Abstract
Pesticides, essential for controlling pests and weeds, significantly boost agricultural productivity. However, their excessive use leads to substantial contamination of environmental matrices, including soil and water. Organophosphorus compounds, which constitute more than 30% of the global use of insecticides and herbicides, are particularly concerning, and their widespread application raises alarms among environmentalists and regulatory agencies due to their high toxicity to aquatic organisms. Therefore, to avoid the spread of these compounds within the environment, the contaminated sites may be treated with various methods. This study explored a soil detoxification procedure utilizing gaseous ozone. As a representative of organophosphorus pesticides, chlorfenvinphos was utilized as soil contaminant. This compound is still reported to occur in a number of environmental matrixes. The method used in this study involved the exposure of the soil matrix in a fluidized state to an ozone-enriched atmosphere. The ozonation procedure enabled the removal of the pesticide from the soil matrix. During its oxidation, some degradation products were detected; in particular, they included 2,4-dichlorobenzoic acid and 2-chloro-1-(2,4-dichloro-phenyl)-ethanone, whose presence was confirmed by a GC-MS system and the NIST database. However, they also underwent degradation. Moreover, on the basis of stereoselective reaction of Z and E isomers, the pesticide degradation pathway was proposed. Additionally, the efficacy of this detoxication method was evaluated using a combination of chronic and acute toxicity tests, employing Eisenia foetida earthworms as bioindicators. On the basis of the obtained results, it can be concluded that organophosphorus herbicides containing unsaturated bonds in their structure, including glyphosate, can be removed using this method.
1. Introduction
The rapid increase in the world population results in pressure being exerted to increase the quantity of food products, which in turn has an impact on agriculture. For the protection of various plant products, several insecticides and herbicides were developed and are applied worldwide [1,2]. Among the pesticides commonly used in agriculture, more than 30% are organophosphorus compounds [3]. This group of compounds may include pesticides such as chlorfenvinphos insecticide or the glyphosate herbicide. Substances from this group of pesticides can be toxic to fish and other aquatic animals [4].
Organophosphorus compounds can cause damage to some plants or land animals that were not the original target of those pesticides. Furthermore, as a result of their wide use in agriculture, there is an increased concentration of these compounds in groundwater and other water bodies [4]. Human exposure to these compounds can cause various health effects, including hearing loss or even cancerogenic effects [5,6].
A number of these agents have already been banned for use in agriculture due to side effects on nontarget animals and plants. However, there are still places where soil and water contamination occur. Furthermore, it was observed that in the last decade, residues from this pesticide group, in particular chlorfenvinphos, were still detected in beeswax in Italy [7].
Chlorfenvinphos was mainly used to control pests on plantations and in horticulture, but it could also be utilized for the control of vector borne diseases in areas of high population density. Insecticides from this group were used both in agriculture and by veterinarians for the control of the population of insects such as flies, fleas, and mites in livestock and house animals [8]. Some of the treatment procedures that have been utilized include the use of emulsions to control pests on plants such as Lucerne and potatoes. But they can also be applied using topically sprays, dressings, and baths for cattle, sheep, horses, deer, goats, and dogs in order to kill external parasites.
Chlorfenvinphos toxicity is based on its attack on the nervous system, i.e., by inhibiting the activity of acetylcholinesterase [9]. Humans may be affected by contact with contaminated environmental media, consumption of food, or contact with animals’ fur [10]. It enters the environment through runoff and leaching from hazardous waste sites. Then, it can be present in the soil within the underground water. Furthermore, it can be transported to surface water [10,11].
Chlorfenvinphos in soil was reported to have a half-life ranging from 28 to 210 days [12]. It is mainly degraded by microorganisms. The efficacy of this process can be improved by adding organic fertilizers, for example, pig slurry, cow manure, municipal waste, or mushroom cultivation composts. Such a treatment causes an increase in the insecticide degradation rate, which could be observed by comparison of the half-life of chlorfenvinphos with and without the addition of various fertilizers [10].
Other transformations of chlorfenvinphos can occur due to hydrolysis. This can be catalyzed by H+, Ca2+, Na+, and K+ from the mono-ionic kaolinite and bentonite present in clays. However, it was indicated that the chlorfenvinphos hydrolysis process is less important compared to degradation mediated by microorganisms (National Registration Authority, 2000). This observation is a result of experiments conducted under natural conditions in loamy sands (pH 7.2; 1.6% of organic carbon) and the fertile layer of soil (pH 6.5; 27.8% of organic carbon) [10].
Chlorfenvinphos is degraded in soil to trichloroacetophenone, 2,4-dichloroacetophenone, α-(chloromethyl)-2,4-dichlorobenzyl alcohol, and 1-(2′,4′-dichlorophenyl)-ethan-1-ol (chlorfen toxicity). Also, 2,4-dichlorobenzoic acid, 2-hydroxy-4-chlorobenzoic acid, and 2,4-dihydroxybenzoic acid were identified as metabolites of this compound. All of the mentioned degradation products do not possess the initial properties of their mother compound. The main degradation product of chlorfenvinphos is trichloroacetophenone. This metabolite can undergo further transformation, and, as a result of hydrolysis, oxidation, and decarboxylation, 2,4-dichlorobenzoic acid is generated. Another transformation of trichloroacetophenone is based on its reduction to α-(chloromethyl)-2,4-dichlorobenzyl alcohol. During that process, the chlorine atom is replaced with a hydrogen atom and two products, 2,4-dichloroacetophenone and 1(-2′,4′-dichlorophenyl)-ethan-1-ol, are generated. The transformation of trichloroacetophenone through this pathway is a slow process. Other chlorfenvinphos metabolites, such as 2-hydroxy-4-chlorobenzoic acid and 2,4-dihydroxybenzoic acid, are generated by replacing chlorine atoms with hydroxyl groups in 2,4-dichlorobenzoic acid [10].
Various experiments on chlorfenvinphos degradation in a water matrix by means ozonation, adsorption, Fenton and photo-Fenton, photoelectrocatalysis, and electrochemical advanced oxidation processes were conducted [2,13,14,15,16]. There is a literature gap on the remediation of chlorfenvinphos-contaminated soil with ozone.
Therefore, the goal of this study was to investigate the effectiveness of the ozone-based method for remediation of soil contaminated by chlorfenvinphos. In this method, the contaminated soil is directly exposed to gaseous ozone. This method allows for countering the toxicity effects in roots by the ozone treatment of contaminated soil matrices before any leaching to water resources may occur, and it is capable of removing pesticides from the soil matrix directly at the place of elevated concentrations. The effectiveness of the remediation was verified by monitoring the reduction in the pesticide concentration, which was complemented by tests of acute and chronic toxicity using earthworms as a biomarker [17]. The toxicity tests were necessary to observe the final effect of soil detoxication on the condition of soil organisms.
Chlorfenvinphos was selected as a representative of organophosphorus pesticides characterized by the presence of phosphorus atoms and unsaturated bonds. These structural elements are found in other organophosphate pesticides, such as, e.g., the herbicide glyphosate’s tautomers. Therefore, the method can be potentially applied for dealing with a variety of insecticides and herbicides belonging to different chemical group, e.g., glyphosate or linuron.
2. Materials and Methods
2.1. Reagents
The chlorfenvinphos utilized during our research was purchased from Yick-Vic Chemicals & Pharmaceuticals (H.K.) Ltd. (Hong Kong, China). Methylene chloride (p.a.) and anhydrous magnesium sulfate (p.a.) were purchased from Chempur (Piekary Śląskie, Poland). Acetone (p.a.) was purchased from Honeywell (Warszawa, Poland).
2.2. Analytical Devices
For the determination of the concentration of pesticides and the detection of their degradation products in the soil matrix, a Varian GC-450 chromatograph coupled with a mass spectrometer MS-240 (Santa Clara, CA, USA) was utilized. The chlorfenvinphos analysis was carried out on a capillary column 30 × 0.25 × 0.39−L (m) × ID (mm) × OD (mm), stationary phase: VF-17ms, with a film thickness of 0.25 μm. The temperature of the column oven was 50–300 °C, with a 10 °C/min temperature gradient (20 min isotherm at 300 °C). The injector 1079 PTV was used (split ratio 1:5), the temperature of injection was 200 °C, and the injection volume was 1 μL. The MS detector settings were as follows: scan mode: 50–500 m/z; gas flow rate: 1 mL/min (He) [17].
2.3. Soil Matrix Preparation
The soil used in the pesticide degradation and toxicity tests was artificially composed of sand, silt, and organic matter according to OECD standards [18,19]. Properties of the soil matrix were determined in earlier research by [17,20,21] and included parameters such as grain size distribution, pH, organic matter content, and nitrates content. The soil was spiked with a pesticide solution in acetone. The solution was mixed within the soil sample, and it was allowed to dry before the experiment.
2.4. Quarter-Technical-Scale Fluidized Bed Reactor
For pesticide degradation, laboratory- and quarter-technical-scale fluidized bed reactors (reactor cross section was 15 cm) were utilized. The capacity of the reactors was 200 g for the laboratory-scale reactor and 3 kg for quarter-technical-scale reactor. A more detailed description can be found elsewhere [17]. Within the reactor chamber, a wire net was placed allowing for holding the soil particles in place, while the netting size allowed for gas transfer through the soil matrix which resulted in a fluidized state of the soil exposed to the gaseous ozone-enriched atmosphere.
2.5. Extraction, Calibration, and Degradation Procedures
2.5.1. Extraction of Pesticides and GC-MS Analysis
To extract pesticides from the soil, 10 g soil samples were treated with 10 mL acetone for 1 h. The extract was separated by filtration through a filter of medium density (Chempur, Poland), and residues were extracted again with 5 mL of acetone for 1 h. Then, after the second filtration step, both extracts were mixed together. The obtained extract was placed into a separator flask, and 50 mL of distilled H2O, 3 mL of saturated NaCl solution, and 7 mL of methylene chloride were added. The mixture was shaken for 5 min. After separation of phases, another 7 mL portion of fresh methylene chloride was added to the aqueous layer, and the shaking process was repeated. The organic phases were combined and evaporated in a vacuum evaporator at 60 °C at a pressure of 0.4 bar. The residue was dissolved in 2 mL of acetone and dried over anhydrous sodium sulfate (VI). The resulting solution was injected for GC-MS analysis. The analyses were conducted with the Varian GC-450 gas chromatograph coupled with the mass spectrometer MS-240 (Santa Clara, CA, USA). During the analysis, the electron ionization method at 70 eV in full scan mode in the range of 5–500 m/z was used. The observed pesticide degradation products were identified with the NIST database.
2.5.2. Detector Calibration
To quantify the response of the detector within the expected range of pesticide concentrations in the soil, a series of soil samples were prepared by spiking pesticides at known concentration. This was achieved by mixing the soil with a solution of a pesticide in acetone. The samples were then left for 24 h to let the pesticide adhere to the matrix. After 24 h, the pesticides present in the soil were extracted using the methodology described above. The response was investigated in the range between 0.001 and 0.2% w/w of the pesticide in the soil. The calibration curves obtained from the peak areas were linear, and the determination coefficient for all the compounds was higher than 0.99. Moreover, before each experiment, a calibration was conducted to check for changes in the detector’s response. The estimated limit of quantification (LOQ) was 1 mg/kg, and the limit of detection (LOD) was 0.03 mg/kg.
2.5.3. Investigation of Possibility of Pesticide Desorption
To exclude the possibility of the impact of pesticide reduction due to desorption from the soil surface in the fluidized bed reactor, additional control measurements were performed. For this purpose, a soil sample was spiked with the pesticide, and then it was subjected to fluidization without the addition of ozone. The experiment was carried out for 96 h. The pesticide content in the soil sample was measured in different time intervals using the procedure described in Section 2.5.1. Next, the ozonation experiments were conducted.
2.5.4. Degradation Procedures in Laboratory-Scale Fluidized Bed Reactor
At the first stage, the experiments were conducted in a laboratory-scale fluidized bed reactor. For this purpose, 200 g of soil was contaminated with chlorfenvinphos, and the concentration was 0.05% w/w, and the contaminated soil was placed in the laboratory-scale reactor chamber. The soil was then exposed to the ozone stream (the ozone concentration was 10 ppm, and the gas flow rate was 1.08 m3/h). To monitor the course of the remediation process, soil samples were acquired in regular time intervals until the reduction in pesticide content ceased, maintaining at a constant level.
2.6. Scaling up of the Soil Detoxication Technology from Laboratory to Quarter-Technical Scale
The positive results of the tests conducted in the laboratory scale reactor allowed for the scale-up of the developed method. The linear scale transfer was achieved by enlarging the reactor cross section and the surface of the sieve supporting the bed. The ratio of the weight of the beads to the surface of the sieve was similar, i.e., 10 and 11, for the laboratory- and technical-scale fluidized bed reactors.
2.7. Toxicity Tests
In this study, we utilized acute and chronic toxicity tests based on [18,19] with the modification proposed by [17]. Exposition of the Eisenia foetida biomarker to was conducted to verify the toxicity of chlorfenvinphos-contaminated soil towards soil biota before and after the utilization of an ozone-based detoxification procedure. Soil tests were conducted according to procedures described in [17] concerning the detoxication of linuron herbicide-contaminated soils. For the purpose of toxicity tests, artificially prepared soil was utilized (Section 2.3). The use of artificial soil was dictated by the fact that the necessary proportions of mineral and organic fractions could be selected in a controlled manner, mimicking the natural habitat of soil organisms. The use of native soils was not recommended because their composition is unstable and site-dependent, which would have made it difficult to standardize the tests. In particular, the selection of the soil matrix was mainly based on the recommendations of the OECD that allowed for bioassays to be carried out.
In particular, the acute toxicity test was performed by the determination of earthworms’ survivability in the control, contaminated, and ozonated soil samples. Chronic toxicity tests involved determination of biochemical parameters such as neutral red retention time (NRR-t) and coelomocyte count which was introduced as a pesticide contamination biomarker by [17]. During the trials conducted, the effectiveness of the soil remediation method used for the detoxication of chlorfenvinphos-contaminated soil was positively verified. Research on the removal of pesticides from the soil and research using test organisms were conducted between 2014 and 2022 in a laboratory at the University of Rzeszow.
3. Results
3.1. Pesticide Desorption Investigation
To determine the impact of the desorption process on the process of pesticide removal, the soil contaminated with chlorfenvinphos at a concentration of 0.05% w/w (sum of isomers) was exposed to fluidization in the air stream (Section 2.5.3).
It was observed that the insecticide concentration remained at a constant level throughout the whole time of the experiment. This indicated that the pesticide did not desorb from the soil.
3.2. Kinetics of Pesticide Degradation
In the next step, the kinetics of the degradation of chlorfenvinphos in the contaminated soil was measured in the laboratory-scale fluidized bed reactor. The measurements carried out are presented in Figure 1a,b.
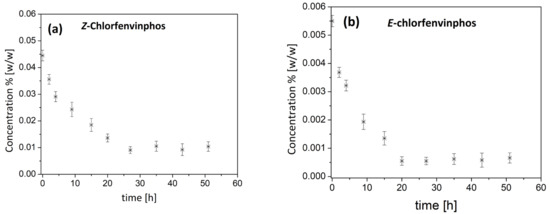
Figure 1.
(a,b). Course of the degradation of (a) Z-chlorfenvinphos and (b) E-chlorfenvinphos in the laboratory scale fluidized bed reactor. The results obtained are presented as a mean value and ± SD from three independent experiments.
After the preliminary success, the process was scaled up. The resulting course of pesticide degradation during the ozonation procedure in the quarter-technical-scale reactor is presented in Figure 2a,b.
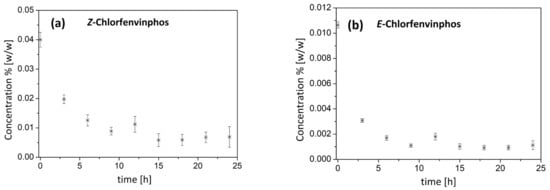
Figure 2.
(a,b). Course of the degradation of (a) Z-chlorfenvinphos and (b) E-chlorfenvinphos during the ozonation procedure in the quarter-technical-scale reactor. The results obtained are presented as mean values and ± SD from three independent experiments.
From Figure 1 and Figure 2, it can be concluded that the efficacy of pesticide reduction depended on the particular isomer type and the reactor construction. To quantitatively describe the kinetics of pesticide degradation with ozone, a model of simple-first-order kinetics was used. The choice of this description was proven by analysis of the ln C/C0 relationship. We detected a high linear relationship of this factor , which was verified using the determination coefficient R2 value. On the basis of the results presented above, the kinetic parameters of chlorfenvinphos degradation could be estimated. The results obtained are summarized in Table 1.

Table 1.
Degradation t ½ of the chlorfenvinphos isomers (E- and Z-chlorfenvinphos) in soil exposed to the air and ozone mixture and in soil during natural degradation.
3.3. Results of Toxicity Tests
Aside from the chemical changes, the main aspects of the conducted investigations were the toxicity assays based on the utilization of earthworms. As presented in Figure 3a–d, chlorfenvinphos toxicity was verified by acute and chronic toxicity tests. The tests included a survivability test, NRR-t assay, body weight measurement, and the number and ratio of celomocytes.
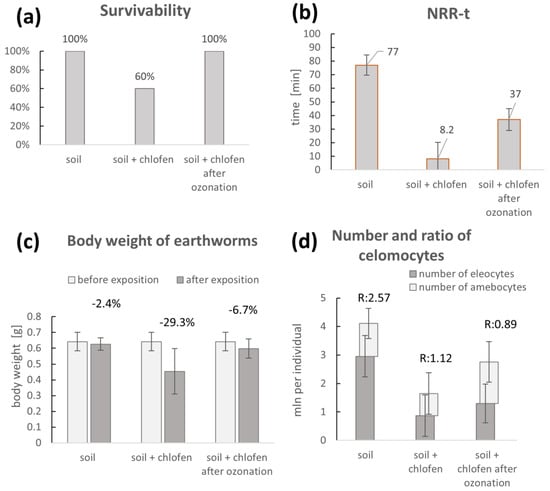
Figure 3.
(a–d). Chronic and acute toxicity results on physical and biochemical parameters of earthworms exposed to the chlorfenvinphos in the soil before and after ozonation.
4. Discussion
4.1. Discussion of the Results of Chlorfenvinphos Degradation in the Soil Matrix
The analysis of the determined kinetics data presented above indicated that the t ½ of the E-chlorfenvinphos isomer is different than the Z-chlorfenvinphos isomer. During the degradation procedures that were performed in the laboratory- and quarter-technical-scale reactors, the Z-chlorfenvinphos isomer was more stable than the E-chlorfenvinphos isomer. On the basis of that observation, we hypothesized that the main pathway of the chlorfenvinphos transformation was the attack of ozone molecules on the unsaturated bond. This is a logical conclusion, since the steric hindrance may hinder the addition of an ozone molecule to the Z-chlorfenvinphos isomer. This effect might be less important in the case of the E-chlorfenvinphos isomer.
The analysis of the detected pesticide degradation products allowed for the conclusion to be drawn that this process can be accompanied by other reactions, e.g., degradation of the ester bond due to the impact of ozone or other reactive individua that are generated from ozone.
Based on the analysis of the pesticide degradation products that were detected with the GC-MS analysis of soil sample extracts acquired in both the laboratory- and quarter-technical-scale degradation experiments, we suggested the following path of pesticide degradation (Figure 4).
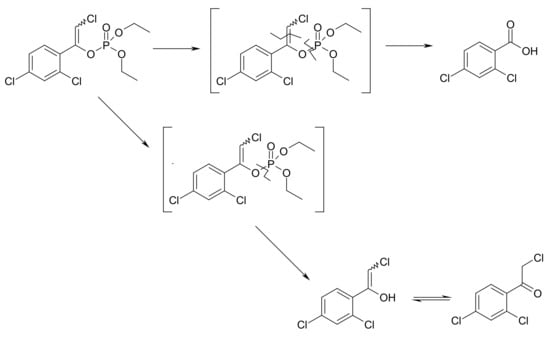
Figure 4.
Pathway of the chlorfenvinphos degradation in soil during ozonation.
The presence of degradation products, such as 2,4-dichloro-benzoic acid and 2-chloro-1-(2,4-dichloro-phenyl)-ethanone, was confirmed by a GC-MS system and NIST database with 90% probability of detection of a particular compound. Degradation products, such as 2,4-dichlorobenzoic acid and its chlorinated derivative, may be caused by the degradation of impurities present in the chlorfenvinphos standard. Uygun observed similar chlorfenvinphos degradation pathways during the storage of carrots [22]. It should be noted that some of the microorganisms, especially fungi, could generate very active enzymatic systems like ligninolytic enzymes, which can degrade very persistent compounds including organophosphorus pesticides utilizing Fenton-like reagents [23]. Fenton-like reagents generate hydroxyl radicals that can react with organic compounds giving analogous degradation products as can be detected during ozonation. The ozonation method, however, provides a fast, safe, and residue-free procedure. Furthermore, its much faster the bioremediation procedures.
Crucial from the scientific point of view is not only the determination of the degradation kinetic parameters but also the susceptibility of organophosphorus pesticides to the degradation method and the determination of pathways for their potential degradation. Particularly important is the discovery of the stereoselectivity of the process, which clearly shows that the limiting step in the process is the attack on the unsaturated bond, which determines the occurrence of the Z and E isomers of chlorfenvinphos. On the basis of the analysis of the degradation products, it was found that the ester bonds that occur directly at the phosphorus atom are also susceptible to the degradation process, which may be useful in predicting the susceptibility to degradation of other compounds belonging to this group. Previously prepared work had failed to discover these phenomena [17].
It should be noted that the maximum degradation level achieved is less than 100%. This is due to the fact that ozone access in the fluid phase is favored over chlorfenvinphos particles on the surface of soil particles. As a heterogeneous material, the soil may consist of highly viscous liquids that absorb chlorfenvinphos, thus limiting ozone access. The concentration of the detected degradation products was very low compared to the initial concentration of the pesticide in the soil. Furthermore, they were also degraded, and no further pesticide degradation products were detected.
4.2. Discussion of the Toxicity Tests
The toxicity tests revealed that the earthworms exposed to the contaminated soil were characterized by lower survivability (Figure 3a). Furthermore, biochemical indicators such as neutral red retention time (NNR-t) showed significant effects on the stability of lysosomes without contamination and before or after treatment of the contaminated soil with ozone. In particular, it was concluded that the retention time of neutral red was much worse in the case of organisms exposed to the toxic factor compared to control organisms. Furthermore, after the detoxication procedure, this parameter of the condition of the earthworms improved (Figure 3b). Another parameter of the earthworm condition that was investigated during the toxicity trials was bodyweight, which was, in general, worse in the case of the exposure of earthworms to contaminated soil in comparison to other conditions, i.e., control soil and contaminated soil after detoxication (Figure 3c). Additionally, the number of coelomocytes was determined in the test organisms exposed to the control, contaminated, and ozonated–contaminated soil. The results of those tests showed that exposure of organisms to contaminated soil caused a reduction in the total count of coelomocytes compared to the control soil. Another observation was the reduction and change in the ratio of coelomocytes, such as eleocytes and amebocytes, in organisms exposed to control, pesticide-contaminated, and ozone-treated contaminated soil (Figure 3d).
5. Conclusions
The experiments conducted in this study revealed that the gaseous ozone causes oxidation of chlorfenvinphos in contaminated soil, which led to pesticide degradation. The stereoisomers of chlorfenvinphos showed different reactivity in the ozone atmosphere, which affected the efficacy of the ozonation procedure. The results of the laboratory and quarter-technical-scale soil ozonation experiments were used to determine parameters of the ozonation kinetics and the degradation products. The reaction kinetics followed a simple-first-order kinetics model with t ½ between 2 and 11 h.
The GC-MS analysis of the degradation products revealed the presence of degradation products such as 2,4-dichloro-benzoic acid and 2-chloro-1-(2,4-dichloro-phenyl)-ethanone. On the basis of the analysis, the degradation pathways of chlorfenvinphos isomers were proposed.
The analysis of the detoxication process efficacy was supported with the toxicity test, for which Eisenia feoetida test organisms were utilized. It was observed that acute toxicity expressed as the percentage of survivability of earthworms, in control soil and chlorfenvinphos-contaminated soil before and after ozonation, indicated a 40% survival ratio in contaminated soil and a 100% survival ratio in control and contaminated soil after the detoxication procedure. The test organisms which survived the exposition to contaminated and contaminated and then ozonated soil samples indicated changes in physical and biochemical parameters. In particular, the body weight, NRR-t parameter, and count of coelomocytes were affected which showed a generally better shape of worms exposed to detoxicated soil in comparison to pesticide-spiked soil before the ozonation procedure. On the basis of those observations, it was concluded that the toxicity of soil after ozonation was significantly lower than in contaminated soil. The results achieved proved that the goal of this study was accomplished; the method used matched its objectives. It allowed for a significant reduction in the pesticide content in the soil matrix and improved living conditions for soil organisms. It can also be anticipated that other organophosphorus insecticides and herbicides containing unsaturated bonds in their structure, including glyphosate tautomers, could be removed using this method.
Author Contributions
Conceptualization, P.A.; Methodology, R.J.; Formal analysis, P.A.; Investigation, P.A. and B.S.; Resources, P.A. and M.B.; Writing—original draft, P.A.; Writing—review and editing, P.A. and K.K.; Visualization, M.B., R.J., and K.K. All authors have read and agreed to the published version of this manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in this article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fu, H.; Tan, P.; Wang, R.; Li, S.; Liu, H.; Yang, Y.; Wu, Z. Advances in organophosphorus pesticides pollution: Current status and challenges in ecotoxicological, sustainable agriculture, and degradation strategies. J. Hazard. Mater. 2022, 424 Pt B, 127494. [Google Scholar] [CrossRef]
- Mora-Gómez, J.; Escribá-Jiménez, S.; Carrillo-Abad, J.; García-Gabaldón, M.; Montañés, M.T.; Mestre, S.; Pérez-Herranz, V. Study of the chlorfenvinphos pesticide removal under different anodic materials and different reactor configuration. Chemosphere 2022, 290, 133294. [Google Scholar] [CrossRef]
- Oladiran, A.T.; Olusakin, O.P.; Ajibade, O.C.; Akinsola, G.O. Organophosphorus pesticides: Impacts, detection and removal strategies. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100655. [Google Scholar] [CrossRef]
- Roselló-Márquez, G.; Fernández-Domene, R.M.; García-Antón, J. Organophosphorus pesticides (chlorfenvinphos, phosmet and fenamiphos) photoelectrodegradation by using WO3 nanostructures as photoanode. J. Electroanal. Chem. 2021, 894, 115366. [Google Scholar] [CrossRef]
- Sun, H.; Sun, M.L.; Barr, D.B. Exposure to organophosphorus insecticides and increased risks of health and cancer in US women. Environ. Toxicol. Pharmacol. 2020, 80, 103474. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Huang, M.; Zhang, J.; Chen, R. Exploring the effects and mechanisms of organophosphorus pesticide exposure and hearing loss. Front. Public Health 2022, 11, 1001760. [Google Scholar] [CrossRef] [PubMed]
- Perugini, M.; Serena, M.R.; Zezza, T.D.; Fenucci, S.; Conte, A.; Amorena, M. Occurrence of agrochemical residues in beeswax samples collected in Italy during 2013–2015. Sci. Total Environ. 2018, 625, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Biziuk, M. Pestycydy: Występowanie, Oznaczanie i Unieszkodliwianie; Wydawnictwo Naukowo-Techniczne: Warszawa, Poland, 2001; pp. 229–240. [Google Scholar]
- Čadež, T.; Kolić, D.; Šinko, G. Assessment of four organophosphorus pesticides as inhibitors of human acetylcholinesterase and butyrylcholinesterase. Sci. Rep. 2021, 11, 21486. [Google Scholar] [CrossRef] [PubMed]
- National Registration Authority for Agricultural and Veterinary Chemicals. Chlorfenvinphos Interim Report, Environmental Assessment; National Registration Authority for Agricultural and Veterinary Chemicals: Canberra, Australia, 2000. Available online: http://apvma.gov.au/sites/default/files/chlorfenvinphos-phase-6-interim-review-report-vol1.pdf (accessed on 1 January 2015).
- Dorsey, A.S.; Kueberuwa, S.S. Toxicological Profile for Chlorfenvinphos; U.S. Department of Health and Human Services-Public Health Service-Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 1997. [Google Scholar]
- Beynon, K.I.; Hutson, D.H.; Wright, A.N. The metabolism and degradation of vinyl phosphate insecticides. Residue Rev. 1973, 47, 55–142. [Google Scholar] [PubMed]
- Acero, J.L.; Real, F.J.; Benitez, F.J.; González, A. Oxidation of chlorfenvinphos in ultrapure and natural waters by ozonation and photochemical processes. Water Res. 2008, 42, 3198–3206. [Google Scholar] [CrossRef] [PubMed]
- Klamerth, N.; Gernjak, W.; Malato, S.; Agüera, A.; Lendl, B. Photo-Fenton decomposition of chlorfenvinphos: Determination of reaction pathway. Water Res. 2009, 43, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Alves, A.; Madeira, L.M. Treatment of water networks (waters and deposits) contaminated with chlorfenvinphos by oxidation with Fenton’s reagent. Chem. Eng. J. 2014, 241, 190–199. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Roselló-Márquez, G.; Sánchez-Tovar, R.; Lucas-Granados, B.; García-Antón, J. Photoelectrochemical removal of chlorfenvinphos by using WO3 nanorods: Influence of annealing temperature and operation pH. Sep. Purif. Technol. 2019, 212, 458–464. [Google Scholar] [CrossRef]
- Józefczyk, R.; Antos, P.; Pieniążek, M.; Balawejder, M. Procedure for detoxication of linuron contaminated soil based on ozonation and fluidization process. Arch. Environ. Prot. 2022, 48, 48–56. [Google Scholar] [CrossRef]
- OECD. Test No. 207: Earthworm, Acute Toxicity Tests (Eisenia fetida/Eisenia Andrei). In OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, Frence, 1984. [Google Scholar] [CrossRef]
- OECD. Test No. 222: Earthworm Reproduction Test (Eisenia fetida/Eisenia Andrei). In Guideline for Testing of Chemicals; OECD Publishing: Paris, France, 2004; Available online: https://www.oecd.org/env/ehs/testing/Draft-Updated-TestGuildeline-222-Earthworm-reproduction-Test.pdf (accessed on 1 January 2016).
- Balawejder, M.; Antos, P.; Pieniążek, M.; Józefczyk, R.; Piątkowski, W. Metoda remediacji gleby skażonej DDT i ocena skuteczności procesu z wykorzystaniem organizmów testowych. Inżynieria I Apar. Chem. 2014, 53, 219–220. [Google Scholar]
- Balawejder, M.; Józefczyk, R.; Antos, P.; Pieniążek, M. A method for remediation of soil contaminated with simazine. Arch. Environ. Prot. 2016, 42, 41–46. [Google Scholar] [CrossRef][Green Version]
- Uygun, U. Degradation of chlorfenvinphos in carrots during storage. Food Chem. 1997, 60, 459–702. [Google Scholar] [CrossRef]
- Wu, D.; Ren, H.; Xie, L.; Zhang, G.; Zhao, Y.; Wei, Z. Strengthening Fenton-like reactions to improve lignocellulosic degradation performance by increasing lignocellulolytic enzyme core microbes during rice straw composting. Waste Manag. 2023, 161, 72–83. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).