Transcriptome and Metabolome Analysis of Organic and Chemical Fertilizer Effects on Highland Barley Growth and Nutrient Utilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Study of Plant Growth and Root Morphology
2.3. Determination of Nutrient Uptake by Plants
2.4. RNA Extraction and Sequencing
2.5. Transcriptome Analysis
2.6. Metabolite Extraction and UHPLC-MS/MS Analysis
2.7. Combined Transcriptome and Metabolome Analyses
2.8. Assessment of Enzymatic Activities in Nitrogen Metabolism
2.9. Data Analysis
3. Results
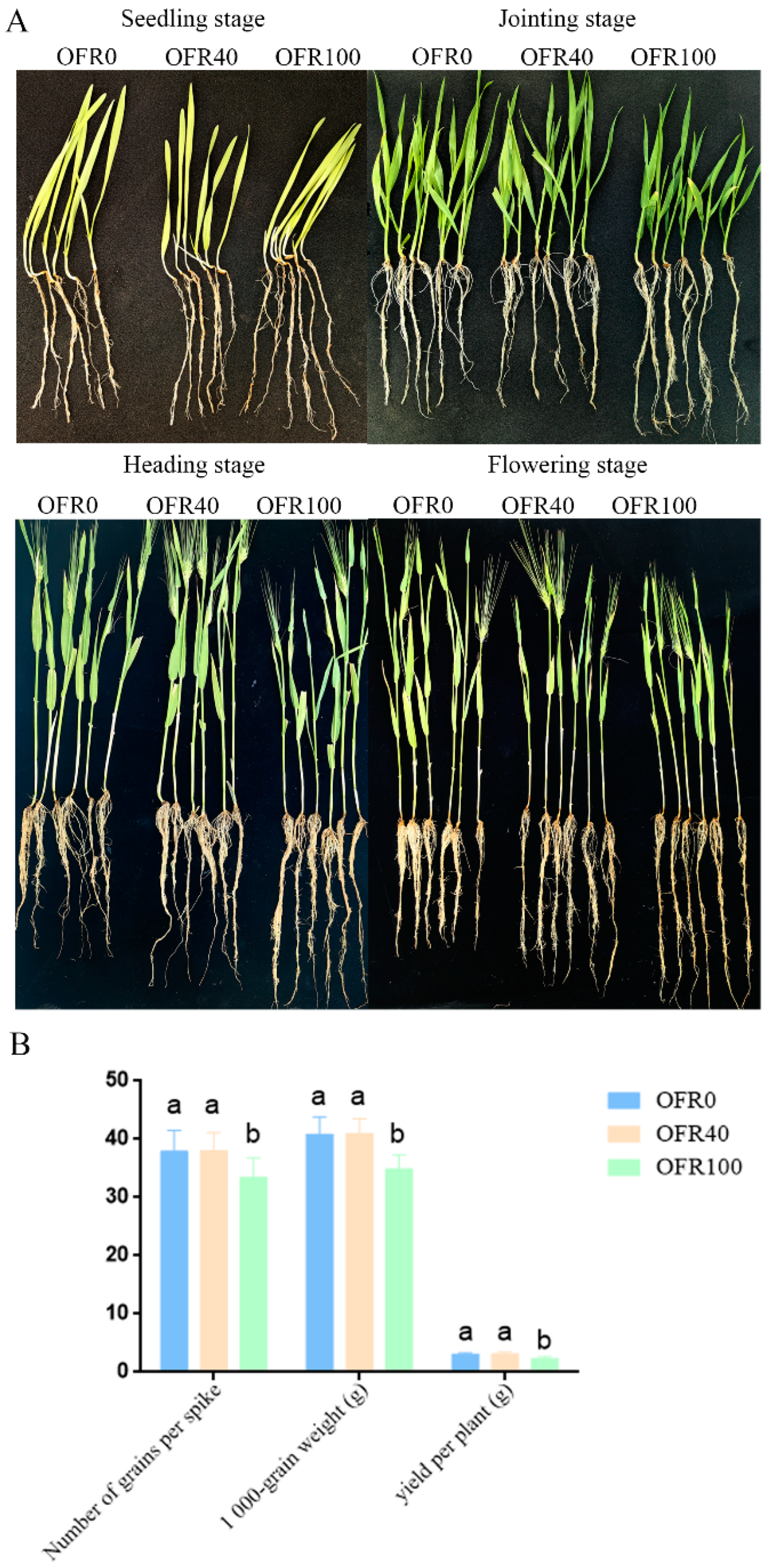
3.1. Growth and Yield
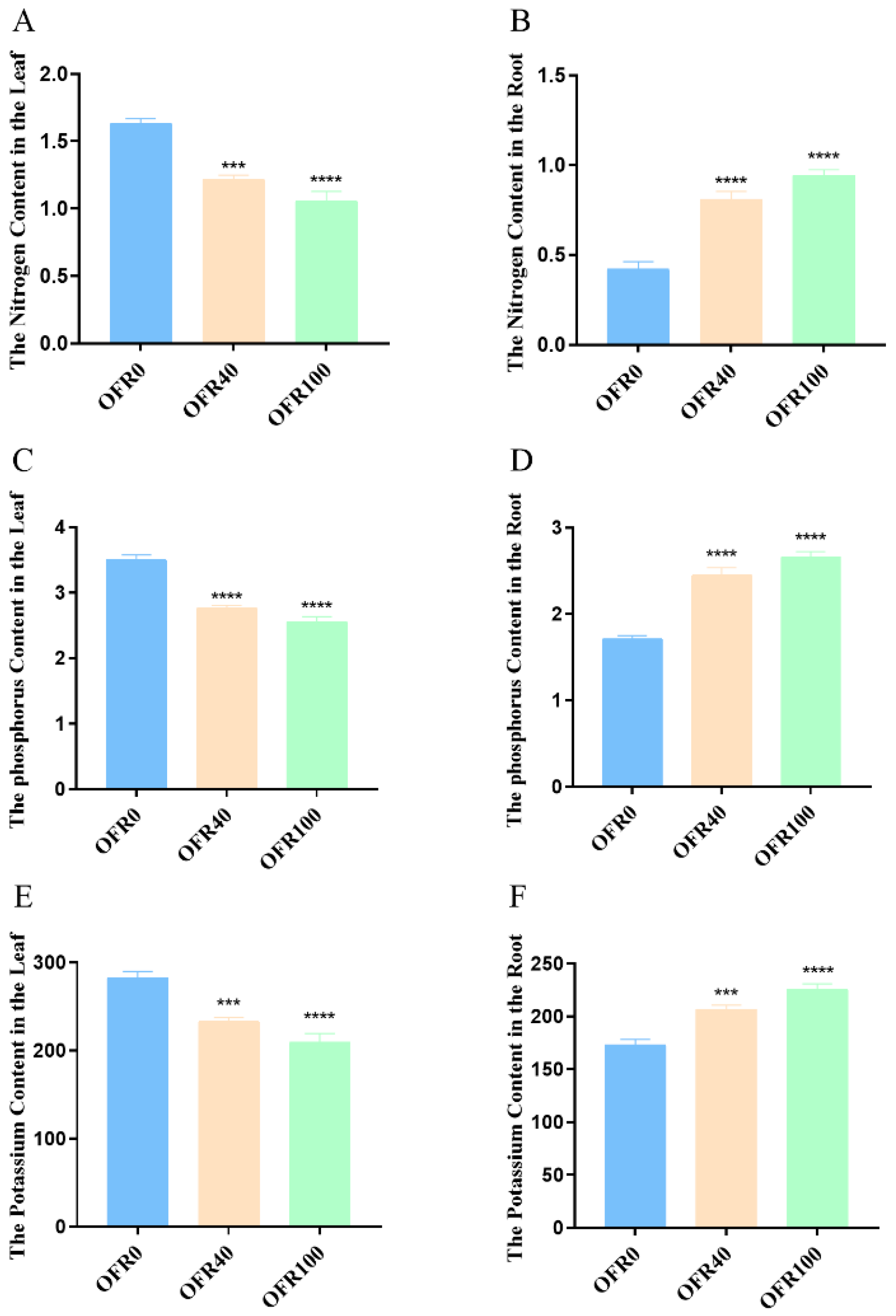
3.2. Nutrient Uptake
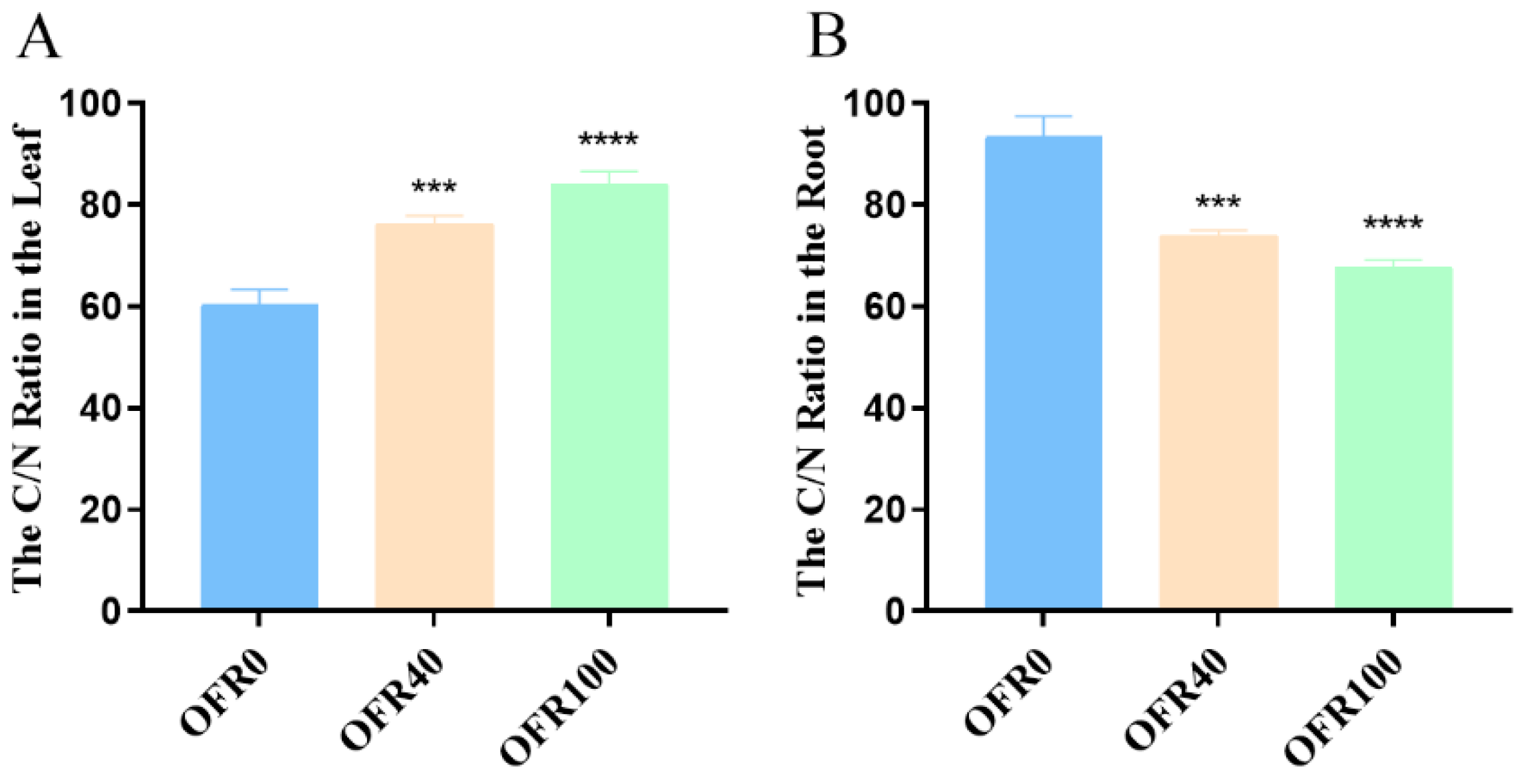
3.3. C/N Ratio
3.4. Transcriptome Analysis
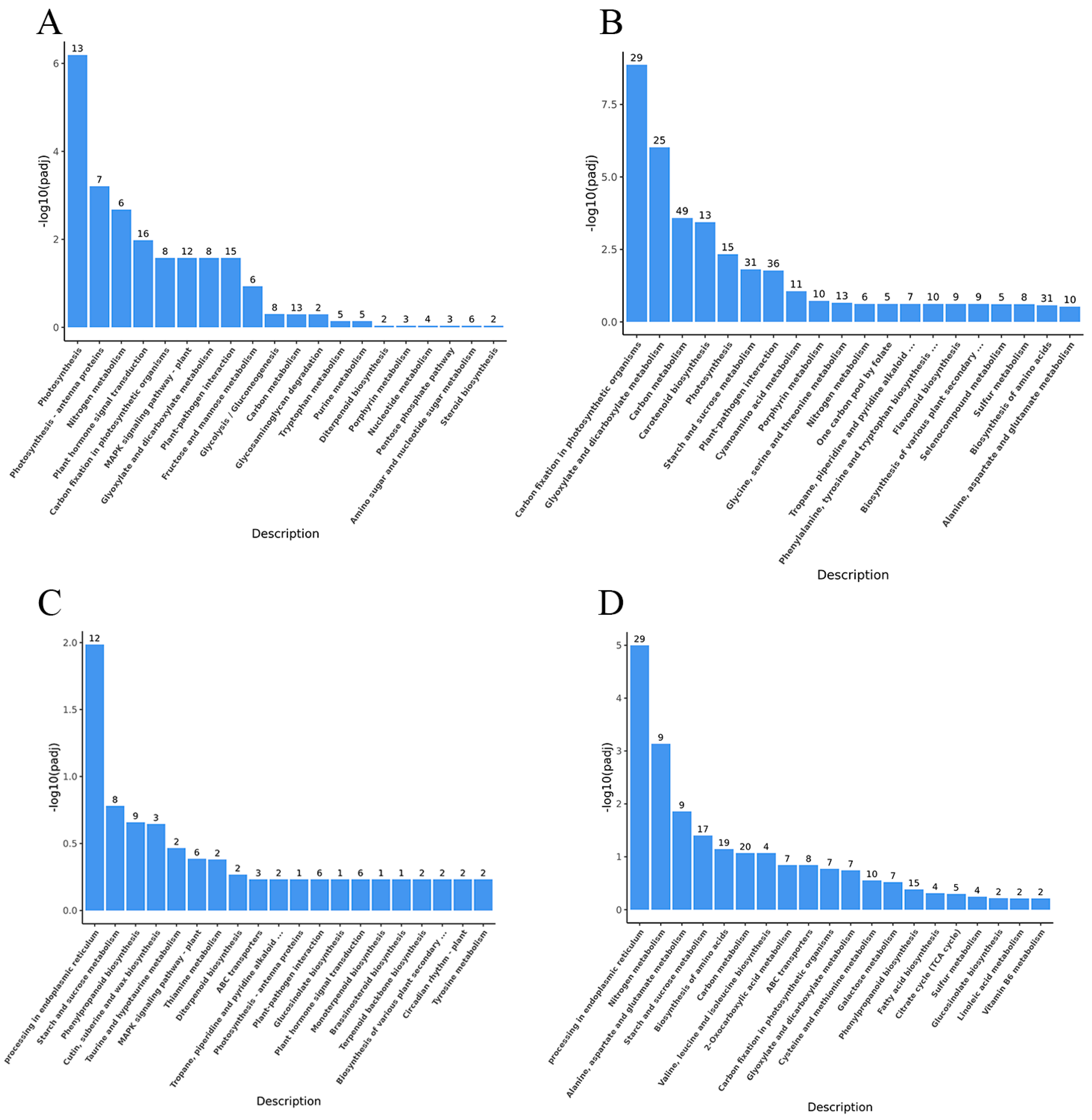
3.5. Metabolomic Insights
3.6. Integrated Analysis of the Transcriptome and Metabolome
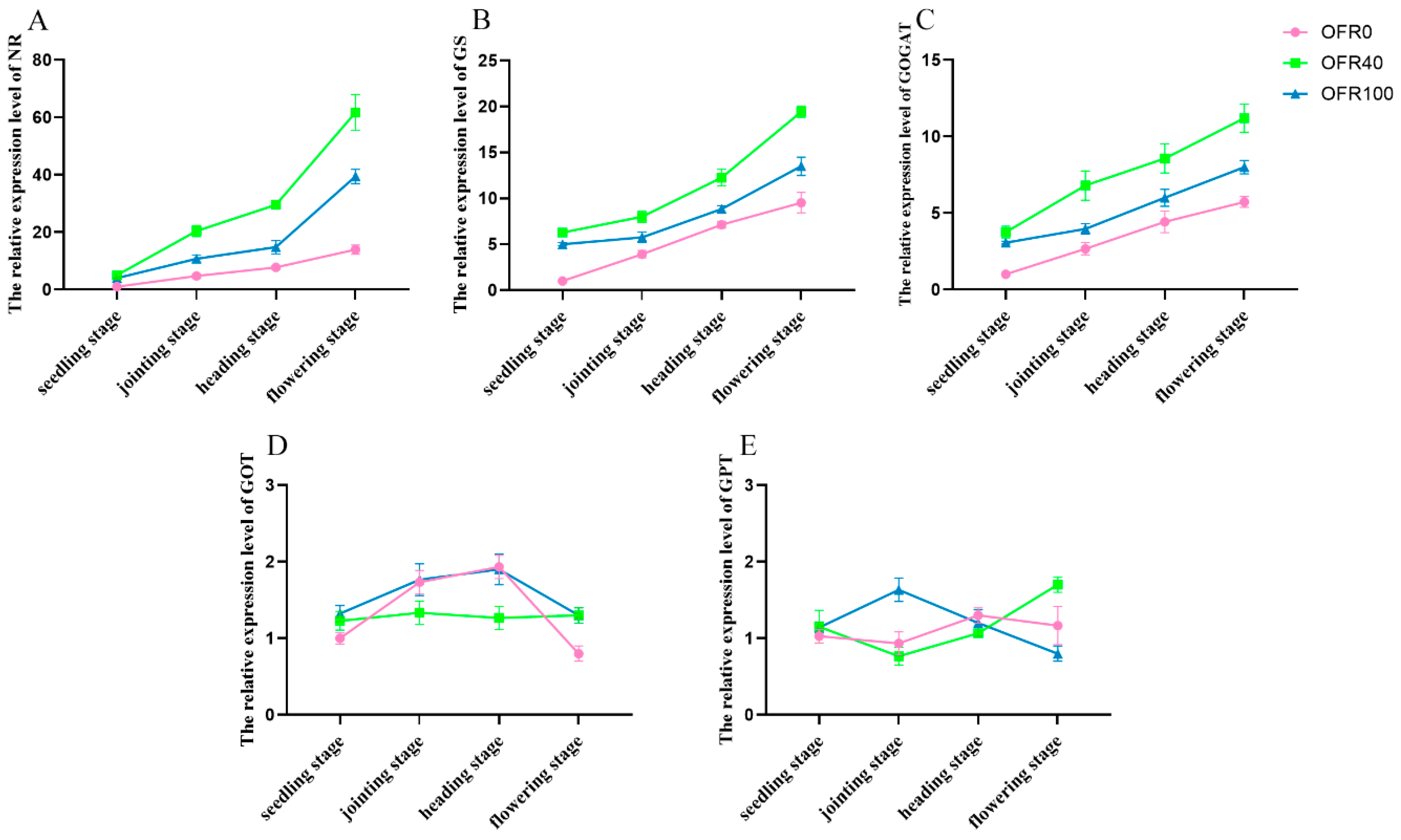
3.7. Nitrogen Metabolism Gene Expression
3.8. Nitrogen Metabolism Enzyme Activities
4. Discussion
4.1. Benefits of Organic Fertilizers
4.2. Root Plasticity and Nutrient Uptake
4.3. Implications for Sustainable Agriculture
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zörb, C.; Ludewig, U.; Hawkesford, M.J. Perspective on Wheat Yield and Quality with Reduced Nitrogen Supply. Trends Plant Sci. 2018, 23, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.H.; Cao, G.J.; Wang, L.C.; Wang, S.H. Effects of equal chemical fertilizer substitutions with organic manure on yield, dry matter, and nitrogen uptake of spring maize and soil nitrogen distribution. PLoS ONE 2019, 14, e0219512. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xue, J.F.; Gao, Z.Q.; Xue, N.W.; Yang, Z.P. Response of yield increase for dryland winter wheat to tillage practice during summer fallow and sowing method in the Loess Plateau of China. J. Integr. Agric. 2018, 17, 817–825. [Google Scholar] [CrossRef]
- Da Costa, P.B.; Beneduzi, A.; de Souza, R.; Schoenfeld, R.; Vargas, L.K.; Passaglia, L.M.P. The effects of different fertilization conditions on bacterial plant growth promoting traits: Guidelines for directed bacterial prospection and testing. Plant Soil 2012, 368, 267–280. [Google Scholar] [CrossRef]
- Caris-Veyrat, C.; Amiot, M.J.; Tyssandier, V.; Grasselly, D.; Buret, M.; Mikolajczak, M.; Guilland, J.C.; Bouteloup-Demange, C.; Borel, P. Influence of organic versus conventional agricultural practice on the antioxidant microconstituent content of tomatoes and derived purees; Consequences on antioxidant plasma status in humans. J. Agric. Food Chem. 2004, 52, 6503–6509. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Medina-Remón, A.; Casals-Ribes, I.; Lamuela-Raventos, R.M. Is there any difference between the phenolic content of organic and conventional tomato juices? Food Chem. 2012, 130, 222–227. [Google Scholar] [CrossRef]
- Karuku, G.N.; Onwonga, R.N.; Chepkemoi, J.; Kathumo, V.M. Effects of tillage practices, cropping systems and organic inputs on soil nutrient content in Machakos County. Afr. J. Agric. Res. 2018, 13, 2618–2630. [Google Scholar] [CrossRef]
- Wang, X.K.; Wang, G.; Guo, T.; Xing, Y.Y.; Mo, F.; Wang, H.D.; Fan, J.L.; Zhang, F.C. Effects of plastic mulch and nitrogen fertilizer on the soil microbial community, enzymatic activity and yield performance in a dryland maize cropping system. Eur. J. Soil Sci. 2021, 72, 400–412. [Google Scholar] [CrossRef]
- Dominchin, M.F.; Verdenelli, R.A.; Berger, M.G.; Aoki, A.; Meriles, J.M. Impact of N-fertilization and peanut shell biochar on soil microbial community structure and enzyme activities in a Typic Haplustoll under different management practices. Eur. J. Soil Biol. 2021, 104, 103298. [Google Scholar] [CrossRef]
- Negi, Y.K.; Sajwan, P.; Uniyal, S.; Mishra, A.C. Enhancement in yield and nutritive qualities of strawberry fruits by the application of organic manures and biofertilizers. Sci. Hortic. 2021, 283, 110038. [Google Scholar] [CrossRef]
- Iqbal, A.; He, L.; Ali, I.; Yuan, P.L.; Khan, A.; Hua, Z.; Wei, S.Q.; Jiang, L.G. Partial Substation of Organic Fertilizer With Chemical Fertilizer Improves Soil Biochemical Attributes, Rice Yields, and Restores Bacterial Community Diversity in a Paddy Field. Front. Plant Sci. 2022, 13, 895230. [Google Scholar] [CrossRef] [PubMed]
- Adekiya, A.O.; Dahunsi, S.O.; Ayeni, J.F.; Aremu, C.; Aboyeji, C.M.; Okunlola, F.; Oyelami, A.E. Organic and in-organic fertilizers effects on the performance of tomato (Solanum lycopersicum) and cucumber (Cucumis sativus) grown on soilless medium. Sci. Rep. 2022, 12, 12212. [Google Scholar] [CrossRef]
- Wang, Z.H.; Yang, T.J.; Mei, X.L.; Wang, N.Q.; Li, X.G.; Yang, Q.S.; Dong, C.X.; Jiang, G.F.; Lin, J.; Xu, Y.C.; et al. Bio-organic fertilizer promotes pear yield by shaping the rhizosphere microbiome composition and functions. Microbiol. Spectr. 2022, 10, e03572-22. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Jin, N.; Wang, S.Y.; Li, J.W.; Meng, X.; Xie, Y.D.; Wu, Y.; Luo, S.L.; Lyu, J.; Yu, J.H. Changes in the microbial structure of the root soil and the yield of chinese baby cabbage by chemical fertilizer reduction with bio-organic fertilizer application. Microbiol. Spectr. 2022, 10, e0121522. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yan, J.; Wang, P.; Wang, H. Impacts of climate change on the reclamation of farmers and herdsmen in the Tibetan Plateau. Acta Ecol. Sin. 2019, 39, 3655–3669. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Superfine grinding improves functional properties and antioxidant capacities of bran dietary fibre from Qingke (hull-less barley) grown in Qinghai-Tibet Plateau, China. J. Cereal Sci. 2015, 65, 43–47. [Google Scholar] [CrossRef]
- Idehen, E.; Tang, Y.; Sang, S.M. Bioactive phytochemicals in barley. J. Food Drug Anal. 2017, 25, 148–161. [Google Scholar] [CrossRef]
- Weng, H.; Yan, J.H.; Guo, L.Z.; Chen, H.Y. Integrated transcriptomic and metabolomic analyses of the molecular mechanisms of two highland barley genotypes with pyroxsulam responses. Front. Plant Sci. 2022, 13, 1030578. [Google Scholar] [CrossRef]
- Sha, A.; Liu, B.; Liu, C.; Sun, Q.; Chen, M.; Peng, L.; Zou, L.; Zhao, C.; Li, Q. Highland barley ELNs and physiological responses to different concentrations of Cr (VI) stress. Ecotoxicol. Environ. Saf. 2024, 288, 117379. [Google Scholar] [CrossRef]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis; Soil Science Society of America: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Twine, J.R.; Williams, C.H. The determination of phosphorus in Kjeldahl digests of plant material by automatic analysis. In Communications in Soil Science and Plant Analysis; Taylor and Francis Ltd.: Milton Park, UK, 1971. [Google Scholar]
- Moreno-Pedraza, A.; Gabriel, J.; Treutler, H.; Winkler, R.; Vergara, F. Effects of water availability in the soil on tropane alkaloid production in Cultivated Datura stramonium. Metabolites 2019, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Y.; Jin, Y.; Kan, L.; Shen, C.; Malladi, A.; Nambeesan, S.; Xu, Y.; Dong, C. Transcriptome Analysis of Pyrus betulaefolia Seedling Root Responses to Short-Term Potassium Deficiency. Int. J. Mol. Sci. 2020, 21, 8857. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Jozefczuk, S.; Klie, S.; Catchpole, G.; Szymanski, J.; Cuadros-Inostroza, A.; Steinhauser, D.; Selbig, J.; Willmitzer, L. Metabolomic and transcriptomic stress response of Escherichia coli. Mol. Syst. Biol. 2010, 6, 364. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-G.; Chen, L.-P.; Yan, W.; Jia, L.; Xu, G.-L.; Tian, L. High temperature at grain-filling stage affects nitrogen metabolism enzyme activities in grains and grain nutritional quality in rice. Rice Sci. 2011, 18, 210–216. [Google Scholar] [CrossRef]
- Nie, W.; Gong, B.; Wen, D.; Qiao, P.; Guo, H.; Shi, Q. Brassinosteroid Enhances Cucumber Stress Tolerance to NaHCO3 by Modulating Nitrogen Metabolism, Ionic Balance and Phytohormonal Response. Plants 2024, 14, 80. [Google Scholar] [CrossRef]
- Yu, X.Z.; Zhang, F.Z. Activities of nitrate reductase and glutamine synthetase in rice seedlings during cyanide metabolism. J. Hazard. Mater. 2012, 225–226, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Sui, J.; Zhang, J. Metabolomics reveals significant variations in metabolites and correlations regarding the maturation of walnuts (Juglans regia L.). Biol. Open 2016, 5, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Liu, Z.X.; Wang, S.S.; Xue, B.; Li, R.D.; Geng, Y.; Yang, T.H.; Li, Y.L.; Dong, H.J.; Luo, Z.H.; Tao, W.K.; et al. Emergy-based indicators of the environmental impacts and driving forces of non-point source pollution from crop production in China. Ecol. Indic. 2021, 121, 107023. [Google Scholar] [CrossRef]
- Velthof, G.; Rietra, R. Nitrous Oxide Emission from Agricultural Soils; 1566–7197; Wageningen Environmental Research: Wageningen, The Netherlands, 2018. [Google Scholar]
- He, L.L.; Zhong, Z.K.; Yang, H.M. Effects on soil quality of biochar and straw amendment in conjunction with chemical fertilizers. J. Integr. Agric. 2017, 16, 704–712. [Google Scholar] [CrossRef]
- Pu, R.F.; Wang, P.P.; Guo, L.P.; Li, M.H.; Cui, X.M.; Wang, C.X.; Liu, Y.; Yang, Y. The remediation effects of microbial organic fertilizer on soil microorganisms after chloropicrin fumigation. Ecotoxicol. Environ. Safe 2022, 231, 113188. [Google Scholar] [CrossRef] [PubMed]
- Lazcano, C.; Zhu-Barker, X.; Decock, C. Effects of Organic Fertilizers on the Soil Microorganisms Responsible for N2O Emissions: A Review. Microorganisms 2021, 9, 983. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.K.; Fan, J.L.; Xing, Y.Y.; Xu, G.C.; Wang, H.D.; Deng, J.; Wang, Y.F.; Zhang, F.C.; Li, P.; Li, Z.B. The Effects of mulch and nitrogen fertilizer on the soil environment of crop plants. Adv. Agron. 2019, 153, 121–173. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Li, B.S.; Zhang, J.L.; Christie, P.; Li, X.L. Organic fertilizer application and Mg fertilizer promote banana yield and quality in an Udic Ferralsol. PLoS ONE 2020, 15, e0230593. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Song, F.; Yin, Z.H.; Chen, P.; Zhang, Z.X.; Qi, Z.J.; Wang, B.; Zheng, E.N. Organic fertilizer substitutions maintain maize yield and mitigate ammonia emissions but increase nitrous oxide emissions. Environ. Sci. Pollut. Res. 2023, 30, 53115–53127. [Google Scholar] [CrossRef]
- He, H.; Peng, M.W.; Lu, W.D.; Hou, Z.N.; Li, J.H. Commercial organic fertilizer substitution increases wheat yield by improving soil quality. Sci. Total Environ. 2022, 851, 158132. [Google Scholar] [CrossRef]
- Den Herder, G.; Van Isterdael, G.; Beeckman, T.; De Smet, I. The roots of a new green revolution. Trends Plant Sci. 2010, 15, 600–607. [Google Scholar] [CrossRef]
- Giehl, R.F.H.; Gruber, B.D.; von Wirén, N. It’s time to make changes: Modulation of root system architecture by nutrient signals. J. Exp. Bot. 2014, 65, 769–778. [Google Scholar] [CrossRef]
- York, L.M.; Nord, E.A.; Lynch, J.P. Integration of root phenes for soil resource acquisition. Front. Plant Sci. 2013, 4, 355. [Google Scholar] [CrossRef]
- Linkohr, B.I.; Williamson, L.C.; Fitter, A.H.; Leyser, H.M.O. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J. 2002, 29, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of root system architecture by DEEPER ROOTING1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef]
- Liu, M.; Linna, C.; Ma, S.; Ma, Q.; Song, W.; Shen, M.; Song, L.; Cui, K.; Zhou, Y.; Wang, L. Biochar combined with organic and inorganic fertilizers promoted the rapeseed nutrient uptake and improved the purple soil quality. Front. Nutr. 2022, 9, 997151. [Google Scholar] [CrossRef]
- Sun, J.B.; Li, W.B.; Li, C.Q.; Chang, W.J.; Zhang, S.Q.; Zeng, Y.B.; Zeng, C.Y.; Peng, M. Effect of different rates of nitrogen fertilization on crop yield, soil properties and leaf physiological attributes in banana under subtropical regions of China. Front. Plant Sci. 2020, 11, 613760. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.; Upadhyay, A.; Kulshreshtha, S. Influence of organic amendments on soil properties, microflora and plant growth. Sustain. Agric. Rev. 2021, 52, 147–191. [Google Scholar] [CrossRef]
- Mikula, K.; Izydorczyk, G.; Skrzypczak, D.; Mironiuk, M.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Controlled release micronutrient fertilizers for precision agriculture—A review. Sci. Total Environ. 2020, 712, 136365. [Google Scholar] [CrossRef] [PubMed]



| Treatment | Nutrient Content in Organic Fertilizer (mg·kg−1) | Nutrient Content in Chemical Fertilizer (mg·kg−1) | ||||
|---|---|---|---|---|---|---|
| N | P2O5 | K2O | N | P2O5 | K2O | |
| OFR0 | 0 | 0 | 0 | 164.5 | 185.5 | 70 |
| OFR40 | 65.8 | 12.28 | 8.94 | 98.7 | 173.2 | 61.06 |
| OFR100 | 164.5 | 30.72 | 22.52 | 0 | 154.78 | 47.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Weng, H.; Hou, L.; Yao, Q.; He, T. Transcriptome and Metabolome Analysis of Organic and Chemical Fertilizer Effects on Highland Barley Growth and Nutrient Utilization. Agronomy 2025, 15, 380. https://doi.org/10.3390/agronomy15020380
Yan J, Weng H, Hou L, Yao Q, He T. Transcriptome and Metabolome Analysis of Organic and Chemical Fertilizer Effects on Highland Barley Growth and Nutrient Utilization. Agronomy. 2025; 15(2):380. https://doi.org/10.3390/agronomy15020380
Chicago/Turabian StyleYan, Jiahui, Hua Weng, Lu Hou, Qiang Yao, and Tao He. 2025. "Transcriptome and Metabolome Analysis of Organic and Chemical Fertilizer Effects on Highland Barley Growth and Nutrient Utilization" Agronomy 15, no. 2: 380. https://doi.org/10.3390/agronomy15020380
APA StyleYan, J., Weng, H., Hou, L., Yao, Q., & He, T. (2025). Transcriptome and Metabolome Analysis of Organic and Chemical Fertilizer Effects on Highland Barley Growth and Nutrient Utilization. Agronomy, 15(2), 380. https://doi.org/10.3390/agronomy15020380






