Lowland Sedge Meadows as a Potential Source of Macro and Micronutrient Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Collection
2.2. Elemental Analysis Method
2.3. Statistical Analysis
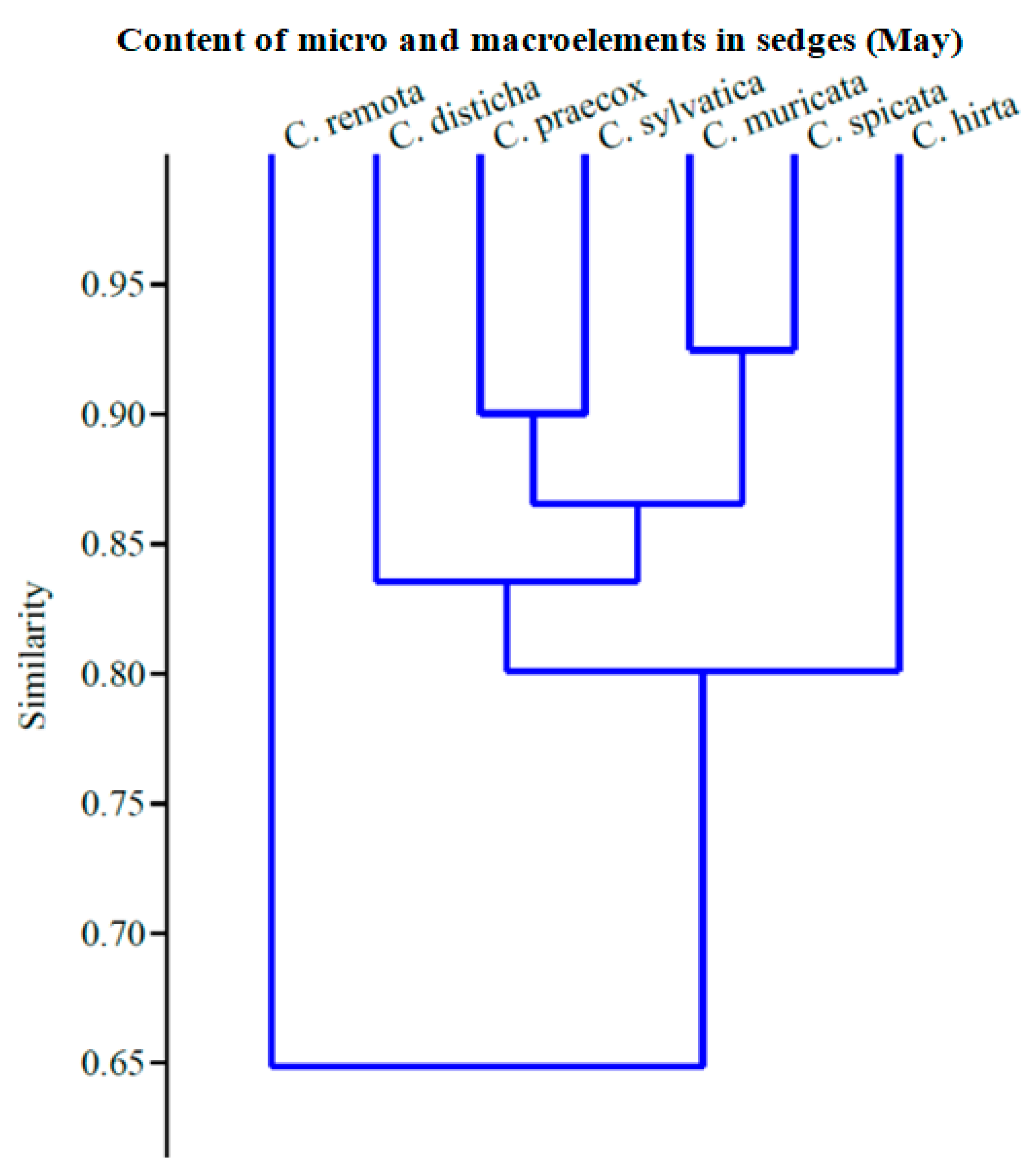
3. Results and Discussion
Micro and Macromineral Concentration in Studied Sedges—May, June, Comparison and Mean Content
4. Conclusions
- The difference in micronutrient content between May and June harvest dates in the dry weight of all sedge species analyzed was statistically confirmed only for copper.
- The timing of harvesting, which is also a reflection of the specific phase of plant development, had a statistically significant effect on the change in the macronutrient content of the dry matter of all the sedge species analyzed and was associated with a decrease in phosphorus, magnesium and calcium, while in the case of silicon, a delay in mowing resulted in an increase in the content of this element.
- The mineral value of sedges, like many other plant species, at the beginning of the growing season is higher compared to the plant material analyzed at the end of the growing season. Species differing in physiognomy and habit were selected for the study. C. spicata, C. muricata and C. remota, are dense-clumped plants, C. sylvatica forms a loose clump. In contrast, C. hirta, C. disticha and C. praecox are plants, with crawling rhizome-producing upright stems at intervals. Additionally, in C. disticha and C. hirta, in contrast to the other species analyzed, usually the leaves begin to die back after the vesicles mature. However, as the analyses showed, these traits generally had no effect on the concentration levels of the individual elements. The only significant exception was C. remota with regard to iron content.
- The results showed that sedge sward is a good and, in most cases, sufficient source of the micro and macronutrients studied in forage, particularly in comparison with the content observed in grass species desirable in meadow communities.
- Sedges appear to be a potentially good source of compounds with beneficial effects on animal organisms, including compounds of a therapeutic nature. They are rich in essential fatty acids, and contained in hair follicles and leaves, making them a good source of supplements. Low-sedge floodplain meadows may be a potential source of macro and micronutrient supplementation, but their nutritional value may depend on a number of environmental factors. The results of our study indicate variation in the mineral content of the dry matter of all analyzed sedge species depending on the harvest date.
- However, it should be noted that the study had some limitations. Firstly, the meadows analyzed represent specific habitat conditions, which may make it difficult to extrapolate the results to other regions. Secondly, the influence of seasonal and hydrological factors on the mineral composition of plants requires further research in a multi-year perspective. Finally, the bioavailability of nutrients to animals was not assessed, which is an important aspect of the practical application of the results.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Egorova, T.V. The Sedges Carex L. of Russia and Adjacent States; State Chemical-Pharmaceutical Academy St Petersburg: St Petersburg, Russia, 1999. [Google Scholar]
- Grzelak, M.; Janyszek, M.; Spychalski, W. Evaluation of the Fodder Value of the Over Ground Parts of Sedges from the Section Muehlenbergianae (L.H. Bailey) KÜK. Rocz. AR Pozn. Bot.-Stec. 2005, 9, 89–95. [Google Scholar]
- Janyszek, M.; Grzelak, M.; Spychalski, W. Nutritive Value of the Over Ground Parts of Sedges from The Section Vulpine (Carey) Christ. at Different Plant Developmental Stages. Rocz. AR Pozn. Bot.-Stec. 2005, 9, 103–109. [Google Scholar]
- Janyszek-Sołtysiak, M.; Grzelak, M.; Gajewski, P.; Jagodziński, A.M.; Gaweł, E.; Wrońska-Pilarek, D. Mineral Contents in Aboveground Biomass of Sedges (Carex L., Cyperaceae). Energies 2021, 14, 8007. [Google Scholar] [CrossRef]
- Grzelak, M.; Gaweł, E.; Janyszek, M.; Wrońska-Pilarek, D.; Janyszek, S.; Murawski, M.; Runowski, S.; Knioła, A. Nature and fodder Value of Grass-Sedge Communities in The Notec Valley in the Natura 2000 Area of Grass-Sedge Communities in The Noteć Valley in The Natura 2000 Area. J. Res. Appl. Agri. Engin. 2017, 62, 135–140. [Google Scholar]
- Grzelak, M.; Majchrzak, L.; Janyszek-Sołtysiak, M.; Gaweł, E.; Wrońska-Pilarek, D. Influence of Selected Habitat Factors on Floristic Diversity, Natural Values and Economic Value of the Black Sedge (Carex nigra Reichard) Community in the Noteć Bystra Valley. Fragm. Agron. 2019, 36, 27–35. (In Polish) [Google Scholar]
- Al-Rowaily, S.L.; Abd-ElGawad, A.M.; Alghanem, S.M.; Al-Taisan, W.A.; El-Amier, Y.A. Nutritional Value, Mineral Composition, Secondary Metabolites, and Antioxidant Activity of Some Wild Geophyte Sedges and Grasses. Plants 2019, 8, 569. [Google Scholar] [CrossRef] [PubMed]
- Vejnovic, J.; Djuric, B.; Lombnæs, P.; Singh, B.R. Concentration of trace and major elements in natural grasslands of Bosnia and Herzegovina in relation to soil properties and plant species. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 68, 243–254. [Google Scholar] [CrossRef]
- Babu, R.H.; Savithramma, N. Studies on mineral analysis of grasses of Poaceae. Int. J. Pharma Sci. 2014, 4, 526–531. [Google Scholar]
- Parzych, A.; Sobisz, Z.; Jonczak, J. Comparing Carex Species of mind-forest spring ecosystems in terms of ability to accumulate macro and microelements. J. Ecolog. Engin. 2017, 18, 125–136. [Google Scholar] [CrossRef][Green Version]
- Veselkin, D.V.; Konoplenko, M.A.; Betekhtina, A.A. Means for soil nutrient uptake in sedges with different ecological strategies. Russ. J. Ecol. 2014, 45, 547–554. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Szteke, B. Pierwiastki śladowe w układzie gleba-roślina. Inż. Ekol. 2005, 26, 28–29. [Google Scholar]
- Parzych, A.; Jonczak, J.; Sobisz, Z. Bioaccumulation of macronutrients in the herbaceous plants of mid-forest spring niches. Baltcic For. 2017, 23, 384–393. [Google Scholar]
- Available online: https://pl.climate-data.org/europa/polska/lubusz-voivodeship/krzesniczka-448942/ (accessed on 2 October 2024).
- Urban, G. Klimat Zielonej Góry; IMGW-PIB: Warszawa, Poland, 2020; pp. 25–36. (In Polish) [Google Scholar]
- Ostrowska, A.; Porębska, G. Skład Chemiczny Roślin Jego Interpretacja i Wykorzystanie w Ochronie Środowiska; Instytut Ochrony Środowiska: Warsaw, Poland, 2002. (In Polish) [Google Scholar]
- Falkowski, M.; Kukułka, I.; Kozłowski, S. Chemical Properties of Meadow Plants; Agriculture University of Poznań: Poznań, Poland, 2000. [Google Scholar]
- Krasicka-Korczyńska, E.; Dembek, R.; Korczyński, M.; Stosik, T. Natural and fodder values of community with Carex nigra in the Bydgoszcz Canal Valley. Acta Sci. Pol. Agric. 2014, 134, 77–91. [Google Scholar]
- Juknevičius, S.; Sabienė, N. The content of mineral elements in some grasses and legumes. Ekologija 2007, 53, 44–52. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 10 October 2024).
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research, R Package Version 1.3-7. 2023. Available online: https://cran.r-project.org/web/packages/agricolae/agricolae.pdf (accessed on 10 October 2024).
- Hammer, Ø. PAST: PAleontological STatistics Version 4.02. Reference manual; Natural History Museum, University of Oslo: Oslo, Norway, 1999–2020; p. 280. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Meehan, M.A.; DeKeyser, E.S.; Sedivec, K.K.; Norland, J.E. Nutrition composition of Sprengel’s sedge Carex sprengelii. Can. J. Plant Sci. 2012, 92, 867–871. [Google Scholar] [CrossRef]
- Alldrege, M.W.; Peek, J.M.; Wall, W.A. Nutritional quality of forages used by elk in Northern Idaho. J. Rang. Manag. 2002, 55, 253–259. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soil and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Kovaces, S.; Kutasy, E.; Csajbok, J. The Multiple Role of Silicon Nutrition in Alleviating Environmental Stresses in Sustainable Crop Production. Plants 2022, 11, 1223. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Mur, L.A.J.; Ruan, J.; Shen, Q.; Shiwe, G. Functions of silicon in plant drought stress responses. Hortic. Res. 2021, 8, 254. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon Uptake and Accumulation in Higher Plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Gralak, M.A.; Bates, D.L.; von Keyserlingk, M.A.G.; Fisher, L.J. Influence of species, cultivar and cut on the microelement content of grass forages. Slovak. J. Anim. Sci. 2006, 39, 84–88. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Biogeochemistry Trace Elements (Pol); Polish Scientific Publishers PWN: Warsaw, Poland, 1993. [Google Scholar]
- Krzywy, E. Nutrition of Plants. West Pomeranian; University of Technology Szczecin Press: Szczecin, Poland, 2007; p. 178. [Google Scholar]
- Samecka-Cymerman, A.; Kempers, A.J. Concentrations of heavy metals and plant nutrients in water, sediments and aquatic macrophytes of anthropogenic lakes (former open cut brown coal mines) differing in stage of acidification. Sci. Tot. Environ. 2001, 281, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Janyszek, M. A Carpological Monograph of Central European Species of the Carex L. (Cyperaceae) Genus; Rozprawy Naukowe; Wydawnictwo UP w Poznaniu: Poznań, Poland, 2014; p. 238. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants III; Academic Press: London, UK, 2011. [Google Scholar]
- Pirhofer-Walzl, K.; Søegaard, K.; Høgh-Jensen, H.; Eriksen, J.; Sanderson, M.A.; Rasmusen, J.; Rasmussen, J. Forage herbs improve mineral composition of grassland herbage. Grass Forage Sci. 2011, 66, 415–423. [Google Scholar] [CrossRef]
- Metson, A.J.; Gibson, E.J.; Hunt, J.L.; Sauders, W.M.H. Seasonal variations in chemical composition of pasture. N. Zealand J. Agric. Res. 1979, 22, 309–318. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Olgun, A. Fatty acid composition in leaf lipids of some Carex L. species from Northeast Anatolia (Turkey). Grasas Y Aceites 2000, 51, 307–310. [Google Scholar] [CrossRef]
- Bogucka-Kocka, A.; Janyszek, M. Fatty acids composition of fruits of selected Central European sedges, Carex L. (Cyperaceae). Grasas Y Aceites 2010, 61, 165–170. [Google Scholar] [CrossRef]
- Van de Staaij, J.; De Bakker, N.V.J.; Oosthoek, A.; Broekman, R.; Van Beem, A.; Stroetenga, M.; Aerts, R.; Rozema, J. Flavonoid concentrations in three grass species and a sedge grown in the field and under controlled environment conditions in response to enhanced UV-B radiation. J. Photochem. Photobiol. 2002, 66, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Bogucka-Kocka, A.; Szewczyk, K.; Janyszek, M.; Janyszek, S.; Cieśla, Ł. RP-HPLC Analysis of Phenolic Acids of Selected Central European Carex L. (Cyperaceae) Species and Its Implication for Taxonomy. J. AOAC Int. 2011, 94, 9–16. [Google Scholar] [CrossRef]
- Rajak, P.; Ghosh, A. RP-HPLC based analysis of different polyphenols in seven species of Carex L. (Cyperaceae Juss.) from West Bengal, India. Biodiversitas 2022, 23, 2329–2341. [Google Scholar] [CrossRef]
- Arraki, K.; Richard, T.; Badac, A.; Pedrat, E.; Bisson, J.; Waffo-Teguo, P.; Mahjaub, A.; Merrillon, J.M.; Decendit, A. Isolation, characterisation and quantification of stilbenes from some Carex species. Rec. Nat. Prod. 2013, 4, 281–291. [Google Scholar]
- David, C.; Hohmann, J.; Vasas, A. Chemistry and Pharmacology of Cyperaceae Stilbenoids: A Review. Molecules 2021, 10, 2794. [Google Scholar] [CrossRef] [PubMed]
- Dávid, C.Z.; Kúsz, N.; Agbadua, O.G.; Berkecz, R.; Kincses, A.; Spengler, G.; Hunyadi, A.; Hohmann, J.; Vasas, A. Phytochemical Investigation of Carex praecox Schreb. and ACE-Inhibitory Activity of Oligomer Stilbenes of the Plant. Molecules 2024, 29, 3427. [Google Scholar] [CrossRef] [PubMed]
- Tříska, J.; Vrchotová, N.; Horník, Š.; Sýkora, J.; Kučerová, A. Stilbenes in Carex acuta and Carex lepidocarpa. Molecules 2024, 29, 3840. [Google Scholar] [CrossRef]




| Species | Carbon (C) | Nitrogen (N) | C−N | |||
|---|---|---|---|---|---|---|
| [g·kg−1 DM] | ||||||
| May | June | May | June | May | June | |
| C. spicata | 434.00 ab ± 6.00 | 440.00 bcd ± 2.00 | 18.88 a ± 1.06 | 19.48 a ± 0.90 | 23.03 d ± 1.14 | 22.62 cd ± 1.50 |
| C. muricata | 426.33 bc ± 4.93 | 435.67 cd ± 4.04 | 19.47 a ± 0.76 | 20.58 a ± 0.57 | 21.93 d ± 0.95 | 21.19 d ± 0.74 |
| C. hirta | 418.33 c ± 1.53 | 424.00 e ± 4.58 | 14.47 b ± 0.88 | 15.93 bc ± 1.89 | 28.99 bc ± 1.72 | 26.83 bc ± 2.72 |
| C. disticha | 439.33 a ± 5.86 | 460.67 a ± 6.66 | 17.70 a ± 0.34 | 18.06 ab ± 0.06 | 24.83 cd ± 0.79 | 25.50 bcd ± 0.33 |
| C. praecox | 436.33 ab ± 2.52 | 446.33 bc ± 2.52 | 9.77 c ± 0.52 | 10.46 d ± 0.73 | 44.76 a ± 2.27 | 42.82 a ± 2.90 |
| C. sylvatica | 434.00 ab ± 5.00 | 448.33 b ± 3.21 | 13.42 b ± 1.07 | 15.22 c ± 0.75 | 32.48 b ± 2.77 | 29.50 b ± 1.50 |
| C. remota | 420.00 c ± 2.65 | 433.33 de ± 3.05 | 14.06 b ± 0.95 | 15.00 c ± 1.00 | 29.98 b ± 2.21 | 28.98 b ± 1.79 |
| Species | C | N | C−N |
|---|---|---|---|
| [g·kg−1 DM] | |||
| Mean ± SD | |||
| C. spicata | 437.00 abc ± 5.18 | 19.18 ab ± 0.94 | 22.82 de ± 0.98 |
| C. muricata | 431.00 bcd ± 6.51 | 20.02 a ± 0.85 | 21.56 e ± 0.86 |
| C. hirta | 421.17 d ± 4.35 | 15.20 c ± 1.54 | 27.91 bc ± 2.35 |
| C. disticha | 450.00 a ± 12.96 | 17.88 b ± 0.29 | 25.17 cd ± 0.65 |
| C. praecox | 441.33 ab ± 5.92 | 10.11 d ± 0.68 | 43.79 a ± 2.56 |
| C. sylvatica | 441.17 ab ± 8.70 | 14.32 c ± 1.29 | 30.99 b ± 2.57 |
| C. remota | 426.67 cd ± 7.74 | 14.53 c ± 1.01 | 29.48 b ± 1.88 |
| Optimal Value | K−Na | K−Ca+Mg | Ca−P | |||
|---|---|---|---|---|---|---|
| Species | May | June | May | June | May | June |
| C. spicata | 10.3:1 | 12.8:1 | 3.8 | 4.8 | 1.4:1 | 1.2:1 |
| C. muricata | 5.6:1 | 6.3:1 | 2.7 | 3.2 | 2.3:1 | 2.3:1 |
| C. hirta | 8.7:1 | 10.3:1 | 2.8 | 3.1 | 3.2:1 | 5.6:1 |
| C. disticha | 2.6:1 | 2.4:1 | 1.3 | 1.4 | 1.7:1 | 1.4:1 |
| C. praecox | 6.1:1 | 7.1:1 | 1.8 | 1.8 | 4.4:1 | 6.5:1 |
| C. sylvatica | 14.5:1 | 16.8:1 | 2.9 | 3.3 | 2.7:1 | 2.6:1 |
| C. remota | 16.6:1 | 26.4:1 | 3.6 | 4.4 | 2.2:1 | 2.1:1 |
| Species | P (2.8–3.6) | K (17.0) | Ca (7.0–10) | Mg (2.0–3.0) | Na (1.5–2.5) | Si (9.0) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [g·kg−1 DM] | ||||||||||||
| May | June | May | June | May | June | May | June | May | June | May | June | |
| C. spicata | 4.17 a ± 0.07 | 3.72 a ± 0.16 | 30.38 b ± 1.09 | 27.71 b ± 0.17 | 5.66 d ± 0.25 | 4.62 c ± 0.22 | 2.34 bc ± 0.48 | 1.11 e ± 0.02 | 2.95 c ± 0.07 | 2.17 e ± 0.05 | 5.10 ab ± 0.17 | 5.53 c ± 0.25 |
| * | * | ** | * | *** | si | |||||||
| C. muricata | 3.20 bc ± 0.22 | 2.67 c ± 0.11 | 27.74 cd ± 0.55 | 26.08 bc ± 0.94 | 7.47 bc ± 0.83 | 6.21 b ± 0.21 | 2.73 b ± 0.07 | 1.85 b ± 0.06 | 4.92 a ± 0.12 | 4.12 b ± 0.04 | 5.70 a ± 0.61 | 6.13 bc ± 0.15 |
| * | si | si | *** | *** | si | |||||||
| C. hirta | 2.10 d ± 0.19 | 1.13 d ± 0.13 | 25.63 d ± 1.18 | 24.91 c ± 1.12 | 6.82 bcd ± 0.21 | 6.28 b ± 0.35 | 2.21 bc ± 0.23 | 1.35 d ± 0.09 | 2.93 c ± 0.02 | 2.43 d ± 0.08 | 5.67 a ± 0.58 | 7.00 a ± 0.20 |
| ** | si | si | ** | *** | * | |||||||
| C. disticha | 3.61 ab ± 0.24 | 3.19 b ± 0.08 | 12.81 f ± 0.23 | 10.52 e ± 0.50 | 6.02 cd ± 0.58 | 4.56 c ± 0.21 | 3.69 a ± 0.25 | 3.18 a ± 0.06 | 4.91 a ± 0.16 | 4.40 a ± 0.08 | 5.10 ab ± 0.17 | 6.63 ab ± 0.60 |
| * | ** | * | * | ** | * | |||||||
| C. praecox | 2.27 d ± 0.25 | 1.41 d ± 0.04 | 21.94 e ± 0.34 | 19.93 d ± 0.06 | 9.90 a ± 0.08 | 9.15 a ± 0.57 | 2.27 bc ± 0.25 | 1.59 c ± 0.03 | 3.62 b ± 0.29 | 2.80 c ± 0.10 | 6.07 a ± 0.11 | 7.00 a ± 0.17 |
| ** | *** | si | ** | * | ** | |||||||
| C. sylvatica | 2.97 c ± 0.24 | 2.58 c ± 0.21 | 28.11 c ± 0.84 | 27.71 b ± 1.57 | 8.14 b ± 0.95 | 6.73 b ± 0.40 | 1.65 c ± 0.31 | 1.41 cd ± 0.15 | 1.94 d ± 0.22 | 1.65 f ± 0.10 | 5.94 a ± 0.21 | 6.77 ab ± 0.21 |
| si | si | si | si | si | ** | |||||||
| C. remota | 3.28 bc ± 0.30 | 2.51 c ± 0.13 | 39.58 a ± 0.53 | 36.48 a ± 0.37 | 7.25 bcd ± 0.66 | 5.19 c ± 0.10 | 3.83 a ± 0.38 | 3.11 a ± 0.02 | 2.38 cd ± 0.36 | 1.38 g ± 0.05 | 4.60 b ± 0.35 | 5.53 c ± 0.23 |
| * | ** | ** | * | ** | * | |||||||
| Species | Cu (10) | Zn (50) | Cr (0.3–5.0) | Ni (0.1–5.0) | Fe (30) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| [mg·kg−1 DM] | ||||||||||
| May | June | May | June | May | June | May | June | May | June | |
| C. spicata | 6.68 b ± 0.37 | 5.68 d ± 0.18 | 33.00 b ± 0.17 | 29.36 b ± 0.64 | 1.22 bcd ± 0.19 | 1.01 c ± 0.02 | 1.50 bc ± 0.42 | 1.28 b ± 0.04 | 18.80 cd ± 0.89 | 15.53 c ± 0.06 |
| * | *** | si | si | ** | ||||||
| C. muricata | 7.13 b ± 0.55 | 5.66 d ± 0.05 | 33.69 b ± 3.66 | 29.35 b ± 1.39 | 1.48 abc ± 0.47 | 1.12 bc ± 0.03 | 1.90 ab ± 0.22 | 1.34 b ± 0.05 | 26.22 b ± 2.43 | 22.88 b ± 0.15 |
| * | si | si | * | si | ||||||
| C. hirta | 9.72 a ± 0.34 | 9.32 a ± 0.42 | 57.18 a ± 4.50 | 52.80 a ± 1.42 | 0.96 cd ± 0.06 | 0.79 d ± 0.10 | 2.76 a ± 0.51 | 2.68 a ± 0.56 | 19.82 c ± 0.15 | 17.66 c ± 0.06 |
| si | si | si | si | *** | ||||||
| C. disticha | 6.70 b ± 0.36 | 6.70 c ± 0.36 | 28.10 bc ± 0.95 | 26.10 c ± 0.95 | 0.80 d ± 0.16 | 0.62 d ± 0.05 | 0.10 d ± 0.06 | 0.05 c ± 0.00 | 15.41 d ± 0.70 | 13.17 d ± 0.06 |
| si | si | si | si | ** | ||||||
| C. pracox | 6.76 b ± 0.25 | 5.62 d ± 0.11 | 21.18 d ± 1.24 | 18.50 e ± 0.89 | 1.29 bcd ± 0.06 | 1.24 b ± 0.05 | 1.57 bc ± 0.37 | 1.33 b ± 0.04 | 15.95 cd ± 0.66 | 12.85 d ± 0.22 |
| ** | * | si | si | ** | ||||||
| C. sylvatica | 8.62 a ± 0.33 | 8.37 b ± 0.40 | 25.93 cd ± 1.09 | 23.73 cd ± 1.10 | 1.76 ab ± 0.15 | 1.63 a ± 0.12 | 1.74 bc ± 0.43 | 1.65 b ± 0.09 | 14.83 d ± 1.91 | 12.03 d ± 0.20 |
| si | si | si | si | si | ||||||
| C. remota | 7.50 b ± 0.49 | 5.83 d ± 0.29 | 23.83 cd ± 1.36 | 22.13 d ± 1.20 | 1.90 a ± 0.13 | 1.64 a ± 0.07 | 0.78 cd ± 0.21 | 0.61 c ± 0.03 | 86.76 a ± 1.98 | 85.20 a ± 1.99 |
| ** | si | * | si | si | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janyszek-Sołtysiak, M.; Murawski, M.; Majchrzak, L.; Waliszewska, B. Lowland Sedge Meadows as a Potential Source of Macro and Micronutrient Supplementation. Agronomy 2025, 15, 539. https://doi.org/10.3390/agronomy15030539
Janyszek-Sołtysiak M, Murawski M, Majchrzak L, Waliszewska B. Lowland Sedge Meadows as a Potential Source of Macro and Micronutrient Supplementation. Agronomy. 2025; 15(3):539. https://doi.org/10.3390/agronomy15030539
Chicago/Turabian StyleJanyszek-Sołtysiak, Magdalena, Maciej Murawski, Leszek Majchrzak, and Bogusława Waliszewska. 2025. "Lowland Sedge Meadows as a Potential Source of Macro and Micronutrient Supplementation" Agronomy 15, no. 3: 539. https://doi.org/10.3390/agronomy15030539
APA StyleJanyszek-Sołtysiak, M., Murawski, M., Majchrzak, L., & Waliszewska, B. (2025). Lowland Sedge Meadows as a Potential Source of Macro and Micronutrient Supplementation. Agronomy, 15(3), 539. https://doi.org/10.3390/agronomy15030539






