Effects of Biostimulant Foliar Applications on Morphological Traits, Yield, Antioxidant Capacity, and Essential Oil Composition of Thymus vulgaris L. Under Field Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Experimental Design
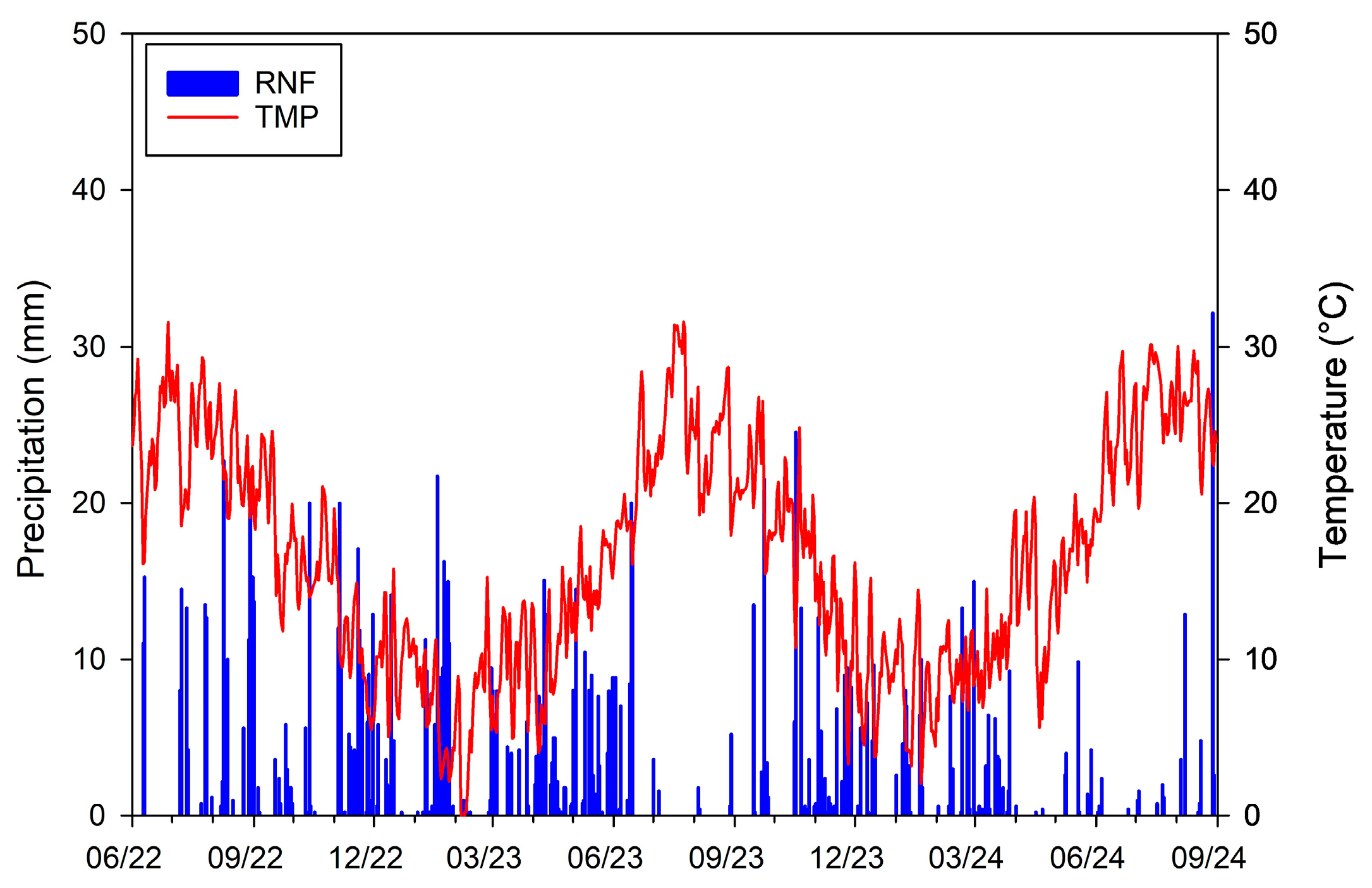
2.2. Climatic Data
2.3. Morphological and Yield Parameters
2.4. Extraction and Analysis of the EOs
2.5. Antioxidant Capacity and Total Phenolic Content (TPC)
2.6. Statistical Analysis
3. Results
3.1. Effects of Year and Biostimulants on Morphological and Yield Parameters of Thyme
3.2. Yield of Essential Oils
3.3. Chemical Composition of Essential Oils
3.3.1. Number of Compounds
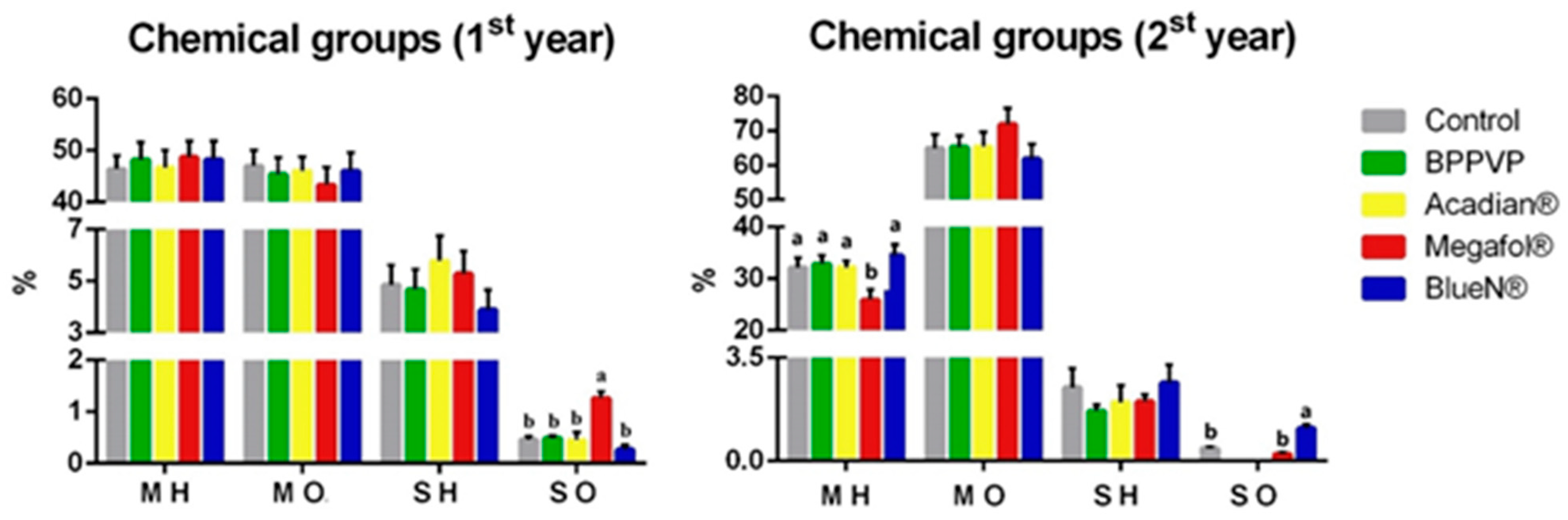
3.3.2. Major Chemical Groups
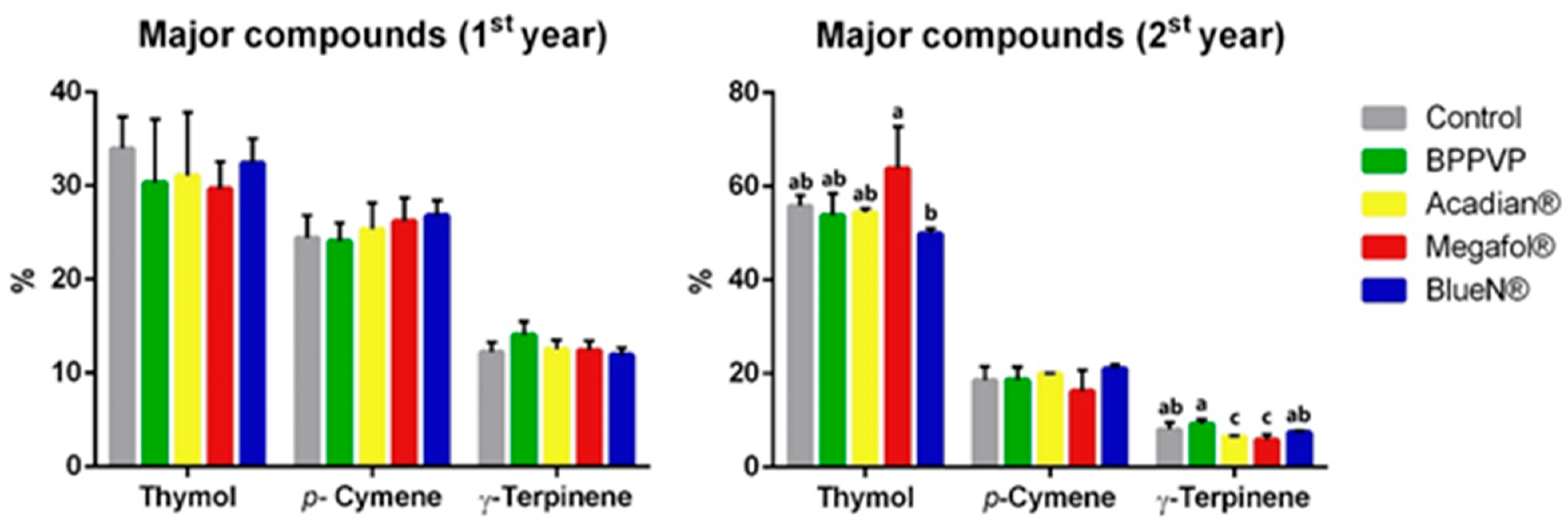
3.3.3. Key Components
3.4. Antioxidant Capacity and Total Phenolic Content
4. Discussion
4.1. Effects of Biostimulants on Morphological and Productive Traits
4.2. Effects of Biostimulants on Essential Oil Composition
4.3. Effects of Biostimulants on Antioxidant Capacity and Total Phenolic Content
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAPs | Medicinal and aromatic plants |
| EO | Essential oil |
References
- Riaz, U.; Iqbal, S.; Sohail, M.I.; Samreen, T.; Ashraf, M.; Akmal, F.; Siddiqui, A.; Ahmad, I.; Naveed, M.; Khan, N.I.; et al. A comprehensive review on emerging importance and economical potential of medicinal and aromatic plants (MAPs) in current scenario. Pak. J. Agric. Res. 2021, 34, 381–392. [Google Scholar] [CrossRef]
- Cadar, R.-L.; Amuza, A.; Dumitras, D.E.; Mihai, M.; Pocol, C.B. Analysing Clusters of Consumers Who Use Medicinal and Aromatic Plant Products. Sustainability 2021, 13, 8648. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Petropoulos, S.A.; Shahrajabian, N. Developing Sustainable Agriculture Systems in Medicinal and Aromatic Plant Production by Using Chitosan and Chitin-Based Biostimulants. Plants 2023, 12, 2469. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2017, 82, 277. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Amato, G.; Cardone, L.; Cicco, N.; Denora, M.; Perniola, M.; Casiello, D.; De Martino, L.; De Feo, V.; Candido, V. Morphological traits, yield, antioxidant activity and essential oil composition of oregano as affected by biostimulant foliar applications. Ind. Crops Prod. 2024, 222, 119702. [Google Scholar] [CrossRef]
- Farruggia, D.; Di Miceli, G.; Licata, M.; Leto, C.; Salamone, F.; Novak, J. Foliar application of various biostimulants produces contrasting response on yield, essential oil and chemical properties of organically grown sage (Salvia officinalis L.). Front. Plant Sci. 2024, 15, 1397489. [Google Scholar] [CrossRef]
- Farruggia, D.; Tortorici, N.; Iacuzzi, N.; Alaimo, F.; Leto, C.; Tuttolomondo, T. Biostimulants Improve Plant Performance of Rosemary Growth in Agricultural Organic System. Agronomy 2024, 14, 158. [Google Scholar] [CrossRef]
- Saia, S.; Corrado, G.; Vitaglione, P.; Colla, G.; Bonini, P.; Giordano, M.; Stasio, E.D.; Raimondi, G.; Sacchi, R.; Rouphael, Y. An endophytic fungi-based biostimulant modulates volatile and non-volatile secondary metabolites and yield of greenhouse basil (Ocimum basilicum L.) through variable mechanisms dependent on salinity stress level. Pathogens 2021, 10, 797. [Google Scholar] [CrossRef]
- Rathore, S.; Kumar, R. Dynamics of phosphorus and biostimulants on agro-morphology, yield, and essential oil profile of German Chamomile (Matricaria chamomilla L.) under acidic soil conditions of the Western Himalaya. Front. Plant Sci. 2022, 13, 917388. [Google Scholar] [CrossRef]
- Machado, V.P.D.O.; Pacheco, A.C.; Carvalho, M.E.A. Effect of biostimulant application on production and flavonoid content of marigold (Calendula officinalis L.). Rev. Ceres 2014, 61, 983–988. [Google Scholar] [CrossRef]
- Rafiee, H.; Badi, H.N.; Mehrafarin, A.; Qaderi, A.; Zarinpanjeh, N.; Sekara, A.; Zand, E. Application of Plant Biostimulants as New Approach to Improve the Biological Responses of Medicinal Plants- A Critical Review. J. Med. Plants 2016, 15, 1–39. [Google Scholar]
- Morales, R. The history, botany and taxonomy of the genus Thymus. In Thyme: The Genus Thymus; Stahl-Biskup, E., Sáez, F., Eds.; Taylor & Francis: London, UK; New York, NY, USA, 2002; pp. 1–43. [Google Scholar]
- Nabavi, S.M.; Marchese, A.; Izadi, M.; Curti, V.; Daglia, M.; Nabavi, S.F. Plants belonging to the genus Thymus as antibacterial agents: From farm to pharmacy. Food Chem. 2015, 173, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G. A review on applications and uses of thymus in the food industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; Mohammadi, M.M.; Siavash Moghaddam, S.; Heydarzadeh, S.; Gitari, H. Effects of stress modifier biostimulants on vegetative growth, nutrients, and antioxidants contents of garden thyme (Thymus vulgaris L.) under water deficit conditions. J. Plant Growth Regul. 2022, 41, 2059–2072. [Google Scholar] [CrossRef]
- Sabry, R.; Salama, A.; Wahba, H.; Mohamed, H.; Abdelhamid, M. The potential of cabbage waste extract as a bio-stimulant for enhancing growth, biochemical constituents, and oil quality of thyme (Thymus vulgaris). Future Food J. Food Agric. Soc. 2022, 11, 1–17. [Google Scholar] [CrossRef]
- Taksera, N.R.; Abu El-Leil, E.F.; Abdel-Fattah, G.M. Impact of bio-fertilizer and humic acid applications on productivity and some biochemical characteristics of thymus vulgais grow in sandy soil. Minia J. Agric. Res. Dev. 2023, 43, 853–872. [Google Scholar] [CrossRef]
- Waly, A.A.; El-Fattah, A.; Hassan, M.A.E.; El-Ghadban, E.M.; Abd Alla, A.S. Enhancing growth, productivity and essential oil percentage of Thymus vulgaris L. plant using seaweeds extract, chitosan and potassium silicate in sandy soil. Sci. J. Flowers Ornam. Plants 2020, 7, 549–562. [Google Scholar] [CrossRef]
- Muetasam Jafr, S.; Rahimi, A.R.; Hashemi, M.; Rokhzadi, A. Influence of N, K, and Seaweed Extract Fertilization on Biomass, Photoisynthetic Pigments, and Essential Oil of Thymus vulgaris: Optimization Study by Response Surface Methodology. Agronomy 2022, 12, 3222. [Google Scholar] [CrossRef]
- Kulbat-Warycha, K.; Nawrocka, J.; Kozłowska, L.; Żyżelewicz, D. Effect of Light Conditions, Trichoderma Fungi and Food Polymers on Growth and Profile of Biologically Active Compounds in Thymus vulgaris and Thymus serpyllum. Int. J. Mol. Sci. 2024, 25, 4846. [Google Scholar] [CrossRef]
- Ghazal, G.M. Growth and oil yield of Thymus vulgaris plant as influenced by some amino acids and ascorbic acid. World J. Pharm. Sci. 2015, 3, 2321–3086. [Google Scholar]
- Council of Europe. European Pharmacopeia, 10th ed.; Council of Europe: Strasbourg Cedex, France, 2020. [Google Scholar]
- Polito, F.; Papaianni, M.; Woo, S.L.; Malaspina, P.; Cornara, L.; De Feo, V. Artemisia arborescens (Vaill.) L.: Micromorphology, Essential Oil Composition, and Its Potential as an Alternative Biocontrol Product. Plants 2024, 13, 825. [Google Scholar] [CrossRef] [PubMed]
- Jennings, W.; Shibamoto, T. Qualitative Analyisis of Flavour and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA, 1980. [Google Scholar] [CrossRef]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicone and Carbowas 20M phases. J. Chromatogr. 1980, 503, 1–24. [Google Scholar] [CrossRef]
- Goodner, K.I. Practical retention index models of OV-101, DB-1, DB-5, and DB-Wax for flavour and fragrance compounds. LWT Food Sci. Technol. 2008, 41, 951–958. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- McLafferty, F.W. The Wiley Registry of Mass Spectral Data, with Nist Spectral Data CD Rom, 7th ed.; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, 1–8. [Google Scholar] [CrossRef]
- Torres Vera, R.; Bernabé García, A.J.; Carmona Álvarez, F.J.; Martínez Ruiz, J.; Fernández Martín, F. Application and Effectiveness of Methylobacterium symbioticum as a Biological Inoculant in Maize and Strawberry Crops. Folia Microbiol. 2024, 69, 121–131. [Google Scholar] [CrossRef]
- Agafonova, N.V.; Kaparullina, E.N.; Doronina, N.V.; Trotsenko, Y.A. Phosphate-solubilizing activity of aerobic Methylobacteria. Microbiology 2013, 82, 864–867. [Google Scholar] [CrossRef]
- Arrobas, M.; Correia, C.M.; Rodrigues, M.Â. Methylobacterium symbioticum Applied as a Foliar Inoculant Was Little Effective in Enhancing Nitrogen Fixation and Lettuce Dry Matter Yield. Sustainability 2024, 16, 4512. [Google Scholar] [CrossRef]
- Rodrigues, M.Â.; Lopes, J.I.; Martins, S.; Brito, C.; Correia, C.M.; Arrobas, M. A Spray Foliar Containing Methylobacterium symbioticum Did Not Increase Nitrogen Concentration in Leaves or Olive Yield Across Three Rainfed Olive Orchards. Horticulturae 2025, 11, 80. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-Based Biostimulants: Sustainable Applications in Agriculture for the Stimulation of Plant Growth, Stress Tolerance, and Disease Management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Golparvar, A.R.; Hadipanah, A. Effect of harvest time on yield and quality of Thymus vulgaris L. essential oil in Isfahan province, Iran. Agric. Conspec. Sci. 2014, 79, 115–118. [Google Scholar]
- Golparvar, A.R.; Bahari, B. Effects of phenological stages on herbage yield and quality/quantity of oil in garden thyme (Thymus vulgaris L.). J. Med. Plants Res. 2011, 5, 6085–6089. [Google Scholar] [CrossRef]
- Gouyon, P.H.; Vernet, P.; Guillerm, J.L.; Valdeyron, G. Polymorphisms and environment: The adaptive value of the oil polymorphism in Thymus vulgaris L. Heredity 1986, 57, 59–66. [Google Scholar] [CrossRef]
- Jordán, M.J.; Martínez, R.M.; Goodner, K.L.; Baldwin, E.A.; Sotomayor, J.A. Seasonal variation of Thymus hyemalis Lange and Spanish Thymus vulgaris L. essential oils composition. Ind. Crops Prod. 2006, 24, 253–263. [Google Scholar] [CrossRef]
- Moisa, C.; Lupitu, A.; Pop, G.; Chambre, D.R.; Popovici, L.; Cioca, G.; Bungau, S.; Popovici, D.M. Variation of the chemical composition of Thymus vulgaris essential oils by phenological stages. Rev. Chim. 2019, 70, 633–637. [Google Scholar] [CrossRef]
- Mugao, L. Factors influencing yield, chemical composition and efficacy of essential oils. Int. J. Multidiscip. Res. Growth Eval. 2004, 5, 169–178. [Google Scholar] [CrossRef]
- Etri, K.; Gosztola, B.; Végvári, G.; Ficzek, G.; Radácsi, P.; Simon, G.; Pluhár, Z. Unravelling the impact of drought and salt stresses on Thymus pannonicus: Morpho-physiological and biochemical insights. Plant Stress 2024, 13, 100557. [Google Scholar] [CrossRef]
- Etri, K.; Pluhár, Z. Exploring Chemical Variability in the Essential Oils of the Thymus Genus. Plants 2024, 13, 1375. [Google Scholar] [CrossRef]
- Mancini, E.; Senatore, F.; Del Monte, D.; De Martino, L.; Grulova, D.; Scognamiglio, M.; Snoussi, M.; De Feo, V. Studies on chemical composition, antimicrobial and antioxidant activities of five Thymus vulgaris L. essential oils. Molecules 2015, 20, 12016–12028. [Google Scholar] [CrossRef]
- Najar, B.; Pistelli, L.; Ferri, B.; Angelini, L.G.; Tavarini, S. Crop yield and essential oil composition of two Thymus vulgaris chemotypes along three years of organic cultivation in a hilly area of central Italy. Molecules 2021, 26, 5109. [Google Scholar] [CrossRef] [PubMed]
- Hudaib, M.; Speroni, E.; Di Pietra, A.M.; Cavrini, V. GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. J. Pharm. Biomed. Anal. 2002, 29, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Shabnum, S.; Wagay, M.G. Essential oil composition of Thymus vulgaris L. and their uses. J. Res. Dev. 2011, 11, 83–94. [Google Scholar]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Čmiková, N.; Kačániová, M. Thymus vulgaris essential oil and its biological activity. Plants 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, W.; Bahri, F.; Romane, A.; Höferl, M.; Wanner, J.; Schmidt, E.; Jirovetz, L. Chemical composition and anti-inflammatory activity of algerian thymus vulgaris essential oil. Nat. Prod. Commun. 2017, 12, 611–614. [Google Scholar] [CrossRef]
- Borugă, O.; Jianu, C.; Mişcă, C.; Goleţ, I.; Gruia, A.T.; Horhat, F.G. Thymus vulgaris essential oil: Chemical composition and antimicrobial activity. J. Med. Life 2014, 7, 56–60. [Google Scholar]
- Satyal, P.; Murray, B.L.; McFeeters, R.L.; Setzer, W.N. Essential oil characterization of Thymus vulgaris from various geographical locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef]
- Preljević, K.; Pašić, I.; Vlaović, M.; Matić, I.Z.; Krivokapić, S.; Petrović, N.; Stanojković, T.; Zivković, V.; Perović, S. Comparative analysis of chemical profiles, antioxidant, antibacterial, and anticancer effects of essential oils of two Thymus species from Montenegro. Fitoterapia 2024, 174, 105871. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Imtara, H.; Al-Waili, N.; Aboulghazi, A.; Abdellaoui, A.; Al-Waili, T.; Lyoussi, B. Chemical composition and antioxidant content of Thymus vulgaris honey and Origanum vulgare essential oil; their effect on carbon tetrachloride-induced toxicity. Vet. World 2021, 14, 292. [Google Scholar] [CrossRef] [PubMed]
- Punya, H.N.; Mehta, N.; Chatli, M.K.; Wagh, R.V.; Panwar, H. In-vitro evaluation of antimicrobial and antioxidant Efficacy of thyme (Thymus vulgaris L.) essential oil. J. Anim. Res. 2019, 3, 443–449. [Google Scholar] [CrossRef]
- Aljabeili, H.S.; Barakat, H.; Abdel-Rahman, H.A. Chemical composition, antibacterial and antioxidant activities of thyme essential oil (Thymus vulgaris). Food Nutr. Sci. 2018, 9, 433–446. [Google Scholar] [CrossRef]
- Lemos, M.F.; Lemos, M.F.; Pacheco, H.P.; Guimarães, A.C.; Fronza, M.; Endringer, D.C.; Scherer, R. Seasonal variation affects the composition and antibacterial and antioxidant activities of Thymus vulgaris. Ind. Crops Prod. 2017, 95, 543–548. [Google Scholar] [CrossRef]
| Biostimulants | Composition | Dose of Application |
|---|---|---|
| BPPVP® | ||
| Biostimol Plus | Algal extract of A. nodosum, total organic carbon (C) of biological origin (1.7%), mannitol (7%), and alginic acid (2–3%). | 2 g L−1 |
| Peptamin-V Plus® | Organic nitrogen (N) (3%) and C of biological origin (10%) and chelates of iron, manganese, and zinc. | 3 g L−1 |
| Acadian® | Algal extract of A. nodosum, N (1%), potassium oxide (K2O) (19%) betaines (0.1%), mannitol (4%), and C of biological origin (20%). | 1 g L−1 |
| Megafol® | Total nitrogen (3%); organic nitrogen (1%); urea nitrogen (2%); K2O (8%); C of biological origin (9%); amino acids (glycin and glutamic acid); betaines; proteins; vitamins (B5, PP, B1, and B6); auxin; gibberellin; cytokine. | 2.5 mL L−1 |
| BlueN® | Bacterial strain Methylobacterium symbioticum (SB23; 3 × 107 UFC/g). | 0.33 g L−1 |
| Treatments | Plant Height (cm) | Plant Width (cm) | Stem Diameter (mm) | Plant Fresh Weight (g) | Branches (n. plant−1) | Branches Weight (g) | Fresh Biomass Yield (g m−2) | Dry Yield (g m−2) |
|---|---|---|---|---|---|---|---|---|
| 1st year (2023) | ||||||||
| Biostimulant | ||||||||
| Control | 25.4 ± 0.10 | 22.6 ± 0.16 | 3.9 ± 0.09 a | 95.7 ± 0.30 ab | 35.3 ± 0.07 a | 2.7 ± 0.13 | 315.8 ± 2.21 b | 102.9 ± 2.43 ab |
| BPPVP® | 26.0 ± 0.08 | 21.9 ± 0.14 | 4.1 ± 0.12 ab | 89.0 ± 0.21 a | 40.8 ± 0.10 b | 2.2 ± 0.10 | 293.7 ± 2.32 ab | 97.5 ± 2.45 a |
| Acadian® | 25.6 ± 0.06 | 20.8 ± 0.12 | 4.2 ± 0.13 ab | 100.8 ± 0.25 b | 41.2 ± 0.11 b | 2.5 ± 0.10 | 332.6 ± 2.33 c | 115.4 ± 2.34 b |
| Megafol® | 25.0 ± 0.10 | 20.8 ± 0.09 | 3.9 ± 0.11 a | 83.8 ± 0.28 a | 41.7 ± 0.09 b | 2.0 ± 0.12 | 276.5 ± 2.35 a | 96.5 ± 2.41 a |
| BlueN® | 25.9 ± 0.09 | 22.9 ± 0.10 | 4.5 ± 0.10 b | 98.8 ± 0.27 ab | 39.2 ± 0.15 ab | 2.5 ± 0.14 | 326.1 ± 2.24 bc | 108.9 ± 2.39 ab |
| ns | ns | * | * | * | ns | ** | * | |
| 2nd year (2024) | ||||||||
| Control | 29.0 ± 0.07 ab | 31.2 ± 0.16 | 2.7 ± 0.17 a | 110.2 ± 0.31 a | 42.3 ± 0.22 a | 2.6 ± 0.07 | 363.7 ± 2.56 a | 128.1 ± 2.54 a |
| BPPVP® | 32.1 ± 0.08 b | 30.2 ± 0.08 | 2.5 ± 0.12 a | 128.4 ± 0.29 ab | 54.9 ± 0.18 ab | 2.3 ± 0.10 | 423.7 ± 2.30 ab | 149.6 ± 2.40 b |
| Acadian® | 29.8 ± 0.05 ab | 32.4 ± 0.11 | 3.0 ± 0.11 ab | 139.7 ± 0.27 b | 58.1 ± 0.14 b | 2.4 ± 0.09 | 461.1 ± 2.38 bc | 160.5 ± 2.38 bc |
| Megafol® | 27.0 ± 0.09 a | 31.1 ± 0.14 | 3.3 ± 0.10 b | 132.7 ± 0.22 b | 48.8 ± 0.11 a | 2.7 ± 0.08 | 437.9 ± 2.40 b | 139.3 ± 2.46 ab |
| BlueN® | 28.7 ± 0.09 a | 31.1 ± 0.08 | 3.0 ± 0.20 ab | 152.1 ± 0.32 c | 57.9 ± 0.25 b | 2.6 ± 0.11 | 501.9 ± 2.44 c | 172.2 ± 2.82 c |
| * | ns | * | ** | * | ns | ** | ** | |
| Yield (%) | ||
|---|---|---|
| 1st Year | 2nd Year | |
| Control | 0.55 ± 0.10 | 0.56 ± 0.11 |
| BPPVP | 0.30 ± 0.08 | 0.47 ± 0.10 |
| Acadian® | 0.37 ± 0.17 | 0.52 ± 0.13 |
| Megafol® | 0.31 ± 0.08 | 0.45 ± 0.09 |
| BlueN® | 0.47 ± 0.09 | 0.53 ± 0.11 |
| Compound | KI a | KI b | % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | BPPVP | Acadian® | Megafol® | BlueN® | Identification c | ||||||||
| 1st Year | 2nd Year | 1st Year | 2nd Year | 1st Year | 2nd Year | 1st Year | 2nd Year | 1st Year | 2nd Year | ||||
| 2.Methyl-butanoic acid, methyl ester | 758 | - | 0.10 ± 0.17 | - | 0.11 ± 0.20 | - | - | - | 0.23 ± 0.21 | - | 0.27 ± 0.23 | - | 1,2 |
| α-Thujene | 858 | 1027 | 2.04 ± 0.12 | 1.33 ± 0.05 | 2.06 ± 0.29 | 1.28 ± 0.17 | 1.78 ± 0.36 | 1.37 ± 0.06 | 2.15 ± 0.41 | 1.09 ± 0.29 | 2.06 ± 0.09 | 1.35 ± 0.02 | 1,2,3 |
| α-Pinene | 862 | 1025 | 1.28 ± 0.07 | 0.93 ± 0.16 | 1.35 ± 0.25 | 0.77 ± 0.08 | 1.22 ± 0.26 | 0.90 ± 0.05 | 1.43 ± 0.22 | 0.48 ± 0.42 | 1.35 ± 0.08 | 0.85 ± 0.06 | 1,2,3 |
| Camphene | 874 | 1068 | 0.98 ± 0.10 | 0.64 ± 0.58 | 1.03 ± 0.11 | 0.22 ± 0.38 | 0.93 ± 0.21 | 0.67 ± 0.10 | 1.07 ± 0.13 | 0.18 ± 0.31 | 0.94 ± 0.08 | 0.51 ± 0.13 | 1,2,3 |
| β-Pinene | 899 | 1110 | 0.46 ± 0.03 | - | 0.57 ± 0.16 | - | 0.37 ± 0.32 | - | 0.50 ± 0.07 | - | 0.46 ± 0.02 | - | 1,2,3 |
| 1-Octen-3-ol | 908 | 1444 | 1.03 ± 0.06 | - | 1.03 ± 0.20 | 0.17 ± 0.29 | 0.96 ± 0.31 | 0.37 ± 0.32 | 0.94 ± 0.12 | - | 1.12 ± 0.07 | - | 1,2 |
| β-Myrcene | 918 | 1161 | 2.07 ± 0.17 | 1.32 ± 0.12 | 2.10 ± 0.12 | 1.33 ± 0.09 | 1.85 ± 0.29 | 1.27 ± 0.07 | 2.08 ± 0.35 | 0.85 ± 0.43 | 2.02 ± 0.13 | 1.38 ± 0.19 | 1,2,3 |
| α-Terpinene | 939 | 1178 | 2.16 ± 0.15 | 1.30 ± 0.32 | 2.24 ± 0.24 | 1.42 ± 0.13 | 1.98 ± 0.23 | 1.19 ± 0.08 | 2.07 ± 0.30 | 1.03 ± 0.16 | 2.00 ± 0.03 | 1.17 ± 0.15 | 1,2,3 |
| p-Cymene | 949 | 1270 | 24.43 ± 2.40 | 18.59 ± 2.94 | 24.04 ± 2.00 | 18.60 ± 2.79 | 25.35 ± 2.81 | 19.95 ± 0.07 | 26.18 ± 2.52 | 16.23 ± 4.46 | 26.77 ± 1.64 | 21.01 ± 0.95 | 1,2,3 |
| Limonene | 951 | 1198 | 0.77 ± 0.04 | - | 0.77 ± 0.05 | - | 0.67 ± 0.07 | 0.36 ± 0.31 | 0.77 ± 0.11 | 0.19 ± 0.33 | 0.72 ± 0.02 | 0.77 ± 0.16 | 1,2,3 |
| Eucalyptol | 952 | 1211 | 0.86 ± 0.11 | 1.31 ± 0.37 | 1.35 ± 0.64 | 1.20 ± 0.53 | 1.46 ± 0.86 | 1.93 ± 0.76 | 1.08 ± 0.08 | 0.43 ± 0.38 | 1.00 ± 0.10 | 1.18 ± 0.33 | 1,2,3 |
| γ-Terpinene | 981 | 1245 | 12.21 ± 1.11 | 8.02 ± 1.50 | 14.06 ± 1.45 | 9.23 ± 0.92 | 12.51 ± 1.02 | 6.52 ± 0.21 | 12.43 ± 0.98 | 5.86 ± 1.12 | 11.93 ± 0.77 | 7.42 ± 0.38 | 1,2,3 |
| cis-Sabinene hydrate | 987 | 1460 | 1.44 ± 0.15 | 0.92 ± 0.03 | 1.20 ± 0.07 | 0.75 ± 0.07 | 1.10 ± 0.13 | 0.98 ± 0.02 | 1.26 ± 0.07 | 0.78 ± 0.03 | 1.30 ± 0.08 | 1.35 ± 0.39 | 1,2 |
| trans-Sabinene hydrate | 1012 | 1549 | 0.25 ± 0.22 | - | 0.10 ± 0.17 | - | 0.21 ± 0.18 | - | 0.34 ± 0.03 | - | 0.24 ± 0.21 | - | 1,2 |
| Linalool | 1019 | 1543 | 2.75 ± 0.06 | 1.70 ± 0.24 | 3.11 ± 0.08 | 2.16 ± 0.12 | 3.00 ± 0.44 | 2.04 ± 0.07 | 2.80 ± 0.12 | 1.99 ± 0.29 | 2.81 ± 0.15 | 1.84 ± 0.05 | 1,2,3 |
| Camphor | 1054 | 1515 | 0.64 ± 0.42 | 0.41 ± 0.72 | 0.71 ± 0.23 | - | 0.65 ± 0.17 | - | 0.98 ± 0.18 | - | 0.59 ± 0.08 | 0.57 ± 0.05 | 1,2,3 |
| Borneol | 1076 | 1700 | 1.95 ± 0.25 | 1.73 ± 0.45 | 1.99 ± 0.11 | 1.48 ± 0.14 | 2.14 ± 0.14 | 1.71 ± 0.40 | 1.90 ± 0.22 | 1.29 ± 0.20 | 1.73 ± 0.10 | 1.26 ± 0.23 | 1,2,3 |
| Verbenone | 1079 | 1720 | - | - | - | - | 0.10 ± 0.17 | - | - | - | - | 0.64 ± 0.06 | 1,2 |
| Terpinen-4-ol | 1088 | 1601 | 0.78 ± 0.01 | - | 0.87 ± 0.03 | 0.61 ± 0.02 | 0.88 ± 0.09 | - | 0.87 ± 0.02 | 0.22 ± 0.39 | 0.70 ± 0.05 | - | 1,2 |
| α-Terpineol | 1097 | 1694 | - | - | 0.10 ± 0.17 | - | 0.12 ± 0.21 | - | 0.10 ± 0.18 | - | - | - | 1,2,3 |
| Thymol methyl ether | 1142 | 1581 | 0.89 ± 0.85 | - | 1.96 ± 0.77 | 1.55 ± 0.51 | 1.80 ± 0.64 | 0.57 ± 0.99 | 1.89 ± 1.25 | 0.23 ± 0.39 | 1.79 ± 0.41 | 0.96 ± 0.07 | 1,2 |
| Carvacrol methyl ether | 1150 | 1614 | 0.73 ± 0.29 | - | 0.88 ± 0.38 | 0.84 ± 0.12 | 0.94 ± 0.43 | 0.78 ± 0.14 | 1.01 ± 0.42 | - | 0.91 ± 0.35 | 1.28 ± 0.25 | 1,2 |
| Bornyl acetate | 1165 | 1575 | - | - | - | - | - | - | - | - | - | 0.52 ± 0.04 | 1,2 |
| Thymol | 1198 | 2164 | 33.95 ± 3.44 | 55.75 ± 2.25 | 30.32 ± 4.28 | 53.76 ± 4.65 | 31.04 ± 6.76 | 54.42 ± 0.89 | 29.64 ± 2.92 | 63.75 ± 8.92 | 32.40 ± 2.66 | 49.93 ± 1.07 | 1,2,3 |
| Carvacrol | 1204 | 2211 | 2.74 ± 0.36 | 3.13 ± 0.15 | 2.43 ± 0.27 | 2.93 ± 0.36 | 2.45 ± 0.37 | 2.93 ± 0.09 | 2.40 ± 0.36 | 3.16 ± 0.20 | 2.55 ± 0.28 | 2.25 ± 0.22 | 1,2,3 |
| Caryophyllene | 1304 | 1598 | 3.80 ± 0.34 | 2.49 ± 0.50 | 3.82 ± 0.48 | 1.69 ± 0.13 | 4.42 ± 0.70 | 1.99 ± 0.06 | 4.03 ± 0.76 | 2.01 ± 0.27 | 3.28 ± 0.20 | 2.65 ± 0.30 | 1,2 |
| Thymoquinol | 1330 | - | - | 0.43 ± 0.38 | 0.11 ± 0.18 | - | - | - | 0.29 ± 0.51 | 0.23 ± 0.40 | - | 0.62 ± 0.08 | 1,2 |
| Germacrene D | 1364 | 1708 | 0.66 ± 0.03 | - | 0.88 ± 0.15 | - | 0.75 ± 0.09 | - | 0.55 ± 0.12 | - | 0.48 ± 0.10 | - | 1,2 |
| γ-Cadinene | 1397 | 1763 | - | - | 0.23 ± 0.21 | - | 0.20 ± 0.17 | - | 0.33 ± 0.06 | - | 0.13 ± 0.23 | - | 1,2 |
| δ-Cadinene | 1402 | 1756 | 0.39 ± 0.03 | - | 0.13 ± 0.23 | - | 0.41 ± 0.06 | - | 0.37 ± 0.12 | - | - | - | 1,2 |
| Caryophyllene oxide | 1457 | 1986 | 0.46 ± 0.04 | - | 0.27 ± 0.24 | - | 0.45 ± 0.03 | - | 0.97 ± 1.12 | - | 0.27 ± 0.23 | 0.49 ± 0.18 | 1,2 |
| τ-Cadinol | 1475 | 2151 | - | - | 0.11 ± 0.18 | - | - | - | - | - | - | - | 1,2 |
| Total | 99.82 ± 0.28 | 99.99 ± 0.01 | 99.93 ± 0.10 | 99.99 ± 0.01 | 99.88 ± 0.10 | 99.96 ± 0.06 | 99.69 ± 0.29 | 100.00 ± 0.00 | 99.82 ± 0.28 | 100.00 ± 0.00 | |||
| Monoterpene hydrocarbons | 46.40 ± 2.65 | 32.13 ± 1.97 | 48.22 ± 3.35 | 32.85 ± 1.67 | 46.66 ± 3.45 | 32.23 ± 1.23 | 48.68 ± 3.18 | 25.91 ± 2.01 | 48.25 ± 3.56 | 34.46 ± 2.17 | |||
| Oxygenated monoterpenes | 46.98 ± 3.04 | 64.94 ± 4.01 | 45.41 ± 3.23 | 65.28 ± 3.25 | 46.03 ± 2.76 | 65.37 ± 4.22 | 43.30 ± 3.45 | 71.85 ± 4.78 | 46.02 ± 3.56 | 61.78 ± 4.35 | |||
| Sesquiterpene hydrocarbons | 4.85 ± 0.78 | 2.49 ± 0.65 | 4.68 ± 0.77 | 1.69 ± 0.22 | 5.78 ± 0.98 | 1.99 ± 0.57 | 5.28 ± 0.88 | 2.01 ± 0.24 | 3.89 ± 0.76 | 2.65 ± 0.62 | |||
| Oxygenated sesquiterpenes | 0.46 ± 0.06 | 0.43 ± 0.05 | 0.49 ± 0.04 | - | 0.45 ± 0.15 | - | 1.26 ± 0.13 | 0.23 ± 0.07 | 0.27 ± 0.09 | 1.11 ± 0.14 | |||
| Others | 1.13 ± 0.11 | - | 1.13 ± 0.13 | 0.17 ± 0.05 | 0.96 ± 0.21 | 0.37 ± 0.09 | 1.17 ± 0.18 | - | 1.39 ± 0.17 | - | |||
| DPPH IC50 (mg/mL) (Mean ± SD) | ABTS TEAC (μmol/g) (Mean ± SD) | TPC mg GAE/g EO (Mean ± SD) | ||||
|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | 1st Year | 2nd Year | 1st Year | 2nd Year | |
| Control | 0.98 ± 0.06 | 2.70 ± 0.04 bc | 21.43 ± 1.23 | 27.56 ± 1.87 | 65.32 ± 2.54 | 43.56 ± 2.28 ab |
| BPPVP | 0.98 ± 0.08 | 2.83 ± 0.05 b | 21.37 ± 1.36 | 26.97 ± 1.58 | 64.77 ± 2.67 | 44.21 ± 2.16 a |
| Acadian® | 0.97 ± 0.06 | 3.41 ± 0.08 a | 22.01 ± 1.65 | 24.43 ± 1.37 | 63.54 ± 2.12 | 37.76 ± 2.54 c |
| Megafol® | 0.97 ± 0.09 | 2.64 ± 0.09 d | 21.87 ± 2.38 | 23.87 ± 2.09 | 63.87 ± 2.97 | 43.33 ± 1.93 ab |
| BlueN® | 0.98 ± 0.08 | 2.20 ± 0.07 e | 22.23 ± 1.12 | 25.01 ± 2.01 | 65.98 ± 2.47 | 45.65 ± 1.91 a |
| ns | * | ns | ns | ns | * | |
| Trolox | 3.45 × 10−3 ± 0.2 × 10−3 | |||||
| Ascorbic acid | 38.3 × 103 ± 0.8 × 103 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardone, L.; Polito, F.; Denora, M.; Casiello, D.; Castronuovo, D.; Cicco, N.; Perniola, M.; De Feo, V.; Candido, V. Effects of Biostimulant Foliar Applications on Morphological Traits, Yield, Antioxidant Capacity, and Essential Oil Composition of Thymus vulgaris L. Under Field Conditions. Agronomy 2025, 15, 442. https://doi.org/10.3390/agronomy15020442
Cardone L, Polito F, Denora M, Casiello D, Castronuovo D, Cicco N, Perniola M, De Feo V, Candido V. Effects of Biostimulant Foliar Applications on Morphological Traits, Yield, Antioxidant Capacity, and Essential Oil Composition of Thymus vulgaris L. Under Field Conditions. Agronomy. 2025; 15(2):442. https://doi.org/10.3390/agronomy15020442
Chicago/Turabian StyleCardone, Loriana, Flavio Polito, Michele Denora, Donato Casiello, Donato Castronuovo, Nunzia Cicco, Michele Perniola, Vincenzo De Feo, and Vincenzo Candido. 2025. "Effects of Biostimulant Foliar Applications on Morphological Traits, Yield, Antioxidant Capacity, and Essential Oil Composition of Thymus vulgaris L. Under Field Conditions" Agronomy 15, no. 2: 442. https://doi.org/10.3390/agronomy15020442
APA StyleCardone, L., Polito, F., Denora, M., Casiello, D., Castronuovo, D., Cicco, N., Perniola, M., De Feo, V., & Candido, V. (2025). Effects of Biostimulant Foliar Applications on Morphological Traits, Yield, Antioxidant Capacity, and Essential Oil Composition of Thymus vulgaris L. Under Field Conditions. Agronomy, 15(2), 442. https://doi.org/10.3390/agronomy15020442










