Optimizing Tomato Seedling Production in the Tropics: Effects of Trichoderma, Arbuscular Mycorrhizal Fungi, and Key Agronomical Factors
Abstract
1. Introduction
2. Materials and Methods
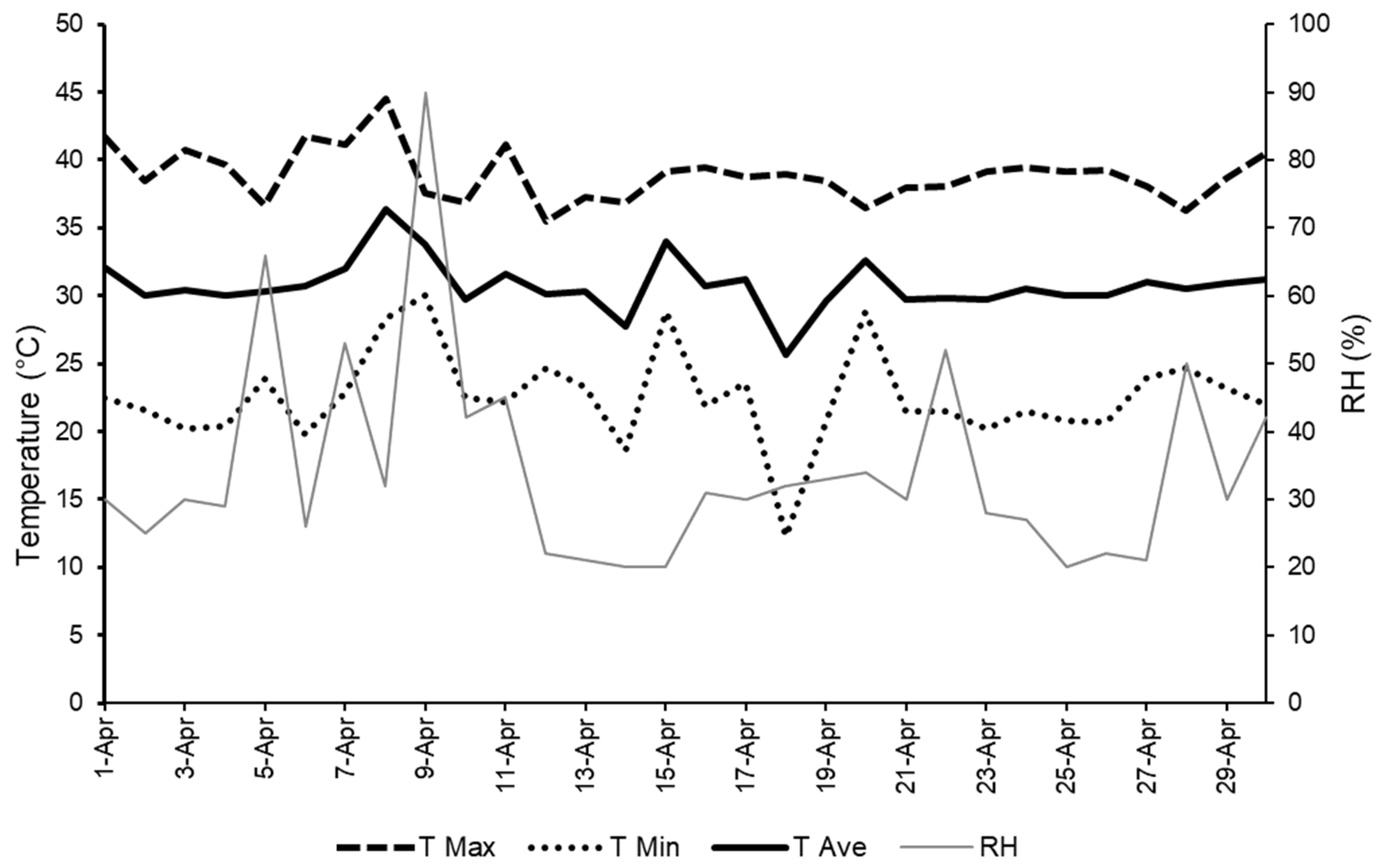
2.1. Location and Climatic Conditions
2.2. Treatments and Experimental Design
2.3. Crop Management
2.4. Biometric Parameters
2.5. Dry Matter Yield and Partitioning
2.6. Leaf Temperature
2.7. Quality Parameters
2.8. Climatic Parameters
2.9. Statistical Analysis
3. Results
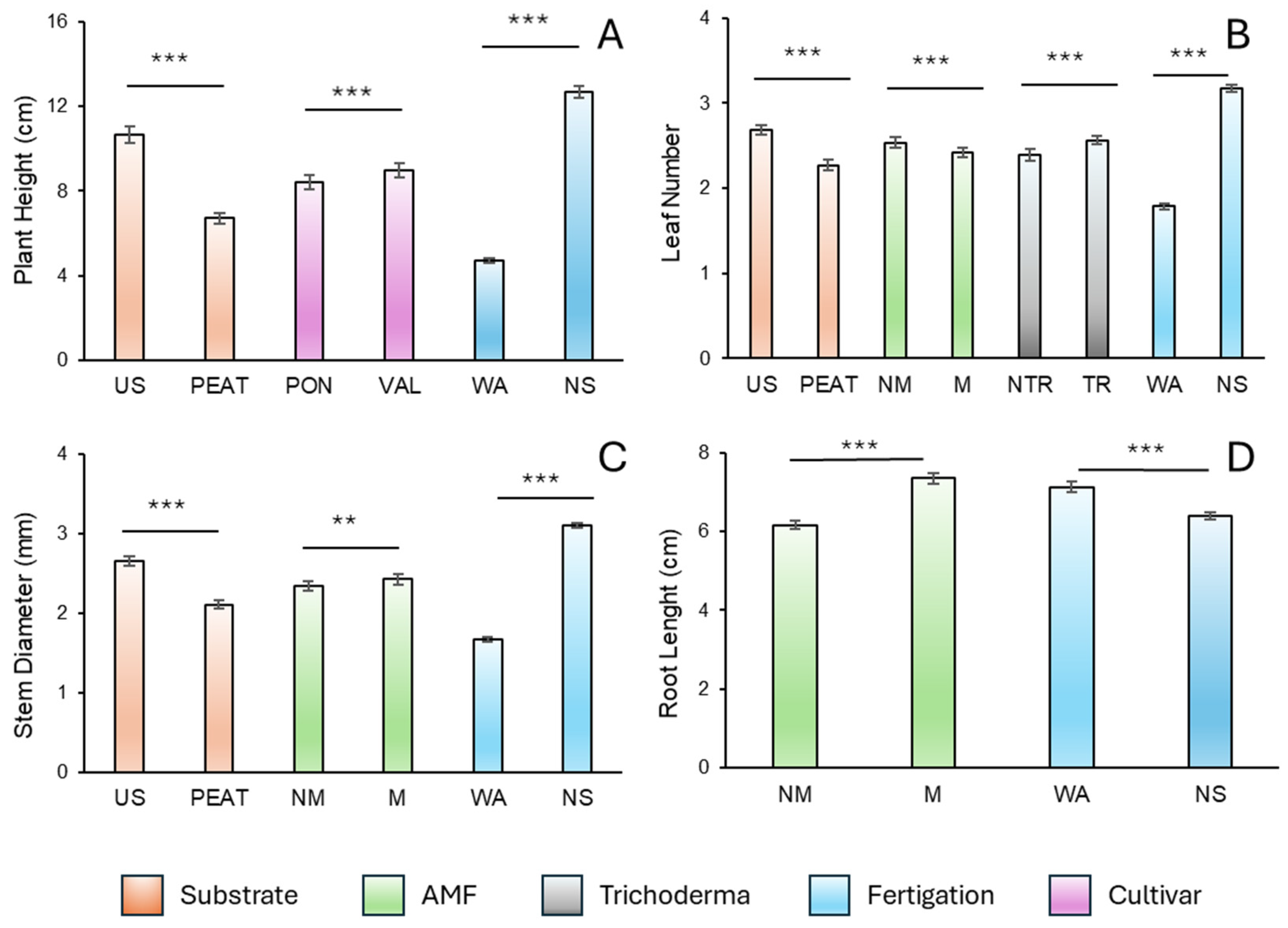
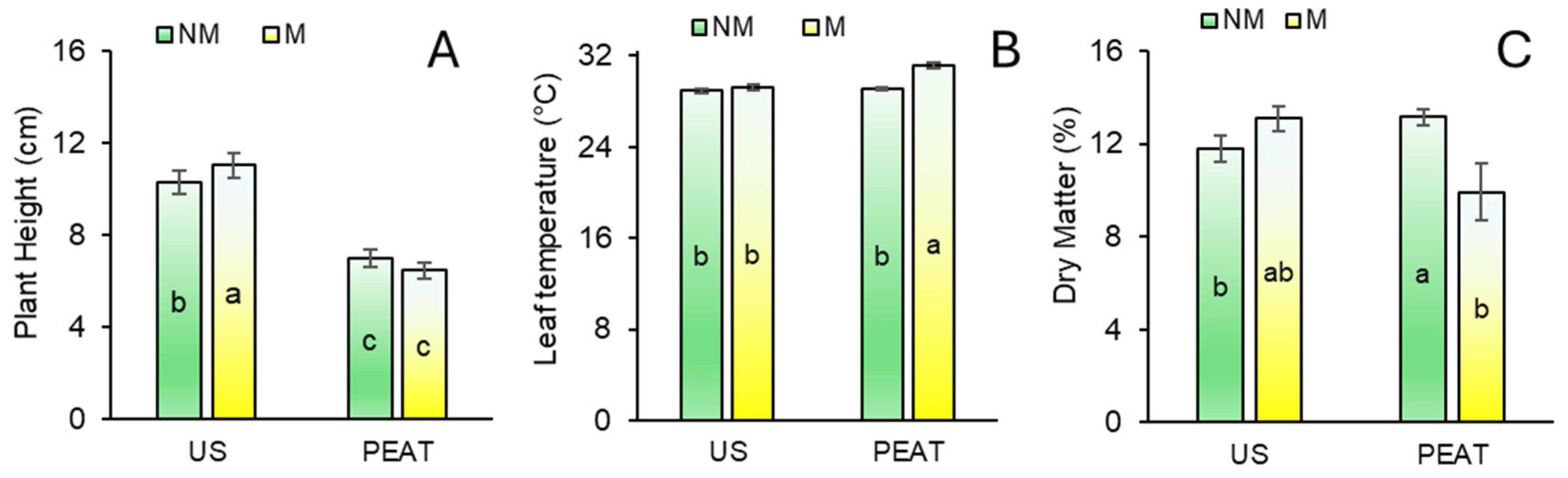
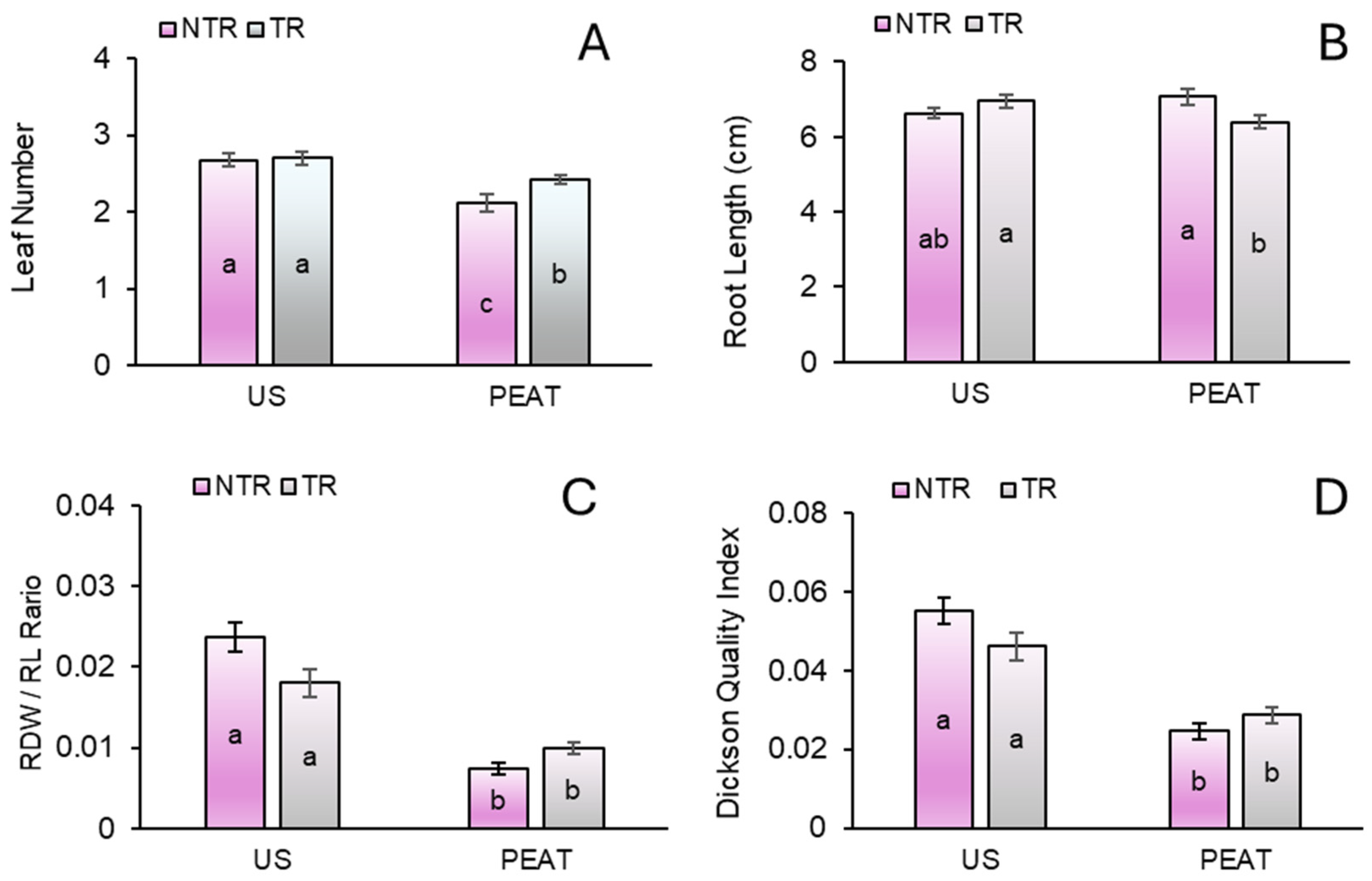
3.1. Responses of Biometric Parameters
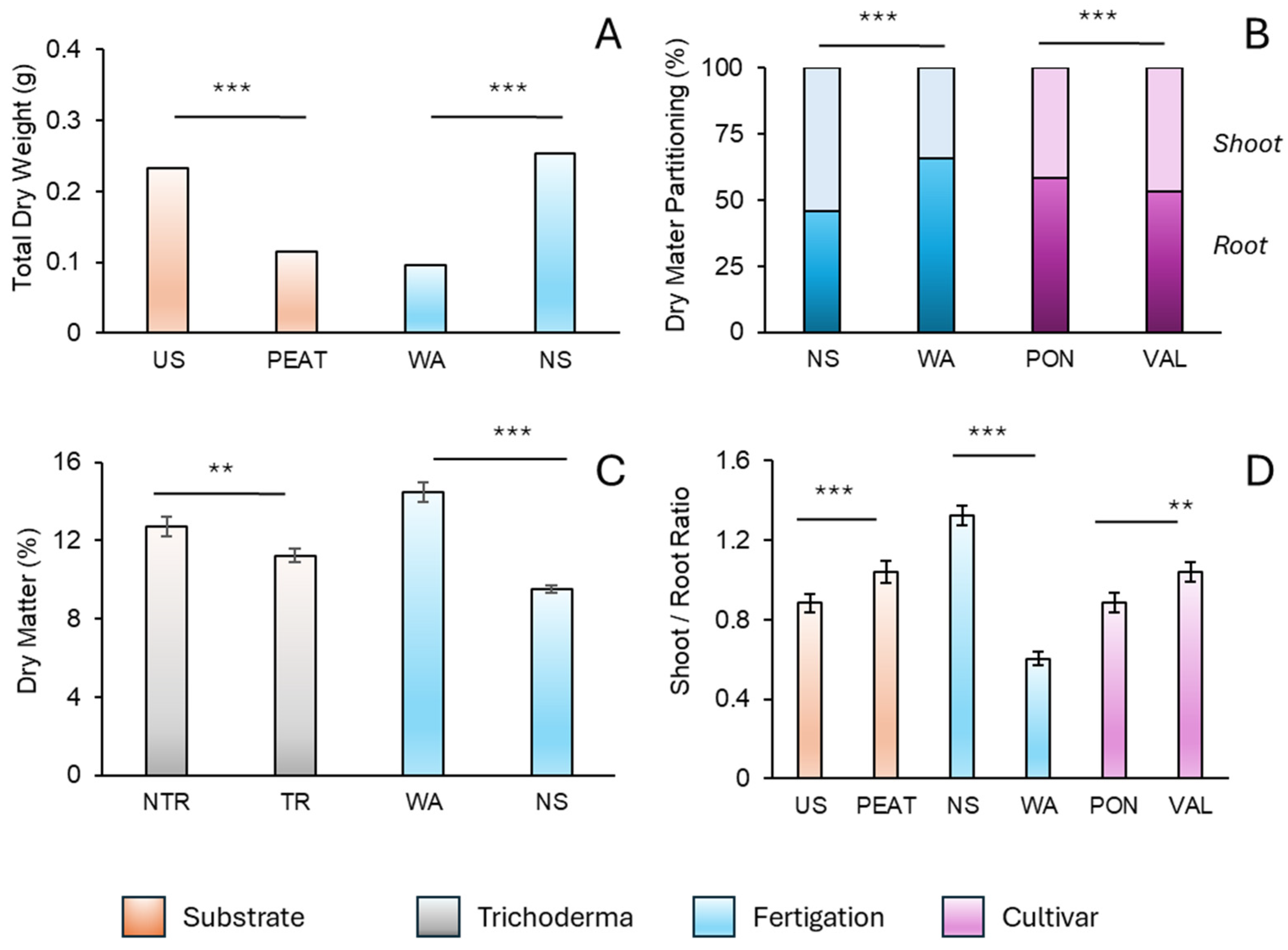
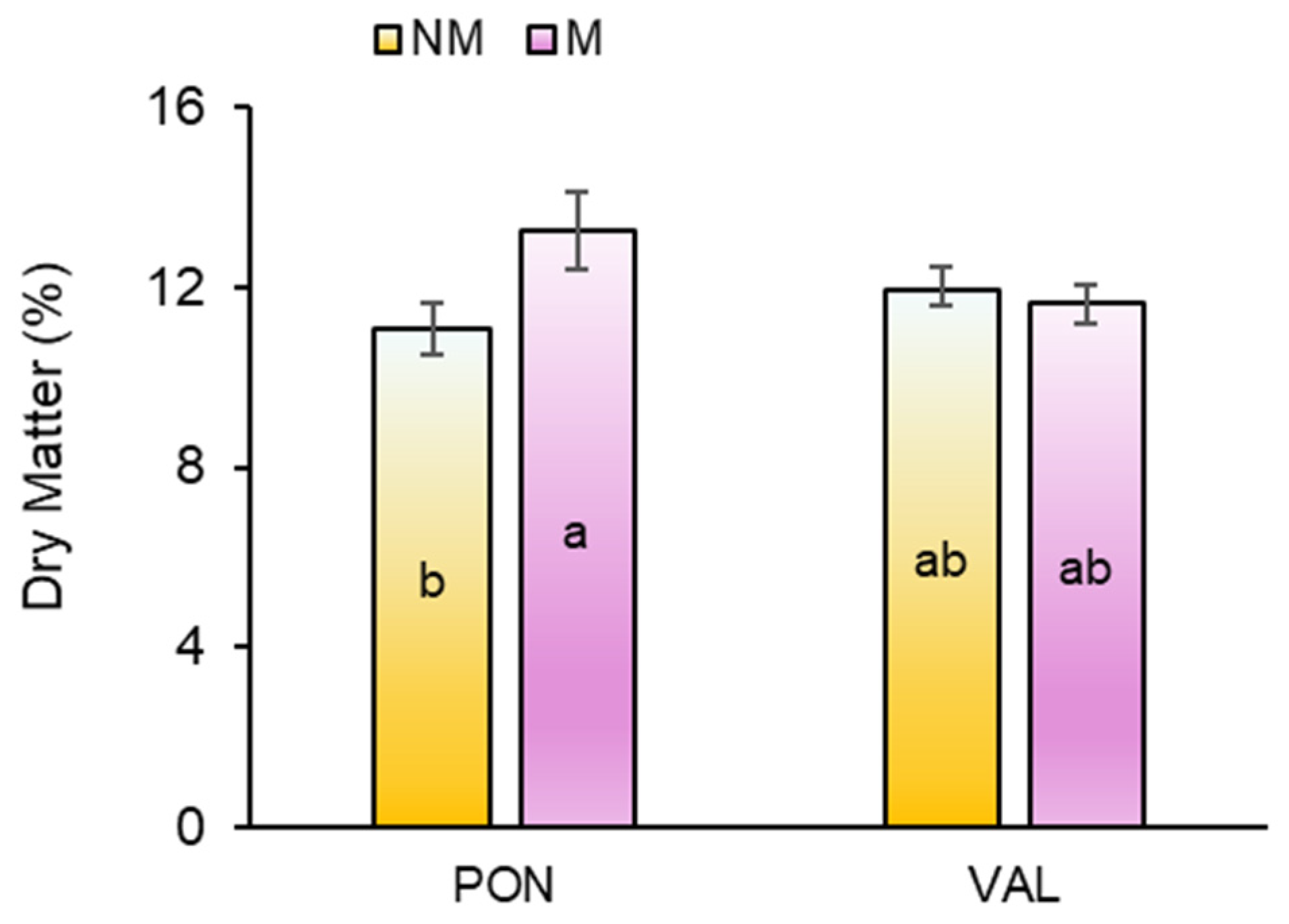
3.2. Biomass Yield and Partitioning
3.3. Leaf Temperature Responses
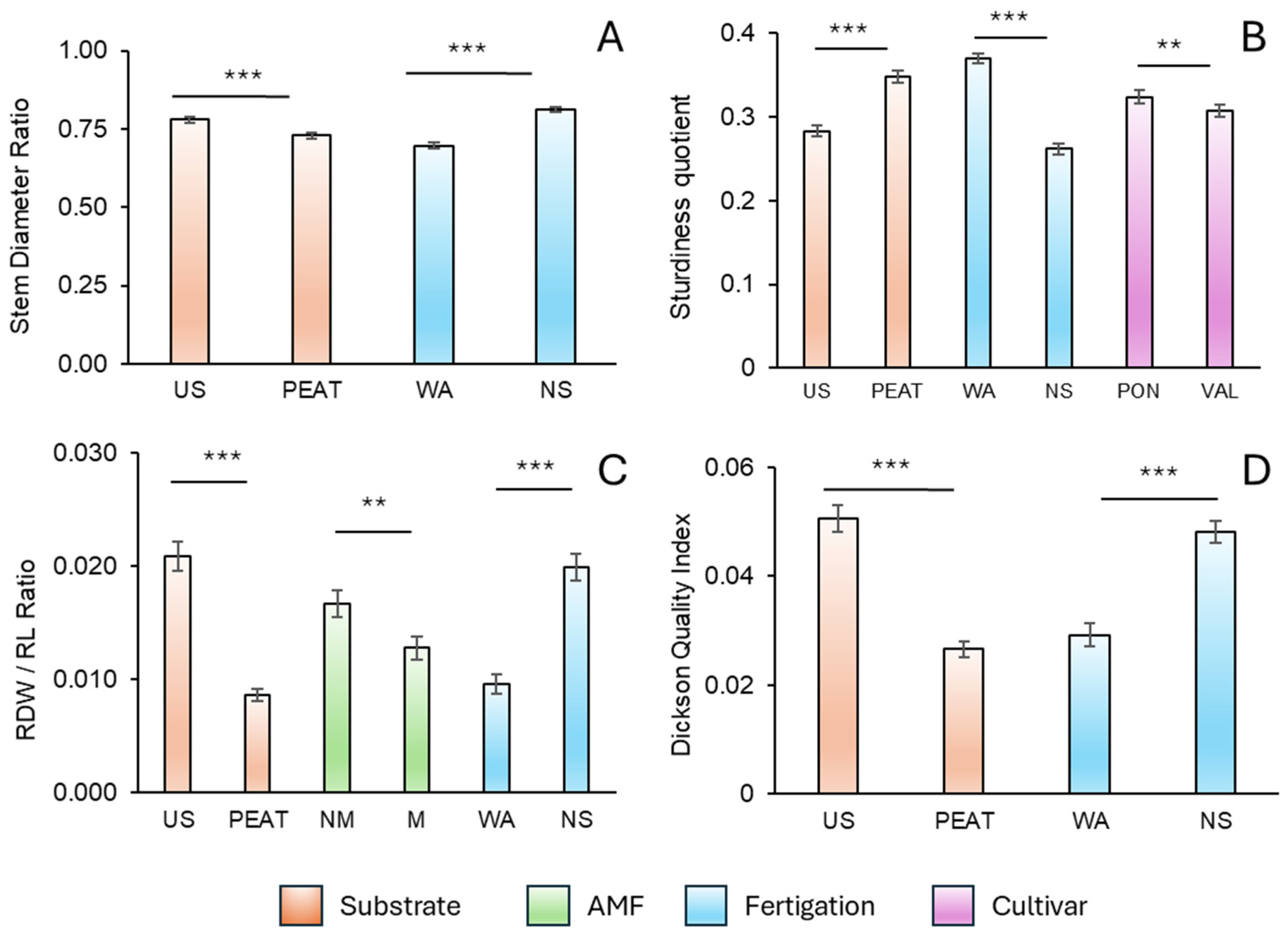
3.4. Qualitative Parameters
3.5. Significant Interactions Among Studied Variables
3.6. Principal Component Analysis (PCA)
4. Discussion
4.1. Fertigation
4.2. Substrates
4.3. Arbuscular Mycorrhiza Fungi
4.4. Trichoderma
4.5. Interactions
4.6. Dickson Quality Index
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peguero, F.; Zapata, S.; Sandoval, L. Agricultural Production of Central America and the Caribbean: Challenges and Opportunities. Choices 2019, 34, 1–10. [Google Scholar] [CrossRef]
- Eckstein, D.; Künzel, V.; Schäfer, L. Global Climate Risk Index 2021; Germanwatch: Bonn, Germany, 2021. [Google Scholar]
- Hammond, J.; Fraval, S.; Van Etten, J.; Suchini, J.G.; Mercado, L.; Pagella, T.; Frelat, R.; Lannerstad, M.; Douxchamps, S.; Teufel, N.; et al. The Rural Household Multi-Indicator Survey (RHoMIS) for rapid characterisation of households to inform climate smart agriculture interventions: Description and applications in East Africa and Central America. Agric. Syst. 2017, 151, 225–233. [Google Scholar] [CrossRef]
- Baca, M.; Läderach, P.; Haggar, J.; Schroth, G.; Ovalle, O. An Integrated Framework for Assessing Vulnerability to Climate Change and Developing Adaptation Strategies for Coffee Growing Families in Mesoamerica. PLoS ONE 2014, 9, e88463. [Google Scholar] [CrossRef]
- Schmidt, A.; Eitzinger, A.; Sonder, K.; Sain, G.; Rodriguez, B. Tortillas on the Roaster (TOR) Central American Maize-Bean Systems and the Changing Climate Full Technical Report; CRS: Baltimore, MD, USA; ICAT: Cali, Colombia; CIMMYT: CIMMYT: Mexico City, Mexico, 2012; p. 127. [Google Scholar]
- FAOSTAT 2024. Food and Agriculture Organization of the United Nations FAO FAOSTAT. Available online: https://www.fao.org/faostat/en/#data (accessed on 25 October 2024).
- Kaveh, H.; Nemati, H.; Farsi, M.; Jartoodeh, S.V. How salinity affect germination and emergence of tomato lines. J. Biol. Environ. Sci. 2011, 5, 159–163. [Google Scholar]
- Li, P.-S.; Kong, W.-L.; Wu, X.-Q. Salt Tolerance Mechanism of the Rhizosphere Bacterium JZ-GX1 and Its Effects on Tomato Seed Germination and Seedling Growth. Front. Microbiol. 2021, 12, 657238. [Google Scholar] [CrossRef]
- Nafees, K.; Kumar, M.; Bose, B. Effect of Different Temperatures on Germination and Seedling Growth of Primed Seeds of Tomato. Russ. J. Plant Physiol. 2019, 66, 778–784. [Google Scholar] [CrossRef]
- Chohan, S.; Perveen, R.; Abid, M.; Naqvi, A.H.; Naz, S. Management of seed borne fungal diseases of tomato: A review. Pak. J. Phytopathol. 2017, 29, 193–200. [Google Scholar]
- Cammarano, D.; Ronga, D.; Di Mola, I.; Mori, M.; Parisi, M. Impact of climate change on water and nitrogen use efficiencies of processing tomato cultivated in Italy. Agric. Water Manag. 2020, 241, 106336. [Google Scholar] [CrossRef]
- Basu, S.; Rabara, R.C.; Negi, S. AMF: The future prospect for sustainable agriculture. Physiol. Mol. Plant Pathol. 2018, 102, 36–45. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.-J.; Hao, Z.-P.; Li, H.; Chen, B.-D. Aquaporin genes GintAQPF1 and GintAQPF2 from Glomus intraradices contribute to plant drought tolerance. Plant Signal. Behav. 2013, 8, e24030. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, Y.; Dalpé, Y.; Séguin, S.; Fernández, K.; Fernández, F.; Rivera, R.A. Glomus cubense sp. nov., an arbuscular mycorrhizal fungus from Cuba. Mycotaxon 2012, 118, 337–347. [Google Scholar] [CrossRef]
- Mujica-Pérez, Y.; Medina-Carmona, A.; Rodríguez-Guerra, E. Inoculation of arbuscular mycorrhizal fungi and plant growth promoting bacteria in peanut crop (Arachis hypogaea L.). Cultiv. Trop. 2017, 38, 15–21. [Google Scholar]
- Mujica-Pérez, Y.; Mena-Echevarría, A.; Medina-Carmona, A.; Rosales Jenquis, P.R. Tomato (Solanum lycopersicum L.) plants response to liquid biofertilization with Glomus cubense. Cultiv. Trop. 2014, 35, 21–26. [Google Scholar]
- Bhandari, S.; Pandey, K.R.; Joshi, Y.R.; Lamichhane, S.K. An overview of multifaceted role of Trichoderma spp. for sustainable agriculture. Arch. Agric. Environ. Sci. 2021, 6, 72–79. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Stracquadanio, C.; Quiles, J.M.; Meca, G.; Cacciola, S.O. Antifungal Activity of Bioactive Metabolites Produced by Trichoderma asperellum and Trichoderma atroviride in Liquid Medium. JoF 2020, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Khoshmanzar, E.; Aliasgharzad, N.; Neyshabouri, M.R.; Khoshru, B.; Arzanlou, M.; Asgari Lajayer, B. Effects of Trichoderma isolates on tomato growth and inducing its tolerance to water-deficit stress. Int. J. Environ. Sci. Technol. 2020, 17, 869–878. [Google Scholar] [CrossRef]
- Raviv, M. The use of mycorrhiza in organically-grown crops under semi arid conditions: A review of benefits, constraints and future challenges. Symbiosis 2010, 52, 65–74. [Google Scholar] [CrossRef]
- Moncada, A.; Vetrano, F.; Esposito, A.; Miceli, A. Fertigation Management and Growth-Promoting Treatments Affect Tomato Transplant Production and Plant Growth after Transplant. Agronomy 2020, 10, 1504. [Google Scholar] [CrossRef]
- Machado-Santos, J.A.; Matos-Andrade, T.; Barreto-Garcez, T. Production of San Marzano tomato seedlings submitted to mixed fertilizer rates and application forms. Rev. Bras. Ciênc. Agrár. 2023, 18, 8. Available online: http://www.agraria.pro.br/ojs32/index.php/RBCA/article/view/v18i3a2967/1564 (accessed on 30 November 2024).
- Kaçiu, S.; Fetahu, S.; Aliu, S.; Ramadani, S.; Rusinovci, I. Influence of different types of substrates in growth intensity of seedlings for different hybrids of tomato (Lycopersicon esculentum Mill.). Sjemenarstvo 2009, 26, 47–54. [Google Scholar]
- Mualchin, I.H.; Verma, D. A Review on Different Types of Media and Their Effects on Tomato Seedling Production. J. Nat. Sci. Res. 2022, 13, 38–43. [Google Scholar] [CrossRef]
- Chowdhury, M.; Espinoza-Ayala, A.; Samarakoon, U.C.; Altland, J.E.; Yang, T. Substrate Comparison for Tomato Propagation under Different Fertigation Protocols. Agriculture 2024, 14, 382. [Google Scholar] [CrossRef]
- Herrera, F.; Castillo, J.E.; Chica, A.F.; López Bellido, L. Use of municipal solid waste compost (MSWC) as a growing medium in the nursery production of tomato plants. Bioresour. Technol. 2008, 99, 287–296. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Metz 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Fonteno, W.C.; Harden, C.T. Procedures for Determining Physical Properties of Horticultural Substrates Using the NCSU Porometer; Horticultural Substrates Laboratory, North Carolina State University: Raleigh, NC, USA, 2003; Available online: https://www.icmag.com/attachments/procedures-for-determining-physical-properties-of-horticultural-substrates-using-the-ncsu-porome-pdf.12600063/ (accessed on 30 November 2024).
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Quality appraisal of white spruce and white pine seedling stock in nurseries. For. Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- Gallegos-Cedillo, V.M.; Diánez, F.; Nájera, C.; Santos, M. Plant Agronomic Features Can Predict Quality and Field Performance: A Bibliometric Analysis. Agronomy 2021, 11, 2305. [Google Scholar] [CrossRef]
- R-Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Length, R.V. emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.7.0. 2021. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 30 November 2024).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. R Package Version 1.1.4. 2023. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 30 November 2024).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Özer, H. The Effects Of Different Seedling Production Systems on Quality of Tomato Plantlets. Acta Sci. Pol. Hortorum Cultus 2018, 17, 15–21. [Google Scholar] [CrossRef]
- Chi, S.H.; Shinohara, Y.; Suzuki, Y. Effect of concentration of nutrient solution and aeration on growth and dry matter partitioning in hydroponically grown young tomato plants. Environ. Control. Bioolyl. 1991, 29, 27–33. [Google Scholar] [CrossRef]
- Santos, S.T.D.; De Oliveira, F.D.A.; De Medeiros Costa, J.P.B.; Souza Neta, M.L.D.; Alves, R.D.C.; Costa, L.P. Qualidade de mudas de cultivares de tomateiro em função de soluções nutritivas de concentrações crescentes. Rev. Agro@ Mbiente On-Line 2017, 10, 326. [Google Scholar] [CrossRef][Green Version]
- Li, C.; Yang, Z.; Zhang, C.; Luo, J.; Zhang, F.; Qiu, R. Effects of Nitrogen Application in Recovery Period after Different High Temperature Stress on Plant Growth of Greenhouse Tomato at Flowering and Fruiting Stages. Agronomy 2023, 13, 1439. [Google Scholar] [CrossRef]
- Luo, J.; Yang, Z.; Zhang, F.; Li, C. Effect of nitrogen application on enhancing high-temperature stress tolerance of tomato plants during the flowering and fruiting stage. Front. Plant Sci. 2023, 14, 1172078. [Google Scholar] [CrossRef]
- Liu, K.; Deng, J.; Lu, J.; Wang, X.; Lu, B.; Tian, X.; Zhang, Y. High Nitrogen Levels Alleviate Yield Loss of Super Hybrid Rice Caused by High Temperatures During the Flowering Stage. Front. Plant Sci. 2019, 10, 357. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, C.; Yan, H.; Li, J.; Ren, J.; Akhlaq, M.; Hameed, M.U.; Disasa, K.N. Physiological Response of Tomato and Cucumber Plants to Micro-Spray in High-Temperature Environment: A Scientific and Effective Means of Alleviating Crop Heat Stress. Agronomy 2023, 13, 2798. [Google Scholar] [CrossRef]
- Lynch, J.; Marschner, P.; Rengel, Z. Effect of Internal and External Factors on Root Growth and Development. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 331–346. ISBN 978-0-12-384905-2. [Google Scholar]
- White, P.J. Ion Uptake Mechanisms of Individual Cells and Roots. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 7–47. ISBN 978-0-12-384905-2. [Google Scholar]
- Campos, C.A.B.; Fernandes, P.D.; Gheyi, H.R.; Blanco, F.F.; Gonçalves, C.B.; Campos, S.A.F. Tomato growth and dry matter partitioning as a function of the irrigation water quality. Rev. Ciên. Agron. 2007, 38, 239–246. [Google Scholar]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- El Ministerio de Agricultura y Ganadería. Guía De Precios De Insumos Agropecuarios 2022; El Ministerio de Agricultura y Ganadería: Santa Tecla, El Salvador, 2022. [Google Scholar]
- Markovic, V.; Djurovka, M.; Ilin, Z. The Effect of Seedling Quality on Tomato Yield, Plant and Fruit Characteristics. Acta Hortic. 1997, 462, 163–170. [Google Scholar] [CrossRef]
- Jawaad Ati, M.; Jellani, G.; Humair Ahm, M.; Saleem, N.; Ullah, H.; Zameer Kha, M.; Ikram, S. Different Growth Media Effect the Germination and Growth of Tomato Seedlings. Sci. Technol. Dev. 2016, 35, 123–127. [Google Scholar] [CrossRef]
- Aleandri, M.P.; Chilosi, G.; Muganu, M.; Vettraino, A.; Marinari, S.; Paolocci, M.; Luccioli, E.; Vannini, A. On farm production of compost from nursery green residues and its use to reduce peat for the production of olive pot plants. Sci. Hortic. 2015, 193, 301–307. [Google Scholar] [CrossRef]
- Fryda, L.; Visser, R.; Schmidt, J. Biochar Replaces Peat in Horticulture: Environmental Impact Assessment of Combined Biochar & Bioenergy Production. Detritus 2018, 5, 1. [Google Scholar] [CrossRef]
- Pokluda, R.; Ragasová, L.; Jurica, M.; Kalisz, A.; Komorowska, M.; Niemiec, M.; Sekara, A. Effects of growth promoting microorganisms on tomato seedlings growing in different media conditions. PLoS ONE 2021, 16, e0259380. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Lorente, B.; Sánchez-Blanco, M.J.; Ortuño, M.F.; Nortes, P.A.; Alarcón, J.J. Influence of Mixed Substrate and Arbuscular Mycorrhizal Fungi on Photosynthetic Efficiency, Nutrient and Water Status and Yield in Tomato Plants Irrigated with Saline Reclaimed Waters. Water 2020, 12, 438. [Google Scholar] [CrossRef]
- Azarmi, R.; Hajieghrari, B.; Giglou, A. Effect of Trichoderma isolates on tomato seedling growth response and nutrient uptake. Afr. J. Biotechnol. 2011, 10, 5850–5855. [Google Scholar] [CrossRef]
- Cornejo-Ríos, K.; Osorno-Suárez, M.D.P.; Hernández-León, S.; Reyes-Santamaría, M.I.; Juárez-Díaz, J.A.; Pérez-España, V.H.; Peláez-Acero, A.; Madariaga-Navarrete, A.; Saucedo-García, M. Impact of Trichoderma asperellum on Chilling and Drought Stress in Tomato (Solanum lycopersicum). Horticulturae 2021, 7, 385. [Google Scholar] [CrossRef]
- Li, Y.-T.; Hwang, S.-G.; Huang, Y.-M.; Huang, C.-H. Effects of Trichoderma asperellum on nutrient uptake and Fusarium wilt of tomato. Crop Prot. 2018, 110, 275–282. [Google Scholar] [CrossRef]
- Cao, Q.; Liang, Y.; Tian, Y.; Lian, H.; Jiang, X.; Li, M. Survival Dynamics of Trichoderma longibrachiatum Tr58 in Conidia- and Chlamydospore-Amended Soils with Different Moisture Levels. Agriculture 2023, 13, 238. [Google Scholar] [CrossRef]
- Begoude, B.A.D.; Lahlali, R.; Friel, D.; Tondje, P.R.; Jijakli, M.H. Response surface methodology study of the combined effects of temperature, pH, and aw on the growth rate of Trichoderma asperellum. J. Appl. Microbiol. 2007, 103, 845–854. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Roldán, A.; Pascual, J.A. Interaction between arbuscular mycorrhizal fungi and Trichoderma harzianum under conventional and low input fertilization field condition in melon crops: Growth response and Fusarium wilt biocontrol. Appl. Soil Ecol. 2011, 47, 98–105. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Horton, T.R. Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. J. Ecol. 2009, 97, 1139–1150. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.T.; Wilson, J.A.; Miller, R.M.; Bowker, M.A. Mycorrhizal phenotypes and the L aw of the M inimum. New Phytol. 2015, 205, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, P.; Wang, X.; Li, Y.; Ji, B. Specific Plant Mycorrhizal Responses Are Linked to Mycorrhizal Fungal Species Interactions. Front. Plant Sci. 2022, 13, 930069. [Google Scholar] [CrossRef] [PubMed]
- Visconti, D.; Fiorentino, N.; Cozzolino, E.; Woo, S.L.; Fagnano, M.; Rouphael, Y. Can Trichoderma-Based Biostimulants Optimize N Use Efficiency and Stimulate Growth of Leafy Vegetables in Greenhouse Intensive Cropping Systems? Agronomy 2020, 10, 121. [Google Scholar] [CrossRef]
- Cai, F.; Chen, W.; Wei, Z.; Pang, G.; Li, R.; Ran, W.; Shen, Q. Colonization of Trichoderma harzianum strain SQR-T037 on tomato roots and its relationship to plant growth, nutrient availability and soil microflora. Plant Soil 2015, 388, 337–350. [Google Scholar] [CrossRef]
- Currey, C.J.; Torres, A.P.; Lopez, R.G.; Jacobs, D.F. The Quality Index—A New Tool for Integrating Quantitative Measurements to Assess Quality of Young Floriculture Plants. Acta Hortic. 2013, 1000, 385–391. [Google Scholar] [CrossRef]
- Nkurunziza, E.; Nyalala, S.; Umuhoza, K.N.J. Effect of seedling quality on growth, yield and quality of tomato (Solanum lycopersicum L.). Int. J. Hortic. Sci. 2022, 28, 64–72. [Google Scholar] [CrossRef]
- Thompson, B.E. Seedling morphological evaluation. In What Can You Tell by Looking; Forest Research Laboratory, Oregon State University: Corvallis, OR, USA, 1985; pp. 59–72. ISBN 978-0-87437-000-0. [Google Scholar]





| Parameter | Unit | Methodology | Peat | Forest Soil |
|---|---|---|---|---|
| Bulk density | g/L | EN 13040 (1999) | 240.6 ± 27.1 | 370.5 ± 34.6 |
| Total pore space | % | NCSU porometer * | 76.8 ± 3.9 | 79.4 ± 3.0 |
| Air-filled porosity | % | NCSU porometer | 36.0 ± 3.2 | 54.2 ± 3.5 |
| Water holding capacity | % | NCSU porometer | 40.8 ± 0.9 | 25.2 ± 1.8 |
| pH | EN 13037 (1999) | 6.17 ± 0.1 | 7.87 ± 0.1 | |
| Electrical conductivity | mS/cm | EN 13038 (1999) | 1.34 ± 0.1 | 3.32 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leuratti, T.; Fellin, L.; Michelon, N.; Palacios Tario, J.B.; Gutiérrez, J.E.S.; Gianquinto, G.; Orsini, F.; Zanin, G. Optimizing Tomato Seedling Production in the Tropics: Effects of Trichoderma, Arbuscular Mycorrhizal Fungi, and Key Agronomical Factors. Agronomy 2025, 15, 392. https://doi.org/10.3390/agronomy15020392
Leuratti T, Fellin L, Michelon N, Palacios Tario JB, Gutiérrez JES, Gianquinto G, Orsini F, Zanin G. Optimizing Tomato Seedling Production in the Tropics: Effects of Trichoderma, Arbuscular Mycorrhizal Fungi, and Key Agronomical Factors. Agronomy. 2025; 15(2):392. https://doi.org/10.3390/agronomy15020392
Chicago/Turabian StyleLeuratti, Teresa, Lorenzo Fellin, Nicola Michelon, Juan Bosco Palacios Tario, Jaime Ernesto Santamaria Gutiérrez, Giorgio Gianquinto, Francesco Orsini, and Giampaolo Zanin. 2025. "Optimizing Tomato Seedling Production in the Tropics: Effects of Trichoderma, Arbuscular Mycorrhizal Fungi, and Key Agronomical Factors" Agronomy 15, no. 2: 392. https://doi.org/10.3390/agronomy15020392
APA StyleLeuratti, T., Fellin, L., Michelon, N., Palacios Tario, J. B., Gutiérrez, J. E. S., Gianquinto, G., Orsini, F., & Zanin, G. (2025). Optimizing Tomato Seedling Production in the Tropics: Effects of Trichoderma, Arbuscular Mycorrhizal Fungi, and Key Agronomical Factors. Agronomy, 15(2), 392. https://doi.org/10.3390/agronomy15020392









