Abstract
Soybean seeds with similar germination rates may exhibit subtle differences in physiological quality, influencing field performance and storage longevity. This study used a shotgun proteomics approach to characterize the proteomic profile of two commercial soybean seed lots (higher- and lower-quality) during germination, aiming to identify biomarkers associated with vigor and deterioration. Proteins were analyzed across three germination phases: imbibition (Phase I, 0.5 h), metabolic activation (Phase II, 20 h), and radicle protrusion (Phase III, 51 h). A total of 777 proteins were identified, and of these differentially abundant proteins (DAPs), the following totals were detected: 12 in Phase I, 17 in Phase II, and 28 in Phase III. In Phase I, ribosomal proteins were more abundant in high-quality seeds, indicating efficient translation and preparation for germination. Conversely, in Phase III, low-quality seeds showed increased levels of storage proteins and stress-response proteins, including alcohol dehydrogenase (ADH), heat shock proteins, and annexins, reflecting delayed germination and more deterioration. These findings highlight the dynamic nature of protein expression during germination and demonstrate the potential of proteomics to detect subtle differences in physiological quality. The identified biomarkers provide insights for seed quality assessment and offer practical applications for improving classification and management of commercial soybean seed lots.
1. Introduction
Soybean [Glycine max (L.) Merrill] is one of the most important crops and commodities worldwide. Soybean grains are a rich source of proteins, oils, anthocyanins, and isoflavones, being used for various food, pharmacological, and cosmetic products [1]. Considering the constant environmental changes in many agricultural production areas around the world, there is a need to carry out research to guarantee crop production and, thus, maintain food security for future generations.
An important factor in increasing soybean crop productivity is the use of seeds with high physiological quality. This characteristic is an important and desired attribute in commercial lots of soybean seeds since this is directly correlated to obtaining an adequate stand of seedlings, homogeneity, and crop productivity [2,3,4]. High germination rates, seed vigor, and longevity are the parameters used in the characterization and evaluation of the physiological quality of seeds [5]. That is, the physiological quality brings together information about germination (viability) and seed vigor [6]. Therefore, a safe evaluation of seed quality allows the identification of seed lots that are more likely to present the desired performance during storage and in the field.
Different seed lots with the same germination rate, obtained by laboratory tests, may show variation in performance in the field, or in storage [6]. This is due to a subtle variation in the level of seed deterioration in these lots. In turn, when it comes to the seed vigor of the lots, these variations will only be noticeable through more sensitive vigor tests and/or molecular techniques [7,8]. Broad and in-depth evaluations of artificially aged soybean seeds have shown changes in protein profiles, which, when identified, were mainly related to primary metabolism and responses to stimuli. Proteins related to an activity in seed nutrient reserves had increased accumulation, while proteins involved in primary metabolisms, such as the carbohydrate metabolism process, protein folding, the glucose metabolism process, and redox activity (ROS detoxification) had a reduced accumulation [9,10]. However, these studies only observed the seeds in terms of the deterioration process and did not analyze them from the perspective of physiological quality, as vigor tests were not performed. In addition, it is not yet clear how this variation occurs during germination.
Therefore, it has not been reported yet in the literature and there are still no studies that elucidate on a molecular level, more specifically with proteomics, the subtle differences that can be found in the physiological quality of different soybean seed lots. In addition to this, an evaluation of the proteomic profile during germination increases the chances of finding biomarkers for seed vigor. This study aimed to characterize the proteomic profile of soybean seeds during germination through an identification of proteins that subtly influence the vigor of different soybean seed lots, as well as to identify protein expression patterns in soybean seed lots with subtle variation in the physiological quality.
Our results indicate that analyses of the proteomic profile can identify subtle differences in the physiological quality of commercial soybean seeds lots, since the proteomic profile of soybean seeds with a subtle difference in the physiological quality varies during different germination stages. In addition, several proteins related to subtle differences in physiological quality are presented, which are potential biomarkers for seed vigor and/or deterioration.
2. Materials and Methods
The experiment was conducted at the State University of Santa Cruz-BA in the phytotechnics laboratories and the Proteomics laboratory of the Center for Biotechnology and Genetics (CBG). Samples from commercial soybean seed lots (2019 harvest) were stored in a refrigerator, and five samples from different soybean seed lots were selected; of these five lots, three were of the M8808 variety (lots B, D, and E), and two from M8374 (lots G and H). Due to the low humidity (~3%) of the stored seed samples, before the tests, the seeds were pre-conditioned for 16 h under an aluminum screen inside plastic boxes (11.0 × 11.0 × 3.5 cm3) with water [11,12]. After physiological characterization, only two seed lots (B and E) were selected for the tetrazolium test, imbibition curve characterization, and proteomic analysis.
2.1. Physiological Characterization of Soybean Seed Lots
2.1.1. Germination and Vigor Tests
The five lots were physiologically characterized through germination and vigor tests:
Water content (WC): Oven method, two replications of 25 seeds in an oven at 105 ± 3 °C for 24 h, the results being expressed in percentage (wet basis), and performed before and after accelerated aging [12].
Germination Test (GE), First Count (FC), and Germination Speed Index (GSI): on paper for seed germination, previously moistened with distilled water at a rate of 2.5 times its dry weight, 50 seeds were distributed in 4 repetitions on two sheets of paper, with one more sheet placed on the seeds and the whole set rolled up. The rolls were kept in a chamber for seed germination at a temperature of 30 °C. The germination counts of normal seedlings were performed on the 5th and 8th days after sowing and the results were expressed as a percentage [12]. Concomitantly, the First Count was determined by the percentage of normal seedlings germinated on the fifth day after the installation of the germination test [12,13]. The germination values of normal seedlings in the first and second counts were used to calculate the germination speed index [14].
Biomass—total fresh mass (TFM) and total dry mass (TDM): the fresh mass of the seedlings obtained in the first germination count was evaluated, with 10 seedlings being weighed per repetition. Then, the seedlings were placed to dry in an oven with forced air circulation at 70 °C, until a constant dry mass weight was obtained. Results are expressed in milligrams (mg).
Seedling biometry—Root length (RL) and seedling (SL): four samples of 20 seeds from each lot were distributed in rolls of paper towels moistened with distilled water at a ratio of 2.5 to 1 (mL of distilled water per dry paper weight in grams) and kept in a germinator at 25 °C for five days [15]. A line was drawn on the moistened paper towel in the upper third, in the longitudinal direction, where the seeds were placed, directing the micropyle downwards. The length of the primary root and of the seedlings considered normal [12] was determined at the end of the fifth day, with the aid of a caliper, obtaining results in centimeters per seedling (cm/SL).
Seedling emergence test in substrate (SE) and Emergence Speed Index (ESI): In plastic trays containing washed sand, 50 seeds were sown in four replications per lot, seedlings that had the cotyledons open were counted, and the counting time was at five, seven, nine, and eleven days after sowing, expressed as a percentage. The ESI was calculated with the values of the three counts [14].
Accelerated aging test (AA): 250 seeds were used for each lot, distributed in plastic boxes (11.0 × 11.0 × 3.5 cm3), with aluminum screens attached to them, on which the seeds were placed in a single layer. In each box, 40 mL of distilled water was added, and the box was kept in an oven at 41 °C for 48 h [15]. After the aging period, the seeds were submitted to the germination test [12] and readings were taken on the fifth day after test installation. In addition, the water content after aging was measured.
Tetrazolium test: 4 replications of 50 seeds were used for lots B and E. The seeds were pre-conditioned for 16 h under an aluminum screen inside plastic boxes (11.0 × 11.0 × 3.5 cm3,) with water, then conditioned on Germitest paper in BOD at 25 °C for 16 h; after conditioning, for coloring, the seeds were soaked in a 0.075% tetrazolium solution in plastic cups at 40 °C in a BOD chamber for three hours, after which the seeds were washed and interpreted. The results were expressed as the percentage of seeds with high vigor and the percentage of viable seeds according to classification [16].
2.1.2. Characterization of the Imbibition Curve During Germination
One hundred seeds were used for each lot (B and E) with 4 repetitions of 25 seeds. The initial WC was determined for each lot and each repetition, and the initial weight was obtained. The seeds were placed to soak in Germitest paper rolls; three sheets were used. The paper was moistened with distilled water at a rate of 2.5 times its dry weight. The rolls with the seeds were placed at a temperature of 25 °C, in BOD. For four days, successive weighing was carried out; for these evaluations the seeds were removed from the paper, rubbed on paper towels to remove excess surface moisture, and weighed on a digital scale to obtain the final weight. When necessary, at each weighing, the paper was moistened. With the values of the initial water content, initial weight, and final weight it was possible to calculate the final water content of each repetition. Thus, a graph was prepared with the imbibition curve of lots B and E, with the x-axis representing the time in hours and the y-axis the water content in percentage. At each weighing, whether there was protrusion of the primary root (germination stricto sensu) in the seeds was also monitored; at 6 days after imbibition, normal and abnormal seedlings were classified.
2.2. Proteomics in Soybean Seeds
2.2.1. Obtaining the Treatments
In this step, samples from lots B (higher quality) and E (lower quality), which were previously physiologically characterized, were utilized. These samples were analyzed alongside the characterization of the imbibition curve. To achieve this, four rolls of moistened Germitest paper, each containing 25 seeds (one per replicate), were prepared following the same methodology as for the imbibition curve characterization. For the first sampling, 24 seeds were removed immediately after sanitization and approximately 0.5 h after the start of imbibition in Germitest paper, forming treatments BI and EI. The second sampling involved removing six seeds from each replicate approximately 20 h after the start of imbibition, forming treatments BII and EII. The third sampling consisted of seeds exhibiting primary root protrusion (stricto sensu germination), collected 51 h after the start of imbibition, with six seeds from each replicate, forming treatments BIII and EIII. The Roman numerals I, II, and III in the treatment nomenclature refer to stages one, two, and three of germination, respectively, while the letters B and E correspond to lots B (higher quality) and E (lower quality).
2.2.2. Superficial Seed Disinfestation and Excision of the Embryonic Axis with Part of the Vascular Region
Immediately after obtaining each treatment (BI, EI, BII, EII, BIII, and EIII), the seeds were superficially disinfected by immersing them in 1.05% NaOCl for 5 min, then in 70% alcohol for 10 s, and then performing 3 washes in water (distilled and autoclaved) for 5 min [17]. After disinfestation, with the aid of a scalpel, the embryonic axis was excised with part of the vascular region with a diagonal cut made in the upper part of the seeds held with tweezers, and the integument was removed (Figure S2) [16] and the cuts were immediately immersed in liquid nitrogen for cryogenization and macerated with liquid nitrogen and Polyvinylpolypyrrolidone (PVPP). Finally, the samples were placed in 2 mL microtubes and placed in an ultrafreezer at −80 °C until use.
2.2.3. Protein Extraction
Protein extraction was performed in triplicate according to the protocol with minor modifications. TCA/acetone e Phenol/dense SDS [18].
2.2.4. Protein Quantification and SDS-PAGE
For quantification, the standard protocol of the commercial product 2-D Quant Kit, manufactured by GE Healthcare Life Sciences (Amersham, UK) was used. Subsequently, 30 µg of protein from each sample were analyzed by SDS-PAGE (Sodium Dodecyl Sulfate—Polyacrylamide Gel Electrophoresis) 12.5% in an electrophoresis mini-vat (Mighty Small II Mini Vertical Electrophoresis Unit SE250, Hoefer, Bridgewater, MA, USA). The gel was stained with 0.08% Comassie Blue G-250 then destained with distilled water and scanned using ImageScanner II (GE Healthcare, Amersham, England).
2.2.5. Gel-Free Mass Spectrometry
A mass of 100 μg of proteins from each sample was used for tryptic digestion with some modifications [19]. Soon after, the samples were diluted in water at a 1:1 ratio, followed by protein reduction with dithiothreitol (DTT) and alkylation with iodoacetamide (IAA). Then, the samples were diluted in a 1:5 ratio with 50 mM ammonium bicarbonate, with the addition of CaCl2. Subsequently, they were incubated for 16–24 h with trypsin overnight at 37 °C. The reaction was immediately stopped by adding trifluoroacetic acid (TFA) until the pH was below 2. Subsequently, the samples were desalted using tips with C18 resin (10 µL; Millipore® Ziptips C18, Merck, Darmstadt, Germany). The peptides were eluted in 50 µL of solution containing 75% acetonitrile, 25% water, and 0.1% formic acid.
The peptides were analyzed in a liquid chromatography system (Agilent 1290 Infinity II HPLC, Agilent, Santa Clara, CA, USA) coupled to a quadrupole/Time-of-Flight mass spectrometer (Agilent 6545 LC/QTOF, Agilent, Santa Clara, CA, USA). Eight microliters of each sample were injected in technical triplicate. The peptides were separated using a reversed-phase column (C18; AdvanceBio Peptide Mapping 2.1 × 250 mm2; Agilent, Santa Clara, CA, USA), maintaining a temperature of 55 °C. A 20 min gradient was applied with mobile phases A (H2O with 0.1% formic acid) and B (acetonitrile with 0.1% formic acid). The percentages of phase B along the gradient were 5% to 35% (1–10 min), 35% to 70% (11–14 min), 70% to 100% (16–18 min), and 100% (16–20 min). To stabilize the pressure, a final period of 5 min with 5% phase B was maintained.
The samples were injected into QTOF through an electrospray source, using the AutoMS/MS acquisition mode, with a maximum selection of 10 precursors per cycle. The parameters for the selection of precursors were threshold of 1000, 10,000 counts/spectrum, stringency of 100% purity, cut-off of 30% purity, peptide isotopic model, charge preference of 2, 3, >3, and unknown. The collision energy (in V) was set to be applied according to the following formula: (slope) × (m/z)/100 + Offset, where m/z represents the precursor mass/charge ratio and the slope and offset range from 3.1 to 5 and from −4.8 to 10, respectively, depending on whether the precursor charge is 2, 3, >3, or unknown. The instrument parameters used were gas temperature of 325 °C, gas flow of 13 L/min, capillary voltage of 4000 V, and skimmer voltage of 56 V. Nitrogen gas was used for the induced dissociation collision. Instrument control (HPLC and QTOF) and parameter configuration were performed using the Agilent MassHunter Acquisition software version 12.0.
2.2.6. Protein Identification
The resulting spectra were processed in triplicate for peptide identification using Spectrum Mill software (Rev BI.07.08.214; Agilent). The parameters for spectra extraction were as follows: MSNoiseThreshold of 10 counts, fixed modifications of carbamidomethylation, MH+ precursor from 200 to 6000 Da, retention time tolerance of ±60 s, tolerance of m/z ± 1.4, and find precursor load. After extracting the MS/MS spectra, a database search was performed. The database used was Glycine max, downloaded from UniProt (https://www.uniprot.org). The parameters for comparing MS/MS spectra in the protein bank were as follows: maximum number of cleavages missed: 2; fixed modifications: carbamidomethylation (C); variable modifications: Oxidized methionine (M), pyroglutamic acid (N-termQ), deamidated (N), phosphorylated S (S), phosphorylated T (T), phosphorylated Y (Y); minimum combined peak intensity: 10%; precursor mass tolerance: ±20 ppm; product mass tolerance: ±50 ppm. The Spectrum-peptide matches (PSMs) from the search were validated and filtered, using the Spectrum Mill Autovalidation tool, for those that had a false discovery rate (FDR) lower than 1%. For subsequent comparative statistical analysis, proteins containing peptides with a score > 5 and Scored Peak Intensity (SPI) > 60% were selected. Proteins that passed the filters were exported in protein–protein comparison mode in the MPP APR file format for subsequent comparative analysis.
2.3. Statistical Analysis and Identification of Differentially Abundant Proteins
The tests for physiological characterization were carried out in a completely randomized design (DIC) with four replications for each lot. With the aid of R software version 4.4.2, for the comparison of five lots, analysis of variance (ANOVA) was performed followed by Tukey’s test (α = 0.05), before the variables were tested for normality (Shapiro–Wilk) and homoscedasticity (Bartlett), meeting the ANOVA prerequisites. For the comparison between two lots, the t test was used (α = 0.05).
Statistical analyses for identification of differentially abundant proteins were performed using Mass Profiler Professional 15.1 software (MPP; Agilent). The abundance of each protein was calculated using the median of the spectral intensity of the identified peptides. Protein abundance per treatment was calculated by averaging the abundance of proteins in the technical replicates. A frequency filter was added: only proteins that were present in at least two of the three technical replicates were considered for the analysis of unique entities and statistical significance.
Statistical significance analyses of comparisons between the treatment and control of each lot were performed using the unpaired t-test. The p-value calculation was asymptotic and multi-test correction was performed using the Benjamini–Hochberg method. Only proteins common between treatment and control that had a p-value < 0.05 and fold-change ≥1.5 were considered differentially abundant. Single entity analysis was performed on proteins that passed the frequency filter to identify proteins exclusive to each treatment.
3. Results
3.1. Physiological Characterization Identifies a Subtle Variation in the Physiological Quality of Soybean Seed Lots
The physiological characterization (germination and vigor) of five lots was carried out with the intention of selecting two lots with subtle but perceptible differences by some vigor tests, for a later evaluation of the proteomic profiles of these lots. Seed vigor is not a single measurable variable, but a concept that describes several characteristics [11,20].
It is worth mentioning that the different seed lot samples were stored in a refrigerator with a water content (WC) of approximately 3% to prevent rapid deterioration. However, to avoid damage by imbibition and eventual errors in the evaluations of the physiological quality of the seed lots, before conducting the tests, a pre-conditioning procedure was adopted, which increased the water content of all seeds, to standardize the samples (Table 1). Sensitivity damage to rapid imbibition that can occur in the germination test on soybean seedlings, as described in the Rules for Seed Analysis [12], may also occur during seed preparation for the tetrazolium test. Typically, this damage occurs when the water content of the seeds is below 12% [21]. After the pre-conditioning of all samples, the WC was around 12% and did not show great variation between lots. Less than a percentage point between lots was observed, providing great reliability to the tests used for a comparison between the different seed lots.

Table 1.
Physiological characterization of soybean cultivar M8808 (B, D, and E) and M8349 (G and H) seed lots. Water Content (WC), Germination (GE), First Count (FC), Germination Speed Index (GSI), Total Fresh Mass (TFM), Total Dry Mass (TDM), Root Length (RL), and Seedling Length (SL).
The germination (GE), first count (FC), and germination speed index (GSI) tests (Table 1) statistically showed the same stratification of the lots, in which only lot G was considered of low physiological quality according to these variables (GE = 76%, FC = 64%, and GSI = 11.2). FC and GSI showed low sensitivity, as it was only able to stratify the lots into two groups, even though vigor tests are variables derived from germination and this test overestimates the physiological quality of a seed lot [5].
The total fresh mass (TFM) stratified the seed lots into three groups; lot H (TFM = 22.5 g) showed a greater mass accumulation when compared to lot E, which presented a lower accumulation (TFM = 15.4 g). Lots B, D, and G were not different statistically (Table 1). For total dry mass (TDM), seed lot H also presented the highest performance, and lot E numerically had the smallest accumulation (TDM = 2.41 g), but, again, this difference was not statistically significant by the Tukey test (α = 0.05), with lot E not differing from the other lots of the same soybean cultivar (B and D). The results suggest that vigorous seeds provided greater mass transfer from their reserve tissues (cotyledon) to the embryonic axis in the germination phase, originating seedlings with greater fresh and dry weight, due to the greater accumulation of matter, some LEAs and storage mRNAs used in germination [13,21,22,23].
The seedling length test (SL) estimates the vigor of a seed lot, as more vigorous seeds provide seedlings with high growth rates from the great supply of storage tissue reserves [23]. The root length (RL) and seedling length (SL) tests did not show statistical differences between the lots. RL values were in the range between 83.4 mm and 118.05 mm, while SL values were between 170.4 mm and 212 mm (Table 1). These are tests that evaluate the performance of normal seedlings and fail to statistically differ seed lots from each other.
The seedling emergence test in the substrate (SE) and its derived emergence speed index (EVI) (Table 2) classified lot G with the lowest quality (SE = 79%), aligning with the other variables evaluated. The EVI for lot D was superior to lot E. However, it was equal to lots B and H. The EVI evaluates the seedling emergence speed in field conditions, that is, uncontrolled conditions. In this way, the faster the emergence of seedlings, the greater the vigor of the seeds [13].

Table 2.
Physiological characterization of soybean cultivar M8808 (B, D, and E) and M8349 (G and H) seed lots. Seedling Emergence (SE), Emergence Speed Index (EVI), Water Content after Accelerated Aging (WCAA), and Accelerated Aging (AA).
The accelerated aging test (AA) (Table 2) stratified the lots into three levels of vigor. Among the soybean cultivar M8808 seed lots, lot B showed germination after 48 h of aging equal to AA = 63%, higher than 39.5% and 41.5% for lots D and E, respectively. This shows a greater ability to resist deterioration, with lot B presenting greater vigor than the other two (D and E) for this soybean cultivar. The accelerated aging test is a test that subjects seeds to high temperature and humidity conditions, accelerating the deterioration process to simulate a long period of storage [24]. In addition, this test can also simulate the biochemical and molecular events that occur during the natural aging of seeds [25]. The AA test has been widely used by seed companies to test the vigor of different seed cultivars [24,25]. For cultivar M8374, the results for the AA test followed the same pattern of reduction in germination after accelerated aging. Lot H was once again superior to lot G, showing a germination rate after aging equal to AA = 57%, while for lot G it was 9%. It was possible to notice a severe reduction in the germination rate for lot G after aging, which aligns with other vigor tests and with the germination test. For lot H, the synergy of rapid deterioration was observed, as happens in lots with a low initial germination rate when the effects of deterioration are intensified by the deterioration process, which was already present even before the accelerated aging [5].
At this point, it was already possible to infer that those seeds from lot B had a subtly higher physiological quality than lot E, because even with the same GE, the TFM variables and especially the AA test showed differences in seed vigor.
3.2. The Tetrazolium Test Confirms a Subtle Difference in the Physiological Quality of Seed Lots
The tetrazolium test confirmed the difference between lots B and E (Figure 1). A significant difference (t-test, p < 0.01) was observed between lots B and E for vigor, viability, and moisture damage. However, no significant difference was found for mechanical damage and damage caused by bed bugs. Therefore, the main factor contributing to the reduction in seed lot quality was identified as deterioration due to moisture, as classified by the tetrazolium test [16]. The test indirectly measures the respiratory processes that occur in the mitochondria of the cells that make up the seed tissues. The reductive reaction of the tetrazolium salt solution under the action of dehydrogenase enzymes results in triphenylformazan, which has a crimson-red color. By interpreting the resulting color patterns, seed viability, vigor, and the main problems that affect seed quality are determined [16]. Following the test classification, 74% of the seeds from lot B versus 58.5% from lot E showed vigor between one and three. The viability was 90% for lot B against 82.5% for lot E. Regarding moisture damage, seeds from lot B showed 8.75 percentage points less than lot E. For soybean seeds, the tetrazolium test is the most recommended because, in addition to viability, it provides information on vigor and possible damage to the seed lot, with high reliability. Therefore, the tetrazolium test is used on a large scale for the internal control of soybean seed production [16].
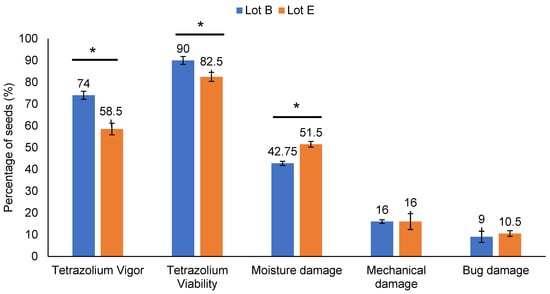
Figure 1.
Tetrazolium test in soybean cultivar M8808 seed lots, quantifying viability, vigor, and moisture, mechanical and stink bug damage in seeds. (*) t test (p < 0.01).
3.3. The Imbibition Curve During Germination Shows a Three-Phase Pattern for Different Soybean Seed Lots
The imbibition curve (Figure 2) for soybean seeds showed a three-phase pattern, as described in the literature [7,20,21]. The two lots showed the same pattern. Ranging from approximately 50% of the water content, seeds within the samples began to show root protrusion (germination stricto sensu).
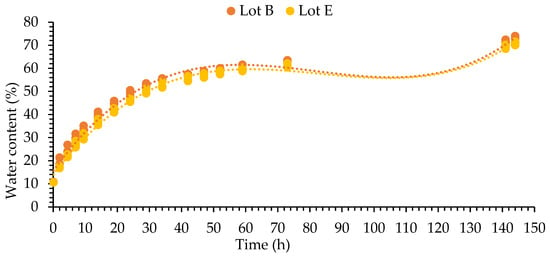
Figure 2.
The imbibition curve of two soybean cultivar M8808 seed lots (B and E).
Following the imbibition curve, the points (times) for sampling and extraction of seed tissue (an embryonic axis with part of the vascular region) were chosen. Phase I was removed 30 min after the start of imbibition, with water content lower than 20%; phase II, 20 h after imbibition, with water content ranging between 40–45%; and phase III, 51 h after imbibition, with water content above 55%, in which the seeds sampled showed protrusion of the primary root. It is worth mentioning that, after 36 h of imbibition, the lots exceeded an average of 10% of seeds with primary root protrusion. The respiration process is activated during seed imbibition only after seeds reach 25% of water content [26].
During the monitoring of the imbibition curve, the germination of the lots was also evaluated (Figure S1). At the level of stricto sensu germination, no statistical difference was observed between lots B and E. However, when the abnormal seedlings were counted 6 days after imbibition, lot E (lower quality) presented 23% of abnormal seedlings, while B only presented 17%. This difference was statistically significant by the t test (p = 0.05). In seedlings, symptoms of moisture damage are detected by the presence of seedling abnormalities [15,16]. These data show the subtle difference in the physiological quality of the selected lots.
3.4. A Proteomic Analysis of Different Seed Lots’ Germination with a Subtle Variation in the Physiological Quality of Soybean Seeds
The quality of the extracted proteins was checked through SDS-PAGE, which showed well-defined bands (Figure S3). The results show that, in total, 1266 proteins were identified in the six soybean seed samples during the germination phases, using the FDR < 1% criterion. After applying the most stringent filter (Score > 5 and Scored Peak Intensity (SPI) > 60%), the identified proteins were reduced to 777 (Figure 3). This represents a significant decrease, indicating that 489 proteins were excluded based on the quality of the spectrometric data. This additional filtering, by maintaining the proteins with higher reliability in the identification, is crucial to ensure the robustness of the conclusions in proteomic studies. Because of the data obtained, almost half of the proteins that possibly have a fine contribution to the physiological quality of seeds need to be studied. In a similar study with soybeans, from seeds that underwent controlled deterioration treatment for 3 and 7 days, in addition to the control, a total of 1626 proteins were identified [10]. This shows that, for soybean seeds, the extraction methods [18] and protein digestion [19] were modified and that the gel-free mass spectrometry analysis employed here was excellent.
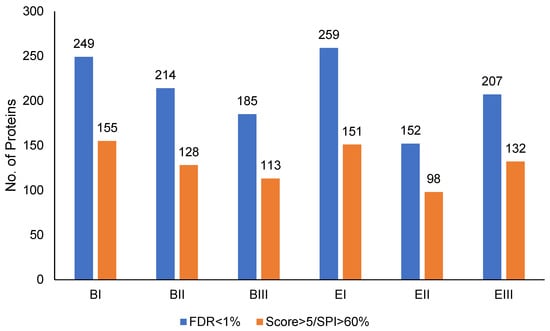
Figure 3.
Proteins identified by mass spectrometry. he number of proteins identified in soybean seeds during the germination stages, which went through the periods of false discovery rate (FDR) < 1%, Score > 5, and Scored Peak Intensity (SPI) > 60%.
To analyze the interdependence of the protein profiles, principal component analyses were carried out at each germination stage for lots B (higher quality) and E (lowest quality) (Figure S4). The variation of the dataset evidenced the difference between lots B and E in all stages of germination. Biplots showed that in phase I of germination, the main component 1 (CP1) explained 28.91% and CP2 only 21.49% of the data variance. In phase II, CP1 and CP2 explained 37.3% and 19.9% of the sample variance, respectively. Finally, in phase III, CP1 and CP2 explained 42.65% and 17.1% of the sample variance, respectively. It is possible to observe that CP1 contains information that possibly separates the set of samples from the two lots at any stage of germination; in addition, as the germination stage advances, the explanatory power of CP1 is relatively greater, for example, CP1 in stage III is higher than CP1 in stage II which is higher than in stage I of germination. In studies with soybean proteomics, on the effect of controlled deterioration over 3 and 7 days on seeds, in PCA analysis, the authors found significant differences for the control dataset, 3, and 7 days of deterioration, in addition to finding that CP1 explained 70% of the variance of the proteomics data [10].
In phase I of germination (0.5 h of imbibition), the contrast between EI (lowest quality) and BI (highest quality) presented 12 differentially abundant proteins, with eight proteins more accumulated in the BI treatment and four more accumulated in the EI treatment (Table S1). Ribosomal proteins are more abundant with better physiological quality during phase I of germination (BI). Five ribosomal proteins were identified that are more accumulated exclusively in the embryonic axis of seeds with higher physiological quality, namely L35 and L13, associated with the 60S portion of the ribosome, and S18, S25, and S30, associated with the 40S portion of the ribosome. In addition to these, an 18 kDa seed maturation protein—GMPM1 (Q01417)—was identified; these six proteins are exclusively more abundant in BI (best quality). The proteins exclusively more abundant in EI (worst quality) are LEA from the seed maturation protein-SMP family (I1LE41) and an Oleosin (K7KTR9). Interestingly, two 5-methyltetrahydropteroyltriglutamate--homocysteine S-methyltransferase (EC 2.1.1.14) enzymes were identified as more abundant in both contrast treatments, in BI the protein Q71EW8 and in EI the I1LXY1; these are variants of proteins probably with the same function, presenting an identity of 88.6% when aligned in Uniprot. In addition to this, proteins P11827 for BI and P0DO15 in EI were more abundant; these proteins are subunit 1 and 2 respectively of the Beta-conglycinin alpha protein with identity according to Uniprot alignment of 87.42%. These proteins have a seed storage function that accumulates during seed development and are hydrolyzed during germination to provide a source of carbon and nitrogen for the developing seedling. Phase I of germination is characterized by the beginning of imbibition, a rapid increase in fresh weight and water content of the seeds. In this phase, all mechanisms of reactivation of metabolism are initiated, including the transcription and translation of new mRNAs as well as the degradation and translation of stored mRNAs. These mechanisms are present in all phases of germination [23].
The contrast between EII (lowest quality) and BII (highest quality) is demonstrated by the fact that, in phase II of germination (20 h of imbibition), 17 differentially abundant proteins were identified, with 12 proteins more abundant in BII and five more abundant in EII (Table S2). In this phase of germination, the ribosomal protein L35 of the 60S subunit (C6SW56) continued to be more abundant, as well as the protein containing the ubiquitin-like domain associated with the 40S ribosome (C6T4R9). In addition, a Ubiquinol oxidase (A0A0R0JTY5), Histone H4 (A0A0R0EX12), and a Kunitz-type trypsin inhibitor (I1KYW3) were also relevant, as they presented greater abundance in the lot with better physiological quality. Furthermore, the other proteins differentially present probable redundancy of molecular function, for example, two Oleosins (I1N747 and K7KTR9, 33.52% identity) were more abundant in BI, and one Oleosin P24 isoform B (P91) (P29531) was more abundant in EII; the identity according to Uniprot for I1N747 × P29531 was 91.89% and K7KTR9 × P29531 was 32.57%. This also occurred with the other proteins identified in this phase; they are Beta-conglycinins subunit 2 (P0DO15) in BII versus subunit 1 (P11827) in EII, for which the identity was described previously; Late embryogenesis abundant protein (MP2) (Q39871) in BII versus LEA-ECP63-like domain-containing protein (I1L957) in EII with 81.09% identity; Cupin type-1 domain-containing protein (I1LHP6 and A0A0R0H2H7, identity = 35.77%) in BII and K7K4G2 in EII, I1LHP6 × K7K4G2 with 83.42% identity; and A0A0R0H2H7 × K7K4G2 with 34.96% identity. There were probable NADPH-dependent aldehyde reductases 1 (A0A0R4J681) in BII and (A0A0R0I4F6) in treatment EII with 96.25% identity. All proteins except C6SW56, I1KYW3, and A0A0R0H2H7 showed unique abundances; these three proteins were expressed in both EII and BII with log2FC of −0.61, −0.71, and −0.75, respectively. These log2FC values will be considered as relevant differences, since the difference between the lots is biologically subtle, so subtle changes in abundance with statistical significance may be relevant and are a reasonable explanation for the difference in vigor in these seed lots.
When comparing lots EIII (lowest quality) with BIII (highest quality) during phase III of germination (51 h of imbibition), 28 differentially abundant proteins were identified, with only four more abundant in BIII and 24 more abundant in EIII (Table S3). Interestingly, in contrast to phases I and II, during phase III of germination, when root protrusion begins (germination stricto sensu), most of the proteins identified were more abundant in the lower-quality EIII. In this EIII versus BIII contrast, three pairs of proteins presented a high degree of identity and were more abundant in both lots: in BIII the protein A0A0R0FER5 and the protein A0A0R0FM97 in EIII are an Elongation factor 1-alpha with 96.87% identity between them; the largest proteins, I7FST9 in BIII and I1KAB7 in EIII, are Protein disulfide-isomerase (EC 5.3.4.1) with 95.83% identity, while two LEAs with 81.29% identities were identified, I1L957 (Late embryogenesis abundant protein ECP63-like domain-containing protein) in BIII and Q39871 (Late embryogenesis abundant protein—MP2) in EIII. Proteins associated with ribosomes and translation were identified; the ribosomal protein L30 ferredoxin-like fold domain-containing (K7MBV6) protein was more abundant in BIII, but five proteins were more abundant in the EIII: Tr-type G domain-containing protein (I1MJ86); 40S ribosomal protein S25 (C6SWX1); Ribosomal protein (I1LMS5); 60S ribosomal protein L12 (C6TMB5); and an EF1Bgamma class glutathione S-transferase (C6TNT2). Proteins that play a storage role in seeds were more abundant in the lowest-quality EIII during phase III of germination, for example, three Cupin type-1 domain-containing proteins (I1LHP6; A0A0R0H2H7; I1L860), and 2S seed storage albumin protein GM2S-1 (P19594). In addition, stress-related proteins, such as the 70 kDa heat shock protein (I1JGR5), were more abundant in the lower quality, as well as an Annexin (I1LLC0), Seed maturation protein PM31 (Q9XET1), and 11-beta-hydroxysteroid dehydrogenase-like 5 (A0A0R0HAM0) with antioxidant activity. Some enzymes involved in varied metabolisms were also more abundant in EIII, an Alcohol dehydrogenase (A0A0R0JL19), Glucose-6-phosphate isomerase EC 5.3.1.9 (I1JV38), a UTP-glucose-1-phosphate uridylyltransferase EC 2.7.7.9 (I1MBR7), Probable bifunctional TENA-E protein EC 3.5.1.-EC 3.5.99.2 (Q9SWB6), 5-methyltetrahydropteroyltriglutamate--homocysteine S-methyltransferase EC 2.1.1.14 (Q71EW8), and a Nucleoside diphosphate kinase EC 2.7.4.6 (I1KJI7).
4. Discussion
Soybean seeds play a fundamental role in agriculture, especially in large-scale cropping systems, where their quality directly impacts productivity and profitability. The physiological quality of seeds is crucial to ensure rapid and uniform seedling emergence, which is essential to establish a population of robust and well-distributed plants in the field [2,4]. High-quality seed lots have greater vigor and germination, characteristics that influence plant resistance to environmental stresses and reduce the need for replanting, optimizing invested resources and increasing agricultural efficiency [4,6]. Obtaining seed lots with high physiological quality is, therefore, of extreme importance for the agronomic sector, as it influences the stability and predictability of production, essential factors to meet the growing demand for food, biofuels, and other soybean-derived products.
We successfully distinguished the three main phases of soybean seed germination, even though biological processes occur across all phases, as reported in other studies with soybean seeds [20,21]. It was possible to identify and characterize differences in the proteomic profile at each phase when comparing commercial seed lots with subtle differences in physiological quality.
Seed germination occurs in three distinct phases. In Phase I, seeds rapidly absorb water through imbibition, reactivating cellular metabolism and repairing damaged membranes without visible growth. In Phase II, water absorption slows down while metabolic activity intensifies, marked by enzyme activation and the initial mobilization of energy reserves stored in the cotyledons. In Phase III, the primary root emerges (germination stricto sensu), accompanied by increased reserve consumption, cellular growth, and tissue differentiation. These phases are interdependent and reflect the seed’s preparation for seedling development [7,20]. Seeds with higher physiological quality exhibit better performance at all stages.
Molecular processes of transcription and translation precede physiological events [7]. The proteins identified in this study have biological significance in explaining, at least partially, the subtle differences in physiological quality. For instance, ribosomal proteins appear to play a prominent role during the early stages of germination, helping to differentiate the subtle quality differences in soybean seeds. This finding aligns with other studies, though these were conducted exclusively on quiescent seeds [9,10,20]. Ribosomal proteins may be associated with higher-quality seeds, aiding in the initiation of germination.
The differentially abundant proteins identified in this study may be significantly influenced by stored mRNAs, which play a crucial role during the early stages of germination. These pre-existing mRNAs enable the translation of essential proteins involved in energy metabolism, cellular repair, and stress response, even before the activation of new gene transcription. In the present study, ribosomal proteins and other translation-associated proteins were more abundant in high-quality seed lots, emphasizing the role of efficient translation as an indicator of seed vigor. Furthermore, the stability and integrity of stored mRNAs are directly linked to seed vigor, as deteriorated seeds exhibit degradation of these mRNAs, impairing the translation of critical proteins [23]. These findings support the idea that the physiological quality of seeds depends not only on genetic factors, but also on the preservation of mRNAs and translation-associated proteins during storage and the early stages of germination. In our study, during the initial stage of germination in Phase I, with approximately 30 min of imbibition, four ribosomal proteins (C6SW56; C6T0A7; I1MLX4; C6SWX1) were more abundant in the seed lot with higher physiological quality (lot BI). In Phase II of germination, 20 h after imbibition, A0A0R0JTY5 was identified as a DAP that is more abundant in the higher-quality seed (lot BII). This protein may also be associated with the reprogramming and utilization of stored mRNAs in seeds, which are essential for supporting protein synthesis and metabolic activation during the early stages of germination. These proteins may be associated with stored mRNAs in seeds, playing a critical role in the translation of key proteins during the early stages of germination.
Ribosomal proteins play a fundamental role in translation and protein synthesis, which are essential processes for early seedling growth and successful seed establishment. In this study, we observed that ribosomal proteins, such as 60S ribosomal protein L12 (C6TMB5) and 40S ribosomal protein S25 (C6SWX1), were more abundant in the lower-quality seed lot during Phase III of germination, after 51 h of imbibition (EIII). This increased abundance may indicate a compensatory response aimed at enhancing protein synthesis to mitigate the effects of accumulated stress, reflecting a heightened metabolic effort to overcome deterioration-related limitations. In contrast, the higher-quality seed lot (BIII) exhibited a balanced presence of ribosomal proteins, suggesting an efficient and well-regulated translational activity characteristic of seeds with higher vigor and reduced impact from deterioration.
Translation-related proteins, including elongation factors, initiation factors, and ribosomal components, play a pivotal role during seed germination. They are highly expressed in Phase II, a stage marked by intense mobilization of reserves and metabolic reactivation [7]. However, these proteins have been shown to be highly sensitive to controlled deterioration (CDT). Previous studies have reported a significant reduction in their abundance in seeds subjected to AA or CDT [9,10], reflecting a slowdown in biosynthetic and primary metabolic processes, which are directly linked to the loss of seed vigor and viability. Additionally, ribosomal proteins have consistently been highlighted as central to soybean germination, underscoring their critical role in protein synthesis and early seedling development [21].
These findings reinforce the hypothesis that translation-related proteins are not only fundamental to the germination process, but also serve as sensitive indicators of seed physiological quality. They have the potential to act as biomarkers for responses to aging and oxidative stress. Overall, our results support the idea that high-quality seed lots exhibit more stable metabolic activity, while lower-quality seeds redirect metabolic resources toward adaptive processes. This underscores the importance of ribosomal proteins as potential markers of seed vigor and physiological health.
Maturation proteins, such as Seed Maturation Protein (GMPM1), belong to the LEA (Late Embryogenesis Abundant) family [22] and play essential roles in cell protection during the final stages of seed development, contributing to their viability and vigor. Although not directly mentioned in the studies analyzed, the role of proteins related to cell maturation and stabilization is evident. The importance of storage proteins, such as globulins, in mobilizing reserves and supporting early seedling growth, processes that are critical for efficient germination [21]. Additionally, significant changes in protein abundance during controlled deterioration treatment, including those associated with primary metabolism and stress response [9,10], reinforcing the relevance of protective proteins for seed physiological quality. This evidence suggests that maturation proteins, such as GMPM1, can act as sensitive biomarkers of vigor, contributing to the identification of seed lots with greater resilience and better performance, especially under adverse conditions, highlighting their potential for applications in quality control and sustainable agricultural production.
It has been reported that ADH is a marker of seed deterioration and vigor [24,25,27,28,29], which corroborates the protein found to be more abundant in the lower-quality lot in phase III of germination. The protein alcohol dehydrogenase (ADH) is widely recognized for its role in fermentative metabolism, activated under conditions of low oxygen availability during seed imbibition. Recent studies suggest a direct relationship between ADH activity and seed vigor, as well as their deterioration, due to the association with energy metabolism and the production of ethanol as a byproduct. In the context of our study, the greater abundance of ADH in EIII, which was of lower physiological quality, is in line with the literature that demonstrates an increase in the expression and activity of this protein in seeds subjected to stresses, such as aging and hypoxia during germination. These results suggest that lower-vigor seeds depend more on fermentative metabolism as a compensatory pathway for energy generation, which may result in greater ethanol production, as observed in other crops such as corn and melon. On the other hand, higher quality lots, such as BIII, show less dependence on this metabolic pathway, possibly due to greater aerobic respiration efficiency and lower mitochondrial deterioration. These findings corroborate the use of ADH as a potential marker to evaluate seed vigor and deterioration, indicating that its abundance may reflect an unbalanced metabolic state in lower-quality lots.
Throughout the three germination phases, a progressive increase in the number of differentially abundant proteins was observed, reflecting the intensification of metabolic and molecular activities as the germination process progressed. In phase I, most of the differentially abundant proteins were more abundant in lot B, which was of higher quality, indicating an efficient preparation for initial metabolic activation. In phase II, this trend remained, with lot B showing a greater abundance of proteins related to translation and storage, which are important for initial seedling growth. However, in phase III, a significant change occurred, with most of the differentially abundant proteins being more expressed in lot E, which was of lower quality. This pattern suggests that lower-quality seeds activate compensatory processes, including stress responses and late energy mobilization, while lot B maintained a more stable and efficient protein profile. These results reinforce the notion that the physiological quality of seeds influences not only the quantity but also the dynamics of protein expression during germination.
5. Conclusions
The use of the shotgun proteomics approach has proven to be a highly sensitive tool for identifying subtle differences in physiological quality between commercial soybean seed lots. Proteomic analysis detected significant variations in the protein expression profile at different germination stages, highlighting the central role of translation-related proteins, such as ribosomal proteins.
Ribosomal proteins are potential biomarkers of soybean seed vigor. They are more abundant in Phase I of germination in higher-quality seed lots, indicating efficient translational activity, and, in Phase III, they signal delayed germination in lower-quality seed lots.
Phase III of germination (approximately 50 h) is the stage where subtle differences in the physiological quality of soybean seeds with similar germination potential are most evident. Storage proteins and translation-related proteins indicate delayed germination, while ADH, Heat Shock Protein, Annexin, and Seed Maturation Protein PM31 may reflect seed deterioration processes and the consequent loss of vigor in soybean seed lots.
These findings highlight not only the potential of proteomics to discriminate lots with subtle physiological differences, but also the identification of protein biomarkers (Table 3) with practical applications in quality control and improvement of commercial seeds.

Table 3.
Potential sensitive proteins as biomarkers of physiological seed quality or deterioration of commercial lots of soybean seeds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15030609/s1, Figure S1: Percentage of strictu-senso germination (GS), at 48, 51 and 144 h and percentage of abnormal seedlings (AS), at the sixth day of imbibition, in two lots of soybean seeds, Lot B (LB) and Lot E (LE), cultivar M8808. Figure S2: (A) Schematic of the section of the embryonic axis with part of the vascular region of soybean seeds represented by the dashed red line and the structure of a soybean seed soaked without the tegument (adapted from, FRANÇA-NETO; KRZYZANOWSKI, 1998). (B) Preparation of soybean seeds at each stage of germination for excised embryonic axis. Figure S3: Protein profile, SDS-PAGE, of total proteins of two lots of soybean seeds, cultivar M8808, during germination, 0.5 h in imbibition (Phase I), 20 h in imbibition (Phase II) and 51 h in imbibition (Phase III). The marker used was from 14 kDa to 97 kDa. Figure S4: Analysis of principal components of two lots of soybean seeds, cultivar M8808, through data ob-tained by mass spectrometry (QTOF-MS/MS). In A) Phase I of germination (0.5 h in imbibition); B) Phase II of germination (20 h in imbibition) and C) Phase III of germination (51 h in imbibition). Red and yellow squares are lot B and E, respectively. Table S1: Differentially abundant proteins (DAP) in phase I of germination between two lots of soybean seeds with subtle differences in vigor. Lot B was used as a control. Table S2: Differentially abundant proteins in phase II of germination between two lots of soybean seeds with subtle differences in vigor. Lot B was used as a control. Table S3: Differentially abundant proteins in phase III of germination between two lots of soybean seeds with subtle differences in vigor. Lot B was used as a control.
Author Contributions
Conceptualization, F.R.S. and C.P.P.; Data curation, F.R.S., I.Y.M.-O., K.R.O., F.D.A.S. and R.M.B.; Formal analysis, F.R.S. and I.Y.M.-O.; Investigation, F.R.S., I.Y.M.-O., C.P.P. and R.M.B.; Methodology, F.R.S., I.Y.M.-O., C.P.P. and R.M.B.; Software, I.Y.M.-O.; Validation and Visualization, F.R.S., I.Y.M.-O., F.D.A.S. and K.R.O.; Writing—original draft, F.R.S.; Writing—review and editing, all authors; Funding acquisition, C.P.P. and R.M.B.; Resources, C.P.P.; Project administration, R.M.B.; Supervision, R.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Universidade Estadual de Santa Cruz and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kim, E.-H.; Kim, S.-L.; Kim, S.-H.; Chung, I.-M. Comparison of Isoflavones and Anthocyanins in Soybean [Glycine max (L.) Merrill] Seeds of Different Planting Dates. J. Agric. Food Chem. 2012, 60, 10196–10202. [Google Scholar] [CrossRef] [PubMed]
- Marcos Filho, J. Fisiologia de Sementes de Plantas Cultivadas; Abrates: Londrina, Brazil, 2005. [Google Scholar]
- Oliveira, K.R.; Sampaio, F.R.; Siqueira, G.S.; Galvão, Í.M.; Bennett, S.J.; Gratão, P.L.; Barbosa, R.M. Physiological quality of soybean seeds grown under different low altitude field environments and storage time. Plant Soil Environ. 2021, 67, 92–98. [Google Scholar] [CrossRef]
- Ebone, L.A.; Caverzan, A.; Tagliari, A.; Chiomento, J.L.T.; Silveira, D.C.; Chavarria, G. Soybean seed vigor: Uniformity and growth as key factors to improve yield. Agronomy 2020, 10, 545. [Google Scholar] [CrossRef]
- Marcos-Filho, J. Seed vigor testing: An overview of the past, present and future perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef]
- Caverzan, A.; Giacomin, R.; Müller, M.; Biazus, C.; Lângaro, N.C.; Chavarria, G. How does seed vigor affect soybean yield components? Agron. J. 2018, 110, 1318–1327. [Google Scholar] [CrossRef]
- Rajjou, I.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed Germination and Vigor. Annu. Rev. Plant Biol. 2012, 63, 507–540. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, X.; Li, L.; Zhao, H.; Liu, S.; Yu, X.; Shen, Y.; Zhou, Y.; Zhu, Y.; Shu, Y.; et al. Quantitative proteomic, physiological and biochemical analysis of cotyledon, embryo, leaf and pod reveals the effects of high temperature and humidity stress on seed vigor formation in soybean. BMC Plant Biol. 2020, 20, 127. [Google Scholar] [CrossRef]
- Min, C.W.; Kim, Y.J.; Gupta, R.; Kim, S.W.; Han, W.Y.; Ko, J.M.; Kang, H.W.; Yoon, W.B.; Choung, M.G.; Kim, Y.C.; et al. High-throughput proteome analysis reveals changes of primary metabolism and energy production under artificial aging treatment in Glycine max seeds. Appl. Biol. Chem. 2016, 59, 841–853. [Google Scholar] [CrossRef]
- Min, C.W.; Lee, S.H.; Cheon, Y.E.; Han, W.Y.; Ko, J.M.; Kang, H.W.; Kim, Y.C.; Agrawal, G.K.; Rakwal, R.; Gupta, R.; et al. In-depth proteomic analysis of Glycine max seeds during controlled deterioration treatment reveals a shift in seed metabolism. J. Proteom. 2017, 169, 125–135. [Google Scholar] [CrossRef] [PubMed]
- International Seed Testing Association. International Rules for Seed Testing: Rules 1993, Adopted at the Twenty-Third International Seed Testing Congress, Argentina 1992, to Become Effective on 1 July 1993; International Seed Testing Association: Zurich, Switzerland, 1999; ISBN 3906549275. [Google Scholar]
- Ministério da Agricultura. Regras para Análise de Sementes; Ministério da Agricultura: Brasília, Brazil, 2009. [Google Scholar]
- Krzyzanowski, F.C.; Nakagawa, J.; Neto, J.B.F.; Vieira, R.D. Testes de vigor baseados no desempenho das plântulas. In Vigor Sementes Conceitos e Testes; Abrates: Londrina, Brazil, 1999; Volume 2, pp. 1–21. [Google Scholar]
- Maguire, J.D. Speed of germination-Aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Marcos Filho, J. Testes de vigor: Importância e utilização In Vigor Sementes Conceitos e Testes; Krzyzanowski, F.C., Vieira, R.D., França Neto, J.B., Eds.; Abrates: Londrina, Brazil, 1999; p. 1. [Google Scholar]
- França Neto, J.D.B.; Krzyzanowski, F.C.; Da Costa, N.P. The Tetrazolium Test for Soybean Seeds; Embrapa Soja-Documentos (INFOTECA-E); Embrapa: Londrina, Brazil, 1998. [Google Scholar]
- Henning, A.A. Guia Prático para Identificação de Fungos Mais Frequentes em Sementes de Soja; Embrapa Soja: Londrina, Brazil, 2015; ISBN 857035441X. [Google Scholar]
- Pirovani, C.P.; Carvalho, H.A.S.; Machado, R.C.R.; Gomes, D.S.; Alvim, F.C.; Pomella, A.W.V.; Gramacho, K.P.; Cascardo, J.C.d.M.; Pereira, G.A.G.; Micheli, F. Protein extraction for proteome analysis from cacao leaves and meristems, organs infected by Moniliophthora perniciosa, the causal agent of the witches’ broom disease. Electrophoresis 2008, 29, 2391–2401. [Google Scholar] [CrossRef] [PubMed]
- Villén, J.; Gygi, S.P. The SCX/IMAC enrichment approach for global phosphorylation analysis by mass spectrometry. Nat. Protoc. 2008, 3, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination-still a mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
- Han, C.; Yin, X.; He, D.; Yang, P. Analysis of Proteome Profile in Germinating Soybean Seed, and Its Comparison with Rice Showing the Styles of Reserves Mobilization in Different Crops. PLoS ONE 2013, 8, e56947. [Google Scholar] [CrossRef]
- Chen, M.-H.; Lin, Y.; Hsieh, J.; Kuang, L.; Chow, T.; Lee, P.; Hsing, Y. Two Genes Encoding GmPM, A Soybean Seed Maturation Protein. J. Genet. Mol. Biol. 2002, 13, 177–187. [Google Scholar]
- Naoto, S.; Loïc, R.; North, H.M. Lost in translation: Physiological roles of stored mRNAs in seed germination. Plants 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Harnowo, D.; Prayogo, Y. The role of PME and ADH enzymes in seed deterioration and its implication for producing high quality soybean seed. IOP Conf. Ser. Earth Environ. Sci. 2023, 1246, 012021. [Google Scholar] [CrossRef]
- Carvalho, E.R.; Carvalho Penido, A.; Kelli Rocha, D.; Vilela Reis, L.; Ferreira dos Santos, S.; dos Santos, H.O. Monitoramento fisiológico e enzimático de sementes tratadas de cultivares de soja durante o armazenamento. Rev. Bras. De Ciências Agrárias 2022, 17, e2077. [Google Scholar] [CrossRef]
- Vertucci, C.W.; Leopold, C. Oxidative processes in soybean and pea seeds. Plant Physiol. 1987, 84, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, J.A.; Gadotti, G.I.; Pinheiro, R.D.M.; Silva, R.N.O.D.; Oliveira, F.K.D.; Moraes, D.M.D. Vigor and anaerobic metabolism of soybean seeds evaluated by ethanol test. J. Seed Sci. 2023, 45, e202345007. [Google Scholar] [CrossRef]
- Chaengsakul, C.; Onwimol, D.; Kongsil, P.; Suwannarat, S. Ethanol production and mitochondrial-related gene expression of maize (Zea mays) seed during storage. J. Integr. Agric. 2019, 18, 2435–2445. [Google Scholar] [CrossRef]
- Ornellas, F.L.S.; de Sousa, A.O.; Pirovani, C.P.; do Nascimento Araújo, M.; da Costa, D.S.; Dantas, B.F.; Barbosa, R.M. Gene expression, biochemical and physiological activities in evaluating melon seed vigor through ethanol release. Sci. Hortic. 2020, 261, 108884. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
