The Effects of Nitrogen Reduction and Sheep Manure Incorporation on the Soil Characteristics and Microbial Community of Korla Fragrant Pear Orchards
Abstract
1. Introduction
2. Materials and Methods
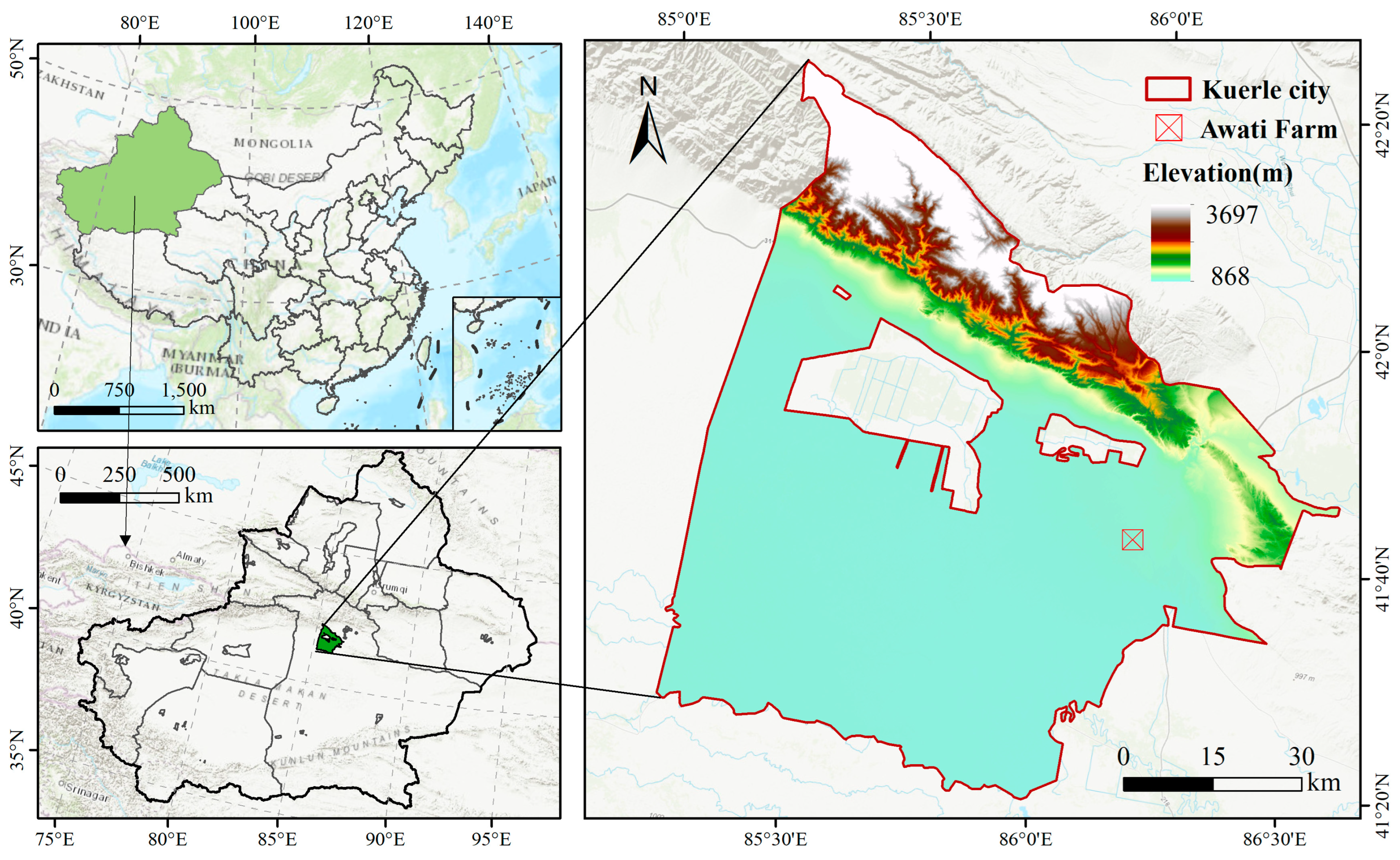
2.1. Experimental Site Description
2.2. Experimental Design
2.3. Test Determination Method
2.3.1. Soil Sample Collection and Processing
2.3.2. Soil Physicochemical Analysis
2.3.3. Soil Microbial Biomass
2.3.4. Measurement of Soil Microbial Communities
2.3.5. Yield Determination
2.4. Statistical Analysis
3. Results
3.1. Soil Physicochemical and Biological Properties
3.1.1. Soil Physicochemical Properties
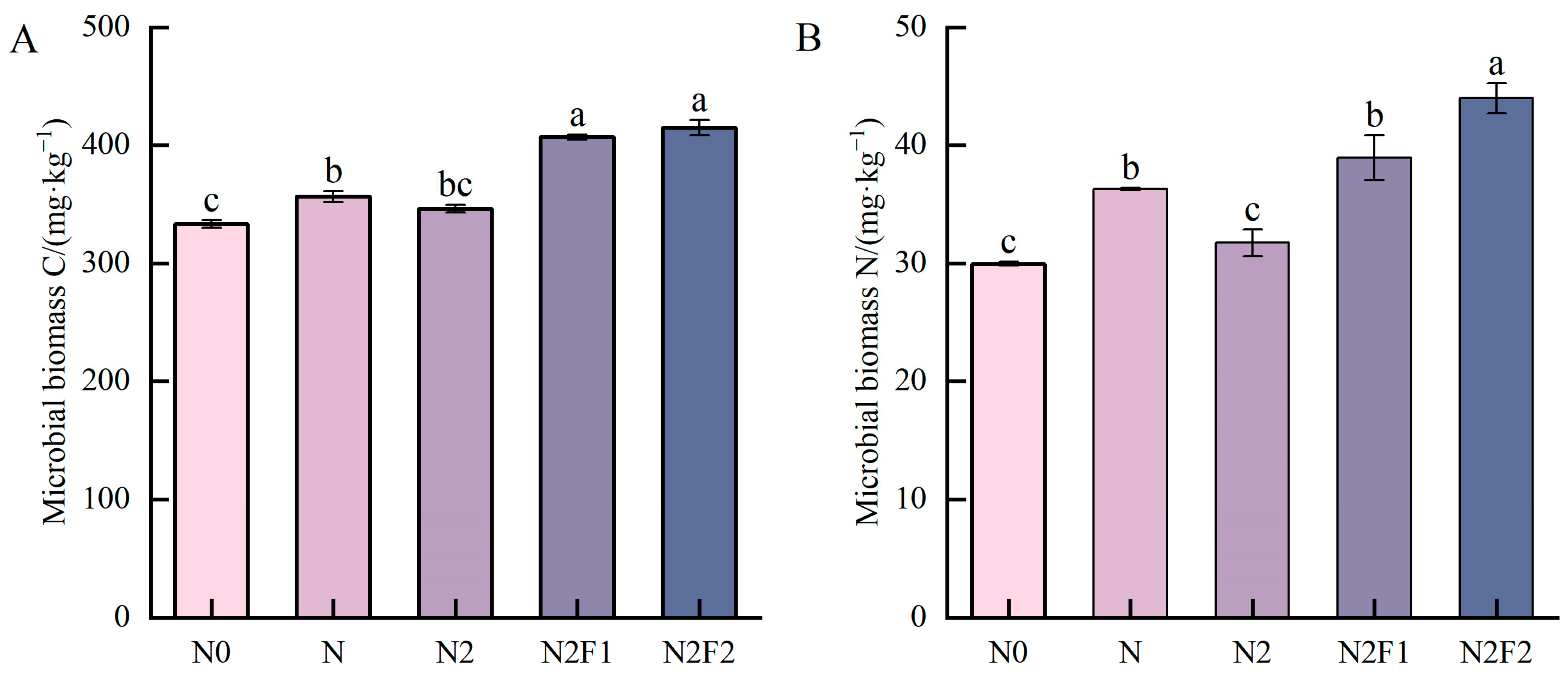
3.1.2. Soil Biological Properties
3.2. Soil Bacterial and Fungal Community Diversity
3.2.1. Alpha Diversity of Soil Bacteria and Fungi
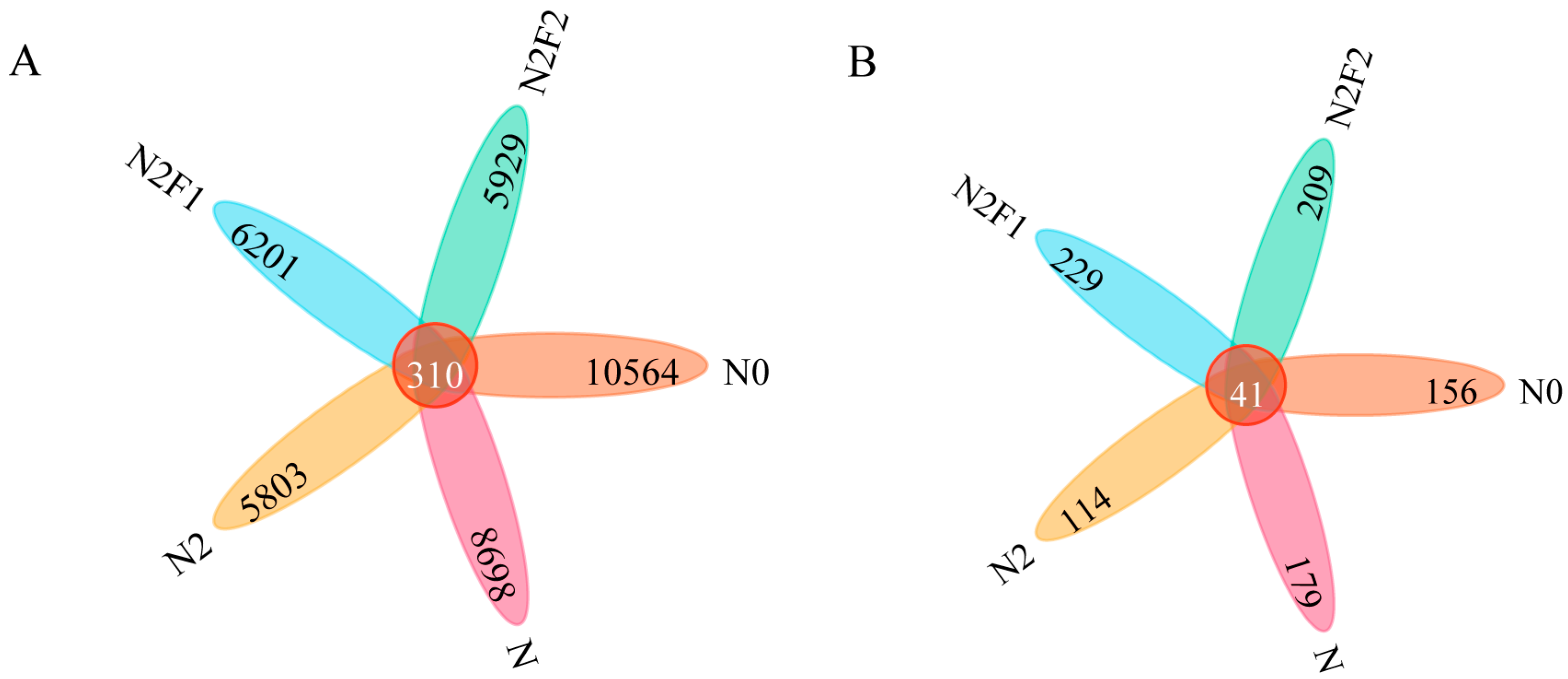
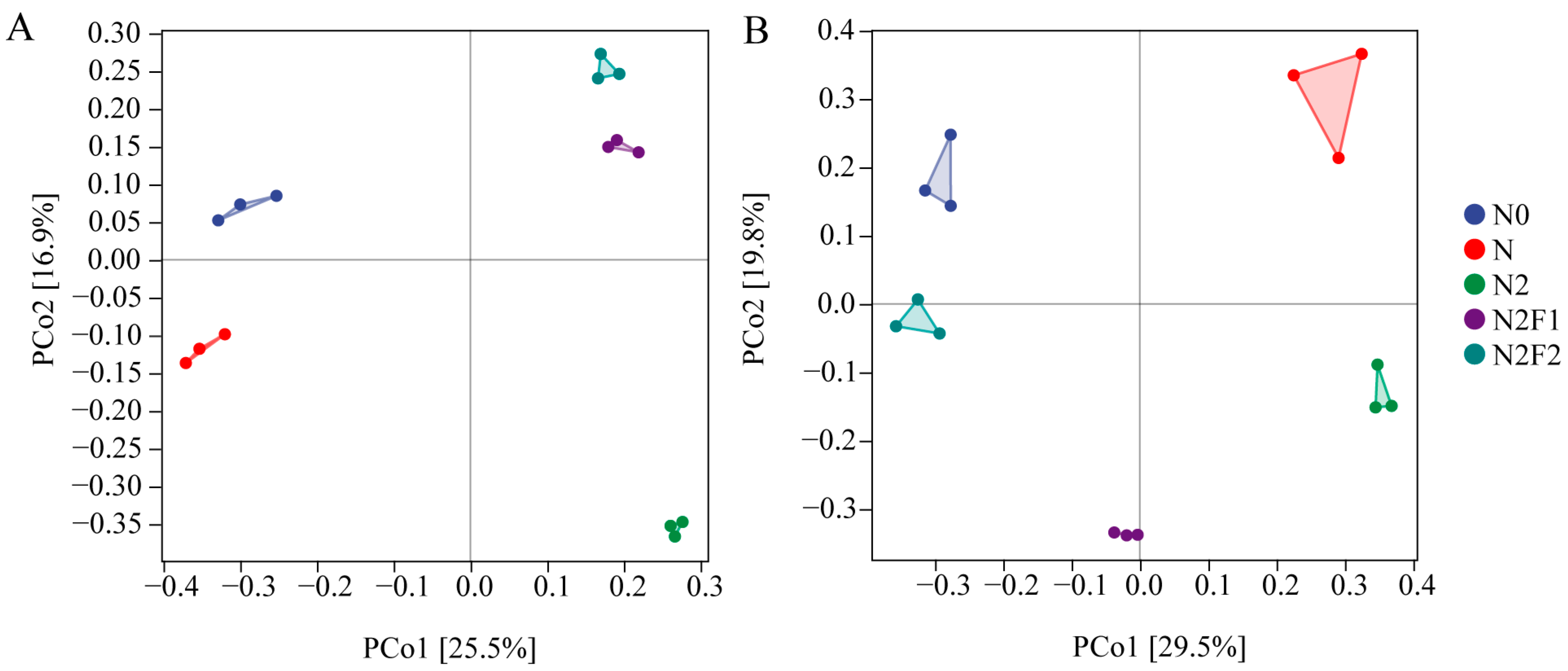
3.2.2. OTU Number and β Diversity of Soil Bacteria and Fungi
3.3. Soil Bacterial and Fungal Microbial Community Composition
3.3.1. Analysis of Soil Bacteria and Taxonomic Composition
3.3.2. LEfSe Hierarchical Tree and LDA Discriminant Results of Soil Bacteria and Fungi
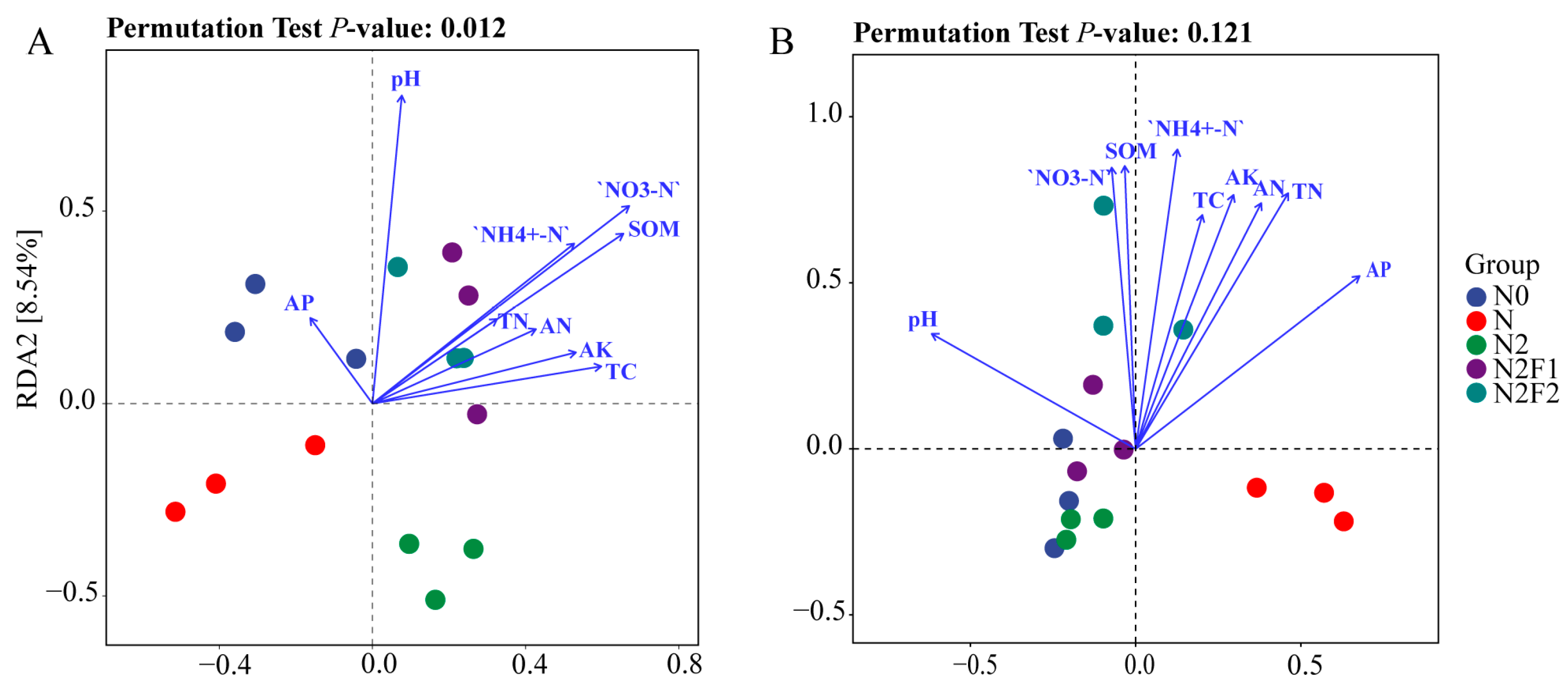
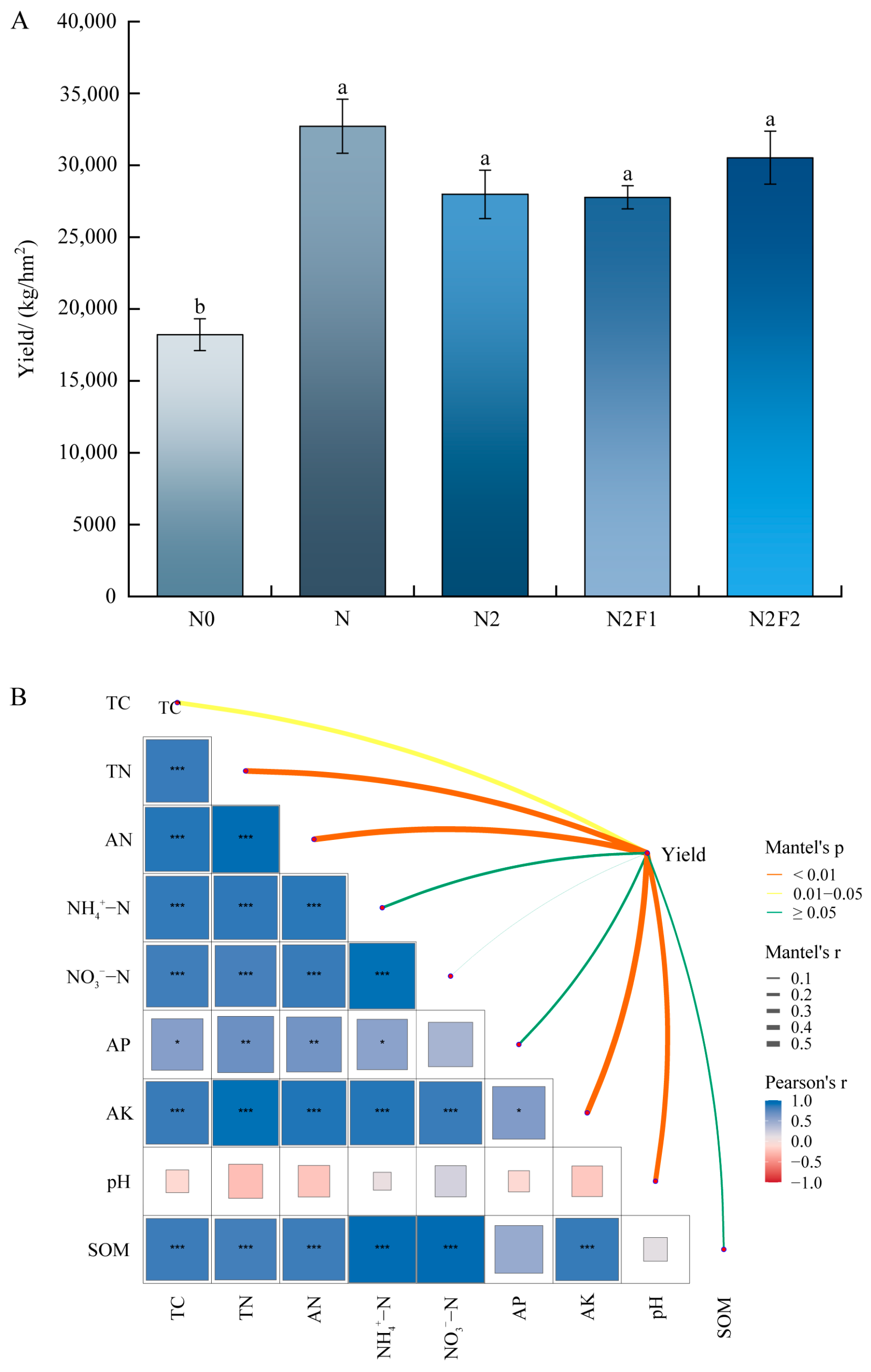
3.4. Correlation Analysis of Soil Environmental Factors, Microbial Community and Yield
3.4.1. Correlation Analysis Between Soil Environmental Factors and Microbial Community
3.4.2. Correlation Analysis Between Yield and Soil Environmental Factors
4. Discussion
4.1. Effects of Nitrogen Reduction Combined with Sheep Manure on Soil Physicochemical Properties and Biological Characteristics
4.2. Effects of Nitrogen Reduction Combined with Sheep Manure on Soil Microbial Community Diversity
4.3. Effects of Nitrogen Reduction Combined with Sheep Manure on Soil Microbial Community Characteristics
4.4. Correlation Analysis Between the Yield and Soil Physical and Chemical Properties of the Microbial Community
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miao, Y.; Stewart, B.A.; Zhang, F. Long-Term Experiments for Sustainable Nutrient Management in China. A Review. Agron. Sustain. Dev. 2011, 31, 397–414. [Google Scholar] [CrossRef]
- Raza, S.; Miao, N.; Wang, P.; Ju, X.; Chen, Z.; Zhou, J.; Kuzyakov, Y. Dramatic Loss of Inorganic Carbon by Nitrogen-Induced Soil Acidification in Chinese Croplands. Glob. Change Biol. 2020, 26, 3738–3751. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent Effects of Nitrogen Amendments on Soil Microbial Communities and Processes across Biomes. Glob. Change Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen Additions and Microbial Biomass: A Meta-Analysis of Ecosystem Studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Girmay, G.; Singh, B.R.; Mitiku, H.; Borresen, T.; Lal, R. Carbon Stocks in Ethiopian Soils in Relation to Land Use and Soil Management. Land Degrad. Dev. 2008, 19, 351–367. [Google Scholar] [CrossRef]
- Khan, A.; Wang, Z.; Chen, Z.; Bu, J.; Adnan, M.; Zhang, M. Investigation of Soil Nutrients and Associated Rhizobacterial Communities in Different Sugarcane Genotypes in Relation to Sugar Content. Chem. Biol. Technol. Agric. 2021, 8, 59. [Google Scholar] [CrossRef]
- Kamaa, M.; Mburu, H.; Blanchart, E.; Chibole, L.; Chotte, J.-L.; Kibunja, C.; Lesueur, D. Effects of Organic and Inorganic Fertilization on Soil Bacterial and Fungal Microbial Diversity in the Kabete Long-Term Trial, Kenya. Biol. Fertil. Soils 2011, 47, 315–321. [Google Scholar] [CrossRef]
- Department of Land and Water Research Center, Agriculture Research Corporation (ARC). Sudan, Elobied Research Station.; Ibrahim, Kh.H.M.; Fadni, O.A.S. Effect of Organic Fertilizers Application on Growth, Yield and Quality of Tomatoes in North Kordofan (Sandy Soil) Western Sudan. Greener J. Agric. Sci. 2013, 3, 299–304. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Li, B.; Chen, L.; Liang, J.; Huang, R.; Tang, X.; Zhang, X.; Wang, C. Partial Substitution of Chemical Fertilizer with Organic Fertilizer over Seven Years Increases Yields and Restores Soil Bacterial Community Diversity in Wheat–Rice Rotation. Eur. J. Agron. 2022, 133, 126445. [Google Scholar] [CrossRef]
- Epule, E.T.; Bryant, C.R.; Akkari, C.; Daouda, O. Can Organic Fertilizers Set the Pace for a Greener Arable Agricultural Revolution in Africa? Analysis, Synthesis and Way Forward. Land Use Policy 2015, 47, 179–187. [Google Scholar] [CrossRef]
- Hagner, M.; Penttinen, O.-P.; Tiilikkala, K.; Setälä, H. The Effects of Biochar, Wood Vinegar and Plants on Glyphosate Leaching and Degradation. Eur. J. Soil Biol. 2013, 58, 1–7. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, X.; Wang, S.; Xing, G.; Zhou, Y. Effects of the Addition of Rice-Straw-Based Biochar on Leaching and Retention of Fertilizer N in Highly Fertilized Cropland Soils. Soil Sci. Plant Nutr. 2013, 59, 771–782. [Google Scholar] [CrossRef]
- Han, J.; Dong, Y.; Zhang, M. Chemical Fertilizer Reduction with Organic Fertilizer Effectively Improve Soil Fertility and Microbial Community from Newly Cultivated Land in the Loess Plateau of China. Appl. Soil Ecol. 2021, 165, 103966. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Wang, B.-R.; Xu, M.-G.; Fan, T.-L. Crop Yield and Soil Responses to Long-Term Fertilization on a Red Soil in Southern China*1. Pedosphere 2009, 19, 199–207. [Google Scholar] [CrossRef]
- Lazcano, C.; Gómez-Brandón, M.; Revilla, P.; Domínguez, J. Short-Term Effects of Organic and Inorganic Fertilizers on Soil Microbial Community Structure and Function: A Field Study with Sweet Corn. Biol. Fertil. Soils 2013, 49, 723–733. [Google Scholar] [CrossRef]
- Wan, J.; Wang, X.; Yang, T.; Wei, Z.; Banerjee, S.; Friman, V.-P.; Mei, X.; Xu, Y.; Shen, Q. Livestock Manure Type Affects Microbial Community Composition and Assembly During Composting. Front. Microbiol. 2021, 12, 621126. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Chatzissavvidis, C.; Papaioannou, A.; Papadakis, I.E. Independent or Combinational Application of Sheep Manure and Litter from Indigenous Field Vegetation of Quercus Sp. Influences Nutrient Uptake, Photosynthesis, Intrinsic Water Use Efficiency, and Foliar Sugar Concentrations in Olive Plants (Olea Europaea L., Cv. “Koroneiki”). Appl. Sci. 2023, 13, 1127. [Google Scholar] [CrossRef]
- Fikry, A.M.; Radhi, K.S.; Abourehab, M.A.S.; Abou Sayed-Ahmed, T.A.M.; Ibrahim, M.M.; Mohsen, F.S.; Abdou, N.A.; Omar, A.A.; Elesawi, I.E.; El-Saadony, M.T. Effect of Inorganic and Organic Nitrogen Sources and Biofertilizer on Murcott Mandarin Fruit Quality. Life 2022, 12, 2120. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Kavvadias, V.; Sotiropoulos, T.; Papadakis, I.E. Organic Fertilization and Tree Orchards. Agriculture 2021, 11, 692. [Google Scholar] [CrossRef]
- Van der Bom, F.; Nunes, I.; Raymond, N.S.; Hansen, V.; Bonnichsen, L.; Magid, J.; Nybroe, O.; Jensen, L.S. Long-Term Fertilisation Form, Level and Duration Affect the Diversity, Structure and Functioning of Soil Microbial Communities in the Field. Soil Biol. Biochem. 2018, 122, 91–103. [Google Scholar] [CrossRef]
- Maltas, A.; Charles, R.; Jeangros, B.; Sinaj, S. Effect of Organic Fertilizers and Reduced-Tillage on Soil Properties, Crop Nitrogen Response and Crop Yield: Results of a 12-Year Experiment in Changins, Switzerland. Soil Tillage Res. 2013, 126, 11–18. [Google Scholar] [CrossRef]
- Blanchet, G.; Gavazov, K.; Bragazza, L.; Sinaj, S. Responses of Soil Properties and Crop Yields to Different Inorganic and Organic Amendments in a Swiss Conventional Farming System. Agric. Ecosyst. Environ. 2016, 230, 116–126. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; Agriculture Press: Beijing, China, 2005. [Google Scholar]
- Horwath, W.R.; Paul, E.A. Microbial Biomass. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1994; pp. 753–773. ISBN 978-0-89118-865-0. [Google Scholar]
- Ding, B.; Bai, Y.; Guo, S.; He, Z.; Wang, B.; Liu, H.; Zhai, J.; Cao, H. Effect of Irrigation Water Salinity on Soil Characteristics and Microbial Communities in Cotton Fields in Southern Xinjiang, China. Agronomy 2023, 13, 1679. [Google Scholar] [CrossRef]
- Wang, H.; Sheng, Y.; Jiang, W.; Pan, F.; Wang, M.; Chen, X.; Shen, X.; Yin, C.; Mao, Z. The Effects of Crop Rotation Combinations on the Soil Quality of Old Apple Orchard. Hortic. Plant J. 2022, 8, 1–10. [Google Scholar] [CrossRef]
- Tang, L.; Hamid, Y.; Chen, Z.; Lin, Q.; Shohag, M.J.I.; He, Z.; Yang, X. A Phytoremediation Coupled with Agro-Production Mode Suppresses Fusarium Wilt Disease and Alleviates Cadmium Phytotoxicity of Cucumber (Cucumis Sativus L.) in Continuous Cropping Greenhouse Soil. Chemosphere 2021, 270, 128634. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Jiang, X.; Guan, D.; Zhao, B.; Ma, M.; Zhou, B.; Cao, F.; Yang, X.; Li, L.; Li, J. Influence of Inorganic Fertilizer and Organic Manure Application on Fungal Communities in a Long-Term Field Experiment of Chinese Mollisols. Appl. Soil Ecol. 2017, 111, 114–122. [Google Scholar] [CrossRef]
- García-Gil, J.C.; Ceppi, S.B.; Velasco, M.I.; Polo, A.; Senesi, N. Long-Term Effects of Amendment with Municipal Solid Waste Compost on the Elemental and Acidic Functional Group Composition and pH-Buffer Capacity of Soil Humic Acids. Geoderma 2004, 121, 135–142. [Google Scholar] [CrossRef]
- De Vries, F.T.; Hoffland, E.; van Eekeren, N.; Brussaard, L.; Bloem, J. Fungal/Bacterial Ratios in Grasslands with Contrasting Nitrogen Management. Soil Biol. Biochem. 2006, 38, 2092–2103. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, T.; Zhao, J.; Wang, L.; Yang, D.; Li, G.; Xiu, W. Variation of Soil Bacterial and Fungal Communities from Fluvo-Aquic Soil Under Chemical Fertilizer Reduction Combined with Organic Materials in North China Plain. J. Soil Sci. Plant Nutr. 2021, 21, 349–363. [Google Scholar] [CrossRef]
- Gao, R.; Duan, Y.; Zhang, J.; Ren, Y.; Li, H.; Liu, X.; Zhao, P.; Jing, Y. Effects of Long-Term Application of Organic Manure and Chemical Fertilizer on Soil Properties and Microbial Communities in the Agro-Pastoral Ecotone of North China. Front. Environ. Sci. 2022, 10, 993973. [Google Scholar] [CrossRef]
- Bakker, E.S.; Olff, H.; Boekhoff, M.; Gleichman, J.M.; Berendse, F. Impact of Herbivores on Nitrogen Cycling: Contrasting Effects of Small and Large Species. Oecologia 2004, 138, 91–101. [Google Scholar] [CrossRef]
- Knops, J.M.H.; Bradley, K.L.; Wedin, D.A. Mechanisms of Plant Species Impacts on Ecosystem Nitrogen Cycling. Ecol. Lett. 2002, 5, 454–466. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Liu, L.; Li, T.; Dou, Y.; Qiao, J.; Wang, Y.; An, S.; Chang, S.X. Nitrogen Fertilization Weakens the Linkage between Soil Carbon and Microbial Diversity: A Global Meta-analysis. Glob. Change Biol. 2022, 28, 6446–6461. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Long-term Nitrogen Fertilization Decreases Bacterial Diversity and Favors the Growth of Actinobacteria and Proteobacteria in Agro-ecosystems across the Globe. Glob. Change Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Guan, D.; Zhou, B.; Zhao, B.; Ma, M.; Qin, J.; Jiang, X.; Chen, S.; Cao, F.; Shen, D.; et al. Influence of 34-Years of Fertilization on Bacterial Communities in an Intensively Cultivated Black Soil in Northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Bulluck, L.R.; Brosius, M.; Evanylo, G.K.; Ristaino, J.B. Organic and Synthetic Fertility Amendments Influence Soil Microbial, Physical and Chemical Properties on Organic and Conventional Farms. Appl. Soil Ecol. 2002, 19, 147–160. [Google Scholar] [CrossRef]
- Scotti, R.; Bonanomi, G.; Scelza, R.; Zoina, A.; Rao, M.A. Organic Amendments as Sustainable Tool to Recovery Fertility in Intensive Agricultural Systems. J. Soil Sci. Plant Nutr. 2015, 15, 333–352. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Ma, T.; Raza, W.; Li, J.; Howland, J.G.; Huang, Q.; Shen, Q. Impacts of Inorganic and Organic Fertilization Treatments on Bacterial and Fungal Communities in a Paddy Soil. Appl. Soil Ecol. 2017, 112, 42–50. [Google Scholar] [CrossRef]
- Edwards, I.P.; Zak, D.R.; Kellner, H.; Eisenlord, S.D.; Pregitzer, K.S. Simulated Atmospheric N Deposition Alters Fungal Community Composition and Suppresses Ligninolytic Gene Expression in a Northern Hardwood Forest. PLoS ONE 2011, 6, e20421. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Yeoh, Y.K.; Kasinadhuni, N.R.P.; Lonhienne, T.G.A.; Robinson, N.; Hugenholtz, P.; Ragan, M.A.; Schmidt, S. Nitrogen Fertilizer Dose Alters Fungal Communities in Sugarcane Soil and Rhizosphere. Sci. Rep. 2015, 5, 8678. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Han, X.; Wang, Y.; Han, M.; Shi, H.; Liu, N.; Bai, H. Influence of Long-Term Fertilization on Soil Microbial Biomass, Dehydrogenase Activity, and Bacterial and Fungal Community Structure in a Brown Soil of Northeast China. Ann. Microbiol. 2015, 65, 533–542. [Google Scholar] [CrossRef]
- He, J.-Z.; Zheng, Y.; Chen, C.-R.; He, Y.-Q.; Zhang, L.-M. Microbial Composition and Diversity of an Upland Red Soil under Long-Term Fertilization Treatments as Revealed by Culture-Dependent and Culture-Independent Approaches. J. Soils Sediments 2008, 5, 349–358. [Google Scholar] [CrossRef]
- Jiang, S.; An, X.; Shao, Y.; Kang, Y.; Chen, T.; Mei, X.; Dong, C.; Xu, Y.; Shen, Q. Responses of Arbuscular Mycorrhizal Fungi Occurrence to Organic Fertilizer: A Meta-Analysis of Field Studies. Plant Soil 2021, 469, 89–105. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Husband, R.; Daniell, T.J.; Watson, I.J.; Duck, J.M.; Fitter, A.H.; Young, J.P.W. Arbuscular Mycorrhizal Community Composition Associated with Two Plant Species in a Grassland Ecosystem. Mol. Ecol. 2002, 11, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, A. Soil Microorganisms; Universityy of Mysore: Mysore, India, 2017. [Google Scholar]
- Hermans, S.M.; Buckley, H.L.; Case, B.S.; Curran-Cournane, F.; Taylor, M.; Lear, G. Bacteria as Emerging Indicators of Soil Condition. Appl. Environ. Microbiol. 2017, 83, e02826-16. [Google Scholar] [CrossRef]
- Brito, I.; Goss, M.J.; de Carvalho, M.; Chatagnier, O.; van Tuinen, D. Impact of Tillage System on Arbuscular Mycorrhiza Fungal Communities in the Soil under Mediterranean Conditions. Soil Tillage Res. 2012, 121, 63–67. [Google Scholar] [CrossRef]
- Ali, I.; Yuan, P.; Ullah, S.; Iqbal, A.; Zhao, Q.; Liang, H.; Khan, A.; Imran; Zhang, H.; Wu, X.; et al. Biochar Amendment and Nitrogen Fertilizer Contribute to the Changes in Soil Properties and Microbial Communities in a Paddy Field. Front. Microbiol. 2022, 13, 834751. [Google Scholar] [CrossRef]
- Boer, W.D.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a Fungal World: Impact of Fungi on Soil Bacterial Niche Development. FEMS Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef]
- Yu, L.; Nicolaisen, M.; Larsen, J.; Ravnskov, S. Molecular Characterization of Root-Associated Fungal Communities in Relation to Health Status of Pisum Sativum Using Barcoded Pyrosequencing. Plant Soil 2012, 357, 395–405. [Google Scholar] [CrossRef]
- Song, W.; Shu, A.; Liu, J.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S.; et al. Effects of Long-Term Fertilization with Different Substitution Ratios of Organic Fertilizer on Paddy Soil. Pedosphere 2022, 32, 637–648. [Google Scholar] [CrossRef]
- Liu, J.; Dang, P.; Gao, Y.; Zhu, H.; Zhu, H.; Zhao, F.; Zhao, Z. Effects of Tree Species and Soil Properties on the Composition and Diversity of the Soil Bacterial Community Following Afforestation. For. Ecol. Manag. 2018, 427, 342–349. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-Term Effects of Mineral Fertilizers on Soil Microorganisms—A Review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Han, S.H.; An, J.Y.; Hwang, J.; Kim, S.B.; Park, B.B. The Effects of Organic Manure and Chemical Fertilizer on the Growth and Nutrient Concentrations of Yellow Poplar (Liriodendron Tulipifera Lin.) in a Nursery System. For. Sci. Technol. 2016, 12, 137–143. [Google Scholar] [CrossRef]
- Fuentes, M.; Govaerts, B.; De León, F.; Hidalgo, C.; Dendooven, L.; Sayre, K.D.; Etchevers, J. Fourteen Years of Applying Zero and Conventional Tillage, Crop Rotation and Residue Management Systems and Its Effect on Physical and Chemical Soil Quality. Eur. J. Agron. 2009, 30, 228–237. [Google Scholar] [CrossRef]
- Yu, H.; Ling, N.; Wang, T.; Zhu, C.; Wang, Y.; Wang, S.; Gao, Q. Responses of Soil Biological Traits and Bacterial Communities to Nitrogen Fertilization Mediate Maize Yields across Three Soil Types. Soil Tillage Res. 2019, 185, 61–69. [Google Scholar] [CrossRef]
- Dawe, D.; Dobermann, A.; Ladha, J.K.; Yadav, R.L.; Bao, L.; Gupta, R.K.; Lal, P.; Panaullah, G.; Sariam, O.; Singh, Y.; et al. Do Organic Amendments Improve Yield Trends and Profitability in Intensive Rice Systems? Field Crops Res. 2003, 83, 191–213. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, D.; Niu, Z.; Yan, J.; Zhou, X.; Kang, X. Effects of Combined Organic/Inorganic Fertilizer Application on Growth, Photosynthetic Characteristics, Yield and Fruit Quality of Actinidia Chinesis Cv ‘Hongyang’. Glob. Ecol. Conserv. 2020, 22, e00997. [Google Scholar] [CrossRef]
- Vanotti, M.B.; Leclerc, S.A.; Bundy, L.G. Short-Term Effects of Nitrogen Fertilization on Soil Organic Nitrogen Availability. Soil Sci. Soc. Am. J. 1995, 59, 1350–1359. [Google Scholar] [CrossRef]
- Carey, P.L.; Benge, J.R.; Haynes, R.J. Comparison of Soil Quality and Nutrient Budgets between Organic and Conventional Kiwifruit Orchards. Agric. Ecosyst. Environ. 2009, 132, 7–15. [Google Scholar] [CrossRef]
- Del Amor, F.M. Yield and Fruit Quality Response of Sweet Pepper to Organic and Mineral Fertilization. Renew. Agric. Food Syst. 2007, 22, 233–238. [Google Scholar] [CrossRef]
- Olsen, R.J.; Hensler, R.F.; Attoe, O.J. Effect of Manure Application, Aeration, and Soil pH on Soil Nitrogen Transformations and on Certain Soil Test Values. Soil Sci. Soc. Am. J. 1970, 34, 222–225. [Google Scholar] [CrossRef]
- Chalmers, A.G. A Review of Fertilizer, Lime and Organic Manure Use on Farm Crops in Great Britain from 1983 to 1987. Soil Use Manag. 2001, 17, 254–262. [Google Scholar] [CrossRef]
- Ma, T.; He, X.; Chen, S.; Li, Y.; Huang, Q.; Xue, C.; Shen, Q. Long-Term Organic–Inorganic Fertilization Regimes Alter Bacterial and Fungal Communities and Rice Yields in Paddy Soil. Front. Microbiol. 2022, 13, 890712. [Google Scholar] [CrossRef] [PubMed]
- Megyes, M.; Borsodi, A.K.; Árendás, T.; Márialigeti, K. Variations in the Diversity of Soil Bacterial and Archaeal Communities in Response to Different Long-Term Fertilization Regimes in Maize Fields. Appl. Soil Ecol. 2021, 168, 104120. [Google Scholar] [CrossRef]
- Sivojiene, D.; Kacergius, A.; Baksiene, E.; Maseviciene, A.; Zickiene, L. The Influence of Organic Fertilizers on the Abundance of Soil Microorganism Communities, Agrochemical Indicators, and Yield in East Lithuanian Light Soils. Plants 2021, 10, 2648. [Google Scholar] [CrossRef] [PubMed]
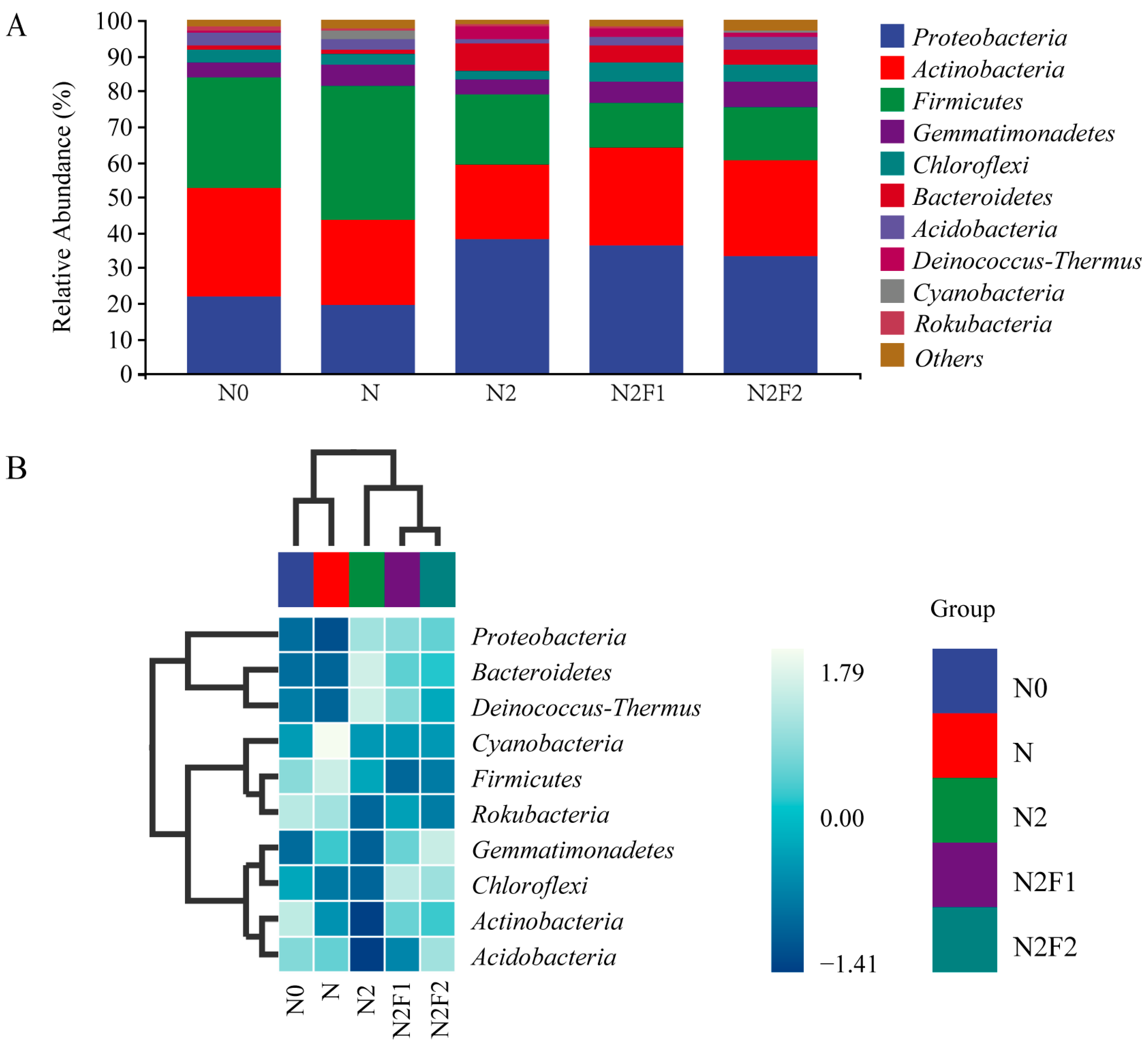
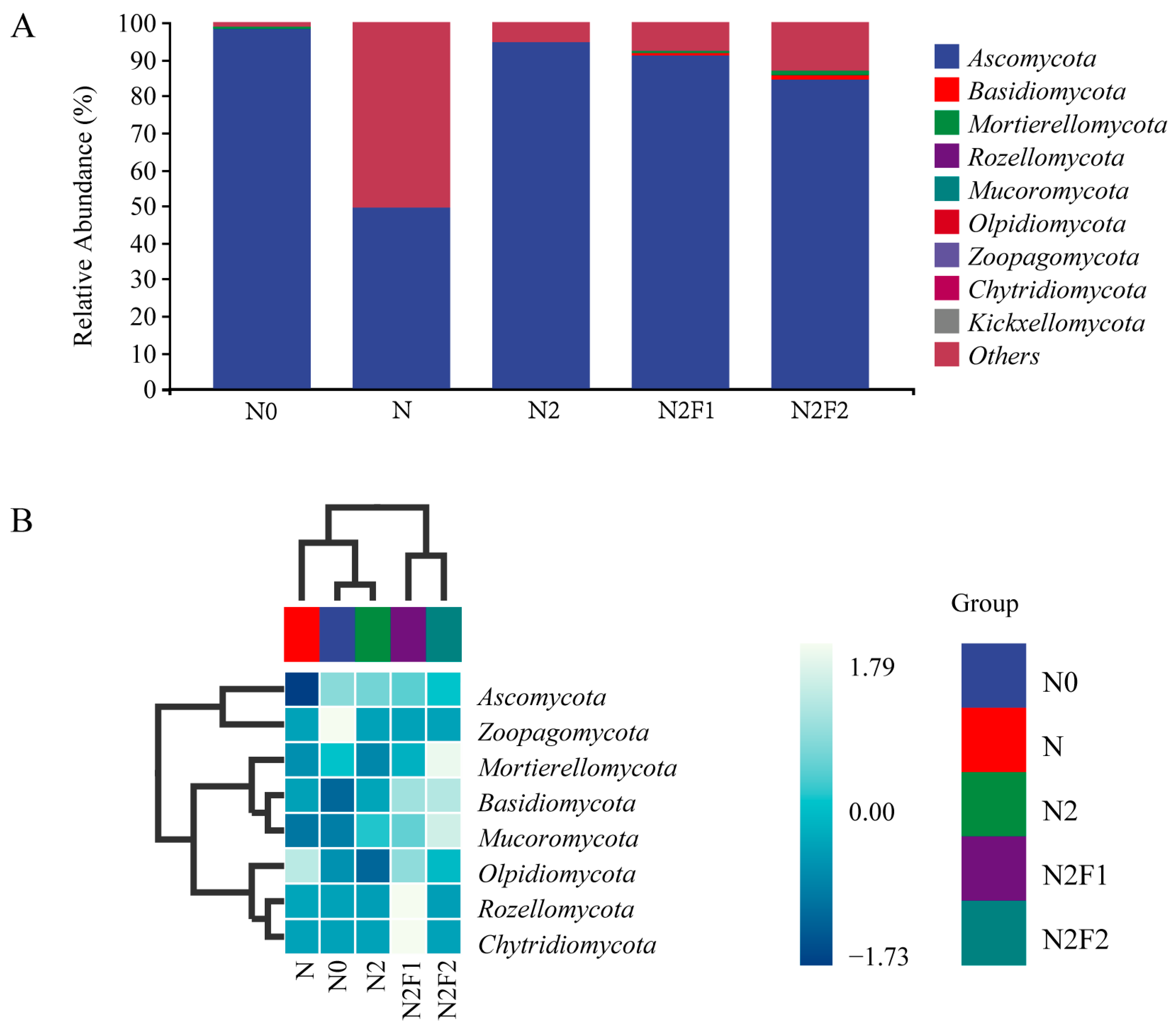
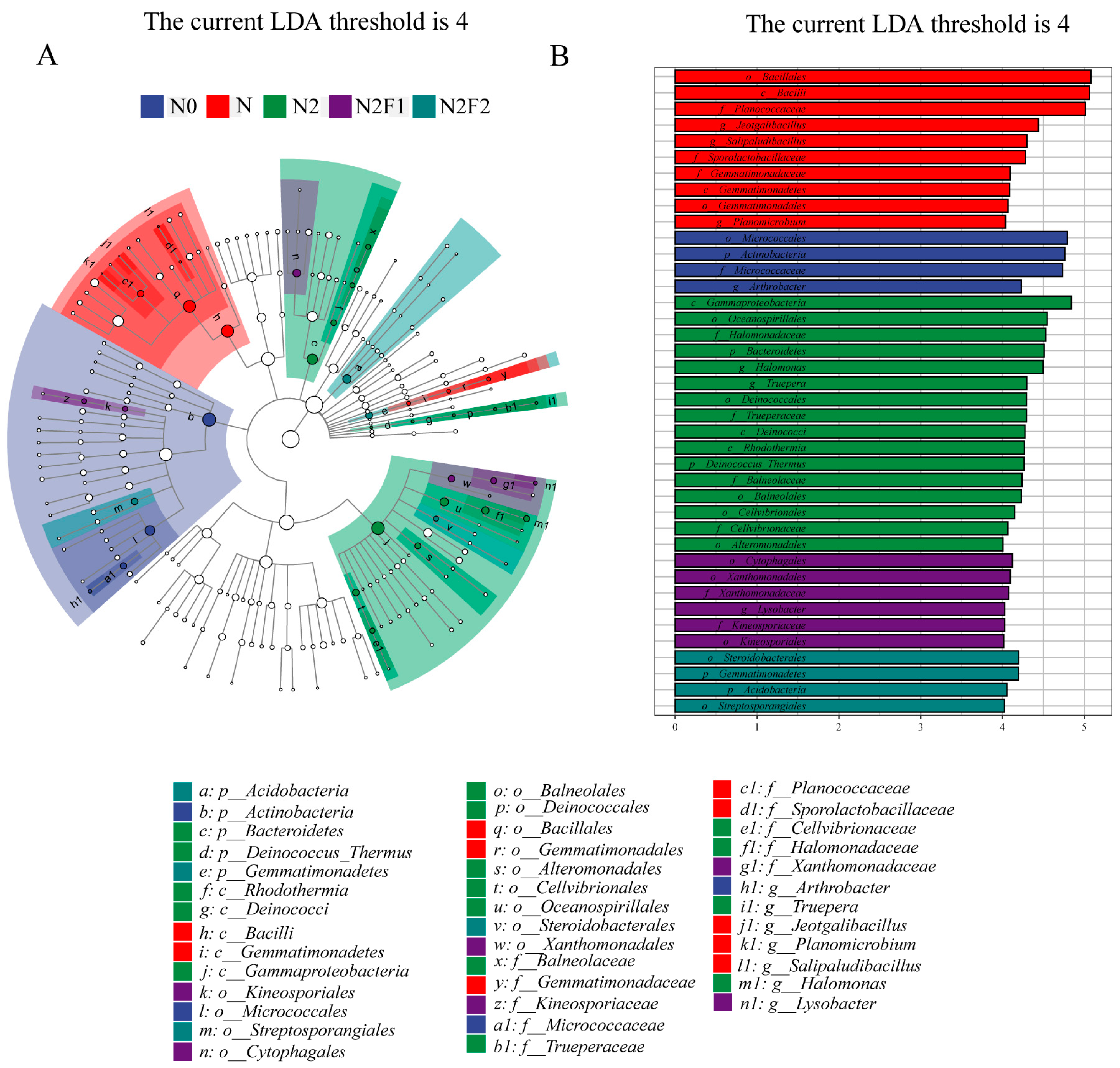
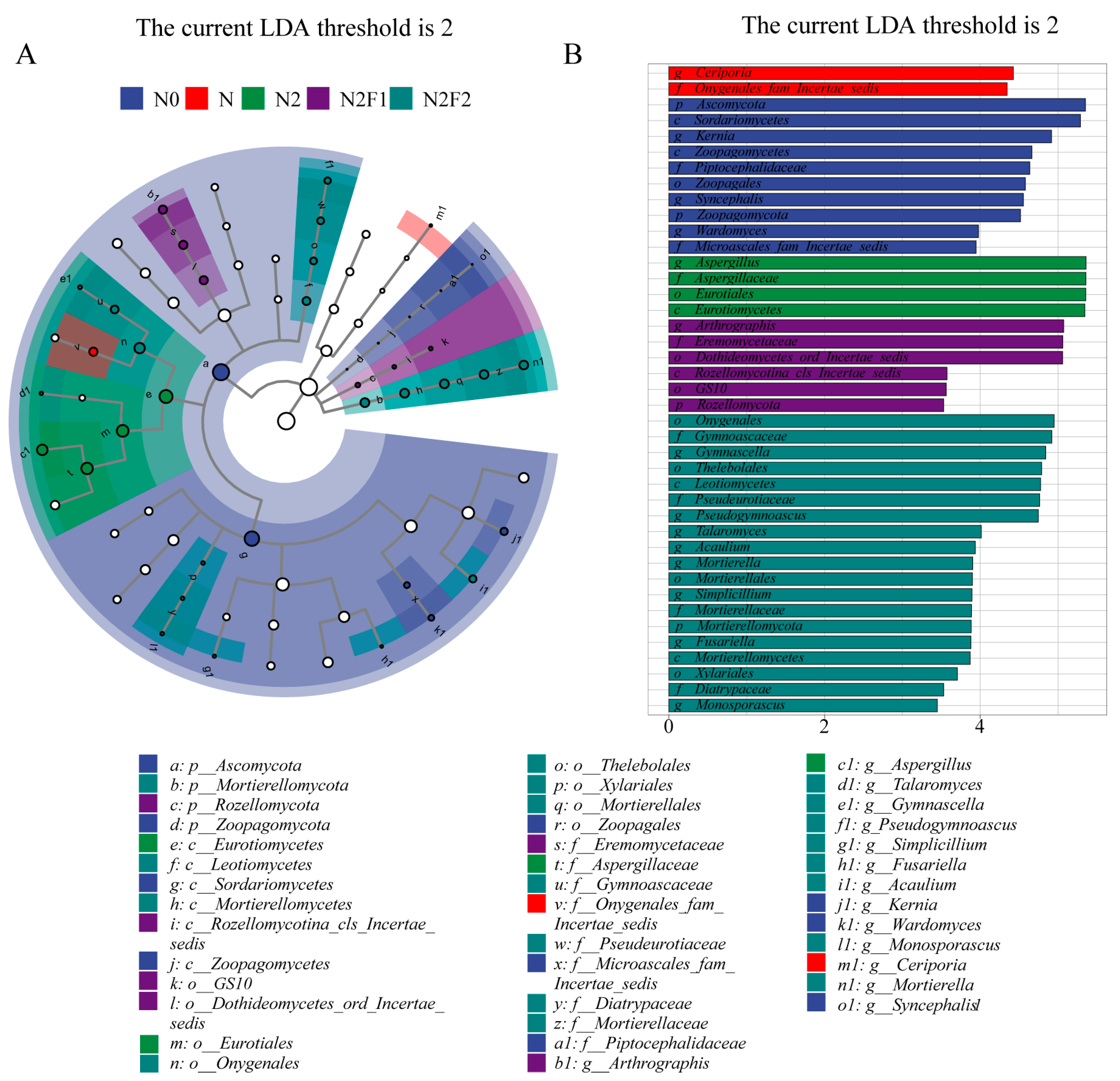
| Treatments | Fertilization Dosage (kg·hm−2) | Fertilization Dosage (kg·plant−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | P2O5 | K2O | Sheep Manure | N | P2O5 | K2O | Sheep Manure | ||
| 1 | N0 | 0 | 300 | 75 | 0 | 0 | 0.267 | 0.067 | 0 |
| 2 | N | 300 | 300 | 75 | 0 | 0.267 | 0.267 | 0.067 | 0 |
| 3 | N2 | 240 | 300 | 75 | 0 | 0.213 | 0.267 | 0.067 | 0 |
| 4 | N2F1 | 240 | 300 | 75 | 22,500 | 0.213 | 0.267 | 0.067 | 20 |
| 5 | N2F2 | 240 | 300 | 75 | 33,750 | 0.213 | 0.267 | 0.067 | 30 |
| Treatment | TC | TN | NO3−-N | NH4+-N | AN | AP | AK | SOM | pH |
|---|---|---|---|---|---|---|---|---|---|
| N0 | 17.65 ± 0.50 c | 0.67 ± 0.02 d | 33.48 ± 1.09 c | 4.19 ± 0.27 d | 41.18 ± 1.63 d | 61.04 ± 1.02 b | 197.67 ± 4.33 c | 20.08 ± 0.41 c | 8.00 + 0.02 a |
| N | 19.79 ± 0.29 b | 0.91 ± 0.01 b | 39.25 ± 1.85 b | 6.52 ± 0.20 c | 62.25 ± 1.46 b | 69.66 ± 2.63 a | 221.00 ± 4.51 b | 21.02 ± 0.35 bc | 7.57 + 0.04 c |
| N2 | 19.29 ± 0.50 b | 0.78 ± 0.03 c | 39.23 ± 2.30 b | 5.79 ± 0.33 c | 51.62 ± 1.98 c | 59.76 ± 2.16 b | 217.33 ± 2.73 b | 21.15 ± 0.19 b | 7.62 + 0.01 c |
| N2F1 | 20.47 ± 0.59 ab | 0.93 ± 0.01 ab | 61.83 ± 0.99 a | 8.42 ± 0.43 b | 66.05 ± 1.00 b | 65.10 ± 2.52 ab | 230.00 ± 2.08 ab | 23.10 ± 0.16 a | 7.81 + 0.03 b |
| N2F2 | 21.96 ± 0.28 a | 1.01 ± 0.04 a | 66.87 ± 0.57 a | 11.67 ± 0.60 a | 73.28 ± 1.19 a | 69.43 ± 0.35 a | 240.67 ± 4.91 a | 24.07 ± 0.25 a | 7.87 + 0.03 b |
| Treatment | Chao1 | Observed_Species | Pielou_e | Shannon | Simpson | |
|---|---|---|---|---|---|---|
| Bacteria | N0 | 6450.95 ± 174.51 a | 5361.80 ± 114.37a | 0.855 ± 0.007 bc | 10.59 ± 0.11 a | 0.9961 ± 0.0008 bc |
| N | 5205.87 ± 130.23 b | 4474.67 ± 100.77 b | 0.835 ± 0.012 c | 10.13 ± 0.16 b | 0.9943 ± 0.0009 c | |
| N2 | 3661.74 ± 129.12 c | 3269.87 ± 101.31 c | 0.862 ± 0.014 abc | 10.06 ± 0.14 b | 0.9971 ± 0.0008 ab | |
| N2F1 | 4234.44 ± 312.26 bc | 3777.50 ± 305.04 c | 0.891 ± 0.002 a | 10.58 ± 0.09 a | 0.9984 ± 0.0001 a | |
| N2F2 | 4322.9 ± 488.34 bc | 3661.27 ± 274.73 c | 0.881 ± 0.009 ab | 10.42 ± 0.10 ab | 0.9976 ± 0.0003 ab | |
| Fungi | N0 | 189.45 ± 19.72 ab | 186.53 ± 18.98 ab | 0.615 ± 0.054 bc | 4.64 ± 0.47 a | 0.8901 ± 0.0553 ab |
| N | 185.53 ± 24.54 ab | 183.67 ± 23.47 ab | 0.635 ± 0.005 ab | 4.76 ± 0.14 a | 0.9171 ± 0.0083 ab | |
| N2 | 157.17 ± 14.05 b | 155.20 ± 13.56 b | 0.517 ± 0.021 c | 3.75 ± 0.14 b | 0.8122 ± 0.0286 b | |
| N2F1 | 241.75 ± 18.64 a | 238.47 ± 18.89 a | 0.623 ± 0.011 ab | 4.92 ± 0.15 a | 0.9081 ± 0.0169 ab | |
| N2F2 | 196.16 ± 27.34 ab | 195.23 ± 27.28 ab | 0.719 ± 0.026 a | 5.44 ± 0.12 a | 0.9523 ± 0.0034a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, W.; Shen, X.; Li, W.; Yan, L.; Li, J.; Ding, B.; Chai, Z. The Effects of Nitrogen Reduction and Sheep Manure Incorporation on the Soil Characteristics and Microbial Community of Korla Fragrant Pear Orchards. Agronomy 2025, 15, 545. https://doi.org/10.3390/agronomy15030545
Xie W, Shen X, Li W, Yan L, Li J, Ding B, Chai Z. The Effects of Nitrogen Reduction and Sheep Manure Incorporation on the Soil Characteristics and Microbial Community of Korla Fragrant Pear Orchards. Agronomy. 2025; 15(3):545. https://doi.org/10.3390/agronomy15030545
Chicago/Turabian StyleXie, Wenge, Xing Shen, Wei Li, Linsen Yan, Jie Li, Bangxin Ding, and Zhongping Chai. 2025. "The Effects of Nitrogen Reduction and Sheep Manure Incorporation on the Soil Characteristics and Microbial Community of Korla Fragrant Pear Orchards" Agronomy 15, no. 3: 545. https://doi.org/10.3390/agronomy15030545
APA StyleXie, W., Shen, X., Li, W., Yan, L., Li, J., Ding, B., & Chai, Z. (2025). The Effects of Nitrogen Reduction and Sheep Manure Incorporation on the Soil Characteristics and Microbial Community of Korla Fragrant Pear Orchards. Agronomy, 15(3), 545. https://doi.org/10.3390/agronomy15030545





