The Influence of Planting Method and Short-Term Organic Amendments on Rhizosphere Microbial Communities in Paddies: Preliminary Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Soil Sample Collection
2.3. Analyses of Physicochemical Parameters, Enzyme Activities, and Microbial Biomass
2.4. DNA Sequencing
2.5. Statistical Analyses
3. Results
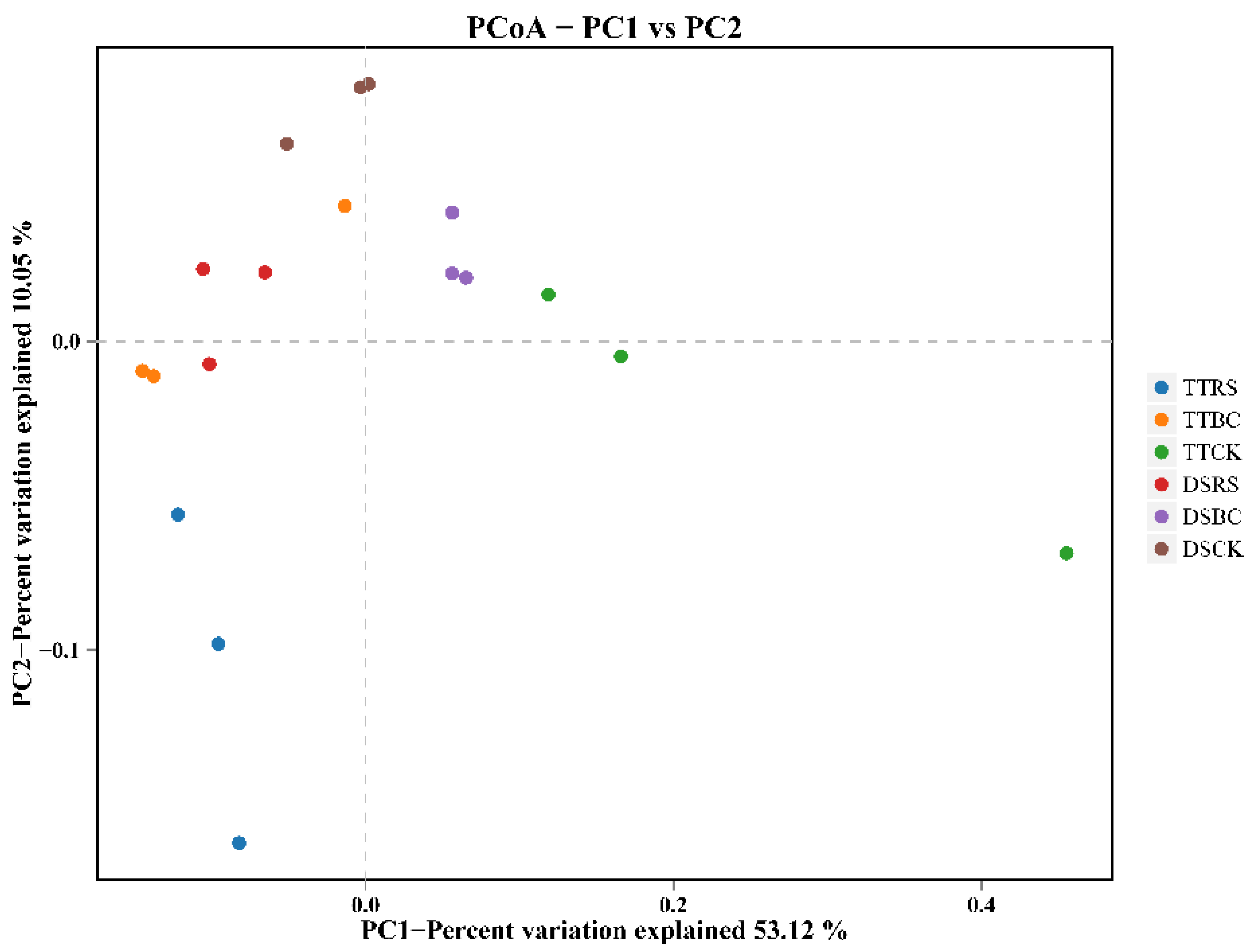
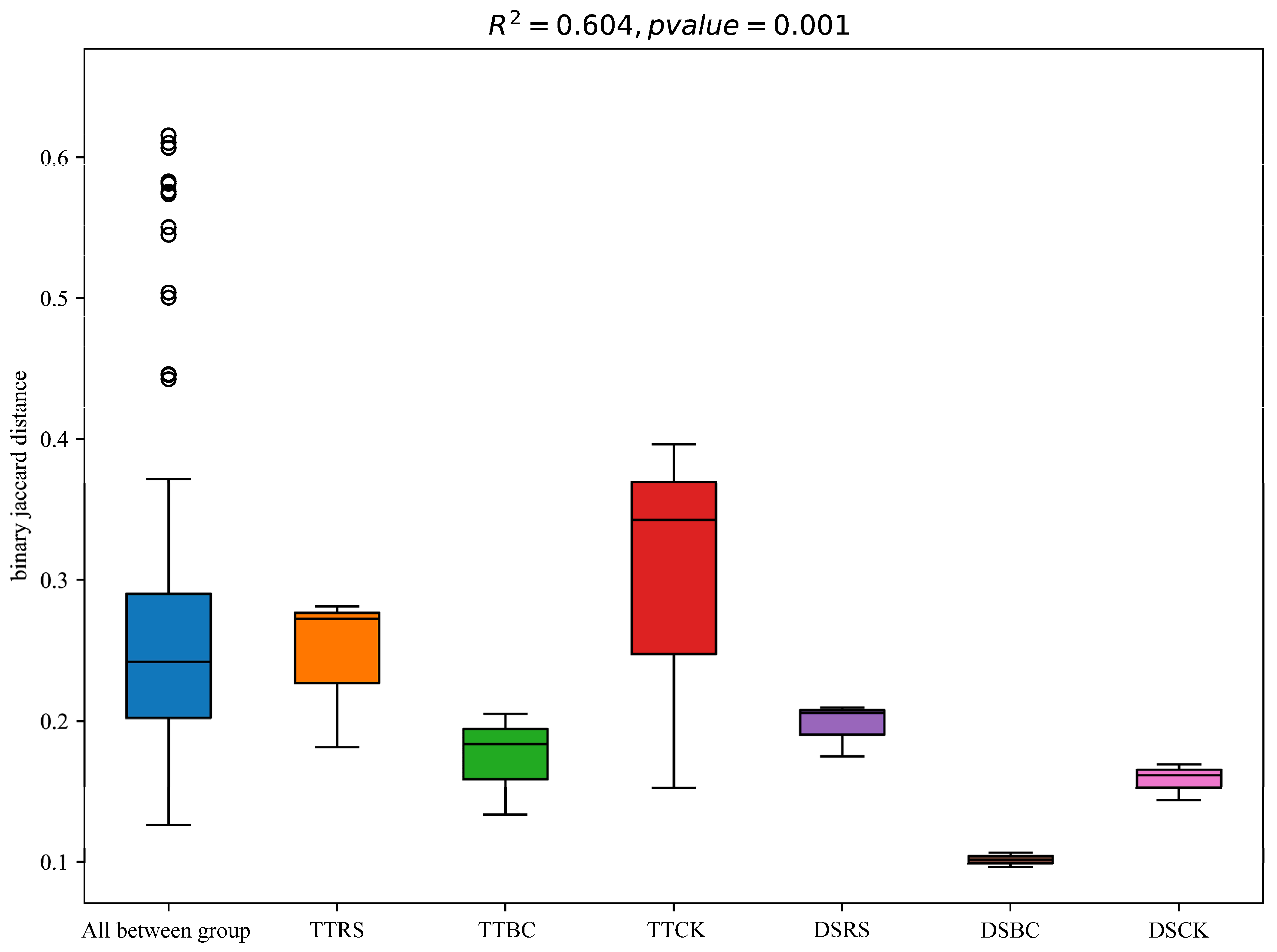
3.1. Microbial Community Diversity
3.2. Soil Bacterial Community Structure and Composition
3.3. Activities of Soil Enzymes and Microbial Biomass
3.4. Soil Physicochemical Properties
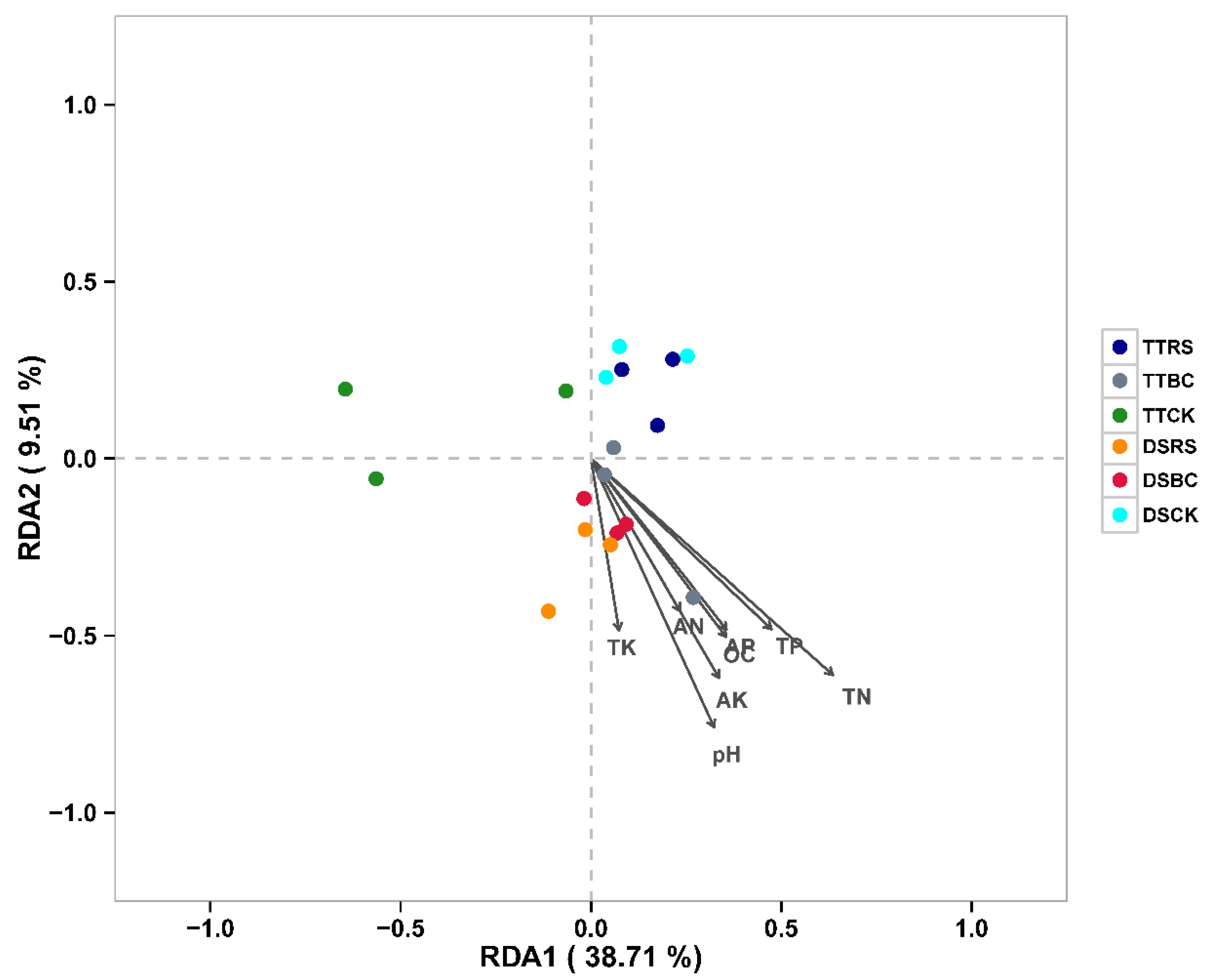
3.5. Associations Between Microbial Communities and Physiochemical Parameters in the Soil
3.6. Associations Between Enzyme Activities, Physicochemical Parameters, and Abundant Phyla
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Susilawati, H.L.; Kartikawati, R.; Setyanto, P. Response of planting methods to rice productivity and greenhouse gas emissions. IOP Conf. Ser. Earth Environ. Sci. 2021, 648, 012121. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, L.; He, N.; Liu, Z.; Ma, Z.; Fu, L.; Wang, H.; Wang, C.; Sui, G.; Zheng, W. Influence of planting methods and organic amendments on rice yield and bacterial communities in the rhizosphere soil. Front. Microbiol. 2022, 13, 918986. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.L.; Xu, F.F.; Han, C.; Qiu, S.; Ge, J.L.; Xu, J.; Zhang, H.C.; Wei, H.Y. Effects of planting methods on yield and quality of different types of japonica rice in northern Jiangsu plain, China. J. Integr. Agric. 2018, 17, 2624–2635. [Google Scholar] [CrossRef]
- Yan, S.; Liu, C.; Li, J.; Li, J.; Cui, C.; Fan, J.; Gong, Z.; Zhang, Z.; Yan, C. Changes in the Soil Phosphorus Supply with Rice Straw Return in Cold Region. Agronomy 2023, 13, 2214. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.X.; Zhang, N.; Hu, B.; Jin, T.; Xu, H.; Qin, Y.; Yan, P.; Zhang, X.; Guo, X.; et al. NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 2019, 37, 676–684. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Tang, Z.; He, N.; Zhang, L.; Wang, L.; Gong, D.; Wang, C.; Wang, H.; Sui, G.; Zheng, W. Straw and Biochar Application Alters the Structure of Rhizosphere Microbial Communities in Direct-Seeded Rice (Oryza sativa L.) Paddies. Agronomy 2024, 14, 316. [Google Scholar] [CrossRef]
- Kumar, D.S.; Kumar, G.G.; Ravikant, A.; Choudhury, B.U.; Mishra, V.K.; Kundu, M.C.; Roy, A.; Mondal, T.; Lama, A.; Dhakre, D.S. Organic nutrient sources and biochar technology on microbial biomass carbon and soil enzyme activity in maize-black gram cropping system. Biomass Conv. Bioref. 2023, 13, 9277–9287. [Google Scholar]
- Zhao, J.; Ni, T.; Xun, W.; Huang, X.; Huang, Q.; Ran, W.; Shen, B.; Zhang, R.; Shen, Q. Influence of straw incorporation with and without straw decomposer on soil bacterial community structure and function in a rice-wheat cropping system. Appl. Microbiol. Biotechnol. 2017, 101, 4761–4773. [Google Scholar] [CrossRef]
- Wang, P.; Kong, X.; Chen, H.; Xiao, Y.; Liu, H.; Li, X.; Zhang, Z.; Tan, X.; Wang, D.; Jin, D.; et al. Exploration of Intrinsic Microbial Community Modulators in the Rice Endosphere Indicates a Key Role of Distinct Bacterial Taxa Across Different Cultivars. Front. Microbiol. 2021, 12, 629852. [Google Scholar] [CrossRef]
- Zhong, Y.; Hu, J.; Xia, Q.; Zhang, S.; Li, X.; Pan, X.; Zhao, R.; Wang, R.; Yan, W.; Shangguan, Z. Soil microbial mechanisms promoting ultrahigh rice yield. Soil Biol. Biochem. 2020, 143, 107741. [Google Scholar] [CrossRef]
- Novak, J.M.; Cantrell, K.B.; Watts, D.W.; Busscher, W.J.; Johnson, M.G. Designing relevant biochars as soil amendments using lignocellulosic based and manure-based feedstocks. Soils Sediments 2014, 14, 330–343. [Google Scholar] [CrossRef]
- Jia, R.; Qu, Z.; You, P.; Qu, D. Effect of biochar on photosynthetic microorganism growth and iron cycling in paddy soil under different phosphate levels. Sci. Total Environ. 2017, 612, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Han, X.; Li, Y.; Yang, J.; Li, N.; An, N. Effects of Biochar and Straw Application on the Physicochemical and Biological Properties of Paddy Soils in Northeast China. Sci. Rep. 2019, 9, 16531. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Guan, S.; Zhang, D.; Zhang, Z. Soil Enzyme and Its Research Methods; China Agriculture Press: Beijing, China, 1986. [Google Scholar]
- Lu, R.K. Soil Agricultural Chemical Analysis Method; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Littell, R.; Milliken, G.; Stroup, W.; Wolfinger, R.; Schabenberger, O. SAS for Mixed Models, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2006. [Google Scholar]
- The R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Yang, H.S.; Fang, C.; Meng, Y.; Dai, Y.J.; Liu, J. Long-term ditch-buried straw return increases the functionality of soil microbial communities. Catena 2021, 202, 105316. [Google Scholar] [CrossRef]
- Wu, L.P.; Ma, H.; Zhao, Q.L.; Zhang, S.R.; Wei, W.L.; Ding, X.D. Changes in soil bacterial community and enzyme activity under five years straw returning in paddy soil. Eur. J. Soil Biol. 2020, 100, 103215. [Google Scholar] [CrossRef]
- Bu, R.Y.; Ren, T.; Lei, M.J.; Liu, B.; Li, X.K.; Cong, R.H. Tillage and straw-returning practices effect on soil dissolved organic matter, aggregate fraction and bacteria community under a rice-rice-rapeseed rotation system. Agric. Ecosyst. Environ. 2020, 287, 106681. [Google Scholar] [CrossRef]
- Win, K.T.; Okazaki, K.; Ohkama-Ohtsu, N.; Yokoyama, T.; Ohwaki, Y. Short-term effects of biochar and Bacillus pumilus TUAT-1 on the growth of forage rice and its associated soil microbial community and soil properties. Biol. Fertil. Soils 2020, 56, 481–497. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, R.; Xue, C.; Xun, W.; Sun, L.; Xu, Y.; Shen, Q. Pyrosequencing Reveals Contrasting Soil Bacterial Diversity and Community Structure of Two Main Winter Wheat Cropping Systems in China. Microb. Ecol. 2013, 67, 443–453. [Google Scholar] [CrossRef]
- Mao, Q.; Hu, B.; Agathokleous, E.; Wang, L.; Koike, T.; Ma, M.; Rennenberg, H. Biochar application improves karstic lime soil physicochemical properties and enzymes activity and enhances sweet tea seedlings physiological performance. Sci. Total Environ. 2022, 830, 154815. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, L.; He, N.; Gong, D.; Gao, H.; Ma, Z.; Fu, L.; Zhao, M.; Wang, H.; Wang, C.; et al. Soil bacterial community as impacted by the addition of rice straw and biochar. Sci. Rep. 2021, 11, 22185. [Google Scholar] [CrossRef]
- Zhao, S.C.; Qiu, S.J.; Xu, X.P.; Ciampitti, I.A.; Zhang, S.Q.; He, P. Change in straw decomposition rate and soil microbial community composition after straw addition in different long-term fertilization soils. Appl. Soil Ecol. 2019, 138, 123–133. [Google Scholar] [CrossRef]
- Sun, W.M.; Xiao, E.Z.; Pu, Z.L.; Krumins, V.; Dong, Y.R.; Li, B.Q. Paddy soil microbial communities driven by environment- and microbe-microbe interactions: A case study of elevation-resolved microbial communities in a rice terrace. Sci. Total Environ. 2018, 612, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.H.; Chen, X.B.; Zheng, X.D.; Deng, S.H.; Hu, Y.J.; Zheng, S.M. Preferential uptake of hydrophilic and hydrophobic compounds by bacteria and fungi in upland and paddy soils. Soil Biol. Biochem. 2020, 148, 107879. [Google Scholar] [CrossRef]
- Ahn, J.H.; Song, J.; Kim, B.Y.; Kim, M.S.; Joa, J.H.; Weon, H.Y. Characterization of the Bacterial and Archaeal Communities in Rice Field Soils Subjected to Long-Term Fertilization Practices. J. Microbiol. 2012, 50, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.J.; You, J.H.; Zhu, C.; Wang, B.L.; Qu, D. Diversity, dynamic and abundance of Geobacteraceae species in paddy soil following slurry incubation. Eur. J. Soil Biol. 2013, 56, 11–18. [Google Scholar] [CrossRef]
- Wisawapipat, W.; Chooaiem, N.; Aramrak, S.; Chittamart, N.; Nookabkaew, S.; Rangkadilok, N. Sulfur amendments to soil decrease inorganic arsenic accumulation in rice grain under flooded and nonflooded conditions: Insights from temporal dynamics of porewater chemistry and solid-phase arsenic solubility. Sci. Total Environ. 2021, 779, 146352. [Google Scholar] [CrossRef]
- Raio, A.; Puopolo, G. Pseudomonas chlororaphis metabolites as biocontrol promoters of plant health and improved crop yield. World J. Microbiol. Biotechnol. 2021, 37, 99. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Cao, P.; Gao, Z.; Chen, A.J.; Han, J. Microbial Community Changes in the Rhizosphere Soil of Healthy and Rusty Panax Ginseng and Discovery of Pivotal Fungal Genera Associated with Rusty Roots. Biomed. Res. Int. 2020, 2020, 8018525. [Google Scholar] [CrossRef]
- Wang, E.; Lin, X.; Tian, L.; Wang, X.; Ji, L.; Jin, F.; Tian, C. Effects of Short-Term Rice Straw Return on the Soil Microbial Community. Agriculture 2021, 11, 561. [Google Scholar] [CrossRef]
- Wolinska, A.; Kuzniar, A.; Zielenkiewicz, U.; Izak, D.; Szafranek-Nakonieczna, A.; Banach, A. Bacteroidetes as a sensitive biological indicator of agricultural soil usage revealed by a culture-independent approach. Appl. Soil Ecol. 2017, 119, 128–137. [Google Scholar] [CrossRef]
- Yuan, C.L.; Na, S.; Li, F.B.; Hu, H.W. Impact of sulphate and iron oxide on bacterial community dynamics in paddy soil under alternate watering conditions. J. Hazard. Mater. 2021, 408, 124417. [Google Scholar] [CrossRef]
- Mathew, R.P.; Feng, Y.; Githinji, L.; Ankumah, R.; Balkcom, K.S. Impact of No-tillage and conventional tillage on soil microbial communities. Appl. Environ. Soil Sci. 2012, 2012, 548620. [Google Scholar] [CrossRef]
- Ren, H.; Guo, H.; Shafiqul Islam, M.; Zaki, H.E.M.; Wang, Z.; Wang, H.; Qi, X.; Guo, J.; Sun, L.; Wang, Q.; et al. Improvement effect of biochar on soil microbial community structure and metabolites of decline disease bayberry. Front. Microbiol. 2023, 14, 1154886. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Xu, X.; Qiu, X.; Zhang, J. Biochar influences the succession of microbial communities and the metabolic functions during rice straw composting with pig manure. Bioresour. Technol. 2019, 272, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Renella, G.; Puglisi, E.; Ceccanti, B.; Masciandaro, G.; Fornasier, F.; Moscatelli, M.; Marinari, S. Soil enzymology: Classical and molecular approaches. Biol. Fertil. Soils 2012, 48, 743–762. [Google Scholar] [CrossRef]
- Hansen, V.; Müller-Stöver, D.; Munkholm, L.J.; Peltre, C.; Hauggaard-Nielsen, H.; Jensen, L.S. The effect of straw and wood gasification biochar on carbon sequestration, selected soil fertility indicators and functional groups in soil: An incubation study. Geoderma 2016, 269, 99–107. [Google Scholar] [CrossRef]
- Singh, N.S.; Mukherjee, I.; Das, S.K.; Varghese, E. Leaching of clothianidin in two different Indian soils: Effect of the organic amendment. Bull. Environ. Contam. Toxicol. 2018, 100, 553–559. [Google Scholar] [CrossRef]
- Das, S.K.; Avasthe, R.K.; Singh, M.; Yadav, A. Soil health improvement using biochar application in Sikkim: A success story. Innov. Farming 2018, 3, 48–50. [Google Scholar]
- Das, S.K.; Ghosh, G.K. Soil hydro-physical environment as influenced by different biochar amendments. Int. J. Bio-Resour. Stress Manag. 2017, 8, 668–673. [Google Scholar] [CrossRef]
- Hagemann, N.; Joseph, S.; Schmidt, H.P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.; Elliott, K.W.; et al. The organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat. Commun. 2017, 8, 1089. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and Soil Physical Properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Ali, I.; Yuan, P.; Ullah, S.; Iqbal, A.; Zhao, Q.; Liang, H.; Khan, A.; Imran; Zhang, H.; Wu, X.; et al. Biochar Amendment and Nitrogen Fertilizer Contribute to the Changes in Soil Properties and Microbial Communities in a Paddy Field. Front. Microbiol. 2022, 13, 834751. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Chang Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Takaichi, S.; Maoka, M.; Takasaki, K.; Hanada, S. Carotenoids of Gemmatimonas aurantiaca (Gemmatimonadetes): Identification of a novel carotenoid, deoxyoscillol 2-rhamnoside, and proposed biosynthetic pathway of oscillol 2, 29-dirhamnoside. Microbiology 2010, 156, 757–763. [Google Scholar] [CrossRef]

| Planting Methods | Organic Amendments | ACE Index | Chao 1 Index | Simpson Index | Shannon Index |
|---|---|---|---|---|---|
| Transplanting (TT) | RS | 1333 | 1366 | 0.99 | 8.14 |
| BC | 1396 | 1411 | 0.994 | 8.75 | |
| CK | 1332 | 1355 | 0.977 | 7.34 | |
| Direct seeding (DS) | RS | 1434 | 1459 | 0.994 | 8.78 |
| BC | 1629 | 1637 | 0.995 | 8.88 | |
| CK | 1556 | 1571 | 0.993 | 8.56 | |
| LSD(0.05) | 91.8 | 120.9 | <0.01 | 0.65 | |
| Planting methods | |||||
| TT | 1354 ± 41.2 b | 1377 ± 43.9 b | 0.987 ± 0.01 | 8.07 ± 0.67 b | |
| DS | 1539 ± 88.8 a | 1556 ± 83.7 a | 0.994 ± 0.01 | 8.74 ± 0.15 a | |
| Organic amendments | |||||
| RS | 1383 ± 60.6 c | 1413 ± 60.5 c | 0.992 ± 0.002 a | 8.45 ± 0.37 b | |
| BC | 1512 ± 129.4 a | 1524 ± 128.1 a | 0.995 ± 0.001 a | 8.81 ± 0.12 a | |
| CK | 1444 ± 126.5 b | 1463 ± 124.0 b | 0.985 ± 0.010 b | 7.95 ± 0.74 c | |
| Analysis of variance | |||||
| Planting methods (PM) | * | * | NS | * | |
| Organic amendments (OA) | ** | ** | ** | ** | |
| PM × OA | ** | ** | * | ** |
| Planting Methods | Organic Amendments | Microbial Biomass N (mg kg−1) | Microbial Biomass C (g kg−1) | Urease Activity (mg g−1day−1) | Cellulase Activity (mg g−1day−1) |
|---|---|---|---|---|---|
| Transplanting (TT) | RS | 132.9 | 1.4 | 8.4 | 1.2 |
| BC | 134.9 | 1.4 | 8.6 | 1.1 | |
| CK | 129.1 | 1.3 | 7.9 | 1.0 | |
| Direct seeding (DS) | RS | 138.5 | 1.4 | 8.1 | 1.3 |
| BC | 141.4 | 1.4 | 8.2 | 1.1 | |
| CK | 134.9 | 1.3 | 7.9 | 1.1 | |
| LSD(0.05) | 1.63 | <0.01 | 0.1 | 0.02 | |
| Planting methods | |||||
| TT | 132.3 ± 2.64 b | 1.36 ± 0.02 b | 8.3 ± 0.31 a | 1.1 ± 0.08 b | |
| DS | 138.3 ± 2.86 a | 1.38 ± 0.03 a | 8.1 ± 0.11 b | 1.6 ± 0.08 a | |
| Organic amendments | |||||
| RS | 135.7 ± 3.12 b | 1.38 ± 0.01 b | 8.3 ± 0.19 b | 1.23 ± 0.03 a | |
| BC | 138.1 ± 3.63 a | 1.39 ± 0.01 a | 8.4 ± 0.24 a | 1.10 ± 0.01 b | |
| CK | 132.0 ± 3.23 b | 1.34 ± 0.01 c | 7.9 ± 0.01 c | 1.06 ± 0.03 c | |
| Analysis of variance | |||||
| Planting methods (PM) | ** | ** | ** | ** | |
| Organic amendments (OA) | ** | ** | ** | ** | |
| PM × OA | NS | ** | ** | NS |
| Planting Methods | Organic Amendments | pH | OC (g kg−1) | TN (g kg−1) | TP (g kg−1) | TK (g kg−1) | AN (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| Transplanting (TT) | RS | 5.8 | 13.1 | 1.36 | 1.29 | 22.5 | 127.3 | 34.4 | 136 |
| BC | 6.0 | 13.5 | 1.37 | 1.24 | 22.5 | 120.6 | 30.2 | 137.4 | |
| CK | 5.8 | 12.2 | 1.24 | 1.16 | 21.8 | 119.6 | 25.5 | 129.8 | |
| Direct seeding (DS) | RS | 5.9 | 13.4 | 1.39 | 1.35 | 22.5 | 130.7 | 33.8 | 140.1 |
| BC | 6.0 | 12.8 | 1.41 | 1.34 | 22.9 | 124.9 | 34.8 | 149.2 | |
| CK | 5.7 | 12.4 | 1.28 | 1.23 | 21.2 | 119.7 | 25.2 | 131.6 | |
| LSD(0.05) | 0.07 | 0.74 | 0.06 | 0.06 | 0.91 | 0.93 | 1.55 | 5.32 | |
| Planting methods | |||||||||
| TT | 5.8 ± 0.09 | 12.9 ± 0.69 | 1.36 ± 0.07 | 1.23 ± 0.06 b | 22.3 ± 0.42 | 122.5 ± 3.67 b | 31.3 ± 3.85 | 134.4 ± 3.85 b | |
| DS | 5.8 ± 0.10 | 12.9 ± 0.52 | 1.32 ± 0.06 | 1.31 ± 0.06 a | 22.2 ± 0.82 | 125.1 ± 4.78 a | 30.0 ± 4.59 | 140.3 ± 7.77 a | |
| Organic amendments | |||||||||
| RS | 5.8 ± 0.03 b | 13.2 ± 0.29 a | 1.37 ± 0.02 a | 1.32 ± 0.04 a | 22.5 ± 0.29 a | 129.0 ± 1.86 a | 34.1 ± 0.48 a | 138.1 ± 2.46 b | |
| BC | 6.0 ± 0.04 a | 13.2 ± 0.42 a | 1.39 ± 0.03 a | 1.29 ± 0.06 a | 22.7 ± 0.27 a | 122.8 ± 2.50 b | 32.5 ± 2.56 b | 143.3 ± 6.83 a | |
| CK | 5.7 ± 0.03 c | 12.2 ± 0.45 b | 1.26 ± 0.03 b | 1.20 ± 0.04 b | 21.5 ± 0.49 b | 119.6 ± 0.38 c | 25.4 ± 0.41 c | 130.7 ± 1.73 c | |
| Analysis of variance | |||||||||
| Planting methods (PM) | NS | NS | NS | * | NS | ** | NS | ** | |
| Organic amendments (OA) | ** | ** | ** | ** | ** | ** | ** | ** | |
| PM × OA | NS | NS | NS | NS | NS | ** | ** | ** |
| p H | OC | TN | TP | TK | AN | AK | AP | |
|---|---|---|---|---|---|---|---|---|
| MBN | 0.612 ** | 0.387 | 0.772 ** | 0.837 ** | 0.495 * | 0.496 * | 0.873 ** | 0.636 ** |
| MBC | 0.862 ** | 0.619 ** | 0.926 ** | 0.795 ** | 0.835 ** | 0.557 * | 0.935 ** | 0.860 ** |
| UA | 0.651 ** | 0.682 ** | 0.577 * | 0.245 | 0.565 * | 0.152 | 0.307 | 0.51 * |
| CA | 0.219 | 0.546 * | 0.591 ** | 0.753 ** | 0.38 | 0.937 ** | 0.349 | 0.734 ** |
| pH | OC | TN | TP | TK | AN | AK | AP | MBN | MBC | UA | CA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acidobacteria | 0.058 | 0.061 | 0.163 | 0.261 | 0.011 | 0.088 | 0.267 | 0.249 | 0.428 | 0.36 | 0.131 | 0.252 |
| Actinobacteria | 0.081 | 0.432 | 0.117 | 0.117 | −0.135 | −0.053 | 0.143 | −0.205 | 0.309 | 0.185 | 0.305 | 0.124 |
| Bacteroidetes | 0.145 | −0.131 | −0.025 | −0.183 | 0.009 | −0.251 | −0.02 | −0.135 | −0.059 | −0.094 | −0.307 | −0.387 |
| Chloroflexi | 0.384 | 0.641 ** | 0.37 | 0.335 | 0.042 | 0.23 | 0.385 | −0.051 | 0.54 * | 0.470 * | 0.275 | 0.35 |
| Firmicutes | −0.156 | −0.635 ** | −0.163 | −0.271 | −0.162 | −0.484 * | −0.168 | −0.263 | 0.022 | −0.193 | −0.692 ** | −0.534 * |
| Gemmatimonadetes | 0.207 | 0.595 ** | 0.299 | 0.492 * | 0.075 | 0.441 | 0.346 | 0.16 | 0.474 * | 0.383 | 0.249 | 0.585 * |
| Planctomycetes | −0.021 | 0.094 | 0.14 | 0.185 | 0.013 | 0.024 | 0.174 | 0.224 | 0.286 | 0.176 | 0.231 | 0.169 |
| Proteobacteria | 0.019 | −0.131 | −0.032 | −0.003 | 0.201 | 0.158 | −0.071 | 0.257 | −0.348 | −0.189 | 0.1 | 0.044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Tang, Z.; Wang, L.; Wen, L.; Liang, Y.; Wang, C.; Wang, H. The Influence of Planting Method and Short-Term Organic Amendments on Rhizosphere Microbial Communities in Paddies: Preliminary Results. Agronomy 2025, 15, 540. https://doi.org/10.3390/agronomy15030540
Liu Z, Tang Z, Wang L, Wen L, Liang Y, Wang C, Wang H. The Influence of Planting Method and Short-Term Organic Amendments on Rhizosphere Microbial Communities in Paddies: Preliminary Results. Agronomy. 2025; 15(3):540. https://doi.org/10.3390/agronomy15030540
Chicago/Turabian StyleLiu, Ziqi, Zhiqiang Tang, Lili Wang, Li Wen, Yi Liang, Changhua Wang, and Hui Wang. 2025. "The Influence of Planting Method and Short-Term Organic Amendments on Rhizosphere Microbial Communities in Paddies: Preliminary Results" Agronomy 15, no. 3: 540. https://doi.org/10.3390/agronomy15030540
APA StyleLiu, Z., Tang, Z., Wang, L., Wen, L., Liang, Y., Wang, C., & Wang, H. (2025). The Influence of Planting Method and Short-Term Organic Amendments on Rhizosphere Microbial Communities in Paddies: Preliminary Results. Agronomy, 15(3), 540. https://doi.org/10.3390/agronomy15030540






