Anaerobic Soil Disinfestation as a Tool for Nematode and Weed Management in Organic Sweetpotato
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishment of Nematode Infested Soils
2.2. Preparation of Sweetpotato Slips
2.3. ASD Initiation and Treatment Setup
2.4. Data Collection
2.5. Data Analysis
3. Results
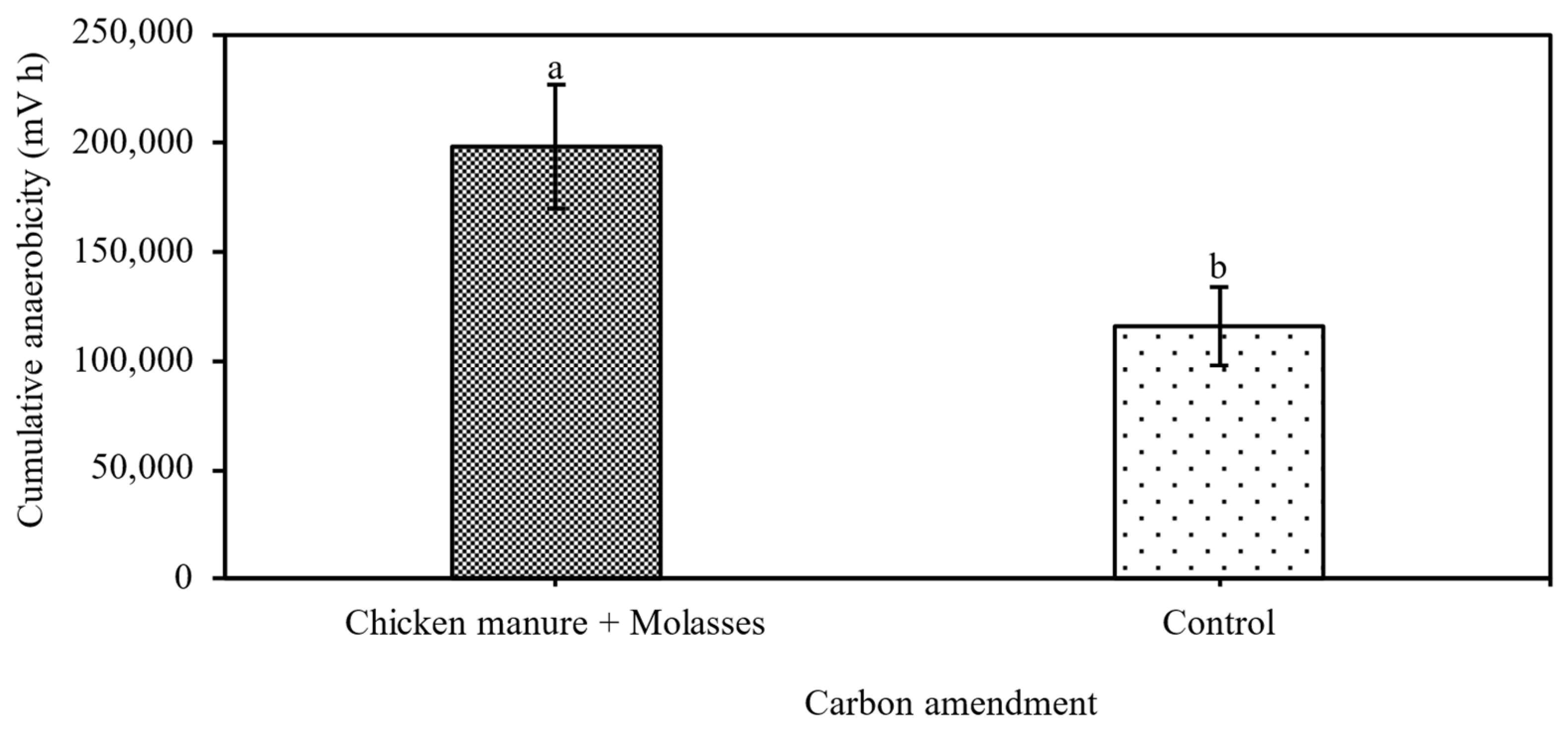
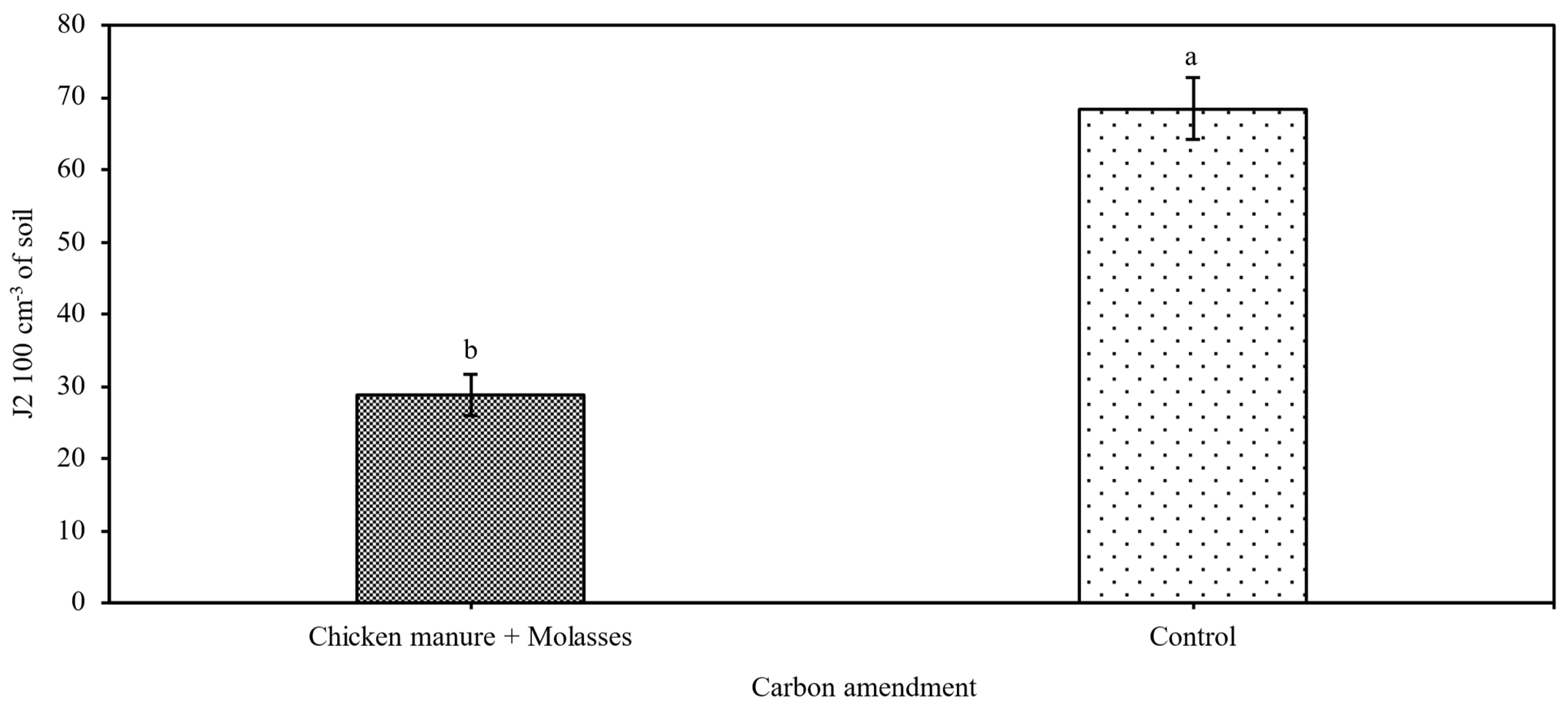
3.1. Impacts of ASD on Anaerobicity, Nematodes and Weeds After Termination of ASD
3.2. Impacts of ASD on Nematodes, Sweetpotato Biomass and Plant Vigor
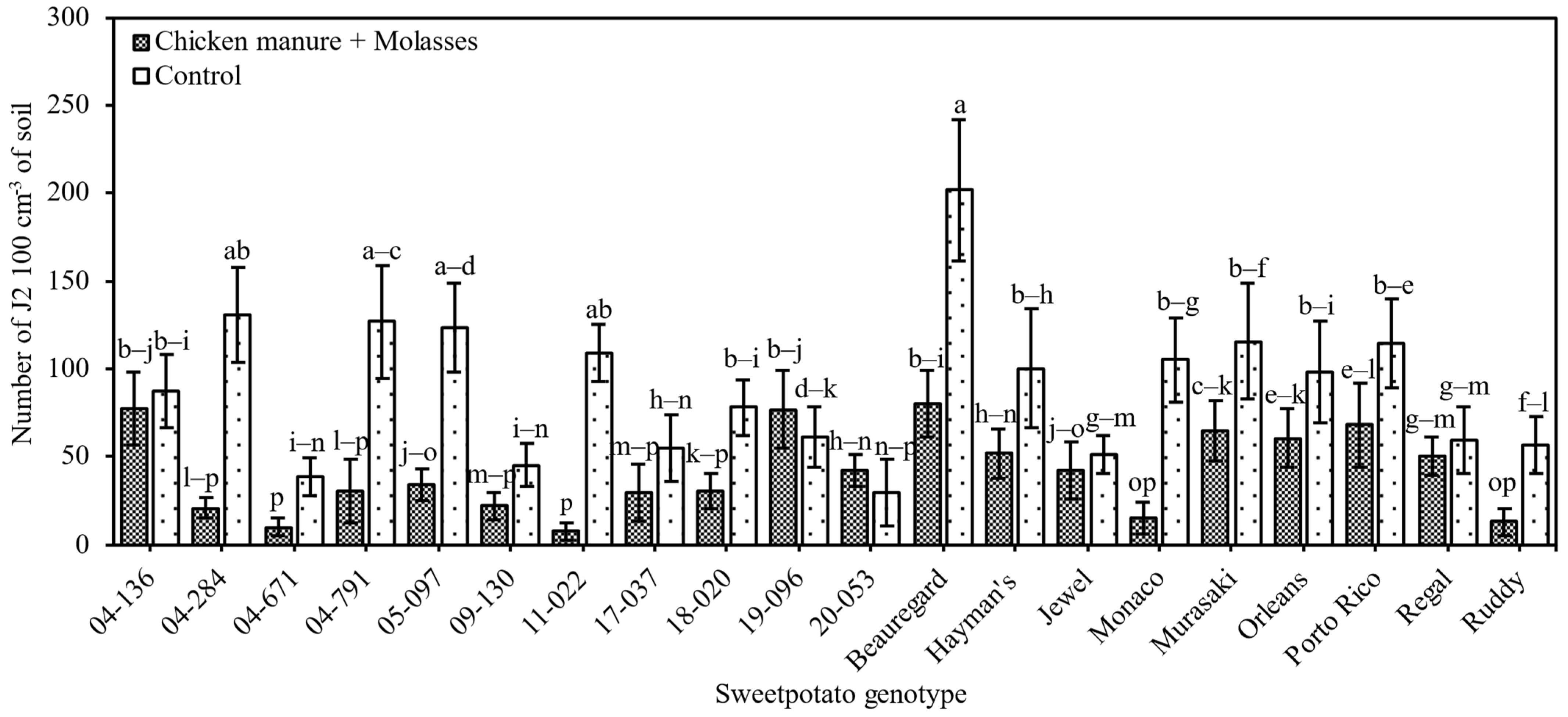
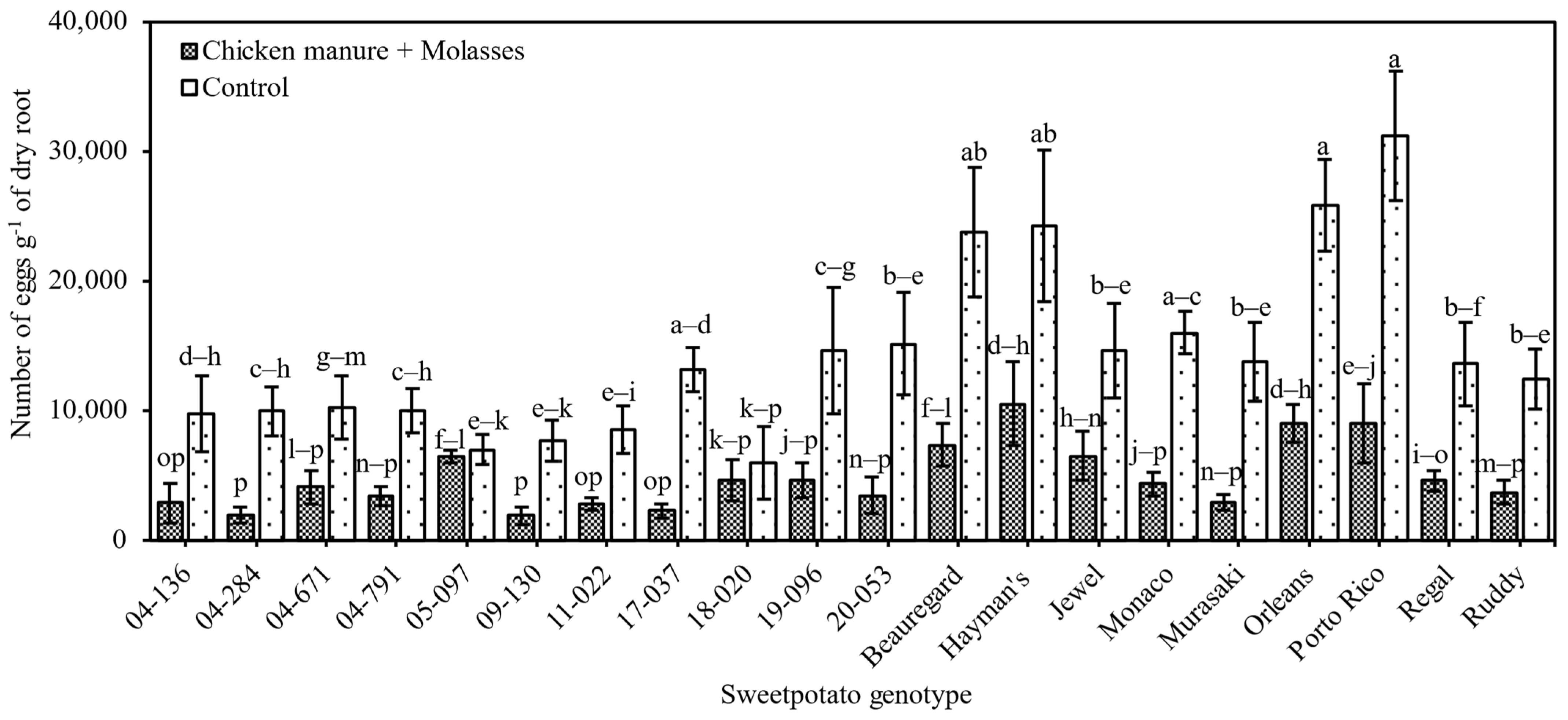
3.2.1. Nematode Reproduction
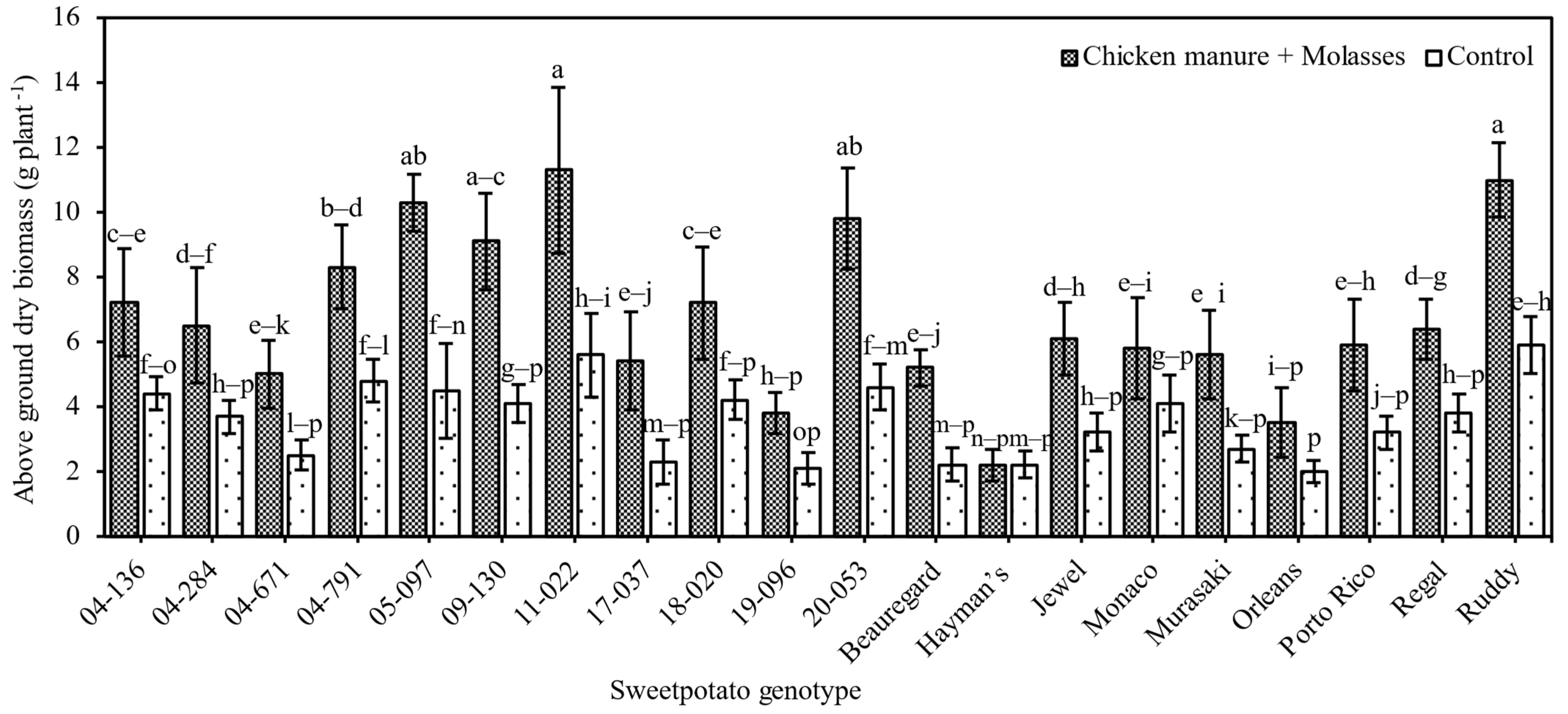
3.2.2. Sweetpotato Dry Above-Ground Biomass
3.2.3. Sweetpotato Dry Below-Ground Biomass
3.2.4. Plant Vigor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Werle, I.S.; Noguera, M.M.; Karaikal, S.K.; Carvalho-Moore, P.; Kouame, K.B.-J.; de Lima, G.H.B.; Roberts, T.L.; Roma-Burgos, N. Integrating weed-suppressive cultivar and cover crops for weed management in organic sweetpotato production. Weed Sci. 2023, 71, 255–264. [Google Scholar] [CrossRef]
- Hotz, C.; Loechl, C.; Lubowa, A.; Tumwine, J.K.; Ndeezi, G.; Masawi, A.N.; Baingana, R.; Carriquiry, A.; Brauw, A.; Meenakshi, J.V.; et al. Introduction of β-carotene–rich orange sweet potato in rural Uganda resulted in increased Vitamin A intakes among children and women and improved vitamin A status among childrens. J. Nutr. 2012, 142, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Low, J.W.; Arimond, M.; Osman, N.; Cunguara, B.; Zano, F.; Tschirley, D. A food-based approach introducing orange-fleshed sweet potatoes increased Vitamin A intake and serum retinol concentrations in young children in rural Mozambique. J. Nutr. 2007, 137, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- USDA-NASS. Organic Survey 2008. Available online: https://agcensus.library.cornell.edu/wp-content/uploads/2007-Organics-Survey-organics_1_23.pdf (accessed on 7 January 2025).
- USDA-NASS. Organic Survey 2022. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/zg64tk92g/2z10z137s/bn99bh97r/cenorg22.pdf (accessed on 7 January 2025).
- Chitwood, B.G. Root-knot nematodes–part I. A revision of the genus Meloidogyne Göldi, 1887. Proc. Helminthol. Soc. Wash. 1949, 16, 90–104. [Google Scholar]
- Kofoid, C.A.; White, W.A. A new nematode infection of man. J. Am. Med. Assoc. 1919, 72, 567–569. [Google Scholar] [CrossRef]
- Feys, J.; Reheul, D.; Smet, D.W.; Clercx, S.; Palmans, S.; Van, V.G.; Cauwer, B. Effect of anaerobic soil disinfestation on tuber vitality of yellow nutsedge (Cyperus esculentus). Agriculture 2023, 13, 1547. [Google Scholar] [CrossRef]
- Clemson University Extension Services 2000. Available online: https://www.clemson.edu/public/regulatory/plant-problem/pdfs/nematode-guidelines-for-south-carolina.pdf (accessed on 21 January 2025).
- Clark, C.A.; Dukes, P.D.; Moyer, J.W. Fifty years of cooperative sweetpotato research. South. Coop. Ser. Bull. 1992, 369, 88–105. [Google Scholar]
- Lawrence, G.W.; Clark, C.A.; Wright, V.L. Influence of Meloidogyne incognita on resistant and susceptible sweetpotato cultivars. J. Nematol. 1986, 18, 59–65. [Google Scholar]
- Khan, M.W. Nematode Interactions, 3rd ed.; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Webster, T.M. Weed survey—Southern states. In Proceedings of the 67th Southern Weed Science Society, Birmingham, AL, USA, 27–29 January 2014; Southern Weed Science Society: Birmingham, AL, USA, 2014; p. 292. [Google Scholar]
- Meyers, S.L.; Shankle, M.W. Interference of yellow nutsedge (Cyperus esculentus) in Beauregard sweet potato (Ipomoea batatas). Weed Technol. 2015, 29, 854–860. [Google Scholar] [CrossRef]
- Oleg, D.; Maren, J.M. Barriers prevent emergence of yellow nutsedge (Cyperus esculentus) in annual plasticulture strawberry (Fragaria × Ananassa). Weed Technol. 2010, 24, 478–482. [Google Scholar] [CrossRef]
- Meyers, S.L.; Shankle, M.W. Postemergence yellow nutsedge management in sweetpotato. Weed Technol. 2016, 30, 148–153. [Google Scholar] [CrossRef]
- Di Gioia, F.; Ozores-Hampton, M.; Zhao, X.; Thomas, J.; Wilson, P.; Li, Z.; Hong, J.; Albano, J.; Swisher, M.; Rosskopf, E. Anaerobic soil disinfestation impact on soil nutrients dynamics and nitrous oxide emissions in fresh-market tomato. Agric. Ecosyst. Environ. 2017, 240, 194–205. [Google Scholar] [CrossRef]
- Butler, D.M.; Kokalis-Burelle, N.; Albano, J.P.; McCollum, T.G.; Muramoto, J.; Shennan, C.; Rosskopf, E.N. Anaerobic soil disinfestation (ASD) combined with soil solarization as a methyl bromide alternative: Vegetable crop performance and soil nutrient dynamics. Plant Soil 2014, 378, 365–381. [Google Scholar] [CrossRef]
- Shrestha, U.; Auge, R.M.; Butler, D.M. A meta-analysis of the impact of anaerobic soil disinfestation on pest suppression and yield of horticultural crops. Front. Plant Sci. 2016, 7, 1254. [Google Scholar] [CrossRef]
- Butler, D.M.; Rosskopf, E.N.; Kokalis-Burelle, N.; Albano, J.P.; Muramoto, J.; Shennan, C. Exploring warm-season cover crops as carbon sources for anaerobic soil disinfestation (ASD). Plant Soil. 2012, 355, 149–165. [Google Scholar] [CrossRef]
- Singh, G.; Ward, B.; Levi, A.; Cutulle, M. Weed management by in situ cover crops and anaerobic soil disinfestation in plasticulture. Agronomy 2022, 12, 2754. [Google Scholar] [CrossRef]
- Blok, W.J.; Lamers, J.G.; Termorshuizen, A.J.; Bollen, G.J. Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology 2000, 90, 253–259. [Google Scholar] [CrossRef]
- Momma, N.; Kobara, Y.; Uematsu, S.; Kita, N.; Shinmura, A. Development of biological soil disinfestations in Japan. Appl. Microbiol. Biotechnol. 2013, 97, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Conn, K.L.; Tenuta, M.; Lazarovits, G.; Cole, E.; Pu, J.; Chung, H.; Quintanilla, M.; Sanabria-Velazquez, A.D.; Testen, A.L.; Khadka, R.B.; et al. Liquid swine manure can kill Verticillium dahliae microsclerotia in soil by volatile fatty acid, nitrous acid, and ammonia toxicity. Phytopathology 2005, 95, 28–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hestmark, K.; Fernández-Bayo, J.; Harrold, D.; Randall, T.; Achmon, Y.; Stapleton, J.; Simmons, C.; VanderGheynst, J. Compost induces the accumulation of biopesticidal organic acids during soil biosolarization. Resour. Conserv. Recycl. 2019, 143, 27–35. [Google Scholar] [CrossRef]
- Momma, N.; Yamamoto, K.; Simandi, P.; Shishido, M. Role of organic acids in the mechanisms of biological soil disinfestation (BSD). J. Gen. Plant Pathol. 2006, 72, 247–252. [Google Scholar] [CrossRef]
- Momma, N. Biological soil disinfestation (BSD) of soilborne pathogens and its possible mechanisms. Jpn. Agr. Res. 2008, 42, 7–12. [Google Scholar] [CrossRef]
- Strauss, S.L.; Greenhut, R.F.; McClean, A.E.; Kluepfel, D.A. Effect of anaerobic soil disinfestation on the bacterial community and key soilborne phytopathogenic agents under walnut tree-crop nursery conditions. Plant Soil 2017, 415, 493–506. [Google Scholar] [CrossRef]
- Singh, G.; Wechter, W.P.; Farmaha, B.S.; Cutulle, M. Integration of halosulfuron and anaerobic soil disinfestation for weed control in tomato. HortTechnology 2022, 32, 401–414. [Google Scholar] [CrossRef]
- Singh, G.; Ward, B.K.; Wechter, W.P.; Katawczik, M.L.; Farmaha, B.S.; Suseela, V.; Cutulle, M.A. Assessment of agro-industrial wastes as a carbon source in anaerobic disinfestation of soil contaminated with weed seeds and phytopathogenic bacterium (Ralstonia solanacearum) in Tomato (Solanum lycopersicum). ACS Agric. Sci. Technol. 2022, 2, 769–779. [Google Scholar] [CrossRef]
- Khadka, R.B.; Sanabria-Velazquez, A.D.; Cardina, J.; Miller, S.A. Evaluation of anaerobic soil disinfestation for environmentally sustainable weed management. Agronomy 2022, 12, 3147. [Google Scholar] [CrossRef]
- Liu, D.; Samtani, J.; Johnson, C.; Zhang, X.; Butler, D.M.; Derr, J. Brewer’s spent grain with yeast amendment shows potential for anaerobic soil disinfestation of weeds and Pythium irregulare. Agronomy 2023, 13, 2081. [Google Scholar] [CrossRef]
- Singh, S.; Rutter, W.; Wadl, P.A.; Campbell, H.T.; Khanal, C.; Cutulle, M. Effectiveness of anaerobic soil disinfestation for weed and nematode management in organic sweetpotato production. Agronomy 2024, 14, 1935. [Google Scholar] [CrossRef]
- Priyashantha, A.K.H.; Attanayake, R.N. Can Anaerobic soil disinfestation (ASD) be a game changer in tropical agriculture? Pathogens 2021, 10, 133. [Google Scholar] [CrossRef]
- Khanal, C.; McGawley, E.C.; Overstreet, C.; Stetina, S.R.; Myers, G.O.; Kularathna, M.T.; McInnes, B.; Godoy, F.M.C. Reproduction and pathogenicity of endemic populations of Rotylenchulus reniformis on cotton. Nematropica 2018, 48, 68–81. [Google Scholar]
- Alam, M.S.; Khanal, C.; Roberts, J.; Rutter, W.; Wadl, P.A. Enhancing reniform nematode management in sweetpotato by complementing host-plant resistance with nonfumigant nematicides. Plant Dis. 2024, 108, 2000–2005. [Google Scholar] [CrossRef]
- Fiedler, S.; Vepraskas, M.J.; Richardson, J.L. Soil redox potential: Importance, field measurements, and observations. Adv. Agron. 2007, 94, 1–54. [Google Scholar] [CrossRef]
- Rabenhorst, M.C.; Castenson, K.L. Temperature effects on iron reduction in a hydric soil. Soil Sci. 2005, 170, 734–742. [Google Scholar] [CrossRef]
- Jenkins, W.R. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Rep. 1964, 48, 692. [Google Scholar]
- Hussey, R.S.; Barker, K.R. A comparison of methods for collecting inocula for Meloidogyne spp. including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Meyers, S.L.; Jennings, K.M.; Monks, D.W. Sweetpotato response to simulated glyphosate wick drip. Weed Technol. 2017, 31, 130–135. Available online: https://www.jstor.org/stable/26567548 (accessed on 10 September 2024). [CrossRef]
- Isaac, R.A. Reference soil test methods for the southern region of the United States. In Southern Cooperative Series Bulletin 289; University of Georgia: Athens, GA, USA, 1983; Available online: https://aesl.ces.uga.edu/sera6/PUB/SCSB289.pdf (accessed on 18 February 2025).
- Donohue, S.J. Reference soil and media diagnostic procedures for the southern region of the United States. In Southern Cooperative Series Bulletin No. 374; Virginia Polytechnic & State University: Blacksburg, VA, USA, 1992; Available online: https://aesl.ces.uga.edu/sera6/PUB/bulletinNo374.pdf (accessed on 18 February 2025).
- Jones, J.B. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Washington, DC, USA, 2001. [Google Scholar]
- Guo, H.; Di Gioia, F.; Zhao, X.; Ozores-Hampton, M.; Swisher, M.E.; Hong, J.; Kokalis-Burelle, N.; DeLong, A.N.; Rosskopf, E.N. Optimizing anaerobic soil disinfestation for fresh market tomato production: Nematode and weed control, yield, and fruit quality. Sci. Hortic. 2017, 218, 105–116. [Google Scholar] [CrossRef]
- Butler, D.M.; Kokalis-Burelle, N.; Muramoto, J.; Shennan, C.; McCollum, T.G.; Rosskopf, E.N. Impact of anaerobic soil disinfestation combined with soil solarization on plant–parasitic nematodes and introduced inoculum of soilborne plant pathogens in raised-bed vegetable production. Crop Prot. 2012, 39, 33–40. [Google Scholar] [CrossRef]
- McCarty, D.G.; Inwood, S.E.E.; Ownley, B.H.; Sams, C.E.; Wszelaki, A.L.; Butler, D.M. Field evaluation of carbon sources for anaerobic soil disinfestation in tomato and bell pepper production in Tennessee. HortScience 2014, 49, 272–280. [Google Scholar] [CrossRef]
- Prescott, K.; Kortman, S.; Duque, J.; Muramoto, J.; Shennan, C.; Greenstein, G.; Haffa, A.L.M. Analysis of trace volatile compounds emitted from flat ground and formed bed anaerobic soil disinfestation in strawberry field trials on California’s central coast. Agronomy 2023, 13, 1190. [Google Scholar] [CrossRef]
- Momma, N. Studies on mechanisms of anaerobicity-mediated biological soil disinfestation and its practical application. J. Gen. Plant Pathol. 2015, 81, 480–482. [Google Scholar] [CrossRef]
- Runia, W.; Thoden, T.; Molendijk, L.; Berg, W.v.D.; Termorshuizen, A.; Streminska, M.; van der Wurff, A.; Feil, H.; Meints, H. Unravelling the mechanism of pathogen inactivation during anaerobic soil disinfestation. Acta Hortic. 2014, 1044, 177–193. [Google Scholar] [CrossRef]
- Hewavitharana, S.S.; Ruddell, D.; Mazzola, M. Carbon source-dependent antifungal and nematicidal volatiles derived during anaerobic soil disinfestation. Eur. J. Plant Pathol. 2014, 140, 39–52. [Google Scholar] [CrossRef]
- Hewavitharana, S.S.; Shennan, C.; Muramoto, J.; Mazzola, M. Anaerobic soil disinfestation disease control performance in strawberry as influenced by environmental variables. Phytopathology 2015, 105, S4.59. [Google Scholar]
- Song, Z.; Massart, S.; Yan, D.; Cheng, H.; Eck, M.; Berhal, C.; Ouyang, C.; Li, Y.; Wang, Q.; Cao, A. Composted Chicken Manure for Anaerobic Soil Disinfestation Increased the Strawberry Yield and Shifted the Soil Microbial Communities. Sustainability 2020, 12, 6313. [Google Scholar] [CrossRef]
- Gilardi, G.; Pugliese, M.; Gullino, M.L.; Garibaldi, A. Evaluation of different carbon sources for anaerobic soil disinfestation against Rhizoctonia solani on lettuce in controlled production systems. Phytopathol. Mediterr. 2020, 59, 77–96. Available online: https://www.jstor.org/stable/27015399 (accessed on 9 September 2024). [CrossRef]
- Faske, T.R.; Mueller, J.; Becker, J.O.; Bernard, E.C.; Bradley, C.; Bond, J.; Desager, J.; Eisenback, J.; Grabau, Z.; Hu, J.; et al. Summarized distribution of the southern root-knot nematode, Meloidogyne incognita, in field crops in the United States. Plant Health Prog. 2023, 24, 522–524. [Google Scholar] [CrossRef]
- Katase, M.; Kubo, C.; Ushio, S.; Ootsuka, E.; Takeuchi, T.; Mizukubo, T. Nematicidal activity of volatile fatty acids generated from wheat bran in reductive soil disinfestation. Nematol. Res. 2009, 39, 53–62. [Google Scholar] [CrossRef]
- Oka, Y. Mechanisms of nematode suppression by organic soil amendments—A review. Appl. Soil Ecol. 2010, 44, 101–115. [Google Scholar] [CrossRef]
- Shennan, C.; Muramoto, J.; Koike, S.; Baird, G.; Fennimore, S.; Samtani, J.; Bolda, M.; Dara, S.; Daugovish, O.; Lazarovits, G.; et al. Anaerobic soil disinfestation is a potential alternative to soil fumigation for control of some soil borne pathogens in strawberry production. Plant Pathol. 2017, 67, 51–66. [Google Scholar] [CrossRef]
- Testen, A.L.; Miller, S.A. Carbon source and soil origin shape soil microbiomes and tomato soilborne pathogen populations during anaerobic soil disinfestation. Phytobiomes J. 2018, 2, 138–150. [Google Scholar] [CrossRef]
- Hu, J.; Wan, L.; Qasim, W.; Lv, H.; Zhao, Y.; Li, G.; Butterbach-Bahl, K.; Lin, S. Anaerobic soil disinfestation promotes soil microbial stability and antagonistic bacteria abundance in greenhouse vegetable production systems. Agronomy 2023, 13, 939. [Google Scholar] [CrossRef]
- Teclu, D.; Tivchev, G.; Laing, M.; Wallis, M. Determination of the elemental composition of molasses and its suitability as carbon source for growth of sulphate-reducing bacteria. J. Hazard. Mater. 2009, 161, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Jacq, V.A.; Fortuner, R. Biological control of rice nematodes using sulphate reducing bacteria. Rev. Nématol 1979, 2, 41–50. [Google Scholar]
- Rodriguez-Kabana, R.; Jordan, J.W.; Hollis, J.P. Nematodes: Biological control in rice fields: Role of hydrogen sulfide. Science 1965, 148, 524–526. [Google Scholar] [CrossRef] [PubMed]
- López-Robles, J.; Olalla, C.; Rad, C.; Díez-Rojo, M.; López-Pérez, J.; Bello, A.; Rodríguez-Kábana, R. The use of liquid swine manure for the control of potato cyst nematode through soil disinfestation in laboratory conditions. Crop Prot. 2013, 49, 1–7. [Google Scholar] [CrossRef]
- Mazzola, M.; Brown, J.; Izzo, A.D.; Cohen, M.F. Mechanism of action and efficiency of seed meal induced pathogen suppression differ in a Brassicaceae species and time-dependent manner. Phytopathology 2007, 97, 454–460. [Google Scholar] [CrossRef]
- Wadl, P.A.; Campbell, H.T.; Rutter, W.B.; Williams, L.H.; Murphey, V.; Culbreath, J.; Cutulle, M. A sustainable approach for weed and insect management in sweetpotato: Breeding for weed and insect tolerant/resistant clones. Weed Technol. 2023, 37, 60–66. [Google Scholar] [CrossRef]
- Webster, T.M.; Nichols, R.L. Changes in the prevalence of weed species in the major agronomic crops of the Southern United States: 1994/1995 to 2008/2009. Weed Sci. 2012, 60, 145–157. [Google Scholar] [CrossRef]
- Adcock, C.W.; Foshee, W.G.; Wehtje, G.R.; Gilliam, C.H. Herbicide combinations in tomato to prevent nutsedge (Cyperus esulentus) punctures in plastic mulch for multi-cropping systems. Weed Technol. 2008, 22, 136–141. [Google Scholar] [CrossRef]
- Santos, B.M.; Morales-Payan, J.P.; Stall, W.M.; Bewick, T.A.; Shilling, D.G. Effects of shading on the growth of nutsedges (Cyperus spp.). Weed Sci. 1997, 45, 670–673. [Google Scholar]
- Norsworthy, J.K.; Oliveira, M.J.; Jha, P.; Malik, M.; Buckelew, J.K.; Jennings, K.M.; Monks, D.W. Palmer amaranth and large crabgrass growth with plasticulture-grown bell pepper. Weed Technol. 2008, 22, 296–302. [Google Scholar] [CrossRef]
- Huang, X.; Liu, L.; Wen, T.; Zhang, J.; Wang, F.; Cai, Z. Changes in the soil microbial community after reductive soil disinfestation and cucumber seedling cultivation. Appl. Microbiol. Biotechnol. 2016, 100, 5581–5593. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Tanji, K.K.; Scardaci, S.C. Impact of rice straw incorporation on soil redox status and sulfide toxicity. Agron. J. 2004, 96, 70–76. [Google Scholar] [CrossRef]
- Ozores-Hampton, M.; Stansly, P.A.; Salame, T.P. Soil chemical, physical, and biological properties of a sandy soil subjected to long-term organic amendments. J. Sustain. Agric. 2011, 35, 243–259. [Google Scholar] [CrossRef]
- Daugovish, O.; Valdes-Berriz, M.; Muramoto, J.; Shennan, C.; Zavatta, M.; Henry, P. Carbon sources for anaerobic soil disinfestation in southern California strawberry. Agronomy 2023, 13, 1635. [Google Scholar] [CrossRef]


| Sweetpotato Genotype | Growth Habit | Subjective Periderm (Skin) Color | Subjective Stele (Flesh) Color | Dry Weight (%) |
|---|---|---|---|---|
| USDA-04-136 | Vining | Red | Orange | 18.75 |
| USDA-04-284 | Vining | Red | Cream | 33.60 |
| USDA-04-671 | Vining | Red | Orange | 24.90 |
| USDA-05-097 | Vining | Purple/red | Orange | 20.30 |
| USDA-09-130 | Vining | Brown | Orange | 23.23 |
| USDA-11-022 | Vining | Purple/red | White | 25.60 |
| USDA-17-037 | Erect | Purple/red | White | 24.60 |
| USDA-18-020 | Erect | Red | Orange | 17.00 |
| USDA-19-096 | Erect | Copper | Orange | 17.40 |
| USDA-20-053 | Vining | Purple/red | Orange | 24.80 |
| ‘Beauregard’ Z | Vining | Rose | Orange | 19.85 |
| ‘Hayman White’ | Vining | Brown/tan | White/cream | 37.80 |
| ‘Jewel’ | Vining | Copper | Orange | 22.93 |
| ‘Monaco’ | Semi-erect | Red | Orange | 21.08 |
| ‘Murasaki-29’ | Vining | Purple | White | 33.30 |
| ‘Orleans’ | Vining | Light rose | Orange | 22.51 |
| ‘Porto Rico’ | Semi-erect | Brown/tan | Orange | 31.60 |
| ‘Regal’ | Vining | Red | Orange | 25.01 |
| ‘Ruddy’ | Vining | Red | Orange | 19.60 |
| Factor | Treatments | Above Ground Biomass (g Plant−1) | Below Ground Biomass (g Plant−1) |
|---|---|---|---|
| Carbon amendment (C) | CM + M | 6.8 a | 0.48 a |
| Control | 3.6 b | 0.36 b | |
| Genotype (G) | USDA-04-136 | 5.8 b–e | 0.44 a–g |
| USDA-04-284 | 5.1 d–f | 0.43 b–h | |
| USDA-04-671 | 3.8 f–i | 0.51 a–e | |
| USDA-05-097 | 6.5 b–d | 0.38 d–i | |
| USDA-09-130 | 7.4 ab | 0.53 a–d | |
| USDA-11-022 | 6.6 b–d | 0.36 d–i | |
| USDA-17-037 | 8.5 a | 0.61 a–c | |
| USDA-18-020 | 3.8 f–i | 0.30 hi | |
| USDA-19-096 | 5.7 c–e | 0.62 a | |
| USDA-20-053 | 2.9 g–i | 0.42 c–h | |
| USDA-04-136 | 7.2 a–c | 0.46 a–f | |
| ‘Beauregard’ z | 3.7 f–i | 0.33 d–i | |
| ‘Hayman White’ | 2.2 i | 0.26 i | |
| ‘Jewel’ | 5.0 d–f | 0.40 d–i | |
| ‘Monaco’ | 4.9 d–f | 0.32 f–i | |
| ‘Murasaki-29’ | 4.2 e–h | 0.43 b–h | |
| ‘Orleans’ | 2.7 hi | 0.32 f–i | |
| ‘Porto Rico’ | 4.6 e–g | 0.36 d–i | |
| ‘Regal’ | 5.1 d–f | 0.32 e–i | |
| ‘Ruddy’ | 8.4 a | 0.61 ab | |
| p-value | C | <0.0001 | <0.0002 |
| G | <0.0001 | <0.0001 | |
| C × G | 0.05 | 0.3304 |
| Treatment | Plant Vigor | ||||||
|---|---|---|---|---|---|---|---|
| 4 WAP | 6 WAP | 8 WAP | |||||
| Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | ||
| Genotype (G) | Carbon Amendment (C) | ||||||
| USDA-04-136 | CM + M | 8.0 a | 7.8 a–g | 7.6 a | 7.6 a–e | 7.9 a | 7.8 a–e |
| control | 6.4 a | 6.8 b–i | 6.8 a | 6.6 b–g | 6.8 a | 7 b–h | |
| USDA-04-284 | CM + M | 7.8 a | 4 l | 7.9 a | 3.6 j | 8.1 a | 4 jk |
| control | 7.3 a | 5.8 g–l | 7.3 a | 6.8 b–g | 7.0 a | 6.5 c–h | |
| USDA-04-671 | CM + M | 8.1 a | 6.5 c–i | 8.0 a | 6.6 b–g | 8.1 a | 6.5 c–h |
| control | 6.8 a | 5.8 g–l | 6.9 a | 6 e–l | 7.0 a | 6.1 d–j | |
| USDA-04-791 | CM + M | 7.5 a | 8.2 a–e | 7.6 a | 8.2 a–d | 7.8 a | 8.5 a–c |
| control | 7.1 a | 7.8 a–g | 7.4 a | 7.1 a–f | 7.1 a | 7.5 a–f | |
| USDA-05-097 | CM + M | 8.8 a | 8.5 a–c | 8.8 a | 7.8 a–e | 9.0 a | 8.5 a–c |
| control | 7.6 a | 6.5 c–i | 7.4 a | 6.4 d–g | 7.1 a | 6.5 c–h | |
| USDA-09-130 | CM + M | 9.0 a | 8.4 a–d | 8.8 a | 8.4 a–c | 8.6 a | 9.2 a |
| control | 6.9 a | 7 a–i | 6.8 a | 7.1 a–f | 7.0 a | 7.2 a–g | |
| USDA-11-022 | CM + M | 9.1 a | 8.7 ab | 8.9 a | 8.5 ab | 9.0 a | 8.8 ab |
| control | 6.8 a | 7.4 a–i | 7.1 a | 7.1 a–f | 6.9 a | 7.2 a–g | |
| USDA-17-037 | CM + M | 6.6 a | 4.4 kl | 6.6 a | 4.4 h–j | 6.9 a | 4.2 i–k |
| control | 5.3 a | 6.8 b–i | 6.1 a | 7 a–f | 5.9 a | 7 b–h | |
| USDA-18-020 | CM + M | 8.8 a | 7.1 a–i | 8.6 a | 6.6 b–g | 9.0 a | 6.8 b–h |
| control | 8.3 a | 6.8 b–i | 8.0 a | 6.9 a–f | 8.3 a | 6.8 b–h | |
| USDA-19-096 | CM + M | 8.3 a | 6.5 c–i | 8.4 a | 6.1 e–h | 8.4 a | 6.25 d–i |
| control | 6.6 a | 6.2 e–k | 6.8 a | 6.1 e–h | 6.8 a | 6 e–j | |
| USDA-20-053 | CM + M | 8.8 a | 8.5 a–c | 8.9 a | 7.9 a–e | 8.6 a | 8.2 a–d |
| control | 7.6 a | 8.1 a–f | 7.5 a | 8.2 a–d | 7.6 a | 8.2 a–d | |
| ‘Beauregard’ Z | CM + M | 8.0 a | 8.5 a–c | 7.6 a | 7.9 a–e | 8.3 a | 8 a–e |
| control | 7.6 a | 6.5 c–i | 7.9 a | 6.1 e–h | 7.6 a | 6 e–j | |
| ‘Hayman’s White’ | CM + M | 7.0 a | 4.5 j–l | 7.0 a | 4.1 ij | 7.3 a | 4.2 i–k |
| control | 6.6 a | 6.1 f–k | 6.9 a | 6.5 c–g | 6.9 a | 6 e–j | |
| ‘Jewel’ | CM + M | 8.4 a | 7.4 a–i | 8.0 a | 6.9 a–f | 8.3 a | 7.5 a–f |
| control | 8.3 a | 6.9 a–i | 8.3 a | 6.9 a–f | 8.1 a | 6.8 b–h | |
| ‘Monaco’ | CM + M | 7.3 a | 7.7 a–h | 7.5 a | 7.6 a–e | 7.9 a | 7.5 a–f |
| control | 7.3 a | 4 l | 7.5 a | 4.1 ij | 7.3 a | 3.8 k | |
| ‘Murasaki-29’ | CM + M | 7.0 a | 6.9 a–i | 7.3 a | 6.1 e–h | 7.4 a | 6.5 c–h |
| control | 5.0 a | 5.6 h–l | 5.8 a | 5.4 f–j | 5.8 a | 5.5 f–k | |
| ‘Orleans’ | CM + M | 7.4 a | 7.2 a–i | 7.8 a | 6.9 a–f | 7.6 a | 6.2 d–i |
| control | 7.3 a | 5.5 i–l | 7.1 a | 5.6 f–l | 6.9 a | 5.2 g–k | |
| ‘Porto Rico’ | CM + M | 8.6 a | 6.4 d–k | 8.5 a | 6.4 d–g | 8.5 a | 6.5 c–h |
| control | 7.8 a | 5.4 i–l | 7.5 a | 4.9 g–j | 7.5 a | 5 h–k | |
| ‘Regal’ | CM + M | 6.5 a | 8.2 a–e | 6.9 a | 8.1 a–d | 7.0 a | 8.5 a–c |
| control | 6.3 a | 6.8 b–i | 6.6 a | 6.6 b–g | 6.5 a | 7 b–h | |
| ‘Ruddy’ | CM + M | 9.0 a | 8.9 a | 9.5 a | 8.8 a | 9.3 a | 8.8 ab |
| control | 7.5 a | 7 a–i | 7.6 a | 6.4 d–g | 7.4 a | 7 b–h | |
| p-value | C | <0.0001 | 0.0014 | <0.0001 | 0.0199 | <0.0001 | 0.0045 |
| G | <0.0001 | <.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| C × G | 0.12 | 0.0394 | 0.06 | 0.0005 | 0.48 | 0.0092 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Cutulle, M.; Rutter, W.; Wadl, P.A.; Ward, B.; Khanal, C. Anaerobic Soil Disinfestation as a Tool for Nematode and Weed Management in Organic Sweetpotato. Agronomy 2025, 15, 548. https://doi.org/10.3390/agronomy15030548
Singh S, Cutulle M, Rutter W, Wadl PA, Ward B, Khanal C. Anaerobic Soil Disinfestation as a Tool for Nematode and Weed Management in Organic Sweetpotato. Agronomy. 2025; 15(3):548. https://doi.org/10.3390/agronomy15030548
Chicago/Turabian StyleSingh, Simardeep, Matthew Cutulle, William Rutter, Phillip A. Wadl, Brian Ward, and Churamani Khanal. 2025. "Anaerobic Soil Disinfestation as a Tool for Nematode and Weed Management in Organic Sweetpotato" Agronomy 15, no. 3: 548. https://doi.org/10.3390/agronomy15030548
APA StyleSingh, S., Cutulle, M., Rutter, W., Wadl, P. A., Ward, B., & Khanal, C. (2025). Anaerobic Soil Disinfestation as a Tool for Nematode and Weed Management in Organic Sweetpotato. Agronomy, 15(3), 548. https://doi.org/10.3390/agronomy15030548








