Contribution of Biological Nitrogen Fixation to the Biomass Productivity of Elephant Grass Grown in Low-Fertility Soil for Energy Purposes
Abstract
1. Introduction
2. Materials and Methods
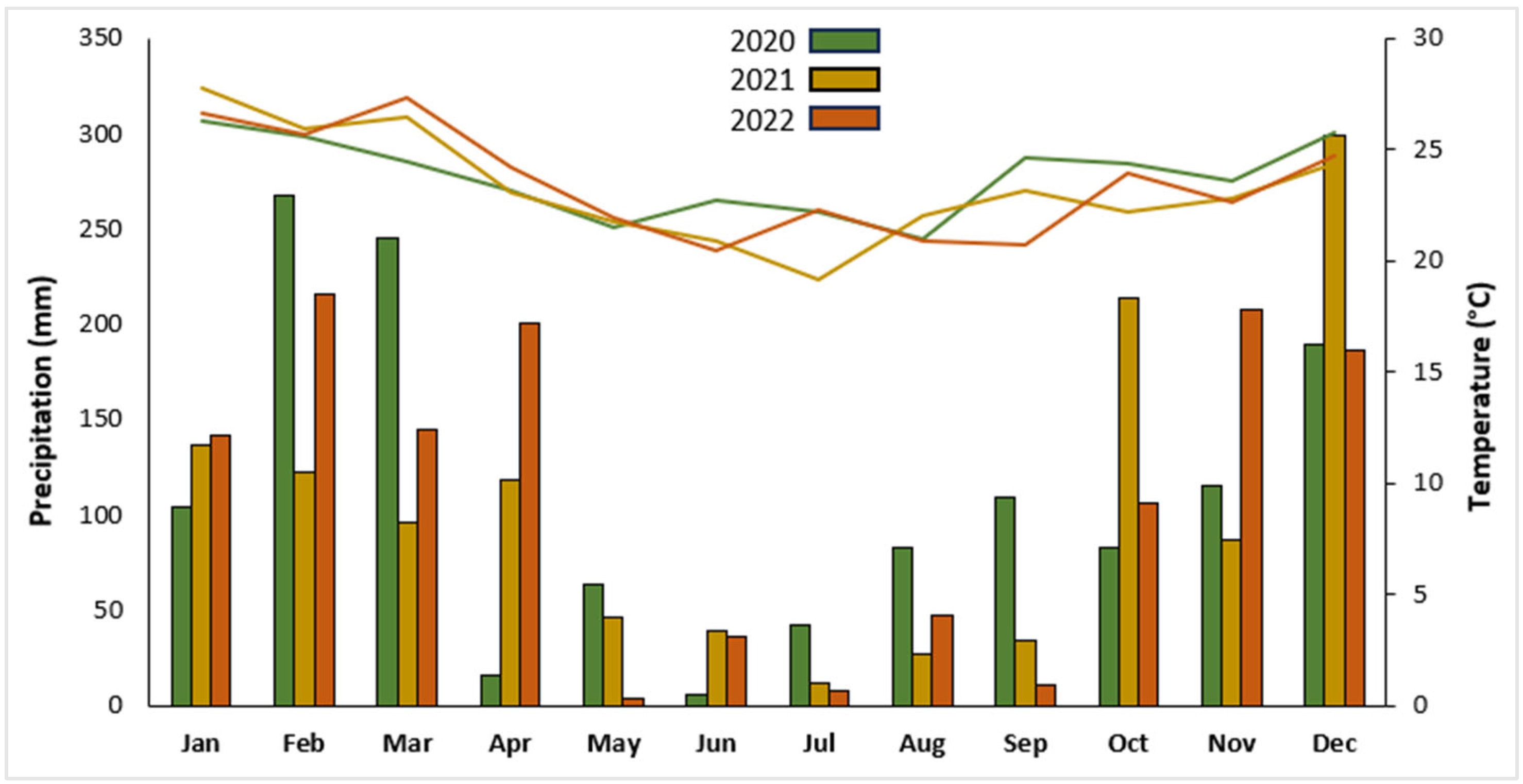
2.1. Plant Material and Experimental Conditions
2.2. Determination of Fresh Mass and Dry Matter Productivity
2.3. Determination of Accumulated N in Plants
2.4. Determination of the Contribution of BNF and BNF-Derived N
2.5. Statistical Analyses
3. Results
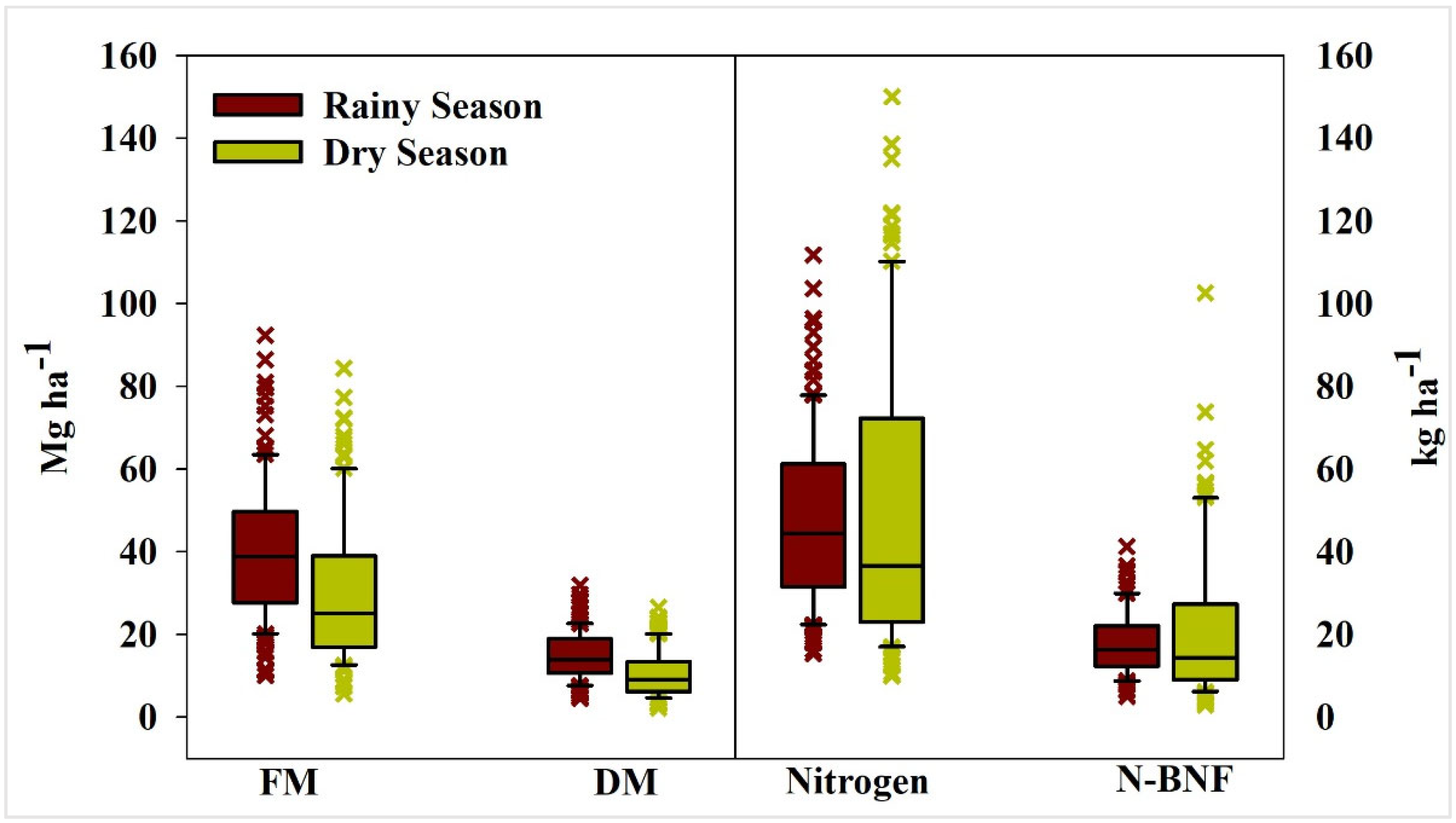
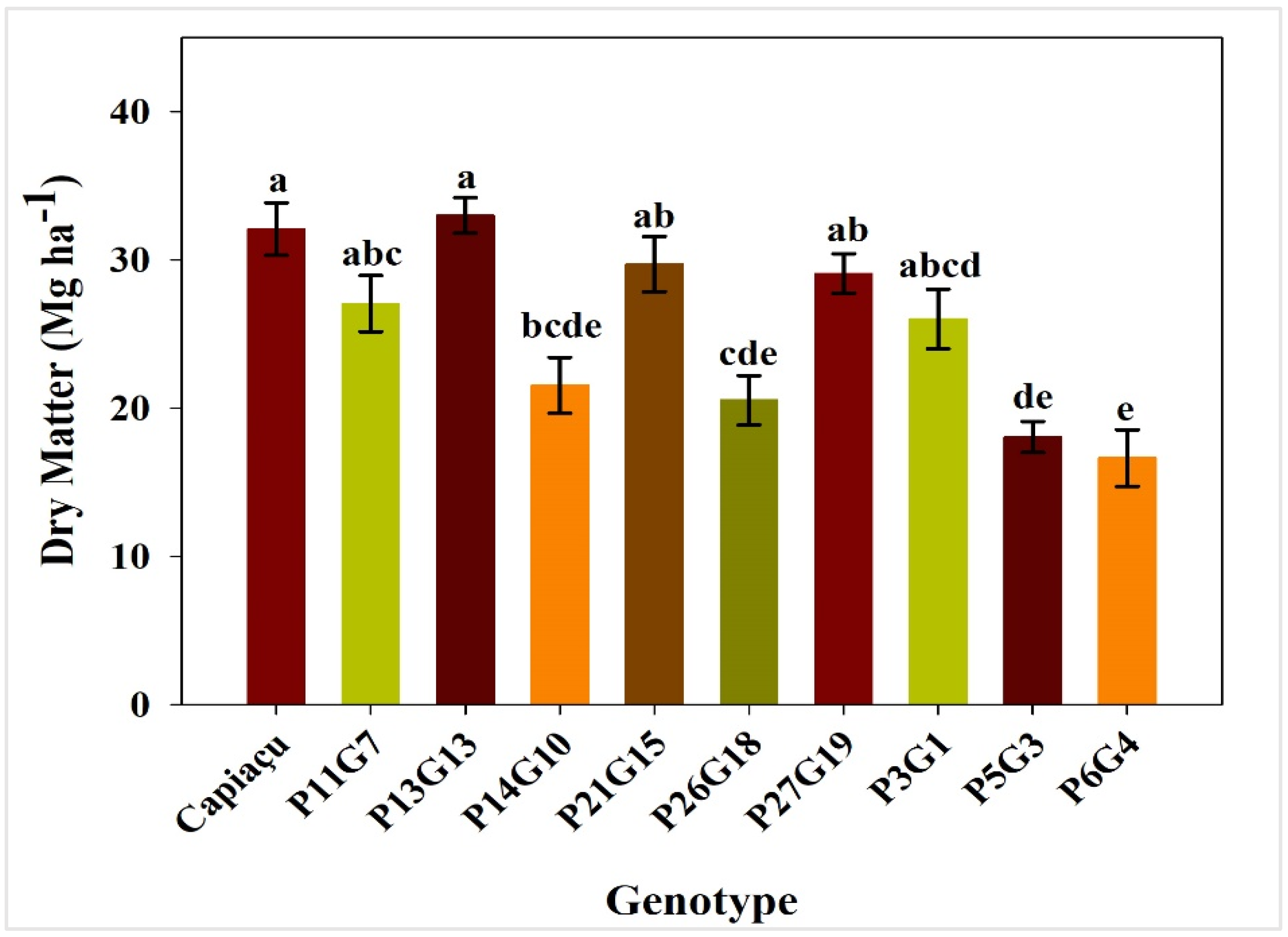
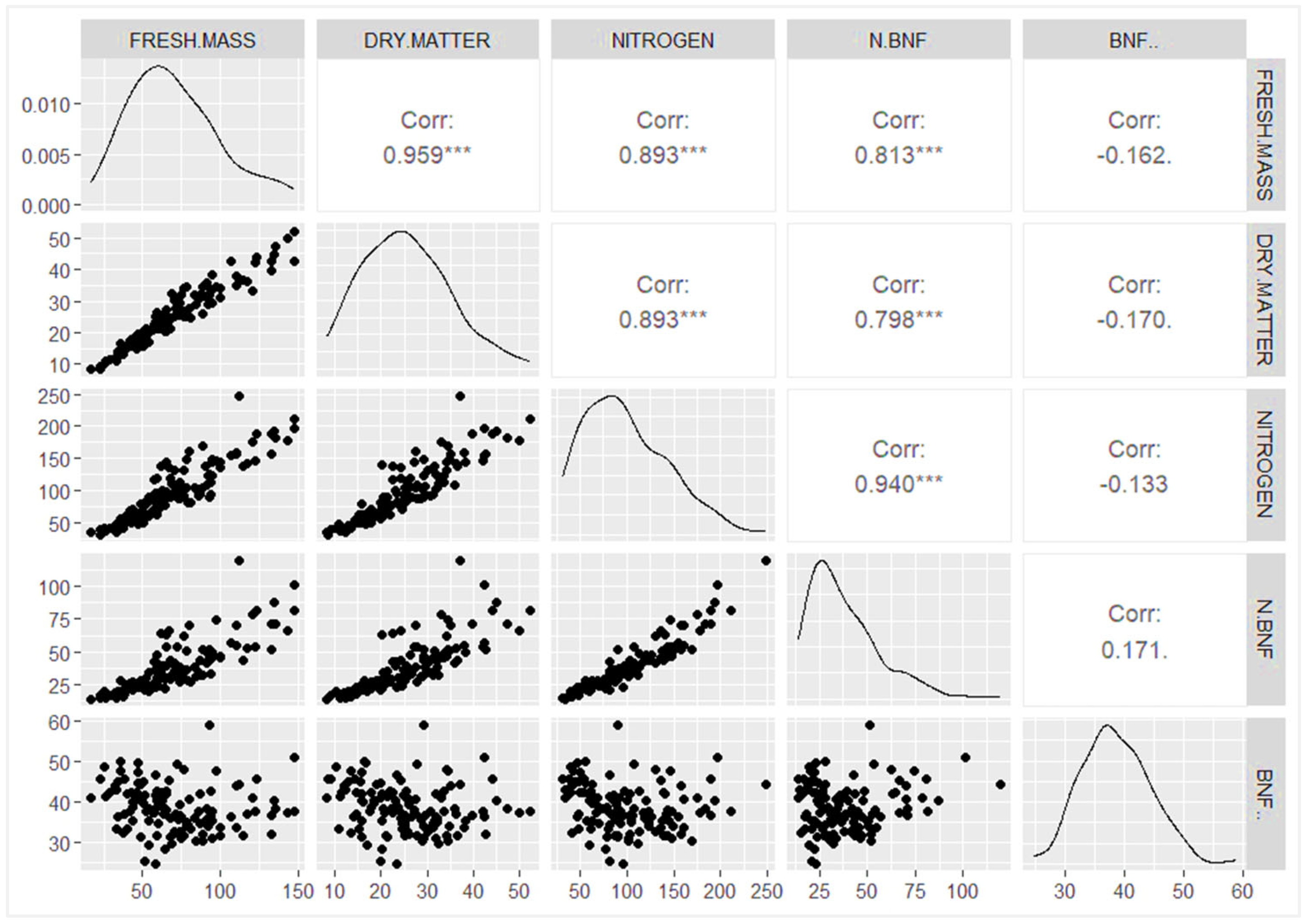
3.1. Fresh Mass Productivity and Dry Matter Productivity
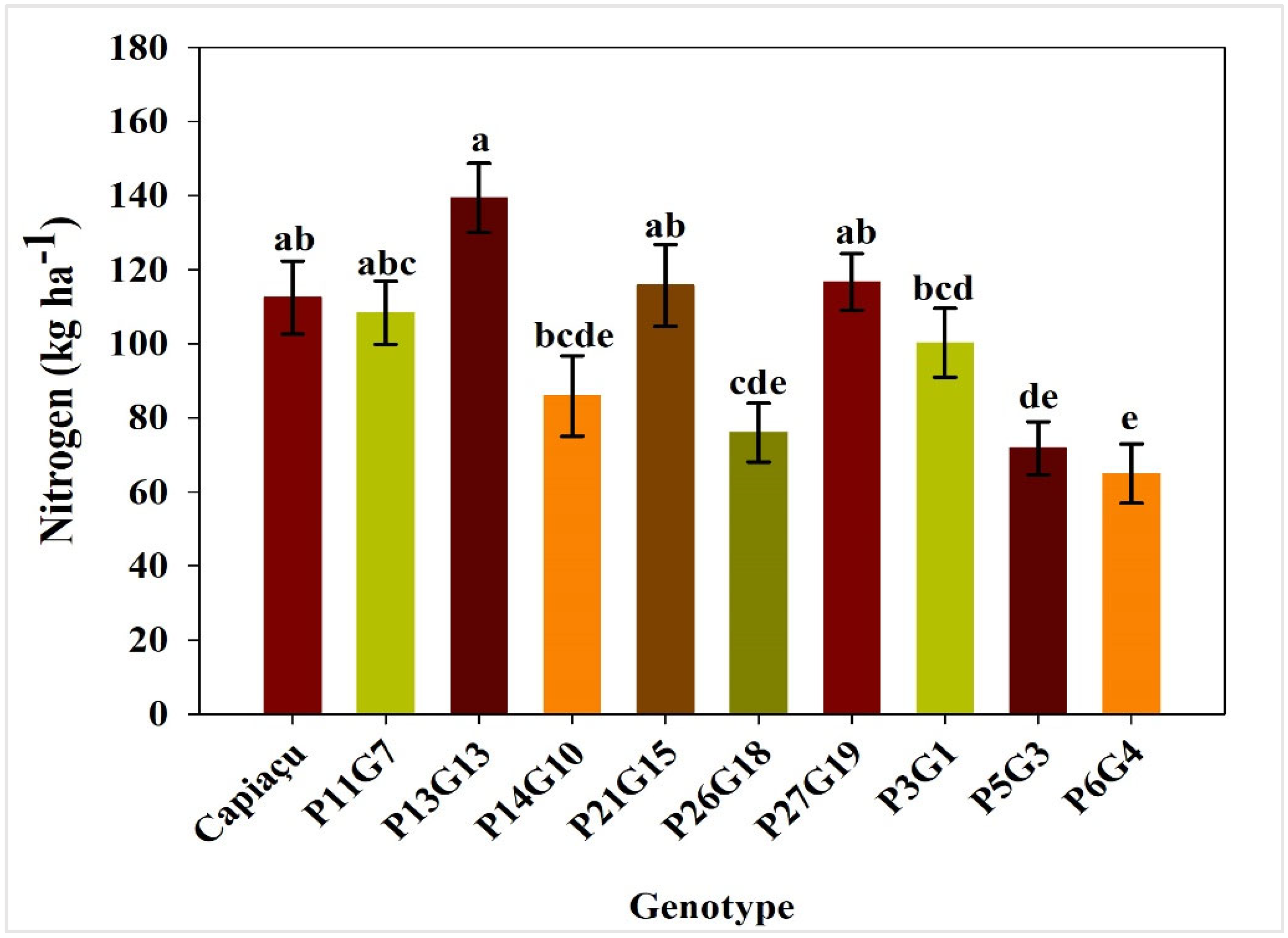
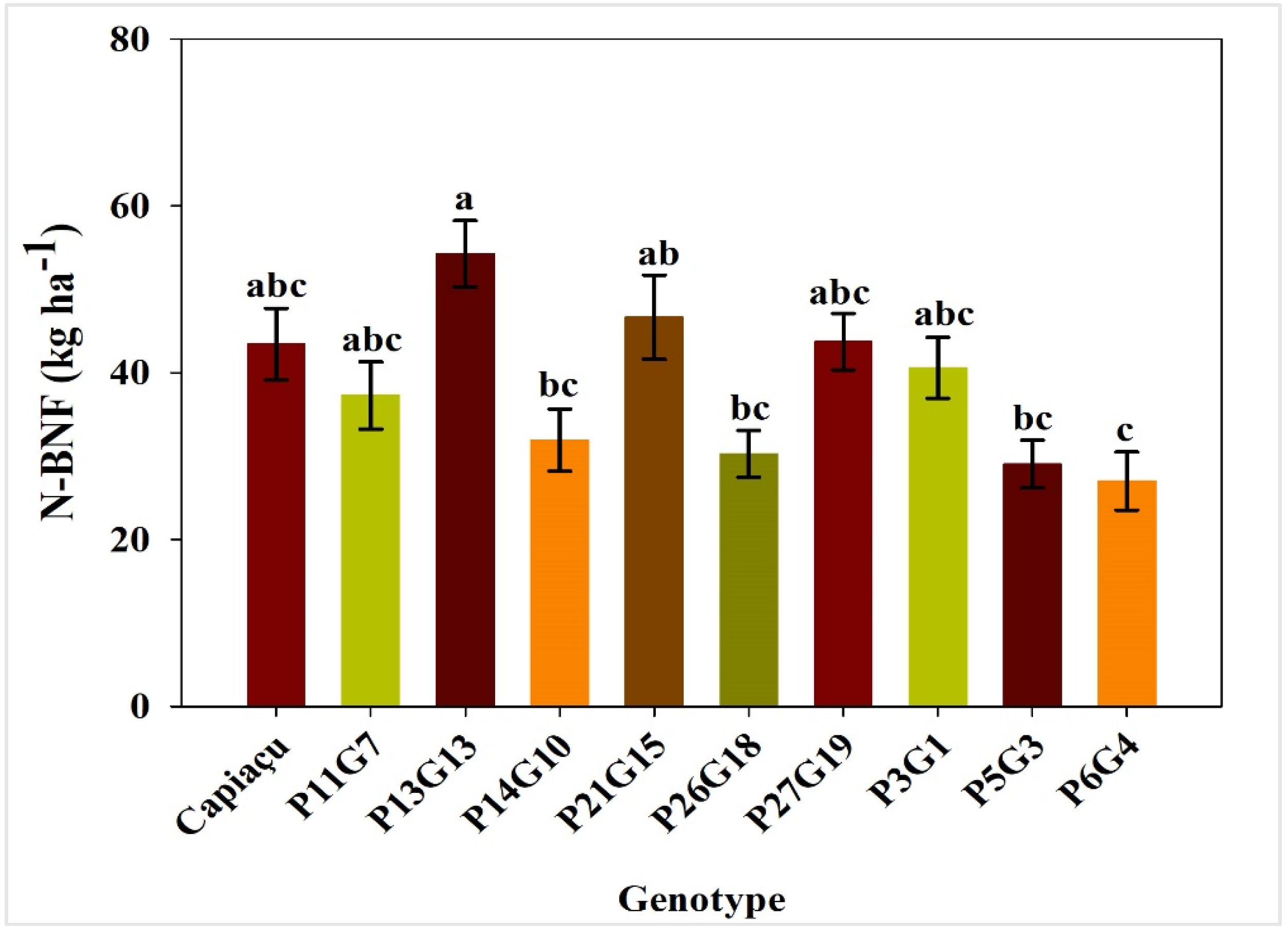
3.2. Accumulated N Content, Contribution of BNF to Nitrogen Nutrition and N Derived from BNF
4. Discussion
4.1. Fresh Mass and Dry Matter Productivity
4.2. Accumulated N Content
4.3. 15N Natural Abundance and Contribution of BNF to Nitrogen Nutrition
4.4. Influence of BNF on Elephant Grass Productivity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribeiro, G.F.; Junior, A.B. The global energy matrix and use of agricultural residues for bioenergy production: A review with inspiring insights that aim to contribute to deliver solutions for society and industrial sectors through suggestions for future research. Waste Manag. Res. 2023, 41, 1283–1304. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.P.M.; Fuganholi, N.S.; Cunha, P.H.; Barelli, V.A.; Bunel, M.P.M.; Novazzi, L.F. Technical and economical analysis of renewable energy. J. Eng. Exact Sci. 2018, 4, 0163–0169. [Google Scholar] [CrossRef]
- Freiberg, A.; Scharfe, J.; Murta, V.C.; Seidler, A. The Use of Biomass for Electricity Generation: A Scoping Review of Health Effects on Humans in Residential and Occupational Settings. Int. J. Environ. Res. Saúde Pública 2018, 15, 354. [Google Scholar] [CrossRef] [PubMed]
- Mullett, J.; Morishige, D.; McCormick, R.; Truong, S.; Hilley, J.; McKinley, B.; Anderson, R.; Olson, S.N.; Rooney, W. Energy sorghum—A genetic model for the design of bioenergy crops. J. Exp. Bot. 2014, 65, 3479–3489. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Ibrahim, M.; Rashid, U.; Nawaz, M.; Ali, S.; Hussain, A.; Gull, M. Biomass production for bioenergy using marginal lands, Sustainable Production and Consumption. Sustain. Prod. Consum. 2017, 9, 3–21. [Google Scholar] [CrossRef]
- Urquiaga, S.; Jantalia, C.P.; Resende, A.D.; Alves, B.J.R.; Boddey, R.M. Contribuição da fixação biológica de nitrogênio na produtividade de sistemas agrícolas na América Latina. Embrapa Informação Tecnológica 2006, 181–200. [Google Scholar]
- Marafon, A. Cultivo dedicado de capim-elefante para uso energético da biomassa. Rev. Biomassa 2022, 6, 60. [Google Scholar]
- Marafon, A.C.; Câmara, T.M.M.; Santiago, A.D.; Rangel, J.D.A. Potencial de produção de biomassa de clones de capim-elefante com fins energéticos na região dos Tabuleiros Costeiros. RenovEnergia’14 2014, 36, 1–3. [Google Scholar]
- Marafon, A.C.; Santiago, A.D.; Amaral, A.F.C.; Bierhals, A.N.; Paiva, H.L.; Guimaraes, V.S. Uso da Biomassa para Geração de Energia. Doc./Embrapa Tabul. Costeiros 2016, 1–29. [Google Scholar] [CrossRef]
- Marafon, A.; Machado, J.; Amaral, A.; Guimarães, V.D.S.; dos Santos, J.P. Frequência de Corte em Genótipos de Capim-Elefante na Produção de Biomassa Para Fins Energéticos. 2019. Available online: https://www.researchgate.net/publication/339284544_Frequencia_de_corte_em_genotipos_de_capim-elefante_na_producao_de_biomassa_para_fins_energeticos (accessed on 21 February 2025). [CrossRef]
- da Silva, E.L.; da Silva, J.F.C.; Vásquez, H.M.; de Andrade, E.M.; Deminicis, B.B.; de Morais, J.P.G.; Costa, D.P.B.; Araújo, S.A.C. Características agronômicas e nutritivas das principais cultivares de capim-elefante do Brasil. Vet. Zootec. 2010, 17, 324–334. [Google Scholar]
- Flores, R.A.; Urquiaga, S.S.; Alves, B.J.R.; Collier, L.S.; Morais, R.F.D.; Prado, R.D.M. Adubação nitrogenada e idade de corte na produção de matéria seca do capim-elefante no Cerrado. Rev. Bras. Eng. Agríc. Ambient. 2012, 16, 1282–1288. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants 2020, 11, 1011. [Google Scholar] [CrossRef] [PubMed]
- Herridge, D.; Peoples, M.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Boddey, R.M.; Polidoro, J.C.; Resende, A.S.; Alves, B.J.R.; Urquiaga, S. Use of the 15N natural abundance technique for the quantification of the contribution of N2 fixation to grasses and cereals. Aust. J. Plant Physiol. 2001, 28, 889–895. [Google Scholar]
- Urquiaga, S.; Xavier, R.P.; de Morais, R.F.; Batista, R.B.; Schultz, N.; Leite, J.M.; Sá, J.M.E.; Barbosa, K.P.; de Resende, A.S.; Alves, B.J.R.; et al. Evidence from field nitrogen balance and 15N natural abundance data for the contribution of biological N2 fixation to Brazilian sugarcane varieties. Plant Soil 2012, 356, 5–21. [Google Scholar] [CrossRef]
- Schultz, N.; Pereira, W.; de Albuquerque Silva, P.; Baldani, J.I.; Boddey, R.M.; Alves, B.J.R.; Urquiaga, S.; Reis, V.M. Yield of sugarcane varieties and their sugar quality grown in different soil types and inoculated with a diazotrophic bacteria consortium. Plant Prod. Sci. 2018, 20, 366–374. [Google Scholar] [CrossRef]
- Martins, D.S.; Reis, V.M.; Schultz, N.; Alves, B.J.; Urquiaga, S.; Pereira, W.; Boddey, R.M. Both the contribution of soil nitrogen and of biological N 2 fixation to sugarcane can increase with the inoculation of diazotrophic bacteria. Plant Soil 2020, 454, 155–169. [Google Scholar] [CrossRef]
- Monteiro, E.C.; Silva, C.G.N.; Martins, M.R.; Reis, V.M.; Boddey, R.M.; Alves, B.J.R.; Urquiaga, S. Strategy for the sampling of sugarcane plants for the reliable quantification of n2 fixation using 15N natural abundance. J. Soil Sci. Plant Nutr. 2021, 21, 2741–2752. [Google Scholar] [CrossRef]
- Fox, A.R.; Soto, G.; Valverde, C.; Russo, D.; Lagares, A., Jr.; Zorreguieta, Á.; Alleva, K.; Pascuan, C.; Frare, R.; Mercado-Blanco, J.; et al. Major cereal crops benefit from biological nitrogen fixation when inoculated with the nitrogen-fixing bacterium Pseudomonas protegens Pf-5 X940. Environ. Microbiol. 2016, 18, 3522–3534. [Google Scholar] [CrossRef]
- Zanetti, J.B.; Morais, R.F.; Alves, B.J.R.; Boddey, R.M.; Urquiaga, S.; Soares, L.H.B. Balanço de energia na produção de capim-elefante em condições experimentais. Bol. Pesqui. Desenvolv. 2010, 71, 18. [Google Scholar]
- Camelo, A.; Barreto, C.P.; Vidal, M.S.; Rouws, J.R.C.; da Silva Lédo, F.J.; Schwab, S.; Baldani, J.I. Field response of two seed propagated elephant grass genotypes to diazotrophic bacterial inoculation and in situ confocal microscopy colonization analyses. Symbiosis 2021, 83, 41–53. [Google Scholar] [CrossRef]
- Samson, R.; Mani, S.; Boddey, R.M.; Sokhansanj, S.; Quesada, D.; Urquiaga, S.; Reis, V.M.; Ho Lem, C. The potential of C4 perennial grasses for developing a Global BIOHEAT industry. Crit Rev Plant Sci. 2005, 24, 461–495. [Google Scholar] [CrossRef]
- Morais, R.F.; Souza, B.J.; Leite, J.M.; Soares, L.H.B.; Alves, B.J.R.; Boddey, R.M.; Urquiaga, S. Elephant grass genotypes for bioenergy production by direct biomass combustion. Pesqui. Agropecu. 2009, 44, 133–140. [Google Scholar] [CrossRef]
- Urquiaga, S.; Cruz, K.H.; Boddey, R.M. Contribution of nitrogen fixation to sugar cane: Nitrogen-15 and nitrogen-balance estimates. Soil Sci. Soc. Am. J. 1992, 56, 105–114. [Google Scholar] [CrossRef]
- Shearer, G.; Kohl, D.H. N2 fixation in field settings: Estimations based on natural 15N abundance. Aust. J. Plant Physiol. 1986, 13, 699–756. [Google Scholar]
- Mistura, C.; Fagundes, J.L.; Fonseca, L.M.; Moreira, C.L.M.; Júnior, D.M.; Júnior, J.R. Disponibilidade e qualidade do capim-elefante com e sem irrigação adubado com nitrogênio e potássio na estação seca. Rev. Bras. Zootec. 2006, 35, 372–379. [Google Scholar] [CrossRef]
- Morais, R.F.; Quesada, D.M.; Reis, V.M.; Urquiaga, S.; Alves, B.J.R.; Boddey, R.M. Contribution of biological nitrogen fixation to Elephant grass (Pennisetum purpureum Schum.). Plant Soil 2012, 356, 23–34. [Google Scholar] [CrossRef]
- de Carvalho, E.X.; Menezes, R.S.C.; Freitas, A.D.S.; Sampaio, E.V.S.; Neto, D.E.S.; Tabosa, J.N.; Primo, D.C.; Queiroz, R.O. The 15N natural abundance technique to assess the potential of biological nitrogen fixation (BNF) in some important C4 grasses. Aust. J. Crop Sci. 2017, 11, 1559–1564. [Google Scholar] [CrossRef]
- Marin, F.R.; Lopes-Assad, M.L.; Assad, E.D.; Vian, C.E.; Santos, M.C. Sugarcane crop efficiency in two growing seasons in São Paulo State, Brazil. Pesqui. Agropecu. 2008, 43, 1449–1455. [Google Scholar] [CrossRef]
- Manhães, C.M.C.; Garcia, R.F.; Francelino, F.M.A.; Francelino, H.O.; Coelho, F.C. Fatores que afetam a brotação e o perfilhamento da cana-de-açúcar. Rev. Vértices 2015, 17, 163–181. [Google Scholar] [CrossRef]
- Bossolani, J.W.; Leite, M.F.; Momesso, L.; Ten Berge, H.; Bloem, J.; Kuramae, E.E. Nitrogen input on organic amendments alters the pattern of soil–microbe-plant co-dependence. Sci. Total Environ. 2023, 890, 164–347. [Google Scholar] [CrossRef]
- Ladha, J.K.; Peoples, M.B.; Reddy, P.M.; Biswas, J.C.; Bennett, A.; Jat, M.L.; Krupnik, T.J. Biological nitrogen fixation and prospects for ecological intensification in cereal-based cropping systems. Field Crops Res. 2022, 283, 108–541. [Google Scholar] [CrossRef] [PubMed]
- Latiri-Souki, K.; Nortcliff, S.; Lawlor, D.W. Nitrogen fertilizer can increase dry matter, grain production and radiation and water use efficiencies for durum wheat under semi-arid conditions. Eur. J. Agron. 1998, 9, 21–34. [Google Scholar] [CrossRef]
- Thiengo, C.C.; Galindo, F.S.; Bernardes, J.V.S.; da Rocha, L.O.; da Silva, C.D.; Burak, D.L.; Lavres, J. Nitrogen fertilization regulates crosstalk between marandu palisadegrass and Herbaspirillum seropedicae: An investigation based on 15N isotopic analysis and root morphology. Environ. Res. 2024, 249, 118–345. [Google Scholar] [CrossRef] [PubMed]
- Quesada, D.M.; Frade, C.; Resende, A.; Polidoro, J.C.; Reis, V.M.; Boddey, R.M.; Alves, B.J.R.; Urquiaga, S.; Xavier, D. A fixação biológica de nitrogênio como suporte para a produção de energia renovável. In Proceedings of the 3. Encontro de Energia No Meio Rural, Campinas, Brazil, 12-15 September 2000. [Google Scholar]
- Steenhoudt, O.; Vanderleyden, J. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: Genetic, biochemical and ecological aspects. FEMS Microbiol. Rev. 2000, 24, 487–506. [Google Scholar] [CrossRef]
- Cassán, F.; Vanderleyden, J.; Spaepen, S. Physiological and agronomical aspects of phytohormone production by model plant-bacteria-promoting rhizobacteria (PGPR) belonging to the genus Azospirillum. J. Plant Growth Regulat. 2014, 33, 440–459. [Google Scholar] [CrossRef]
- Oliveira, A.; Urquiaga, S.; Döbereiner, J.; Baldani, J.I. The effect of inoculating endophytic N2-fixing bacteria on micropropagated sugarcane plants. Plant Soil 2002, 242, 205–215. [Google Scholar] [CrossRef]
- Reis, V.M.; Baldani, J.I.; Urquiaga, S. Recomendação de uma mistura de estirpes de cinco bactérias fixadoras de nitrogênio para inoculação de cana-de-açúcar: Gluconacetobacter diazotrophicus (BR 11281), Herbaspirillum seropedicae (BR 11335), Herbaspirillum rubrisubalbicans (BR 11504), Azospirillum amazonense (BR 11145) e Burkholderia tropica (BR 11366). Circ. Técnica Embrapa 2009, 30, 1–4. [Google Scholar]
- Videira, S.S.; de Oliveira, D.M.; de Morais, R.F.; Borges, W.L.; Baldani, V.L.D.; Baldani, J.I. Genetic diversity and plant growth promoting traits of diazotrophic bacteria isolated from two Pennisetum purpureum Schum. genotypes grown in the field. Plant Soil 2012, 356, 51–66. [Google Scholar] [CrossRef]
- Thiengo, C.C.; Galindo, F.S.; Rodak, B.W.; Bernardes, J.V.S.; da Rocha, L.O.; Gaziola, S.A.; Azevedo, R.A.; Burak, D.L.; Olivares, F.L.; Lavres, J. Harnessing plant growth-promoting bacteria (Herbaspirillum seropedicae) from an optimal mineral nitrogen supply: A study on improving nitrogen use efficiency in marandu palisadegrass. Plant Physiol Biochem. 2025, 10, 109–497. [Google Scholar] [CrossRef]
- Quesada, D.M.; Boddey, R.M.; Reis, V.M.; Urquiaga, S. Parâmetros qualitativos de genótipos de capim elefante (Pennisetum purpureum Schum.) estudados para a produção de energia através da biomassa. Circ. Técnica 2004, 8, 1–4. [Google Scholar]
- Marafon, A.; Machado, J.; dos Santos, J.P.; Guimaraes, V.D.S. Desenvolvimento radicular, produção e qualidade da biomassa em variedades de capim-elefante na Zona da Mata de Alagoas. Bol. Pesqui./Embrapa Tabul. Costeiros 2022. [Google Scholar] [CrossRef]
- Ladha, J.K.; Pathak, H.; Krupnik, T.J.; Six, J.; Van Kessel, C. Efficiency of fertilizer nitrogen in cereal production: Retrospects and prospects. Adv. Agron. 2005, 87, 85–156. [Google Scholar]
- Boddey, R.M.; Sá, J.C.D.M.; Alves, B.J.R.; Urquiaga, S. The contribution of biological nitrogen fixation for sustainable agricultural systems in tropics. Soil Biol. Biochem. 1997, 29, 787–799. [Google Scholar] [CrossRef]
- Tyagi, J.; Ahmad, S.; Malik, M. Nitrogenous fertilizers: Impact on environment sustainability, mitigation strategies, and challenges. Int. J. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]

| GENOTYPE | 2020/21 | 2021/22 | 2022/23 |
|---|---|---|---|
| BRS CAPIAÇU | 29.71481 | 26.150755 | 27.67077 |
| P11G7 | 29.64103 | 27.40141 | 31.52896 |
| P13G13 | 29.547055 | 24.891255 | 27.99227 |
| P14G10 | 30.388415 | 26.943885 | 27.427755 |
| P21G15 | 28.244895 | 25.22509 | 28.67502 |
| P26G18 | 29.77144 | 23.40055 | 26.509755 |
| P27G19 | 28.97286 | 25.914805 | 29.194425 |
| P3G1 | 28.12682 | 23.76335 | 26.551095 |
| P5G3 | 28.753235 | 24.959655 | 26.08045 |
| P6G4 | 29.31908 | 23.72163 | 24.44017 |
| MEAN | 29.247964 | 25.2372385 | 27.607067 |
| SD | 0.72 | 1.37 | 1.94 |
| CV | 2.51 | 5.43 | 7.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, C.A.G.d.; Monteiro, E.d.C.; Souza, W.d.S.; Pio, P.V.A.; Machado, J.C.; Alves, B.J.R.; Boddey, R.M.; Urquiaga, S. Contribution of Biological Nitrogen Fixation to the Biomass Productivity of Elephant Grass Grown in Low-Fertility Soil for Energy Purposes. Agronomy 2025, 15, 605. https://doi.org/10.3390/agronomy15030605
Oliveira CAGd, Monteiro EdC, Souza WdS, Pio PVA, Machado JC, Alves BJR, Boddey RM, Urquiaga S. Contribution of Biological Nitrogen Fixation to the Biomass Productivity of Elephant Grass Grown in Low-Fertility Soil for Energy Purposes. Agronomy. 2025; 15(3):605. https://doi.org/10.3390/agronomy15030605
Chicago/Turabian StyleOliveira, Carolina Almada Gomes de, Edevaldo de Castro Monteiro, Wesley dos Santos Souza, Paulo Vitor Alves Pio, Juarez Campolina Machado, Bruno José Rodrigues Alves, Robert Michael Boddey, and Segundo Urquiaga. 2025. "Contribution of Biological Nitrogen Fixation to the Biomass Productivity of Elephant Grass Grown in Low-Fertility Soil for Energy Purposes" Agronomy 15, no. 3: 605. https://doi.org/10.3390/agronomy15030605
APA StyleOliveira, C. A. G. d., Monteiro, E. d. C., Souza, W. d. S., Pio, P. V. A., Machado, J. C., Alves, B. J. R., Boddey, R. M., & Urquiaga, S. (2025). Contribution of Biological Nitrogen Fixation to the Biomass Productivity of Elephant Grass Grown in Low-Fertility Soil for Energy Purposes. Agronomy, 15(3), 605. https://doi.org/10.3390/agronomy15030605






