, according to Erler et al. [
13], in some cases,
Steinernema affinis and
S. feltiae caused high mortality rates in
A. mellifera larvae, while in other experiments, EPN species such as
H. bacteriophora,
Heterorhabditis taysera, and
Steinernema riobravis did not show any negative effects on the survival of pollinator species, including
A. mellifera,
A. carnica and
A. linguistica. This variability highlights the complexity of EPN–pollinator interactions and suggests that formulation type, application method, and environmental context must all be carefully considered when designing biocontrol programs.
In summary, the results demonstrate that S. feltiae can effectively reduce both F. occidentalis populations and associated fruit damage, suggesting its suitability for integration into IPM programs. In contrast, H. bacteriophora showed limited efficacy under the conditions tested, possibly due to environmental sensitivity. The differential performance of these EPN species emphasizes the need for ongoing field evaluations and adaptive management strategies to optimize pest control outcomes while mitigating risks to non-target organisms. Future research should aim to optimize application methods, explore additional EPN species or strains, and assess long-term impacts on pollinator health and ecosystem services. Additionally, studies focusing on the development of advanced formulations, such as those employing microencapsulation or emulsification technologies, could further enhance the viability of EPNs in diverse agricultural settings.
5. Conclusions
This efficacy trial study provides valuable insights into the potential of S. feltiae as an effective biocontrol agent for managing F. occidentalis populations and reducing fruit damage in nectarine orchards. The significant reduction in thrips numbers per inflorescence, as well as the decreased number of feeding scars on fruits treated with S. feltiae, highlight its suitability for inclusion in integrated pest management (IPM) programs. These findings demonstrate that the application of S. feltiae can contribute not only to improved pest control but also to enhanced crop quality and economic sustainability in nectarine production systems.
In contrast, H. bacteriophora showed limited efficacy in controlling F. occidentalis populations, likely due to its sensitivity to environmental stressors, such as UV radiation and desiccation. This indicates that, while H. bacteriophora may have potential under specific controlled conditions, its performance in commercial orchard settings remains inconsistent, underscoring the need for further optimization of application techniques and environmental modifications.
Furthermore, the laboratory bioassays revealed that S. carpocapsae and, to a lesser extent, S. feltiae, can induce delayed mortality in honeybees under high-exposure conditions. These results align with prior studies on Africanized honeybees, where S. feltiae was found to cause significant mortality over time. Although species of the Heterorhabditis genus generally exhibited lower toxicity to honeybees, these findings emphasize the need for careful species selection and optimization of application methods to mitigate risks to pollinators. In addition, they highlight the importance of considering both immediate and delayed non-target effects when evaluating biocontrol agents for inclusion in IPM programs.
Future research should focus on further investigating the mechanisms underlying EPN toxicity in different honeybee subspecies and exploring mitigation strategies that ensure effective pest control without compromising pollinator health and survival. Such studies could include the development of novel formulations and application protocols (e.g., targeted foliar sprays or soil applications) that maximize pest suppression while minimizing exposure to non-target organisms.
Overall, this study highlights the importance of selecting appropriate EPN species and refining application methods to maximize efficacy against F. occidentalis while safeguarding pollinators. By integrating these biocontrol agents into robust IPM strategies, growers can achieve sustainable pest management that supports both agricultural productivity and environmental conservation.