1. Introduction
Inflorescences of onion plants (
Allium cepa L.) are umbellate cymes, which are surrounded by a spathe that splits before blooming begins. For the sake of brevity, this inflorescence will further be referred to as ’umbel’. Each onion umbel bears between 50 and 2000 perfect hermaphrodite white- to bluish-colored flowers that open scattered on slender pedicels for a period lasting between two and four weeks [
1]. The perianth segments (tepals) of these flowers are six, distributed in two whorls, greenish, expanded, and free. Each flower is made up of six stamens in two whorls. The flower has a single superior pistil with three locules, each of which contains two ovules. Therefore, each single flower produces a maximum of six seeds per capsule [
2]. In onion flowers, nectar and pollen are the most important rewards for insect pollinators. Nectar accumulates at the base of the ovary near the stamen filaments [
2,
3,
4], while pollen grains are abundant in the six bilocular anthers. Pollen is shed in the first 48 h after flower opening, while the stigma only starts to be receptive after the anthers have released their pollen grains. This protandrous dichogamy in individual flowers limits self-pollination and enhances outcrossing [
5,
6]. In this regard, onion strongly relies on pollination carried out by insects for an efficient seed set [
7]. Wind only accounts for 10% of pollination due to the production of sticky pollen grains, which are hardly airborne. Saleh et al. [
8] reported that honeybees (
Apis mellifera L.) are responsible for 87% of the pollination events, with other insect pollinators achieving the remaining 3%. Honeybees are the most efficient and practical pollinators of this crop due to their effectiveness in onion pollen transport [
9], and the seed set in onion is highly correlated with the efficiency of pollination carried out by this insect. After pollination, the seeds develop, and the capsules dry in a couple of weeks, splitting from the apex to down the center of the locule, allowing the mature seeds to fall free to the ground [
10]. Unfortunately, using honeybees as pollinators does not always guarantee profitable seed yield [
11]. Since onion is the third most important horticultural crop in the world after potato and tomato [
12], it is essential to determine the reasons for these failures in the seed set.
Nowadays, most of the onion lines sold by seed companies are F1 hybrids. To obtain these hybrids, it is necessary to cross a male sterile line (MSL) with a male fertile (pollen donor) line (MFL). Synchronous blooming in these parental lines is essential to have fertile pollen available when the pistil of the other line is receptive. This process makes the synchrony of flower phenology and the duration of the effective pollination period (EPP) extremely important. The worldwide use of hybrids is due to their superiority over open-pollinated varieties, as hybrids produce plants with more vigor, better uniformity, and higher bulb quality [
6]. However, these MSLs frequently fail to set, and the seed yield could be up to 40% lower than the MFL yield [
13]. In this sense, and regardless of the MFL used as a pollen donor, some MSLs (pollen recipients) always have low seed yield, even under an abundance of pollinators. Pollination of hybrid seed crops represents a challenge to specialized pollinators, as it requires the movement of pollen between separate lines of plants, which means that such specialization can reduce pollen transfer [
14]. In this regard, adding non-floral resources seems to increase insect visitation rates (flies, beetles, bees, wasps, and ants) to the flowers of some hybrids like carrots, but this is not translated to the yield of those hybrids [
15]. It is still not clear whether poor yields are due to a lack of pollen or other characteristics that have inadvertently been selected during the process of crop breeding and selection or an interaction of plant traits with the environment [
16]. Previous studies have shown that flower morphology and nectar composition reduce the attractiveness of different MSLs to bees [
17]. Due to poor pollination, these MSLs produce low seed yield in quantity and quality [
18,
19].
In addition to the reduced attractiveness of MLS flowers, their flower fertility and duration may be a limiting factor for the seed set. The EPP is defined as the number of days after anthesis during which pollination is effective in producing marketable fruit or seeds [
20]. The duration of the EPP can be calculated as the number of days that the ovules are viable minus the days the pollen tubes take to reach them. In some crops, stigma receptivity can be the limiting factor, and then, the EPP is determined by the days the stigma is able to adhere to pollen grains and allow their germination [
21]. When calculating the duration of the EPP in any crop, it is important to consider that each single flower has its own EPP duration, and therefore, when working with a population of flowers, a variability between flowers must be expected for each single component of the EPP.
To the best of our knowledge, EPPs of different onion lines have not yet been reported. Therefore, the aim of this work is to determine the duration of the EPP in them and the changes in pistil functionality associated with onion protandry to elucidate the factors involved in seed set failures.
2. Materials and Methods
2.1. Experimental Plot and Plant Material
The experiments were carried out using two MSLs (functionally female) and a male fertile line (MFL) (hermaphrodite) during the 2022 and 2023 seasons in an experimental field of the Company Bayer located in Mayor Drummond, Luján de Cuyo, Mendoza, Argentina (32°99′97″ S, 68°86′57″ W). There, onion bulbs were planted at a density of 10 bulbs per meter in rows 1 m apart. A ratio of 4:1 of male sterile/male fertile plants, typical in the commercial production of onion seeds, was used. The male sterile lines and the male fertile were all intermediate-day yellow onions with bulbs with a globe shape. The plants, customarily fertilized and watered by drip irrigation, bloomed during November and December in both years. Pesticides were not used during flowering.
Meteorological data, including air temperature (°C), relative humidity (%), wind speed (m/s), radiation (W/m
2), and rain (mm), were taken from a weather station near the location of the experimental area during the flowering period of both seasons. In addition, the temperature range (i.e., the difference between the highest and lowest temperatures during the day) was taken into account. Data were analyzed from the day the flowers were bagged to the last day of sampling, 8 and 14 DAA in 2022 and 2023, respectively, between the second week of November and the first week of December in both years of study (
Table 1).
2.2. Pollination Procedure
One day before the anthesis of the first flowers, once the thin spathe split and flowering was just about to start a set of umbels was randomly chosen and covered with fabric bags to prevent uncontrolled pollination. The bags were about 1 m long and made of a material with a mesh that allowed air, but not insects, to pass through. The bags were wrapped around the umbel and part of the escape, leaving more than 60 cm above the umbel so as to not affect the temperature of the flowers. Sequential observations of the changes in pistil development and its components (stigma, style, ovary, and ovules) were carried out during two consecutive seasons using as replicates samples of bagged flowers from three umbels, each of them from a different plant; in total, 9 umbels were bagged per line. The observations were performed on different days due to the scattered bloom of the flowers within the umbels but always considering their day of anthesis. To do so, the date at which the experimental flowers were open was annotated, and each of these flowers was labeled with a thread of a different color for each day of the week. MFL flowers were emasculated to avoid self-pollination, removing all the stamens before anther dehiscence.
During the first year, we used honeybees (Apis mellifera L.) as pollinating agents. Bees foraging for more than a few seconds on flowers of MFL were trapped and introduced in a number of 3–4 per umbel inside the bags. Bee introduction inside the bags was performed at anthesis and 2, 3, 4, 6, and 8 days after anthesis (DAA) to force the pollination of MSL flowers of different ages. Tagged flowers were collected 1 day after the introduction of the pollinating bees inside the bags. In the second year, we changed the pollination procedure and performed direct hand pollination of the flowers. In this procedure, anthers were collected from recently open flowers of several umbels of MFL and placed in a Petri dish in the lab, where the released pollen grains were pooled for later use. Bag-isolated flowers from the MSL and MFL were then hand pollinated with a fine camel paint brush, rubbing the stigmas with the pollen previously collected before re-bagging. All open flowers on a day were labeled and pollinated either at anthesis or 2, 4, 6, 8, 10, 12, and 14 DAA and collected 1 day after hand pollination.
2.3. Determination of the Effective Pollination Period
The components of the EPP were determined on flowers pollinated at the prescribed dates and collected one day after their pollination. From them, samples of 20 flowers per date were fixed in FAA (formaldehyde, acetic acid, and 70% ethanol in a ratio of 1:2:17) in the field and taken to the lab, where they were kept in a fridge at 4 °C until further analysis under fluorescence microscopy. Then, the different sets of flowers were placed in Histo-Tek cassettes and washed with running water for four hours, transferred to a 0.8 M NaOH softening solution for another four hours, and then water rinsed overnight. After softening the pistil, the stigma and the style were dissected and separated from the ovary with a transverse section at the end of the style. Finally, the six ovules of the ovary were dissected. The separated pistil organs were placed on a microscope slide and stained with a solution containing 0.1% aniline blue in phosphate buffer [
22]. Finally, the samples were examined under a microscope (Nikon 80i Upright, Japan) equipped with UV excitation filters (DAPI, FITC, Cy3, and Cy5) with NIS-ELEMENTS Software) to measure pollen adhesion and germination, pollen tube growth, fertilization date and levels, and ovule senescence.
2.3.1. Stigma Receptivity Evaluation
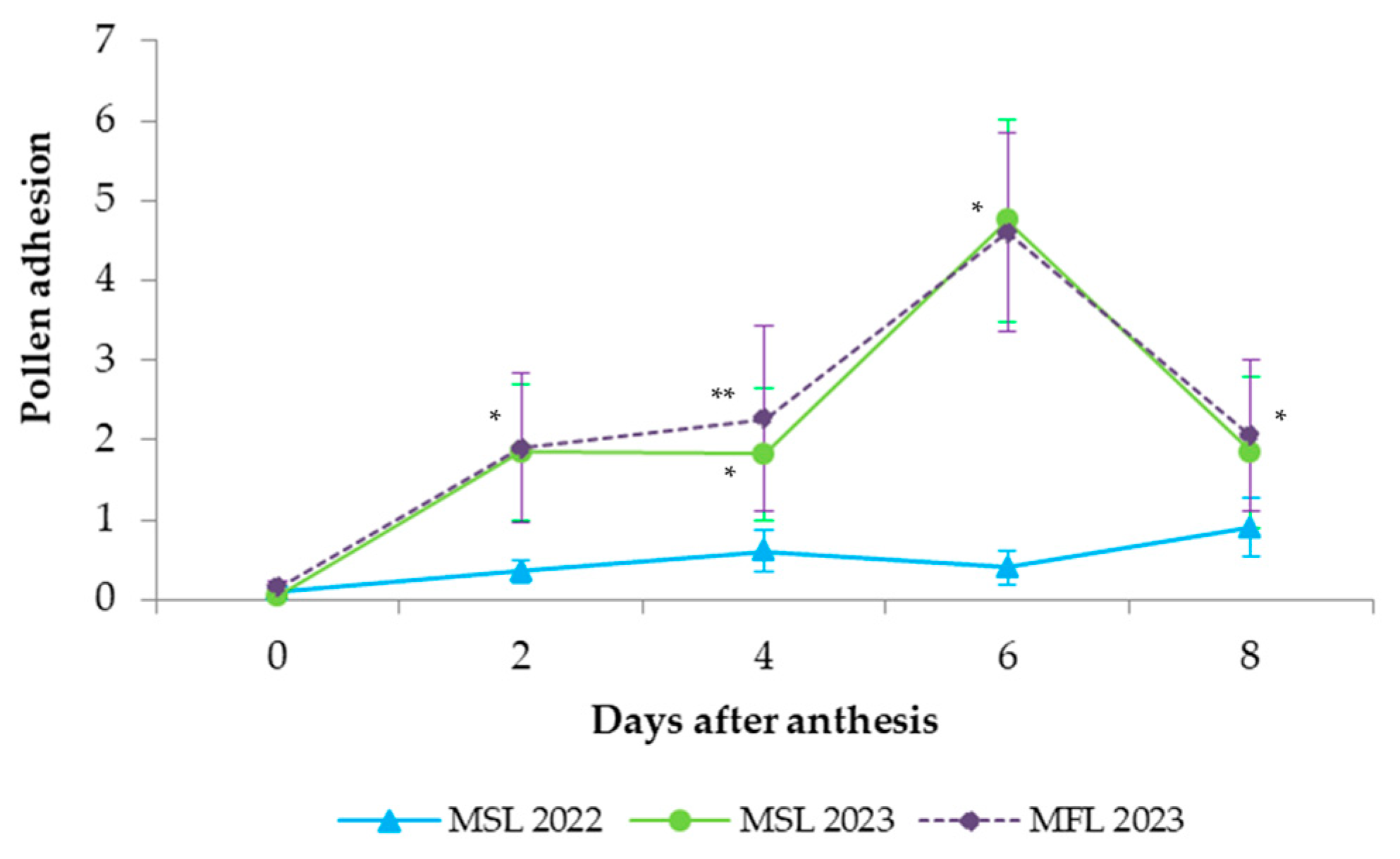
The stigma receptivity was evaluated by means of fluorescence microscopy by determining pollen adhesion and germination on the stigma of virgin flowers that were pollinated at increasing ages (0, 2, 4, 6, and 8 DAA) and sampled one day after. The numbers of adhered pollen grains per flower were counted. Pollen germination was expressed as the percentage of germinated pollen. Changes in the stigma papillae were simultaneously observed.
2.3.2. Pollen Tube Growth Rate
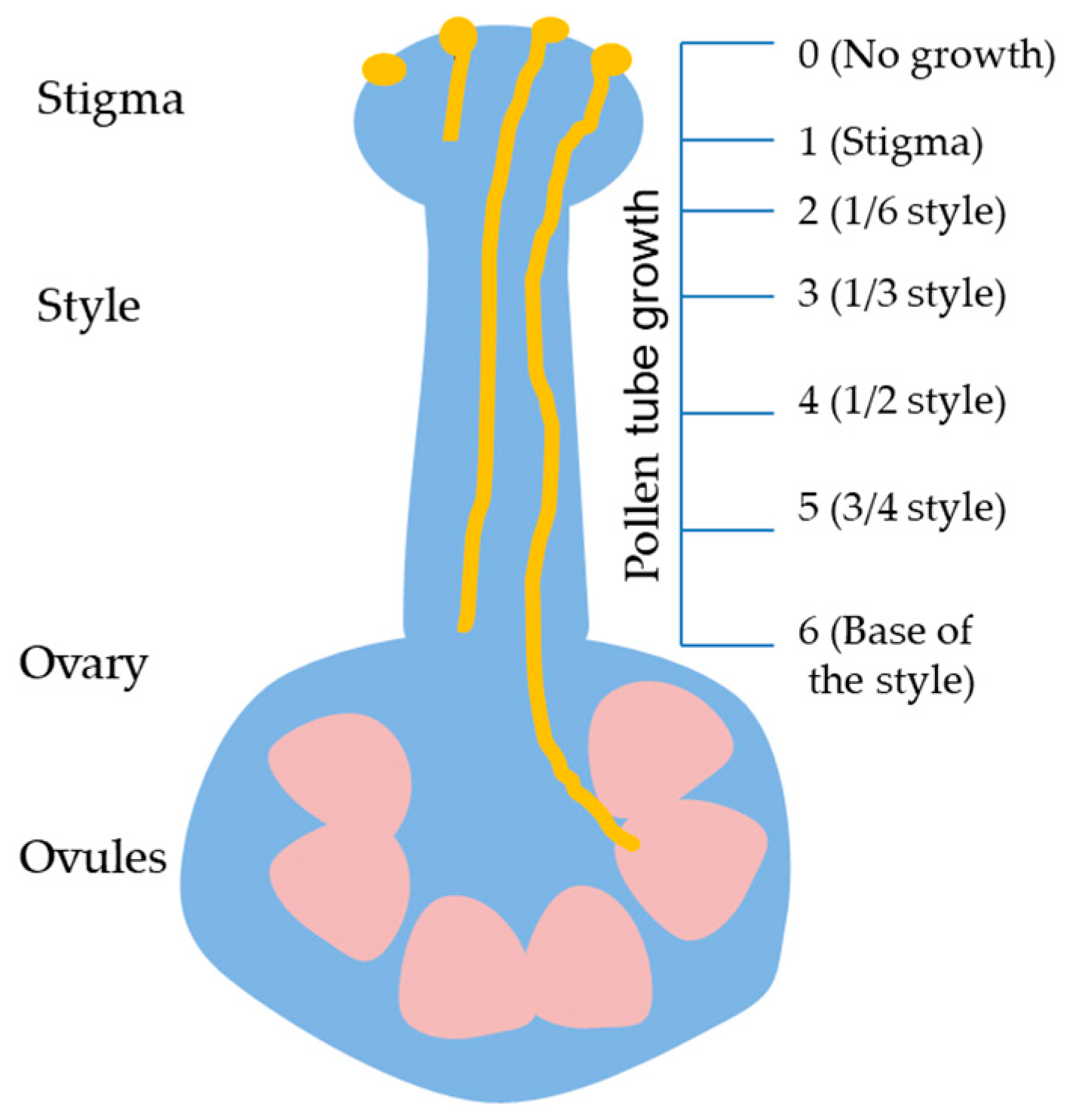
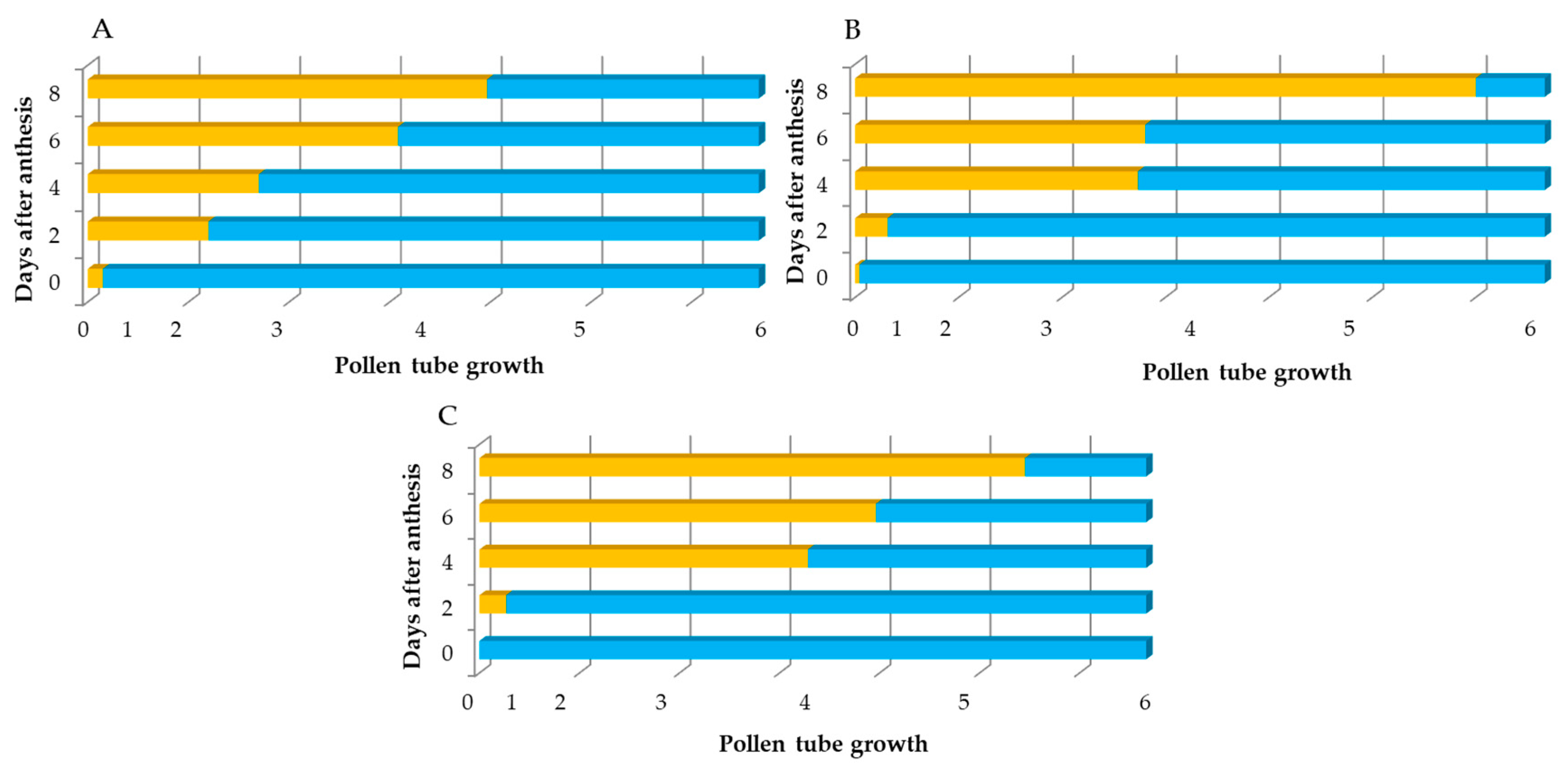
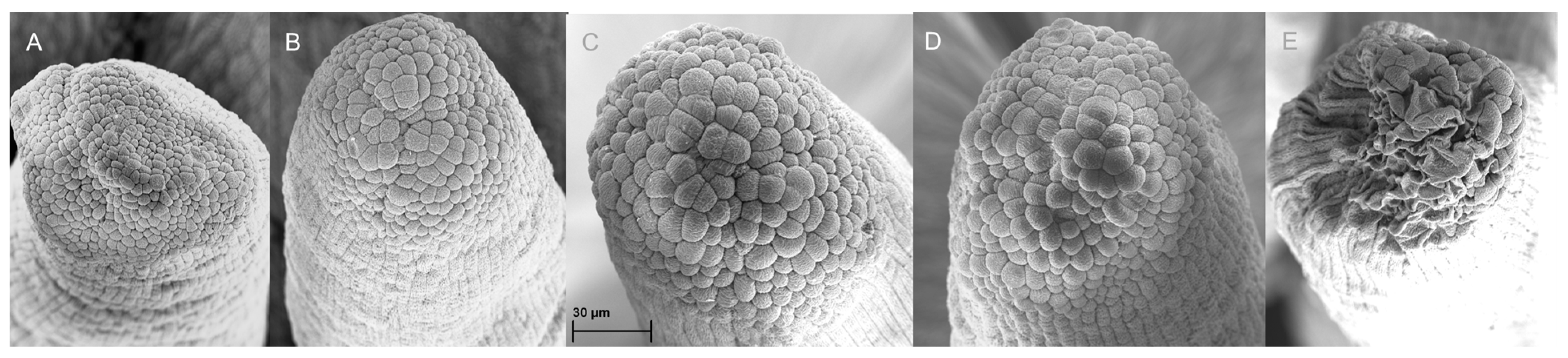
The growth of the pollen tube was analyzed in a different set of flowers (20 per sampling date) that were pollinated at anthesis and collected at increasing periods (days) after pollination. A scale ranging from 0 to 6 was generated to record the maximum length of the pistil traversed by the fastest pollen tube at each date, according to
Figure 1. The value of 0 corresponds to the lack of germination of pollen grains; 1: when pollen tube was emitted but its growth was limited to the stigma surface; 2: when pollen tubes reached 1/6 of the style; 3: pollen tubes reached 1/3 of the style; 4: pollen tubes reached 1/2 of the style; 5: pollen tubes reached 3/4 of the style; and 6: pollen tubes reached the base of the style and entered the ovary (
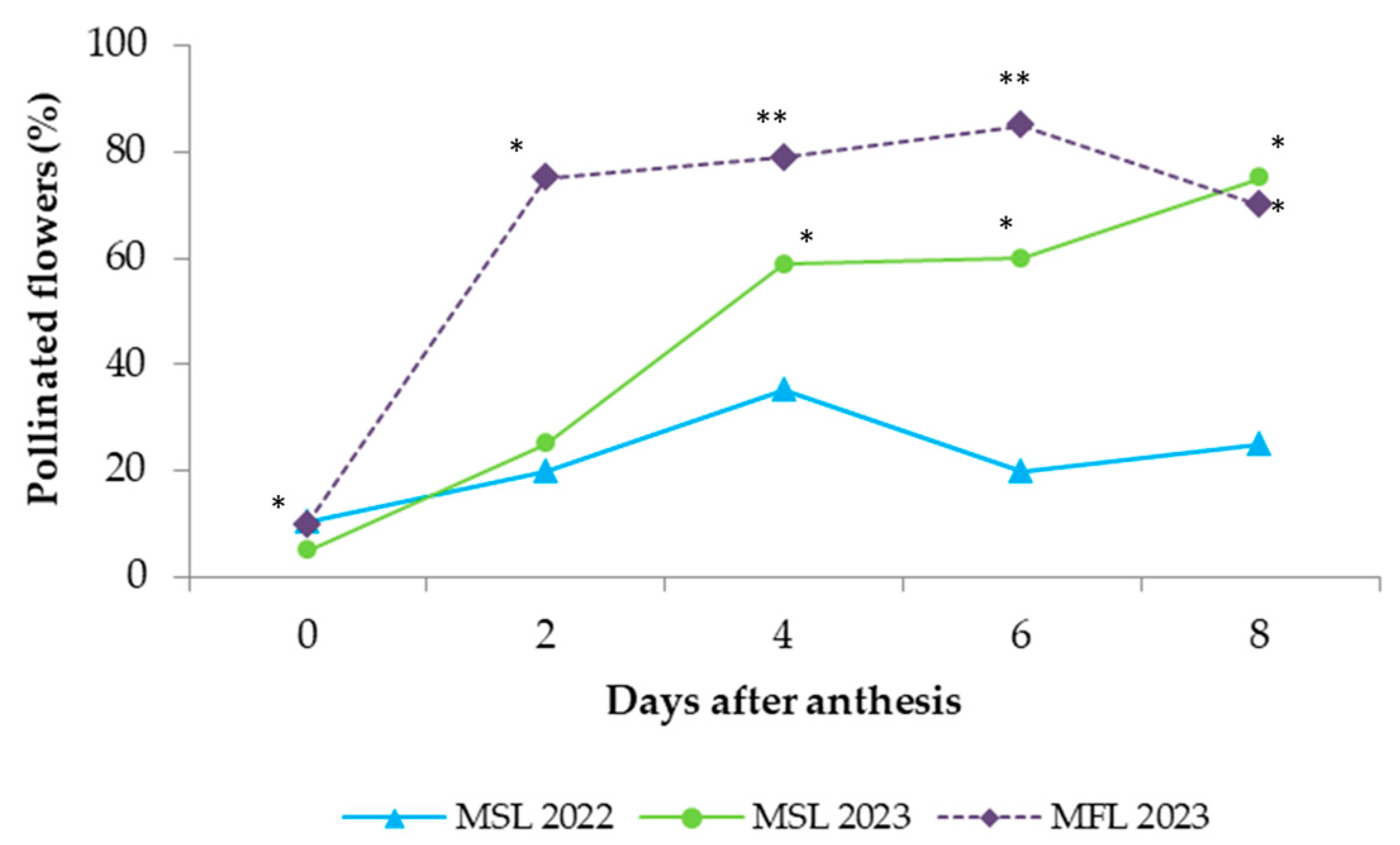
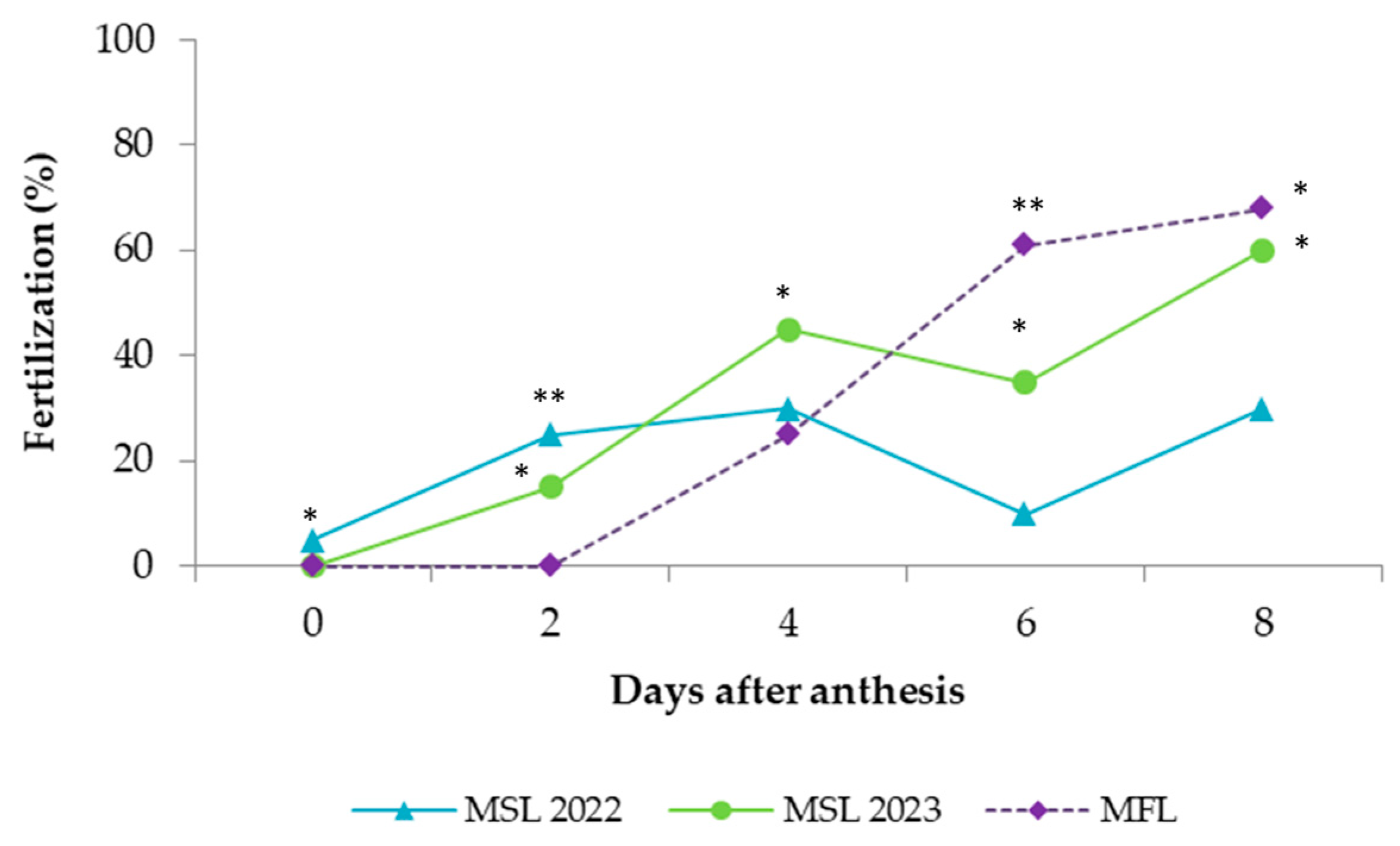
Figure 1). Fertilization was expressed as the percentage of fertilized flowers, with a flower being considered fertilized when at least one ovule (of the six that the ovary contains) was penetrated by a pollen tube. Daily changes in the shape of the style and in its length were observed and recorded.
2.3.3. Ovule Longevity
The viability of the ovules was determined using fluorescence microscopy and the staining of callose by aniline blue as a senescence indicator. Callose is a carbohydrate constituent of the pollen tube, but that also accumulates in the stigma and ovules as senescence progresses [
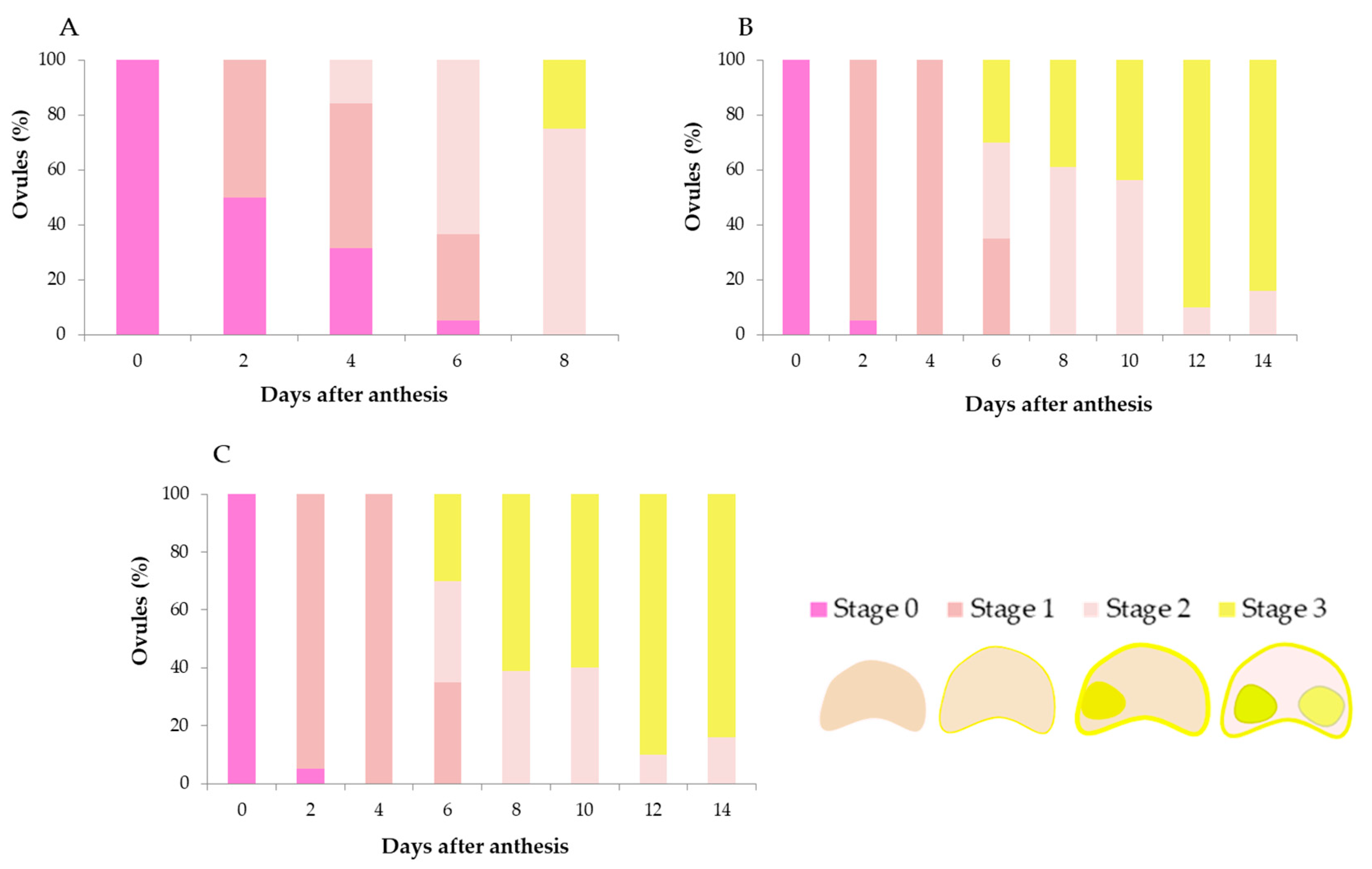
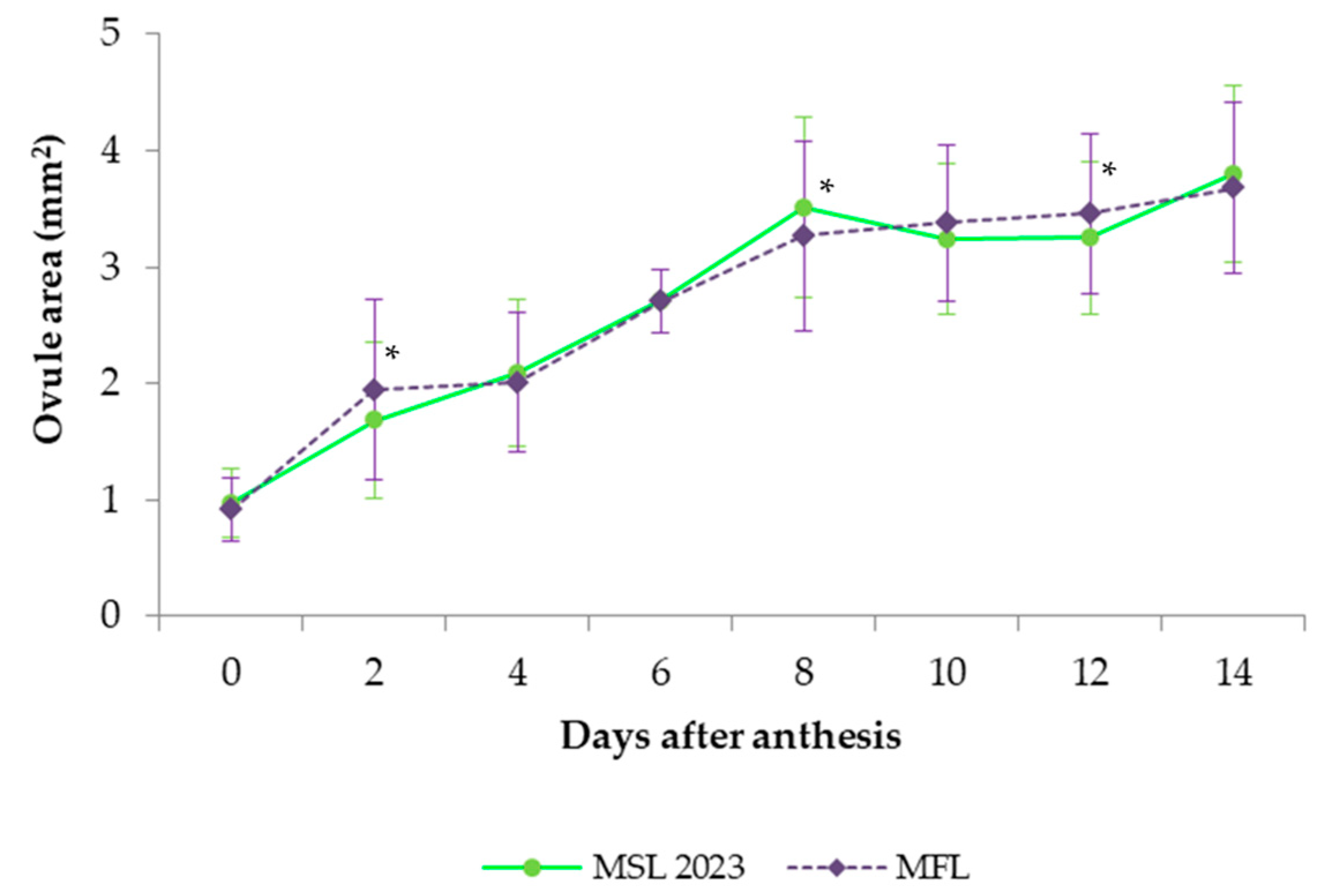
23]. Therefore, callose staining was observed on samples of 20 virgin flowers (120 ovules) of increasing ages. Before staining, the ovaries were dissected, and all the ovules were extracted. Length and width of the ovules were measured at all sampling dates with a 19 mm microscope reticle ruler of 0.05 mm ocular micrometer, and then, after aniline blue staining, each ovule was classified as 0: viable (small pinkish-colored ovule); 1: viable enlarging ovule with a fluorescent area limited to the mycropile; 2: viable ovule with the utmost size showing a fluorescent ring of vascular bundles; or 3: senescent ovule based on the brighter intensity of fluorescence extended over the whole ovule surface. A flower was considered still fertile when at least one of its six ovules remained viable.
2.4. Pistil Development Stages
As onion flowers show a pronounced protandry, a scanning electron microscopy (SEM) study was carried out to observe the developmental changes in the pistil, especially concerning stigma maturation. Before SEM observation, samples of flowers of different ages were completely dehydrated in an ascending series of ethanol until they reached pure ethanol. The dehydrated pistils were then left overnight in 100% absolute ethanol at 4 °C and then changed twice to clean ethanol. For critical point-drying, a BAL-TEC CPD 030 dryer was used and the samples covered with a thin layer of gold with a LEICA EM ACE 200 equipment. Then, the samples were observed with a Field Emission Scanning Electron microscope Zeiss Sigma 300 VP.
2.5. Statistical Analysis
All variables were analyzed using a one-way ANOVA and a Tukey test to compare means using the InfoStat2020 statistical package for Windows (Córdoba, Argentina). The results were considered significant at p ≤ 0.05 unless specified otherwise.
4. Discussion
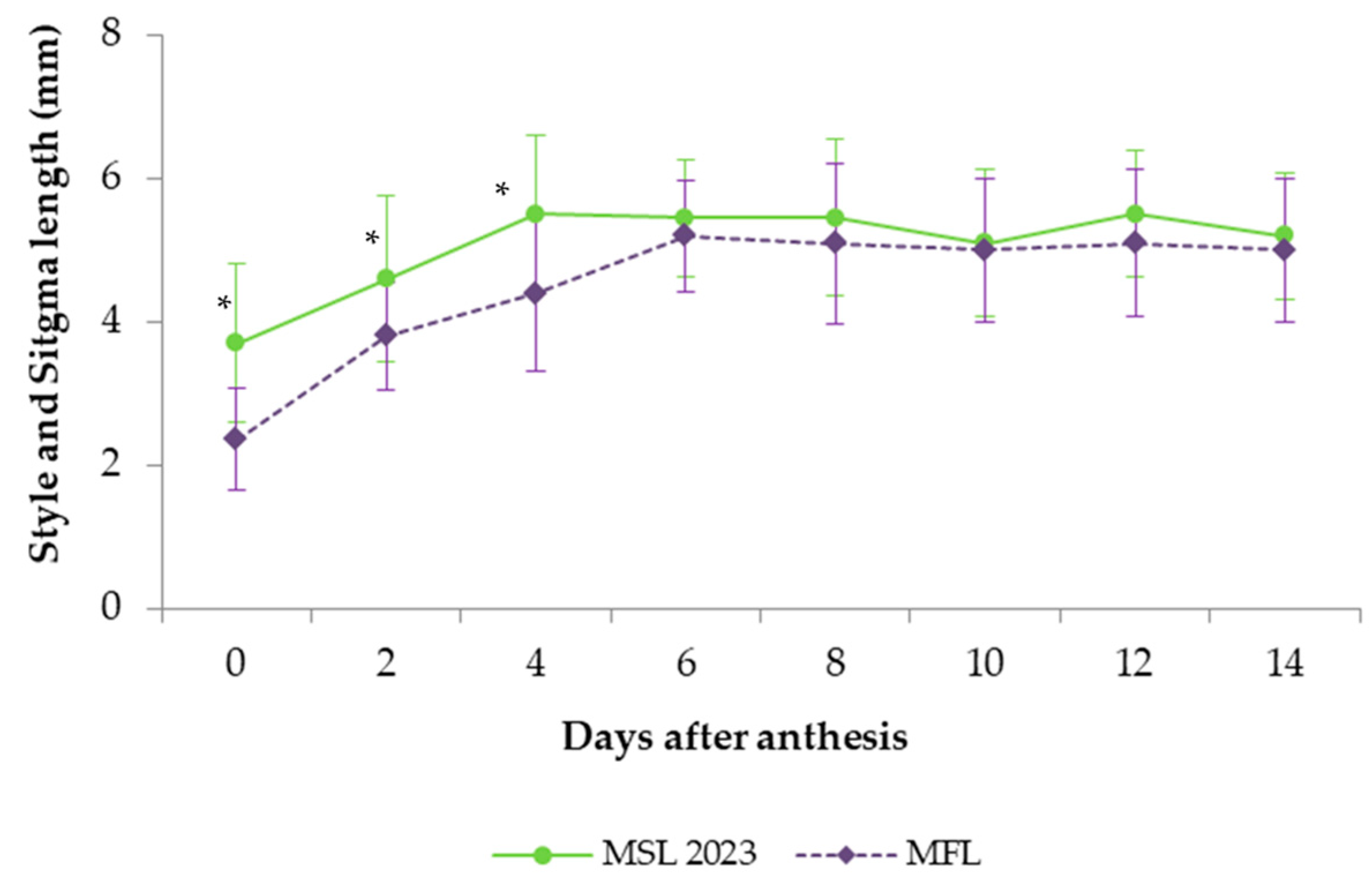
The EPP of onion flowers was determined for two MSLs under natural field conditions in 2022 and 2023 and for a MFL in 2023. Our results showed that the MSL had similar behavior in both years of study, showing a consistency in this type of line. The MFL showed similar results compared to MSL; however, some differences were observed in the development of the pistil. In this regard, our results are in agreement with those of Davi et al. [
4], who reported that the MSL and MFL differed in the characteristics of their style and stigma, with the styles of the MSL being longer, as Ali et al. [
24] also reported.
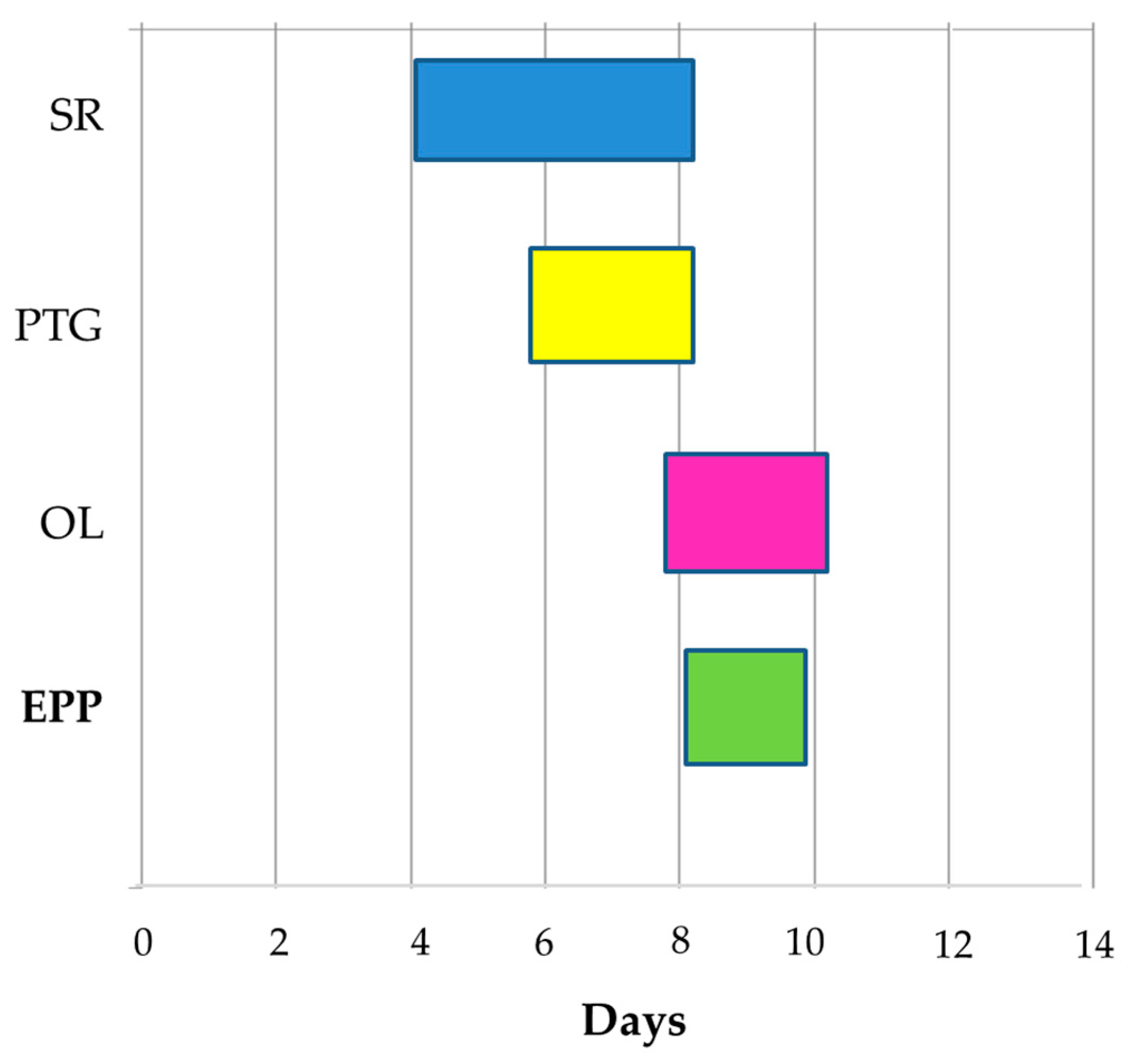
The protandry of the onion flowers was expressed in both lines with a marked delay in pistil development compared to anther dehiscence. Thus, while anther dehiscence takes place soon after tepal opening [
5], the development of the pistil in the flowers was not completed at flower anthesis and continued during the flower’s lifespan. Our observations, in fact, highlight the sequential maturation of the different components of the pistil, with stigmatic papillae maturing before the style reached its final length, and this attained before the ovule reached full size. We understand that attaining full size does not necessarily mean ovule readiness for fertilization since embryo sac development still has to be complete. However, it is clear that a developing and growing ovule is not yet ready for double fertilization. Therefore, we assume that ovules cannot be fertilized by the pollen tube before 8 DAA.
Herrero [
25] reported that the pistil has to reach a certain degree of development to support pollen tube growth and that its developmental stage appears to play a major role in the regulation of pollen tube growth. We observed that the growth of pollen tubes was slow until 4 DAA when the style was close to attaining its final length. Consistent with Herrero [
25], we assume that the developmental changes experienced by the different pistil structures of the flower condition pollen tube growth and fertilization, with the ovules producing secretions guiding the pollen tube toward the embryo sac in its final step. In addition, the growth of the pollen tubes might affect both the ovary and ovule development in a two-way interaction. In some species, the secretions of the ovules to attract the pollen tubes are present at anthesis, and in other species, the attractants are produced when the ovule is mature [
24]. This seems to be the case in onion. The role of the synergids cells in attracting pollen tubes to the embryo sac has been recently confirmed [
26,
27]. Our results showed that the stigma was not receptive immediately after anthesis and became senescent at 8 DAA. Therefore, the stigma seems to be receptive between 2 and 6 DAA. These results are in agreement with Pushpalatha et al. [
19], who reported that the stigma in onion flowers becomes receptive at 4 DAA, and with Moll [
28], who reported that the stigma is receptive after 3 DAA. However, at 2 DAA, the style was still growing and did not reach its final length until 4 DAA, highlighting the sequential maturation of the pistil components. Indeed, the style was very short, around 2 mm long, when the flower opened in the MFL, but it gradually elongated until 6 DAA, whilst in the MSL, it reached its final length sooner (at 4 DAA). These results are in agreement with Currah [
5] and Curra and Ockedon [
29], who reported that the style grew from 1 mm long to its final length after four days, well after the time all pollen has been shed in the first 48 h after anthesis. In addition, Gheshlaghi [
30] reported similar results in kiwifruit. Even though kiwifruit is taxonomically far from onion, it is worth mentioning that the EPP is also short, and its pollination requires more than 4 DAA for a successful fruit set. At the beginning of the flower’s lifespan, the stigma papillae of the kiwi flowers are completely closed and undeveloped, and only after 4 DAA do they seem to be well developed. After 8 DAA, they lost their integrity and collapsed, with the declination of fertilization due to the loss of papillar integrity when they began to rupture. These results are very similar to our findings.
Although the pollen tubes started to grow on the stigmas after 2 DAA, it was not until 4 DAA that there was a significant growth of the pollen tubes in the transmitting tissue of the style. Pollen tubes reached the style base at 6 DAA and fertilized the ovules at 8 DAA, with marked differences between seasons. The decrease in the levels of fertilization observed in 2022 cannot be attributed to any climactic parameters but might reflect the poor pollination performed by bees in that season. Currah [
31] suggested strong competition among pollen tubes to achieve ovule fertilization in onion. However, according to this author, there is a competitive advantage of self-pollen over cross-pollen, despite the fact that cross-pollen sired larger, heavier seeds. The climatic conditions through the flowering period were optimal for pollen germination and pollen tube growth in onion, according to the evaluation carried out by Chang and Struckmeyer [
32,
33,
34].
The ovules appear to not be ready to be fertilized on the first day after anthesis since they are small and continue steadily enlarging until 8 DAA, the day when a significant number of fertilized ovules were observed. This suggests pollen tubes were unable to penetrate the ovules until they reached full maturation. The senescence of ovules started at 6 DAA in a small proportion of flowers. After 8 DAA, the ovules stopped growing, but most of them were still viable. At 10 DAA, the majority of flowers had some, but no many, non-viable ovules, while almost none were viable two days later at 12 DAA. If ovule availability for fertilization is assumed when attaining full size, and pollen tubes need 8 days to reach them, then the window period for successful fertilization is about 2 days (between 8 and 10 DAA). Nonetheless, according to Selak et al. [
35], the assessment of the ovule longevity based on the fluorescence emitted could be an overestimation of the real ovule availability for fertilization, and window time can be even shorter. In addition, ovule size could not be a fully decisive parameter, and the methodology used here could be limiting. More studies about ovule cytology in onion are clearly needed.
Therefore, the limiting factors for successful fertilization in onion appear to be the delay in stigma papillae development and the time required for the pollen tube to reach the ovary before ovule senescence. However, another reason for the low fertilization observed was the low adhesion of pollen. The low number of pollen grains attached to the stigma delayed and limited ovule fertilization. Additionally, we observed that only one or two tubes per flower reached the ovary. Thus, good bee activity, as it is widely known, is needed to increase seed set and yield. Our results clearly showed that our pollination procedures were insufficient for full seed setting. Consistent with Saleh et al. [
8], who evaluated different methods of pollination, we believe that the hand pollination of flowers is a cumbersome job with no profitable results so far; hence, it is not considered a viable option at the commercial level for hybrid seed onion production. In addition, it is important to consider that flowering in MSL or hybrid materials should be synchronized with the MFL, a process called ‘nicking’. Knowing the EPP in these types of lines can be a helpful tool to assure the synchronization of flowering of pistillate and hermaphrodite parents for hybrid seed production [
36]. We understand that onion seed production is risky, because a crop failure can be frequent, and the production process is expensive. The findings of this work suggest that special attention should be paid to the overlap of the MFL with the MSL bloom; otherwise, inefficient pollination could be translated into economic losses.