Improved Phosphorus Bioavailability in Lettuce Crop via Naganishia albida Inoculation of Wastewater-Derived Struvite
Abstract
1. Introduction
2. Materials and Methods
2.1. Struvite Characterization
2.2. Inoculum Preparation
2.3. Phosphate Solubilization Capacity
2.4. Experimental Design and Conditions for Lettuce Growth
2.5. Plant Analysis
2.5.1. Biomass Production, Nitrogen, and Phosphorus Uptake
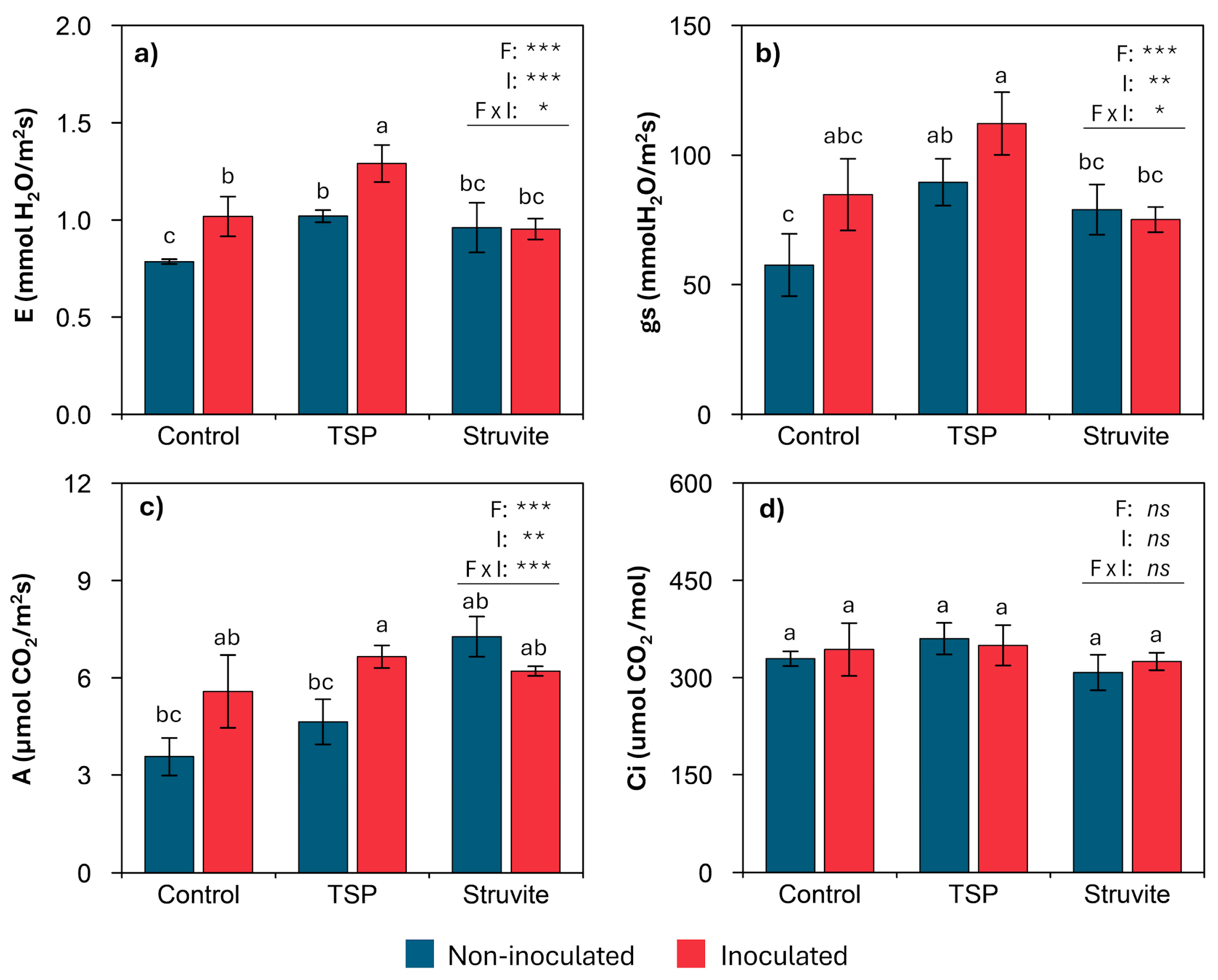
2.5.2. Photosynthetic Traits
2.5.3. Photosynthetic Pigments
2.5.4. Identification and Quantification of Phenolic Compounds and Antioxidant Capacity
2.6. Soil Analysis
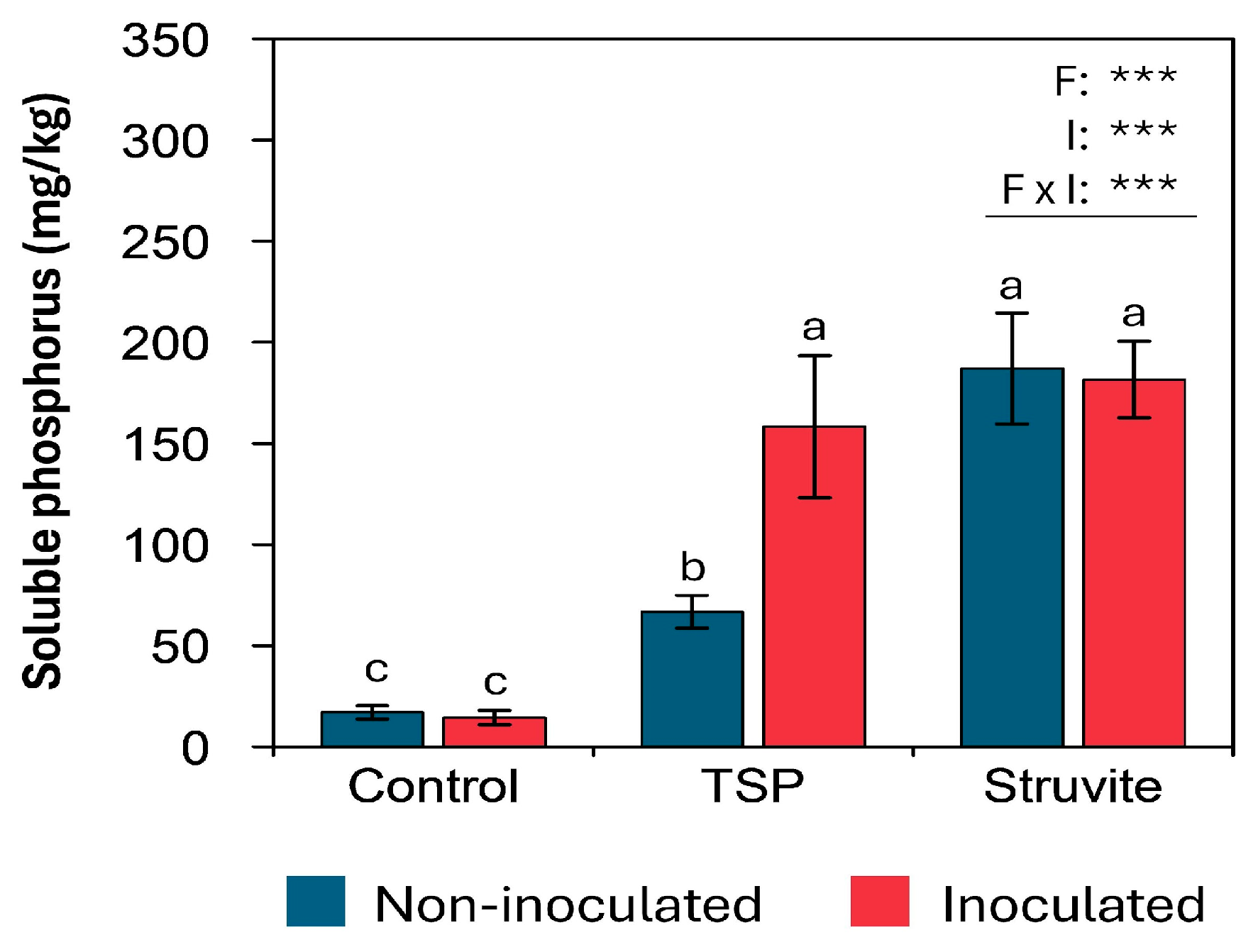
2.6.1. Available Phosphorus
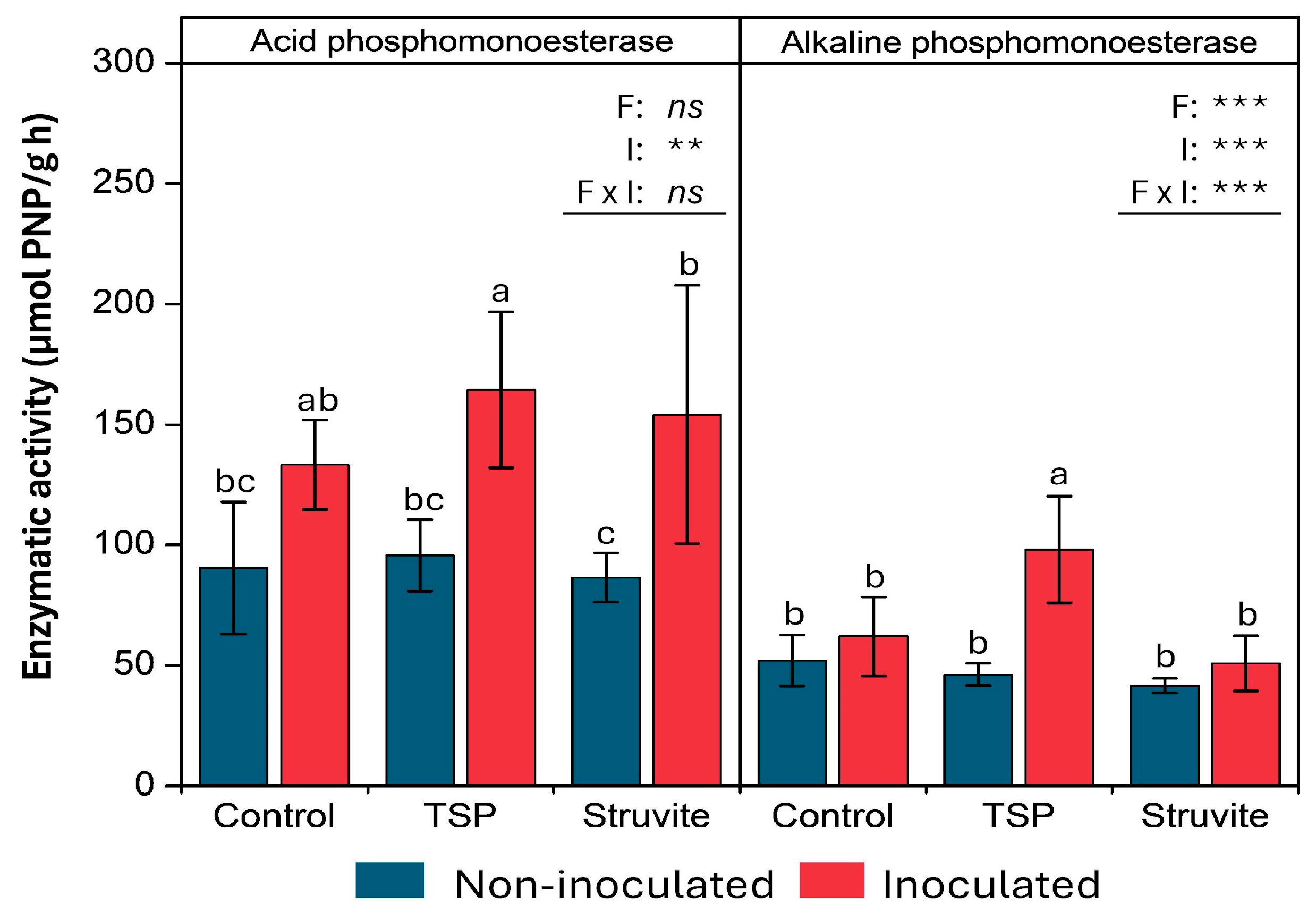
2.6.2. Enzymatic Activity
2.7. Statistical Analysis
3. Results
3.1. Phosphate Solubilization Capacity by Microorganisms
3.2. Biomass Production
3.3. Nitrogen and Phosphorus Uptake
3.4. Photosynthetic Traits and Pigments
3.5. Quantification of Phenolic Compounds and Antioxidant Capacity
3.6. Soluble Phosphorus and Soil Enzymatic Activity
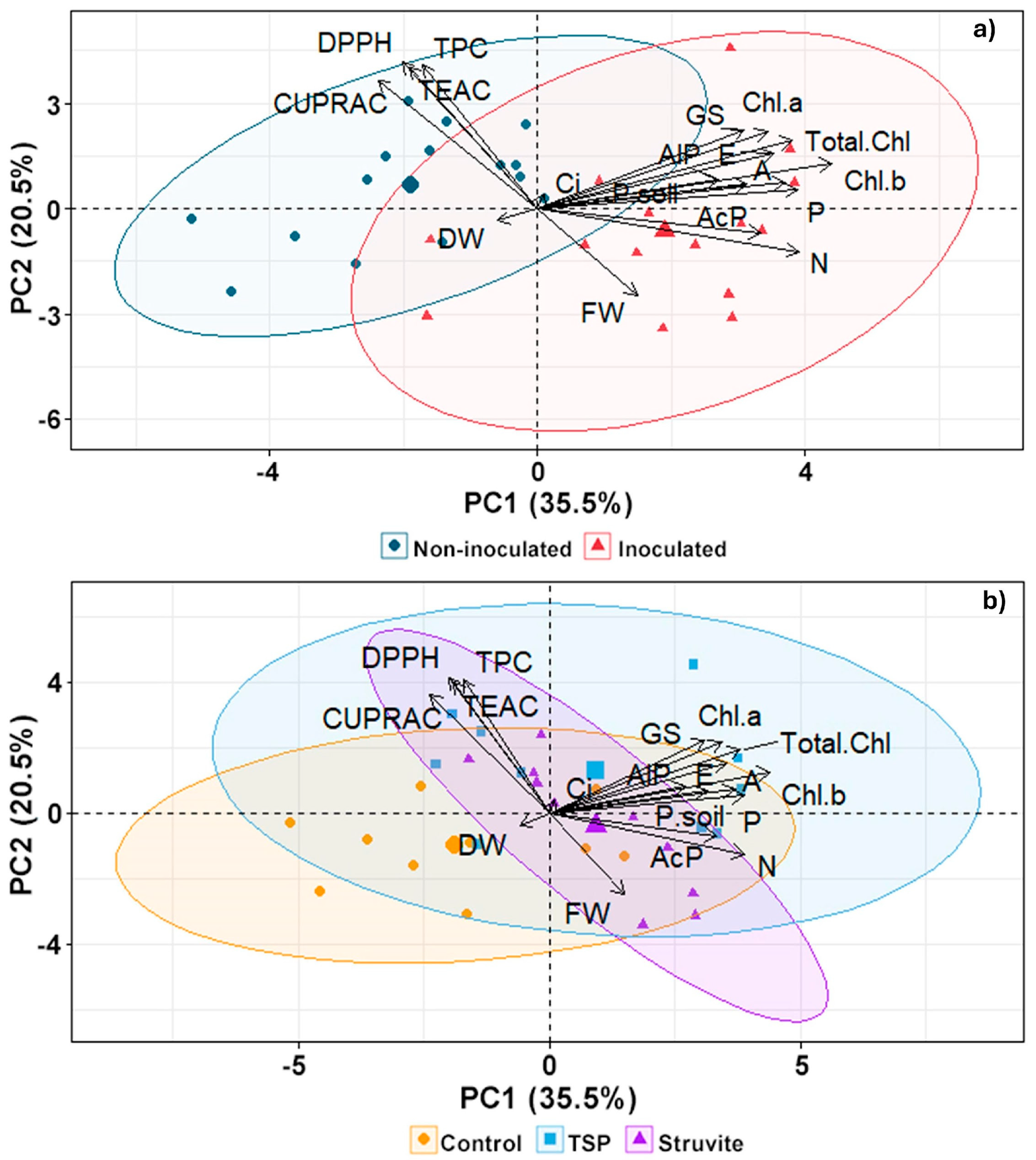
3.7. Multivariate Analysis
4. Discussion
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, L.I.d.; Pereira, M.C.; Carvalho, A.M.X.d.; Buttrós, V.H.; Pasqual, M.; Dória, J. Phosphorus-Solubilizing Microorganisms: A Key to Sustainable Agriculture. Agriculture 2023, 13, 462. [Google Scholar] [CrossRef]
- Zou, X.; Binkley, D.; Doxtader, K.G. A new method for estimating gross phosphorus mineralization and immobilization rates in soils. Plant Soil 1992, 147, 243–250. [Google Scholar] [CrossRef]
- Chowdhury, R.B.; Moore, G.A.; Weatherley, A.J.; Arora, M. Key sustainability challenges for the global phosphorus resource, their implications for global food security, and options for mitigation. J. Clean. Prod. 2017, 140, 945–963. [Google Scholar] [CrossRef]
- Cabeza, R.; Steingrobe, B.; Römer, W.; Claassen, N. Effectiveness of recycled P products as P fertilizers, as evaluated in pot experiments. Nutr. Cycl. Agroecosystems 2011, 91, 173–184. [Google Scholar] [CrossRef]
- Muys, M.; Phukan, R.; Brader, G.; Samad, A.; Moretti, M.; Haiden, B.; Pluchon, S.; Roest, K.; Vlaeminck, S.E.; Spiller, M. A systematic comparison of commercially produced struvite: Quantities, qualities and soil-maize phosphorus availability. Sci. Total Environ. 2021, 756, 143726. [Google Scholar] [CrossRef]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.L.; Withers, P.J.A. Struvite: A slow-release fertiliser for sustainable phosphorus management? Plant Soil 2016, 401, 109–123. [Google Scholar] [CrossRef]
- Hall, R.L.; Boisen Staal, L.; Macintosh, K.A.; McGrath, J.W.; Bailey, J.; Black, L.; Gro Nielsen, U.; Reitzel, K.; Williams, P.N. Phosphorus speciation and fertiliser performance characteristics: A comparison of waste recovered struvites from global sources. Geoderma 2020, 362, 114096. [Google Scholar] [CrossRef]
- Yang, Z.; Ferron, L.M.; Koopmans, G.F.; Sievernich, A.; van Groenigen, J.W. Nitrous oxide emissions after struvite application in relation to soil P status. Plant Soil 2023, 489, 523–537. [Google Scholar] [CrossRef]
- Pérez-Piqueres, A.; Ribó, M.; Rodríguez-Carretero, I.; Quiñones, A.; Canet, R. Struvite as a Sustainable Fertilizer in Mediterranean Soils. Agronomy 2023, 13, 1391. [Google Scholar] [CrossRef]
- Elhaissoufi, W.; Ghoulam, C.; Barakat, A.; Zeroual, Y.; Bargaz, A. Phosphate bacterial solubilization: A key rhizosphere driving force enabling higher P use efficiency and crop productivity. J. Adv. Res. 2022, 38, 13–28. [Google Scholar] [CrossRef]
- Raklami, A.; Babalola, O.O.; Jemo, M.; Nafis, A. Unlocking the plant growth-promoting potential of yeast spp.: Exploring species from the Moroccan extremophilic environment for enhanced plant growth and sustainable farming. FEMS Microbiol. Lett. 2024, 371, fnae015. [Google Scholar] [CrossRef] [PubMed]
- Alzandi, A.A.; Naguib, D.M. Effect of yeast application on soil health and root metabolic status of corn seedlings under drought stress. Arch. Microbiol. 2022, 204, 233. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Gholipour, A.R.; Delattre, C.; Sesdighi, F.; Seveiri, R.M.; Pasdaran, A.; Kheirandish, S.; Pierre, G.; Kozani, P.S.; Kozani, P.S. Production, characterization and biological activities of exopolysaccharides from a new cold-adapted yeast: Rhodotorula mucilaginosa sp. GUMS16. Int. J. Biol. Macromol. 2020, 151, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Perez-Moncada, U.A.; Santander, C.; Ruiz, A.; Vidal, C.; Santos, C.; Cornejo, P. Design of Microbial Consortia Based on Arbuscular Mycorrhizal Fungi, Yeasts, and Bacteria to Improve the Biochemical, Nutritional, and Physiological Status of Strawberry Plants Growing under Water Deficits. Plants 2024, 13, 1556. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2020, 21, 49–68. [Google Scholar] [CrossRef]
- Hernández Jiménez, J.E.; Nyiraneza, J.; Fraser, T.D.; Peach Brown, H.C.; Lopez-Sanchez, I.J.; Botero-Botero, L.R.; Naeth, M.A. Enhancing phosphorus release from struvite with biostimulants. Can. J. Soil Sci. 2021, 101, 22–32. [Google Scholar] [CrossRef]
- Fu, S.-F.; Sun, P.-F.; Lu, H.-Y.; Wei, J.-Y.; Xiao, H.-S.; Fang, W.-T.; Cheng, B.-Y.; Chou, J.-Y. Plant growth-promoting traits of yeasts isolated from the phyllosphere and rhizosphere of Drosera spatulata Lab. Fungal Biol. 2016, 120, 433–448. [Google Scholar] [CrossRef]
- CY, K.; SF, F.; Chou, F.; Chen, R.; Chou, J. Phosphate-solubilizing characteristics of yeasts. Mycosphere 2018, 9, 1117–1131. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Kuo, C.-Y.; Fu, S.-F.; Chou, J.-Y. Plant growth-promoting properties of the phosphate-solubilizing red yeast Rhodosporidium paludigenum. World J. Microbiol. Biotechnol. 2023, 39, 54. [Google Scholar] [CrossRef]
- Perez, R.; Tapia, Y.; Antilen, M.; Ruiz, A.; Pimentel, P.; Santander, C.; Aponte, H.; Gonzalez, F.; Cornejo, P. Beneficial Interactive Effects Provided by an Arbuscular Mycorrhizal Fungi and Yeast on the Growth of Oenothera picensis Established on Cu Mine Tailings. Plants 2023, 12, 4012. [Google Scholar] [CrossRef]
- Santander, C.; Gonzalez, F.; Perez, U.; Ruiz, A.; Aroca, R.; Santos, C.; Cornejo, P.; Vidal, G. Enhancing Water Status and Nutrient Uptake in Drought-Stressed Lettuce Plants (Lactuca sativa L.) via Inoculation with Different Bacillus spp. Isolated from the Atacama Desert. Plants 2024, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Jokkaew, S.; Jantharadej, K.; Pokhum, C.; Chawengkijwanich, C.; Suwannasilp, B.B. Free and Encapsulated Phosphate-Solubilizing Bacteria for the Enhanced Dissolution of Swine Wastewater-Derived Struvite—An Attractive Approach for Green Phosphorus Fertilizer. Sustainability 2022, 14, 2627. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Sniatala, B.; Al-Hazmi, H.E.; Sobotka, D.; Zhai, J.; Makinia, J. Advancing sustainable wastewater management: A comprehensive review of nutrient recovery products and their applications. Sci. Total Environ. 2024, 937, 173446. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, A.; O’Donnell, C.; Robles Aguilar, A.A.; Sigurnjak, I.; Power, N.; Michels, E.; Harrington, J.; Meers, E. Impact of time and phosphorus application rate on phosphorus bioavailability and efficiency of secondary fertilizers recovered from municipal wastewater. Chemosphere 2021, 282, 131017. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, E. The composition of the nutrient solution. In Sand and Water Culture Methods Used in the Study of Plant Nutrition; Commonwealth Agricultural Bureau: Farnham Royal, UK, 1966; Volume 190. [Google Scholar]
- Temminghoff, E.E.; Houba, V.J. (Eds.) Plant Analysis Procedures, 2nd ed.; Agricultural Sciences; Kluwer Academic Publishers: London, UK, 2004; Volume 5. [Google Scholar] [CrossRef]
- Sadzawka, A.; Carrasco, M.; Demanet, R.; Flores, H.; Grez, R.; Mora, M.; Neaman, A. Métodos de análisis de tejidos vegetales. Ser. Actas INIA 2007, 40, 140. [Google Scholar]
- Lichtenthaler, H.K. [34] Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. [Google Scholar] [CrossRef]
- Parada, J.; Valenzuela, T.; Gomez, F.; Tereucan, G.; Garcia, S.; Cornejo, P.; Winterhalter, P.; Ruiz, A. Effect of fertilization and arbuscular mycorrhizal fungal inoculation on antioxidant profiles and activities in Fragaria ananassa fruit. J. Sci. Food Agric. 2019, 99, 1397–1404. [Google Scholar] [CrossRef]
- Olsen, S.R.; Dean, L.A. Phosphorus. In Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1035–1049. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Vetrano, F.; Miceli, C.; Angileri, V.; Frangipane, B.; Moncada, A.; Miceli, A. Effect of bacterial inoculum and fertigation management on nursery and field production of lettuce plants. Agronomy 2020, 10, 1477. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Z.; Wu, G.; Wu, Q.; Zhang, F.; Niu, Z.; Hu, H.-Y. Characteristics of water quality of municipal wastewater treatment plants in China: Implications for resources utilization and management. J. Clean. Prod. 2016, 131, 1–9. [Google Scholar] [CrossRef]
- Rech, I.; Withers, P.J.A.; Jones, D.L.; Pavinato, P.S. Solubility, Diffusion and Crop Uptake of Phosphorus in Three Different Struvites. Sustainability 2018, 11, 134. [Google Scholar] [CrossRef]
- Consentino, B.B.; Aprile, S.; Rouphael, Y.; Ntatsi, G.; De Pasquale, C.; Iapichino, G.; Alibrandi, P.; Sabatino, L. Application of PGPB combined with variable N doses affects growth, yield-related traits, N-fertilizer efficiency and nutritional status of lettuce grown under controlled condition. Agronomy 2022, 12, 236. [Google Scholar] [CrossRef]
- Manzoor, M.; Abbasi, M.K.; Sultan, T. Isolation of phosphate solubilizing bacteria from maize rhizosphere and their potential for rock phosphate solubilization–mineralization and plant growth promotion. Geomicrobiol. J. 2017, 34, 81–95. [Google Scholar] [CrossRef]
- Sun, L.; Wei, B.; Wu, D.; Sun, K.; Jiao, J.; Zhang, W. The effects of struvite on biomass and soil phosphorus availability and uptake in Chinese cabbage, cowpea, and maize. Agronomy 2024, 14, 1852. [Google Scholar] [CrossRef]
- Degryse, F.; Baird, R.; da Silva, R.C.; McLaughlin, M.J. Dissolution rate and agronomic effectiveness of struvite fertilizers–effect of soil pH, granulation and base excess. Plant Soil 2017, 410, 139–152. [Google Scholar] [CrossRef]
- Ryu, H.-D.; Lim, C.-S.; Kang, M.-K.; Lee, S.-I. Evaluation of struvite obtained from semiconductor wastewater as a fertilizer in cultivating Chinese cabbage. J. Hazard. Mater. 2012, 221, 248–255. [Google Scholar] [CrossRef]
- Luan, J.; Fu, Y.; Tang, W.; Yang, F.; Li, X.; Yu, Z. Impact of Interaction between Biochar and Soil Microorganisms on Growth of Chinese Cabbage by Increasing Soil Fertility. Appl. Sci. 2023, 13, 12545. [Google Scholar] [CrossRef]
- Muhie, S.H. Optimization of photosynthesis for sustainable crop production. CABI Agric. Biosci. 2022, 3, 50. [Google Scholar] [CrossRef]
- Griebenow, S.; Zuniga-Feest, A.; Muñoz, G.; Cornejo, P.; Kleinert, A.; Valentine, A. Photosynthetic metabolism during phosphate limitation in a legume from the Mediterranean-type Fynbos ecosystem. J. Plant Physiol. 2019, 243, 153051. [Google Scholar] [CrossRef]
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus plays key roles in regulating plants’ physiological responses to abiotic stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef]
- Santander, C.; Ruiz, A.; García, S.; Aroca, R.; Cumming, J.; Cornejo, P. Efficiency of two arbuscular mycorrhizal fungal inocula to improve saline stress tolerance in lettuce plants by changes of antioxidant defense mechanisms. J. Sci. Food Agric. 2020, 100, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Vaneeckhaute, C.; Janda, J.; Vanrolleghem, P.A.; Filip, M.; Meers, E. Phosphorus use efficiency of bio-based fertilizers: Bioavailability and fractionation. Pedosphere 2016, 26, 310–325. [Google Scholar] [CrossRef]
- Janes-Bassett, V.; Blackwell, M.S.; Blair, G.; Davies, J.; Haygarth, P.M.; Mezeli, M.M.; Stewart, G. A meta-analysis of phosphatase activity in agricultural settings in response to phosphorus deficiency. Soil Biol. Biochem. 2022, 165, 108537. [Google Scholar] [CrossRef]
- Carrillo, V.; Castillo, R.; Magrí, A.; Holzapfel, E.; Vidal, G. Phosphorus recovery from domestic wastewater: A review of the institutional framework. J. Environ. Manag. 2024, 351, 119812. [Google Scholar] [CrossRef]


| Microorganisms | Phosphate Solubilization Index (PSI) | Phosphate Solubilization (mg/mL) | Final pH |
|---|---|---|---|
| Burkholderia caledonica | 1.52 ± 0.28 ab | 1079.17 ± 228.07 a | 6.4 ± 0.07 |
| Meyerozyma guilliermondii | 1.58 ± 0.16 a | 805.09 ± 131.99 b | 6.3 ± 0.02 |
| Naganishia albida | 1.40 ± 0.07 ab | 1186.57 ± 57.80 a | 5.6 ± 0.10 |
| Fresh Weight (g/Plant) | Dry Weight (g/Plant) | |||
|---|---|---|---|---|
| Treatment | Leaves | Roots | Leaves | Roots |
| Not inoculated | ||||
| Control | 35.35 ± 2.18 | 15.85 ± 1.40 | 4.76 ± 0.36 | 2.13 ± 0.21 |
| TSP | 36.42 ± 2.33 | 13.95 ± 0.55 | 4.58 ± 0.32 | 1.75 ± 0.10 |
| Struvite | 34.60 ± 3.13 | 12.13 ± 1.67 | 4.52 ± 0.38 | 1.59 ± 0.21 |
| Inoculated | ||||
| Control | 46.98 ± 4.02 | 18.91 ± 1.20 | 5.08 ± 0.19 | 2.07 ± 0.24 |
| TSP | 39.25 ± 1.72 | 14.46 ± 2.38 | 5.04 ± 0.20 | 1.84 ± 0.22 |
| Struvite | 44.46 ± 1.31 | 14.28 ± 0.62 | 4.47 ± 0.46 | 1.44 ± 0.21 |
| Significance | ||||
| Fertilization | ns | *** | * | *** |
| Inoculation | *** | ** | * | ns |
| Fertilization × Inoculated | *** | ns | ns | ns |
| Treatment | Nitrogen (mg/Plant) | Phosphorus (mg/Plant) | ||
|---|---|---|---|---|
| Leaves | Roots | Leaves | Roots | |
| Not inoculated | ||||
| Control | 41.03 ± 4.90 | 9.35 ± 1.15 | 29.82 ± 2.88 | 11.96 ± 1.48 |
| TSP | 47.27 ± 7.80 | 8.01 ± 0.75 | 34.67 ± 3.72 | 12.73 ± 2.34 |
| Struvite | 54.38 ± 8.73 | 8.18 ± 0.63 | 41.27 ± 3.97 | 14.73 ± 3.21 |
| Inoculated | ||||
| Control | 55.54 ± 3.76 | 9.64 ± 1.14 | 37.66 ± 2.86 | 10.50 ± 2.08 |
| TSP | 60.69 ± 2.49 | 8.74 ± 1.04 | 42.50 ± 3.86 | 14.75 ± 2.95 |
| Struvite | 74.62 ± 6.95 | 7.74 ± 1.18 | 44.41 ± 8.31 | 13.27 ± 2.88 |
| Significance | ||||
| Fertilization | *** | * | *** | ns |
| Inoculation | *** | ns | *** | ns |
| Fertilization × Inoculated | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo, V.; Pérez, R.; González, F.; Santander, C.; Ruiz, A.; Holzapfel, E.; Cornejo, P.; Vidal, G. Improved Phosphorus Bioavailability in Lettuce Crop via Naganishia albida Inoculation of Wastewater-Derived Struvite. Agronomy 2025, 15, 260. https://doi.org/10.3390/agronomy15020260
Carrillo V, Pérez R, González F, Santander C, Ruiz A, Holzapfel E, Cornejo P, Vidal G. Improved Phosphorus Bioavailability in Lettuce Crop via Naganishia albida Inoculation of Wastewater-Derived Struvite. Agronomy. 2025; 15(2):260. https://doi.org/10.3390/agronomy15020260
Chicago/Turabian StyleCarrillo, Valentina, Rodrigo Pérez, Felipe González, Christian Santander, Antonieta Ruiz, Eduardo Holzapfel, Pablo Cornejo, and Gladys Vidal. 2025. "Improved Phosphorus Bioavailability in Lettuce Crop via Naganishia albida Inoculation of Wastewater-Derived Struvite" Agronomy 15, no. 2: 260. https://doi.org/10.3390/agronomy15020260
APA StyleCarrillo, V., Pérez, R., González, F., Santander, C., Ruiz, A., Holzapfel, E., Cornejo, P., & Vidal, G. (2025). Improved Phosphorus Bioavailability in Lettuce Crop via Naganishia albida Inoculation of Wastewater-Derived Struvite. Agronomy, 15(2), 260. https://doi.org/10.3390/agronomy15020260







