Pastureland Soil Organic Carbon Storage Regulated by Pasture Species and Age Under Nitrogen and Water Addition in Northern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Methodology
2.2. Sample Collection
2.3. Indicators and Their Methods
2.4. Data Processing
3. Results
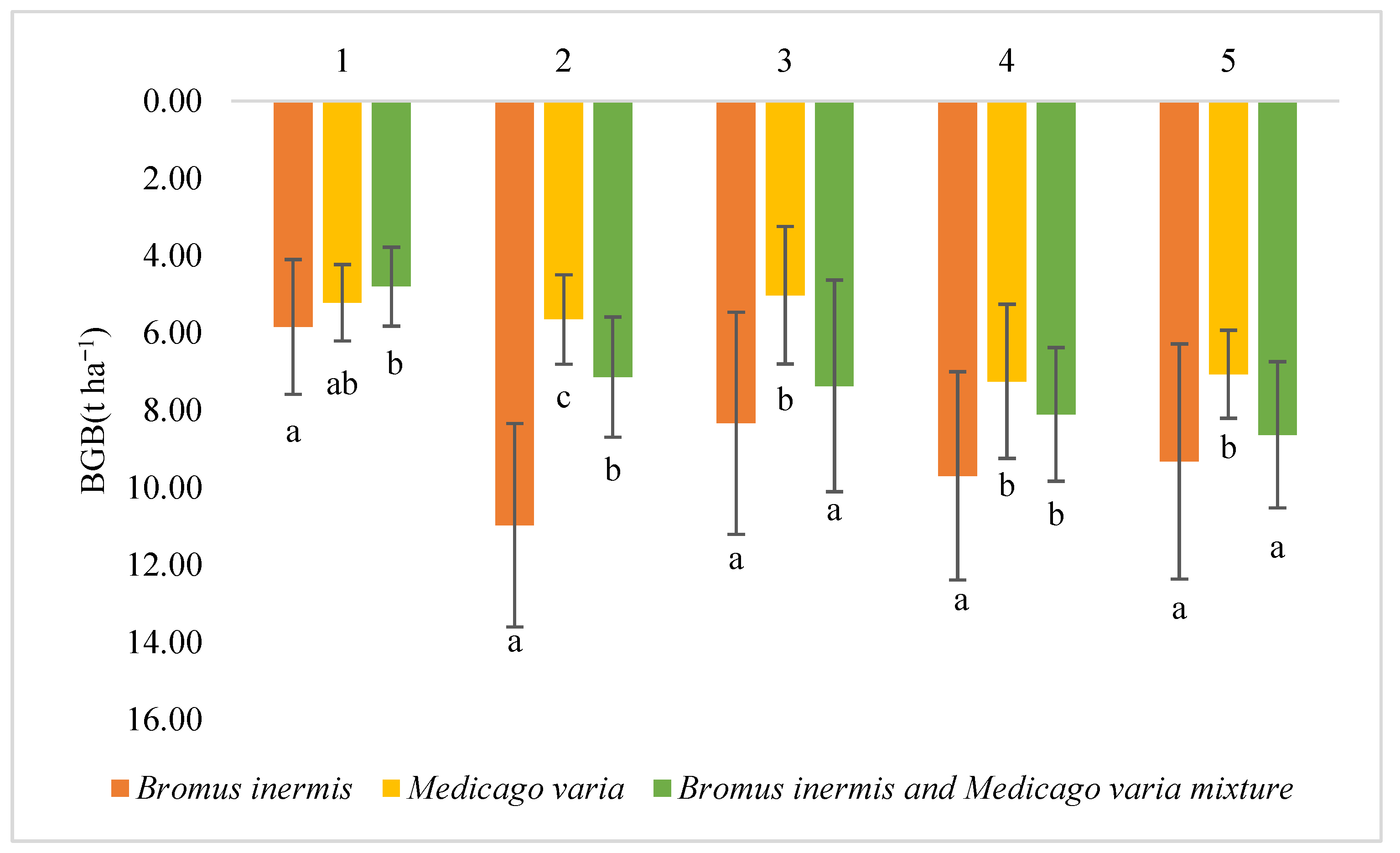
3.1. Below-Ground Biomass
3.2. Changes in Soil Organic Carbon Content
3.3. Changes in Soil Organic Carbon Storage

3.4. Factor Interactions
4. Discussion
4.1. Effects of Pasture–Nitrogen–Water on Below-Ground Biomass of Pastureland with Different Ages
4.2. Effects of Pasture–Nitrogen–Water on Soil Organic Carbon Pools in Pastureland of Different Ages
4.3. Interaction Effects of Factors Influencing Soil Organic Carbon Pools
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bronick, C.J.; Lal, R. Soil Structure and Management: A Review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The Contentious Nature of Soil Organic Matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Hoffland, E.; Kuyper, T.W.; Comans, R.N.J.; Creamer, R.E. Eco-functionality of Organic Matter in Soils. Plant Soil 2020, 455, 1–22. [Google Scholar] [CrossRef]
- Alkharabsheh, H.M.; Seleiman, M.F.; Battaglia, M.L.; Shami, A.; Jalal, R.S.; Alhammad, B.A.; Almutairi, K.F.; Al-Saif, A.M. Biochar and Its Broad Impacts in Soil Quality and Fertility, Nutrient Leaching and Crop Productivity: A Review. Agronomy 2021, 11, 993. [Google Scholar] [CrossRef]
- Lal, R. Soil Carbon Sequestration Impacts on Global Climate Change and Food Security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Kiyas, A.A. The Effectiveness of Perennial Grass in Crop Rotation. Sci. J. Saken Seifullin Kazakh Agrotech. Univ. 2020, 3, 83–90. [Google Scholar]
- He, Y.J.; Bi, Y.X.; Yu, H.Q.; Zhang, Y.J.; Struik, P.C.; Jing, J.Y. Positive Legacy Effects of Grass-Legume Mixture Leys on Phosphorus Uptake and Yield of Maize Weakened over the Growing Season. Field Crops Res. 2024, 314, 109434. [Google Scholar] [CrossRef]
- Malisch, C.S.; Finn, J.A.; Eriksen, J.; Loges, R.; Brophy, C.; Huguenin-Elie, O. The Importance of Multi-Species Grassland Leys to Enhance Ecosystem Services in Crop Rotations. Grass Forage Sci. 2024, 79, 120–134. [Google Scholar] [CrossRef]
- Tan, G.; Liu, Y.J.; Peng, S.G.; Yin, H.Q.; Meng, D.L.; Tao, J.M.; Gu, Y.B.; Li, J.; Yang, S.; Xiao, N.W.; et al. Soil potentials to resist continuous cropping obstacle: Three field cases. Environ. Res. 2021, 200, 111319. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ma, S.Y.; Chen, G.P.; Lu, X.; Chai, Q.; Li, S. Mechanisms and Mitigation Strategies for the Occurrence of Continuous Cropping Obstacles of Legumes in China. Agronomy 2024, 14, 104. [Google Scholar] [CrossRef]
- Ivanov, A.; Ivanova, Z. Efficiency of Precision Fertilization System in Grain-Grass Crop Rotation. Agronomy 2022, 12, 1381. [Google Scholar] [CrossRef]
- Kostensalo, J.; Hyväluoma, J.; Jauhiainen, L.; Keskinen, R.; Nuutinen, V.; Peltonen-Sainio, P.; Heikkinen, J. Diversification of Crop Rotations and Soil Carbon Balance: Impact Assessment Based on National-Scale Monitoring Data. Carbon Manag. 2024, 15, 2298373. [Google Scholar] [CrossRef]
- Yan, Y.F.; Li, H.Y.; Zhang, M.; Liu, X.W.; Zhang, L.X.; Wang, Y.K.; Yang, M.; Cai, R.G. Straw Return or No Tillage? Comprehensive Meta-Analysis Based on Soil Organic Carbon Contents, Carbon Emissions, and Crop Yields in China. Agronomy 2024, 14, 2263. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.L.; Liu, L.X.; Li, T.; Dou, Y.X.; Qian, J.B.; Wang, Y.Q.; An, S.S.; Chang, S.X. Nitrogen Fertilization Weakens the Linkage Between Soil Carbon and Microbial Diversity: A Global Meta-Analysis. Glob. Change Biol. 2022, 28, 6446–6461. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Wang, G.S.; Han, Q.S.; Sun, J.S.; Ning, H.F.; Feng, D. Soil Moisture and Water-Nitrogen Synergy Dominate the Change of Soil Carbon Stock in Farmland. Agric. Water Manag. 2023, 287, 108424. [Google Scholar] [CrossRef]
- Post, W.M.; Kwon, K.C. Soil Carbon Sequestration and Land-Use Change: Processes and Potential. Glob. Change Biol. 2000, 6, 317–327. [Google Scholar] [CrossRef]
- Yang, T.; Adams, J.M.; Shi, Y.; He, J.S.; Jing, X.; Chen, L.T.; Tedersoo, L.; Chu, H.Y. Soil Fungal Diversity in Natural Grasslands of the Tibetan Plateau: Associations with Plant Diversity and Productivity. New Phytol. 2017, 215, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.C.; Wang, M.Y.; Hu, S.J.; Zhang, X.D.; Zhu, O.Y.; Zhang, G.L.; Huang, B.; Zhao, S.W.; Wu, J.S.; Xie, D.T.; et al. Economics and Policy-Driven Organic Carbon Input Enhancement Dominates Soil Organic Carbon Accumulation in Chinese Croplands. Proc. Natl. Acad. Sci. USA 2018, 115, 4045–4050. [Google Scholar] [CrossRef] [PubMed]
- Maenhout, P.; Di Bene, C.; Cayuela, M.L.; Diaz-Pines, E.; Govednik, A.; Keuper, F.; Mavsar, S.; Mihelic, R.; O’Toole, A.; Schwarzmann, A.; et al. Trade-Offs and Synergies of Soil Carbon Sequestration: Addressing Knowledge Gaps Related to Soil Management Strategies. Eur. J. Soil Sci. 2024, 75, e13515. [Google Scholar] [CrossRef]
- Calone, R.; Fiore, A.; Pellis, G.; Cayuela, M.L.; Mongiano, G.; Lagomarsino, A.; Bregaglio, S. A Fuzzy Logic Evaluation of Synergies and Trade-Offs between Agricultural Production and Climate Change Mitigation. J. Clean. Prod. 2024, 442, 140878. [Google Scholar] [CrossRef]
- Condron, L.M.; Hopkins, D.W.; Gregorich, E.G.; Black, A.; Wakelin, S.A. Long-Term Irrigation Effects on Soil Organic Matter under Temperate Grazed Pasture. Eur. J. Soil Sci. 2014, 65, 741–750. [Google Scholar] [CrossRef]
- Parijat, S.P.S.; Baruah, K.K. Carbon Fixation, Partitioning and Storage in Field Planted with Wheat (Triticum Aestivum) at Different Moisture Regimes. Clim. Change Environ. Sustain. 2014, 2, 39–47. [Google Scholar]
- Bai, Y.F.; Cotrufo, M.F. Grassland Soil Carbon Sequestration: Current Understanding, Challenges, and Solutions. Science 2022, 377, 603–608. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K.; Doran, J.W. Aggregation and Soil Organic Matter Accumulation in Cultivated and Native Grassland Soils. Soil Sci. Soc. Am. J. 1998, 62, 1367–1377. [Google Scholar] [CrossRef]
- Sabanci, C.O. Role and Management of Permanent Grasslands. In New Approaches for Grassland Research in a Context of Climate and Socio-Economic Changes; Options Mediterraneennes Serie A, Seminaires Mediterraneens; CIHEAM: Zaragoza, Spain, 2012; pp. 285–293. [Google Scholar]
- Balesdent, J.; Balabane, M. Major Contribution of Roots to Soil Carbon Storage Inferred from Maize Cultivated Soils. Soil Biol. Biochem. 1996, 28, 1261–1263. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Decoupling of Nitrogen and Phosphorus in Terrestrial Plants Associated with Global Changes. Nat. Clim. Change 2015, 5, 465–469. [Google Scholar] [CrossRef]
- Zhang, T.A.; Chen, H.Y.H.; Ruan, H.H. Global Negative Effects of Nitrogen Deposition on Soil Microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, X.K.; Mori, T.; Mao, Q.G.; Zhou, K.J.; Zhou, G.Y.; Nie, Y.X.; Mo, J.M. Responses of Soil Microbial Community to Continuous Experimental Nitrogen Additions for 13 Years in a Nitrogen-Rich Tropical Forest. Soil Biol. Biochem. 2018, 121, 103–112. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Zheng, M.H.; Jiang, L.F.; Luo, Y.Q. Patterns and Mechanisms of Responses by Soil Microbial Communities to Nitrogen Addition. Soil Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. Organic Amendments: Microbial Community Structure, Activity and Abundance of Agriculturally Relevant Microbes Are Driven by Long-Term Fertilization Strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil Moisture is the Major Factor Influencing Microbial Community Structure and Enzyme Activities across Seven Biogeoclimatic Zones in Western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Emde, D.; Hannam, K.D.; Most, I.; Nelson, L.M.; Jones, M.D. Soil Organic Carbon in Irrigated Agricultural Systems: A Meta-Analysis. Glob. Change Biol. 2021, 27, 3898–3910. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.; Jin, L.X.; Ganjegunte, G.; Borrok, D.; Lougheed, V.; Ma, L. Soil Quality Changes due to Flood Irrigation in Agricultural Fields Along the Rio Grande in Western Texas. Appl. Geochem. 2018, 90, 87–100. [Google Scholar] [CrossRef]
- Hakala, K.; Keskitalo, M.; Eriksson, C.; Pitkänen, T. Nutrient uptake and biomass accumulation for eleven different field crops. Agric. Food Sci. 2009, 18, 366–387. [Google Scholar] [CrossRef]
- Singh, K.A. Effect of Age, Cutting Interval, Nitrogen and Farmyard Manure on Root-Growth Parameters of Forages in an Acid Inceptisol. Indian J. Agric. Sci. 1996, 66, 38–44. [Google Scholar]
- Deng, L.; Wang, K.B.; Li, J.P.; Shangguan, Z.P.; Sweeney, S. Carbon Storage Dynamics in Alfalfa (Medicago sativa) Fields in the Hilly-Gully Region of the Loess Plateau, China. Clean-Soil Air Water 2014, 42, 1253–1262. [Google Scholar] [CrossRef]
- Hahn, C.; Lüscher, A.; Ernst-Hasler, S.; Suter, M.; Kahmen, A. Timing of Drought in the Growing Season and Strong Legacy Effects Determine the Annual Productivity of Temperate Grasses in a Changing Climate. Biogeosciences 2021, 18, 585–604. [Google Scholar] [CrossRef]
- Hassan, N.; Zhong, Z.W.; Wang, D.L.; Zhu, Y.; Naeen, I.; Ahungu, A.B.; Wan, H.Y.; Li, X.F. Effects of Long-Term Mowing on Species Diversity, Biomass and Composition of Plant Community in a Semi-Arid Grassland in Northeastern China. Appl. Veg. Sci. 2023, 26, e12743. [Google Scholar] [CrossRef]
- Bama, K.S.; Babu, C. Perennial Forages as a Tool for Sequestering Atmospheric Carbon by Best Management Practices for Better Soil Quality and Environmental Safety. Forage Res. 2016, 42, 149–157. [Google Scholar]
- Somenahally, A.; Mclawrence, J.; DuPont, J.I.; Brady, J.; Sarkar, R.; Rouquette, M. Root-Mycorrhizae Interactions Contributed to Organic Carbon Density in the Sandy Soil Profiles of Adapted Grazing Lands. Appl. Soil Ecol. 2020, 154, 103656. [Google Scholar] [CrossRef]
- Cinar, S.; Hatipoglu, R. Forage Yield and Botanical Composition of Mixtures of Some Perennial Warm Season Grasses with Alfalfa (Medicago sativa) Under Mediterranean Conditions. Turk. J. Field Crops 2014, 19, 13–18. [Google Scholar] [CrossRef]
- Dang, P.F.; Zhang, M.M.; Chen, X.L.; Loreau, M.; Duffy, J.E.; Li, X.E.; Wen, S.Y.; Han, X.Q.; Liao, L.C.; Huang, T.T.; et al. Plant Diversity Decreases Greenhouse Gas Emissions by Increasing Soil and Plant Carbon Storage in Terrestrial Ecosystems. Ecol. Lett. 2024, 27, e14469. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, D.; Islam, M.A. Grass-Legume Mixtures for Improved Soil Health in Cultivated Agroecosystem. Sustainability 2018, 10, 2718. [Google Scholar] [CrossRef]
- Liu, Y.H.; Li, C.; Cai, G.; Sauheitl, L.; Xiao, M.L.; Shibistova, O.; Ge, T.D.; Guggenberger, G. Meta-Analysis on the Effects of Types and Levels of N, P and K Fertilization on Organic Carbon in Cropland Soils. Geoderma 2023, 437, 116580. [Google Scholar] [CrossRef]
- Xu, C.H.; Xu, X.; Ju, C.H.; Chen, H.Y.H.; Wilsey, B.J.; Luo, Y.Q.; Fan, W. Long-Term, Amplified Responses of Soil Organic Carbon to Nitrogen Addition Worldwide. Glob. Change Biol. 2021, 27, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Novais, S.V.; Zenero, M.D.O.; Frade, E.F., Jr.; Novais, R.F. Changes in Organic Matter of an Oxisol with the Addition of Soybean and Corn Residues, Nitrogen and Phosphorus. Commun. Soil Sci. Plant Anal. 2018, 49, 1208–1217. [Google Scholar] [CrossRef]
- Ugarte, C.M.; Kwon, H.; Andrews, S.S.; Wander, M.M. A Meta-Analysis of Soil Organic Matter Response to Soil Management Practices: An Approach to Evaluate Conservation Indicators. J. Soil Water Conserv. 2014, 69, 422–430. [Google Scholar] [CrossRef]
- Zhang, K.P.; Li, Z.X.; Li, Y.F.; Wan, P.X.; Chai, N.; Li, M.; Wei, H.H.; Kuzyakov, Y.; Filimonenko, E.; Alharbi, S.A.; et al. Contrasting Impact of Plastic Film Mulching and Nitrogen Fertilization on Soil Organic Matter Turnover. Geoderma 2023, 440, 116714. [Google Scholar] [CrossRef]
- Cao, H.B.; Xie, J.Y.; Hong, J.; Wang, X.; Hu, W.; Hong, J.P. Organic Matter Fractions within Macroaggregates in Response to Long-Term Fertilization in Calcareous Soil after Reclamation. J. Integr. Agric. 2021, 20, 1636–1648. [Google Scholar] [CrossRef]
- Dullau, S.; Kirmer, A.; Tischew, S.; Holz, F.; Meyer, M.H.; Schmidt, A. Effects of Fertilizer Levels and Drought Conditions on Species Assembly and Biomass Production in the Restoration of A Mesic Temperate Grassland on Ex-Arable Land. Glob. Ecol. Conserv. 2023, 48, e02730. [Google Scholar] [CrossRef]
- van der Wal, A.; Geydan, T.D.; Kuyper, T.W.; de Boer, W. A Thready Affair: Linking Fungal Diversity and Community Dynamics to Terrestrial Decomposition Processes. FEMS Microbiol. Rev. 2013, 37, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.; Schaeffer, S. Microbial Control over Carbon Cycling in Soil. Front. Microbiol. 2012, 3, 348. [Google Scholar] [CrossRef] [PubMed]
- Domeignoz-Horta, L.A.; Pold, G.; Liu, X.J.A.; Frey, S.D.; Melillo, J.M.; DeAngelis, K.M. Microbial Diversity Drives Carbon Use Efficiency in a Model Soil. Nat. Commun. 2020, 11, 3684. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Jiang, X.; Liu, G.; Yao, L.G.; Liu, W.Z.; Pan, Y.T.; Zuo, Y.X. Environmental Factors and Microbial Diversity and Abundance Jointly Regulate Soil Nitrogen and Carbon Biogeochemical Processes in Tibetan Wetlands. Environ. Sci. Technol. 2020, 54, 3267–3277. [Google Scholar] [CrossRef]
- Qiu, Q.Y.; Wu, L.F.; Ouyang, Z.; Li, B.B.; Xu, Y.Y. Different Effects of Plant-Derived Dissolved Organic Matter (DOM) and Urea on the Priming of Soil Organic Carbon. Environ. Sci. Process. Impacts 2016, 18, 330–341. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Zhu, B.; Cheng, W.X. Root Effects on Soil Organic Carbon: A Double-Edged Sword. New Phytol. 2021, 230, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Yu, J.L.; Zhu, L.Y.; Gikas, P.; He, Y.; Xiao, Y.L.; Deng, S.H.; Zhang, Y.Z.; Zhang, S.R.; Zhou, W.; et al. Nitrogen Addition may Promote Soil Organic Carbon Storage and CO2 Emission but Reduce Dissolved Organic Carbon in Zoige Peatland. J. Environ. Manag. 2022, 324, 116376. [Google Scholar] [CrossRef]
- Tripolskaja, L.; Toleikiene, M.; Skersiene, A.; Versuliene, A. Biomass of Shoots and Roots of Multicomponent Grasslands and Their Impact on Soil Carbon Accumulation in Arenosol Rich in Stones. Land 2024, 13, 1098. [Google Scholar] [CrossRef]
- Liu, M.X.; Shang, F.; Lu, X.J.; Huang, X.; Song, Y.; Liu, B.; Zhang, Q.; Liu, X.J.; Cao, J.J.; Xu, T.T.; et al. Unexpected Response of Nitrogen Deposition to Nitrogen Oxide Controls and Implications for Land Carbon Sink. Nat. Commun. 2022, 13, 3126. [Google Scholar] [CrossRef] [PubMed]



| Factors | Acronyms | Treatments Represented |
|---|---|---|
| Pasture species | B | Monocultured Bromus inermis |
| M | Monocultured Medicago varia | |
| BM | Bromus inermis and Medicago varia mixed sowing (1:1) | |
| Nitrogen addition | H | High nitrogen (150 kg N hm−2 y−1) |
| L | Low nitrogen (75 kg N hm−2 y−1) | |
| Z | CK (0 kg N hm−2 y−1) | |
| Water addition | Y | Dry-season supplementation |
| N | No supplementation |
| Soil Layers | SOC (g kg−1) | TN (g kg−1) | TP (g kg−1) | TK (g kg−1) | AHN (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) | pH |
|---|---|---|---|---|---|---|---|---|
| 0–10 cm | 13.62 | 2.20 | 0.52 | 25.76 | 207.04 | 16.25 | 315.96 | 6.77 |
| 10–20 cm | 13.34 | 2.19 | 0.49 | 25.59 | 219.88 | 11.95 | 249.49 | 6.79 |
| 20–40 cm | 10.23 | 1.70 | 0.42 | 24.49 | 161.25 | 8.89 | 192.36 | 7.34 |
| 40–60 cm | 5.08 | 1.02 | 0.35 | 23.59 | 104.78 | 7.68 | 148.77 | 7.94 |
| Factors | F (BGB) |
|---|---|
| Pasture | 58.596 *** |
| Nitrogen | 6.834 ** |
| Water | 3.975 * |
| Age | 29.939 *** |
| Pasture × Nitrogen | 3.924 ** |
| Pasture × Water | 2.023 |
| Pasture × Age | 5.597 *** |
| Nitrogen × Water | 0.256 |
| Nitrogen × Age | 1.76 |
| Water × Age | 0.304 |
| Pasture × Nitrogen × Water | 1.029 |
| Pasture × Nitrogen × Age | 0.524 |
| Pasture × Water × Age | 0.71 |
| Nitrogen × Water × Age | 0.763 |
| Pasture × Nitrogen × Water × Age | 0.801 |
| Factors | F (ΔSOC%) | F (SOCs) |
|---|---|---|
| Pasture | 0.469 | 2.06 |
| Nitrogen | 2.478 | 1.00 |
| Water | 0.746 | 0.10 |
| Age | 45.185 *** | 222.26 *** |
| Soil Depth | 27.014 *** | 1.07 |
| Pasture × Nitrogen | 2.921 * | 1.31 |
| Pasture × Water | 0.002 | 1.12 |
| Pasture × Age | 0.899 | 0.26 |
| Pasture × Soil Depth | 1.371 | 0.16 |
| Nitrogen × Water | 0.115 | 0.39 |
| Nitrogen × Age | 1.717 | 0.24 |
| Nitrogen × Soil Depth | 0.76 | 0.10 |
| Water × Age | 0.825 | 1.71 |
| Water × Soil Depth | 0.024 | 0.62 |
| Age × Soil Depth | 6.449 *** | 9.40 *** |
| Pasture × Nitrogen × Water | 2.3 | 1.19 |
| Pasture × Nitrogen × Age | 1.159 | 0.34 |
| Pasture × Nitrogen × Soil Depth | 2.246 ** | 0.54 |
| Pasture × Water × Age | 0.584 | 0.44 |
| Pasture × Water × Soil Depth | 0.564 | 0.41 |
| Pasture × Age × Soil Depth | 0.522 | 0.66 |
| Nitrogen × Water × Age | 1.62 | 0.44 |
| Nitrogen × Water × Soil Depth | 3.182 ** | 0.32 |
| Nitrogen × Age × Soil Depth | 0.48 | 0.39 |
| Water × Age × Soil Depth | 0.363 | 0.21 |
| Pasture × Nitrogen × Water × Age | 0.473 | 0.58 |
| Pasture × Nitrogen × Water × Soil Depth | 2.284 ** | 0.05 |
| Pasture × Nitrogen × Age × Soil Depth | 0.584 | 0.31 |
| Pasture × Water × Age × Soil Depth | 0.426 | 0.51 |
| Nitrogen × Water × Age × Soil Depth | 0.193 | 0.54 |
| Pasture × Nitrogen × Water × Age × Soil Depth | 0.619 | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuo, M.; Xu, L.; Yuan, B.; Nie, Y.; Wei, J. Pastureland Soil Organic Carbon Storage Regulated by Pasture Species and Age Under Nitrogen and Water Addition in Northern China. Agronomy 2025, 15, 399. https://doi.org/10.3390/agronomy15020399
Cuo M, Xu L, Yuan B, Nie Y, Wei J. Pastureland Soil Organic Carbon Storage Regulated by Pasture Species and Age Under Nitrogen and Water Addition in Northern China. Agronomy. 2025; 15(2):399. https://doi.org/10.3390/agronomy15020399
Chicago/Turabian StyleCuo, Meji, Lijun Xu, Bo Yuan, Yingying Nie, and Jiaqiang Wei. 2025. "Pastureland Soil Organic Carbon Storage Regulated by Pasture Species and Age Under Nitrogen and Water Addition in Northern China" Agronomy 15, no. 2: 399. https://doi.org/10.3390/agronomy15020399
APA StyleCuo, M., Xu, L., Yuan, B., Nie, Y., & Wei, J. (2025). Pastureland Soil Organic Carbon Storage Regulated by Pasture Species and Age Under Nitrogen and Water Addition in Northern China. Agronomy, 15(2), 399. https://doi.org/10.3390/agronomy15020399






